The Synthesis and Evaluation of RGD−Conjugated Chitosan Gel as Daily Supplement for Body Weight Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the RGD−CT Gel
2.2. Fourier-Transform Infrared (FTIR) Spectrometry
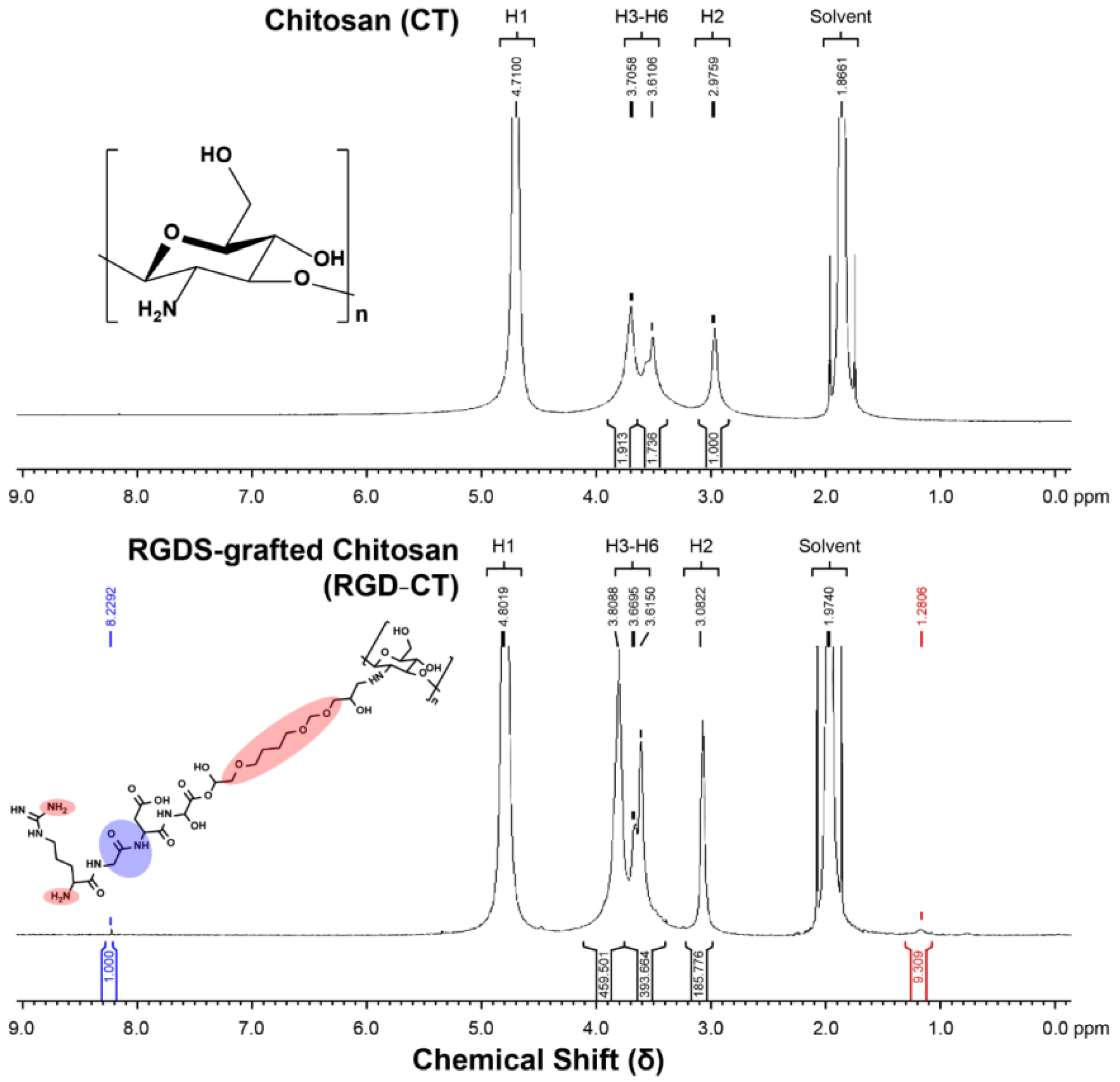
2.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.4. RGD Functionality Test
2.5. Ninhydrin Test
2.6. WST-1 Assay for Cell Proliferation
2.7. Live and Dead Cells Staining
2.8. Dose Optimization
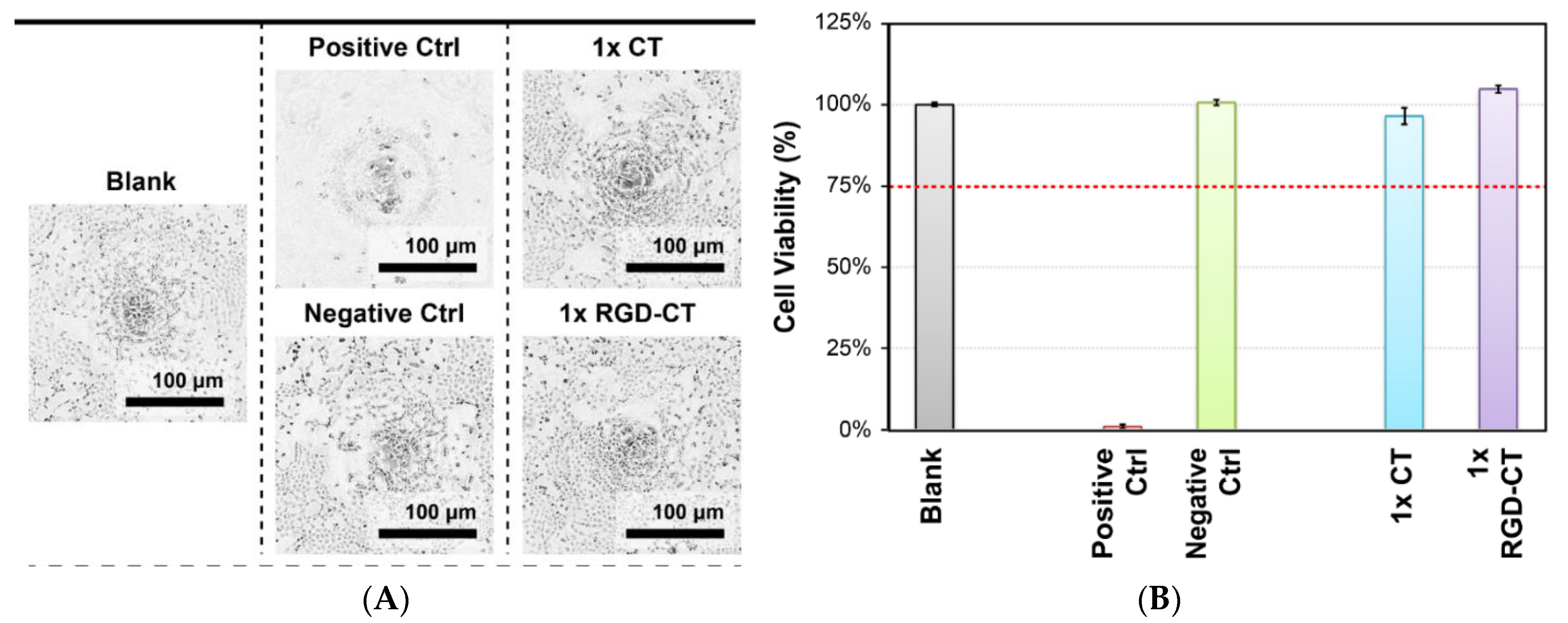
2.9. Cell Adhesion Test
2.10. Animal Model
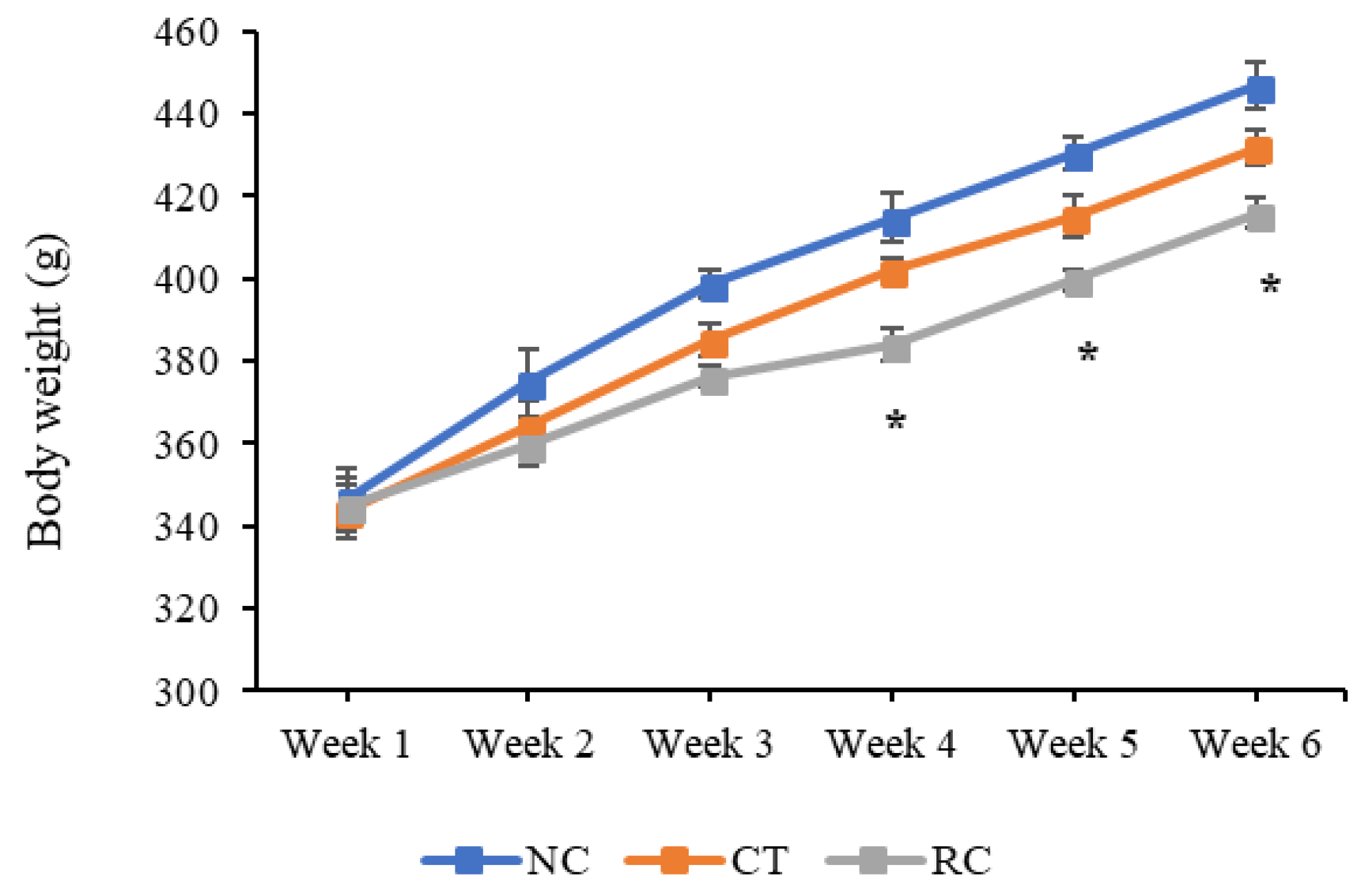
2.11. Change in Body Weight
2.12. Serum Chemistry and Hematology
2.13. Percent Body Fat
2.14. Statistical Methods
3. Results
3.1. Material Analysis
3.2. In Vitro Studies
3.3. In Vivo Studies
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy balance and obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, E.A.; Khavjou, O.A.; Thompson, H.; Trogdon, J.G.; Pan, L.; Sherry, B.; Dietz, W. Obesity and severe obesity forecasts through 2030. Am. J. Prev. Med. 2012, 42, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Swainson, M.G.; Batterham, A.; Tsakirides, C.; Rutherford, Z.; Hind, K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 2017, 12, e0177175. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Hirsch, J.; Gallagher, D.; Leibel, R.L. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am. J. Clin. Nutr. 2008, 88, 906–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.; Still, C.D. Pharmacological management of obesity: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef]
