Boosting Piezo/Photo-Induced Charge Transfer of CNT/Bi4O5I2 Catalyst for Efficient Ultrasound-Assisted Degradation of Rhodamine B
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Catalysts
2.2. Characterization
2.3. Evaluation of Piezo-/Piezophoto-Catalytic Activities
2.4. Carrier Migration Measurement
3. Results and discussion
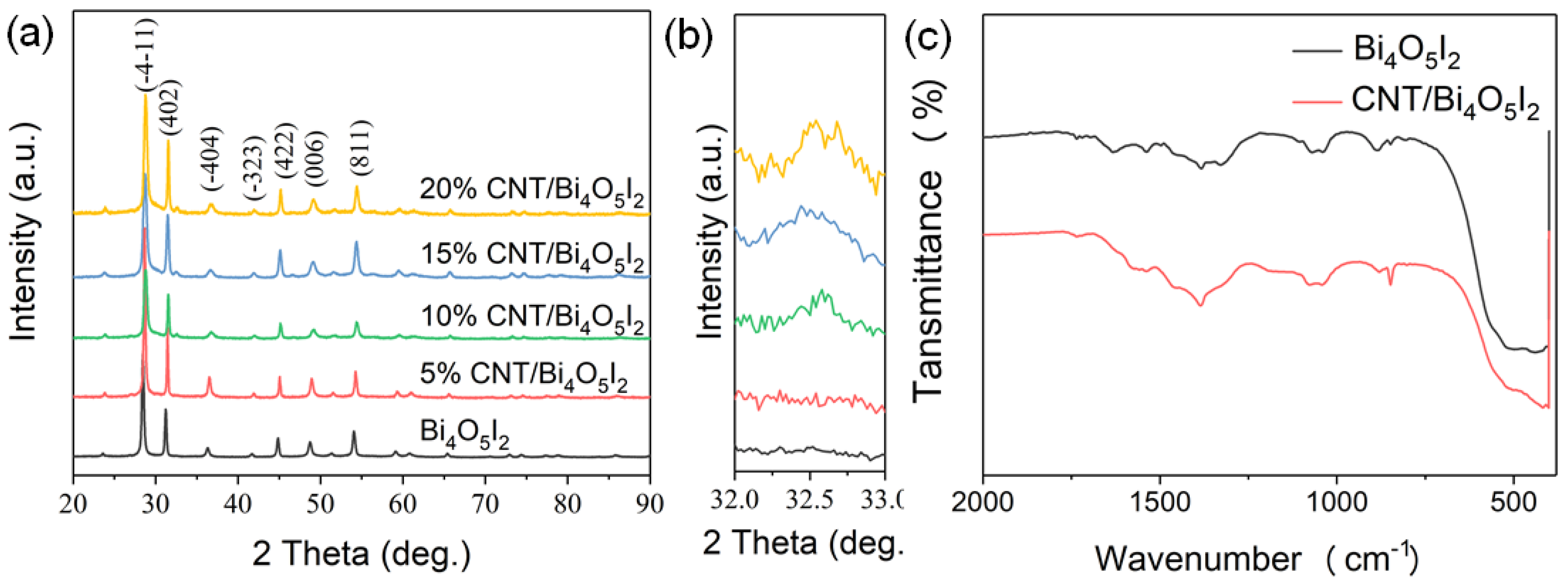
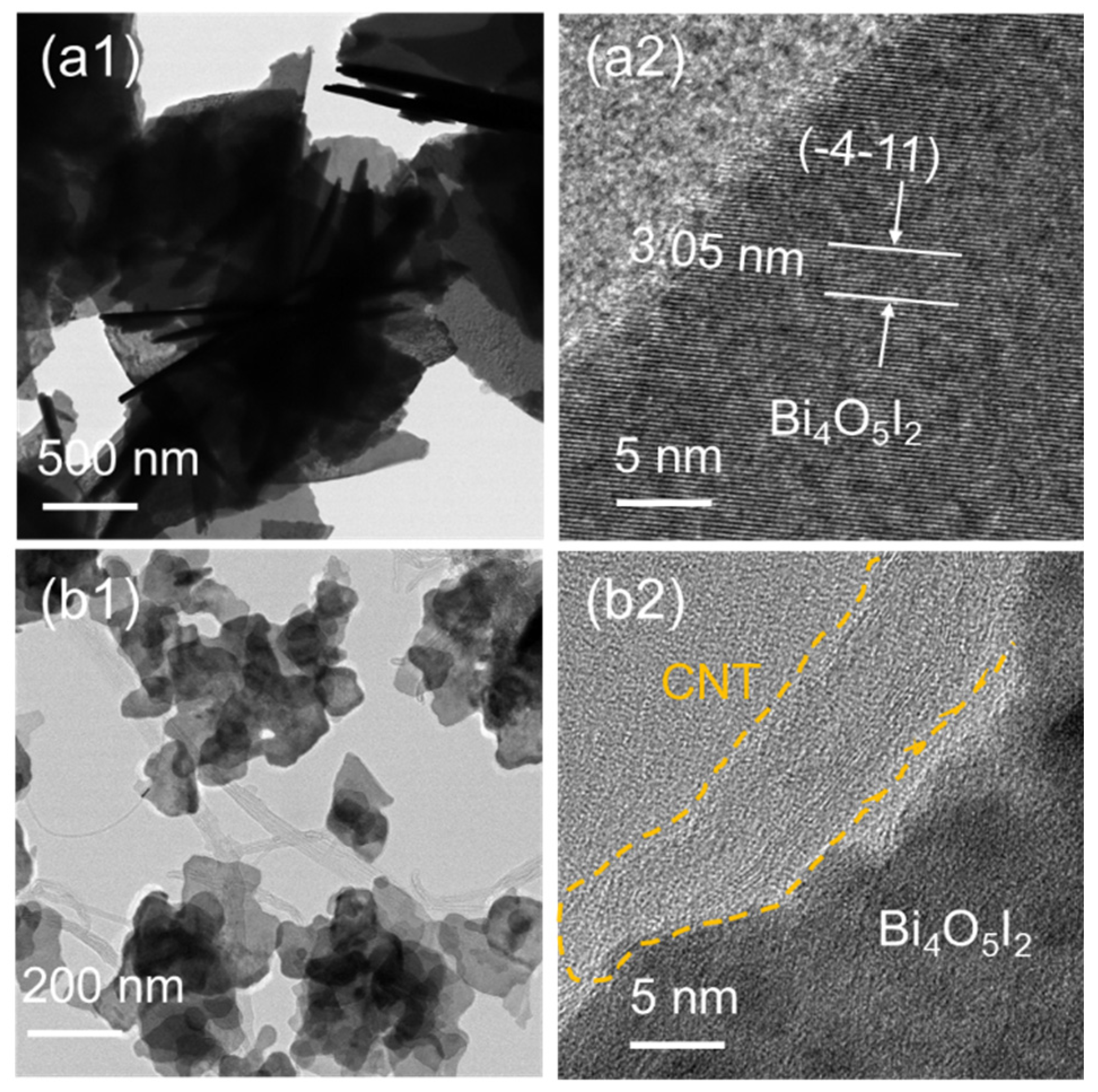
3.1. Characterizations of the As-Synthesized Samples