- Haslam, D. Weight management in obesity–past and present. Int. J. Clin. Pract. 2016, 70, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Betzel, B.; Cooiman, M.I.; Aarts, E.O.; Janssen, I.M.C.; Wahab, P.J.; Groenen, M.J.M.; Drenth, J.P.H.; Berends, F.J. Clinical follow-up on weight loss, glycemic control, and safety aspects of 24 months of duodenal-jejunal bypass liner implantation. Surg. Endosc. 2019, 34, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruban, A.; Ashrafian, H.; Teare, J.P. The EndoBarrier: Duodenal-Jejunal Bypass Liner for Diabetes and Weight Loss. Gastroenterol. Res. Pract. 2018, 2018, 7823182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, A.R.; Cho, S.-J.; Cho, Y.-Y.; Kwon, E.-Y.; Choi, J.-Y.; Lee, J.H.; Han, Y.; Kim, Y.S.; Piao, Z.; Shin, Y.C.; et al. Antiobesity Effects of Short-Chain Chitosan in Diet-Induced Obese Mice. J. Med. Food 2018, 21, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Onishi, H.; Machida, Y. Application of Chitin and Chitosan Derivatives in the Pharmaceutical Field. Curr. Pharm. Biotechnol. 2003, 4, 7. [Google Scholar] [CrossRef]
- Furda, I. Reduction of absorption of dietary lipids and cholesterol by chitosan and its derivatives and special formulation. In Chitosan per Os: From Dietary Supplement to Drug Carrier; Muzzarelli, R.A.A., Ed.; Atec: Grottammare, Italy, 2000; pp. 41–63. [Google Scholar]
- Kanauchi, O.; Deuchi, K.; Imasato, Y.; Shizukuishi, M.; Kobayashi, E. Mechanism for the inhibition of fat digestion by chitosan and for the synergistic effect of ascorbate. Biosci. Biotechnol. Biochem. 1995, 59, 786–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razdan, A.; Pettersson, D. Hypolipidaemic, gastrointestinal and related responses of broiler chickens to chitosans of different viscosity. Br. J. Nutr. 2007, 76, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, M.S.; Albertengo, L.E. Interaction between Chitosan and Oil under Stomach and Duodenal Digestive Chemical Conditions. Biosci. Biotechnol. Biochem. 2005, 69, 2057–2062. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Hong, H.; Matson, V.Z.; Javadi, A.; Xu, W.; Yang, Y.; Zhang, Y.; Engle, J.W.; Nickles, R.J.; Cai, W.; et al. Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and PET Imaging. Theranostics 2012, 2, 757–768. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef] [Green Version]
- Beysseriat, M.; Decker, E.A.; McClements, D.J. Preliminary study of the influence of dietary fiber on the properties of oil-in-water emulsions passing through an in vitro human digestion model. Food Hydrocoll. 2006, 20, 800–809. [Google Scholar] [CrossRef]
- Maschmeyer, I.; Lorenz, A.K.; Schimek, K.; Hasenberg, T.; Ramme, A.P.; Hübner, J.; Lindner, M.; Drewell, C.; Bauer, S.; Thomas, A.; et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 2015, 15, 2688–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.-H.; Wang, D.-M.; Hsieh, H.-J.; Liu, H.-C.; Hsien, T.-Y.; Lai, J.-Y.; Hou, L.-T. Preparation and characterization of RGD−immobilized chitosan scaffolds. Biomaterials 2005, 26, 3197–3206. [Google Scholar] [CrossRef]
- Weinhold, M.X.; Sauvageau, J.C.M.; Keddig, N.; Matzke, M.; Tartsch, B.; Grunwald, I.; Kübel, C.; Jastorff, B.; Thöming, J. Strategy to improve the characterization of chitosan for sustainable biomedical applications: SAR guided multi-dimensional analysis. Green Chem. 2009, 11, 498–509. [Google Scholar] [CrossRef]
- Charhouf, I.; Bennamara, A.; Abourriche, A.; Chenite, A.; Zhu, J.; Berrada, M. Characterization of a dialdehyde chitosan generated by periodate oxidation. Biosensors 2014, 16, 18. [Google Scholar]
- Ni Mhurchu, C.; Dunshea-Mooij, C.; Bennett, D.; Rodgers, A. Effect of chitosan on weight loss in overweight and obese individuals: A systematic review of randomized controlled trials. Obes. Rev. 2005, 6, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Loveless, S.E.; Hoban, D.; Sykes, G.; Frame, S.R.; Everds, N.E. Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicol. Sci. 2008, 105, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Bomberg, E.M.; Ryder, J.; Brundage, R.C.; Straka, R.J.; Fox, C.K.; Gross, A.C.; Oberle, M.; Bramante, C.T.; Sibley, S.D.; Kelly, A.S. Precision medicine in adult and pediatric obesity: A clinical perspective. Ther. Adv. Endocrinol. Metab. 2019, 10. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-Y.; Chen, Y.-T.; Ke, C.-J.; Chen, C.-Y.; Lin, F.-H. The Synthesis and Evaluation of RGD−Conjugated Chitosan Gel as Daily Supplement for Body Weight Control. Materials 2021, 14, 4467. https://doi.org/10.3390/ma14164467
Chen W-Y, Chen Y-T, Ke C-J, Chen C-Y, Lin F-H. The Synthesis and Evaluation of RGD−Conjugated Chitosan Gel as Daily Supplement for Body Weight Control. Materials. 2021; 14(16):4467. https://doi.org/10.3390/ma14164467
Chicago/Turabian StyleChen, Wei-Yao, Yu-Ting Chen, Cherng-Jyh Ke, Ching-Yun Chen, and Feng-Huei Lin. 2021. "The Synthesis and Evaluation of RGD−Conjugated Chitosan Gel as Daily Supplement for Body Weight Control" Materials 14, no. 16: 4467. https://doi.org/10.3390/ma14164467
APA StyleChen, W.-Y., Chen, Y.-T., Ke, C.-J., Chen, C.-Y., & Lin, F.-H. (2021). The Synthesis and Evaluation of RGD−Conjugated Chitosan Gel as Daily Supplement for Body Weight Control. Materials, 14(16), 4467. https://doi.org/10.3390/ma14164467






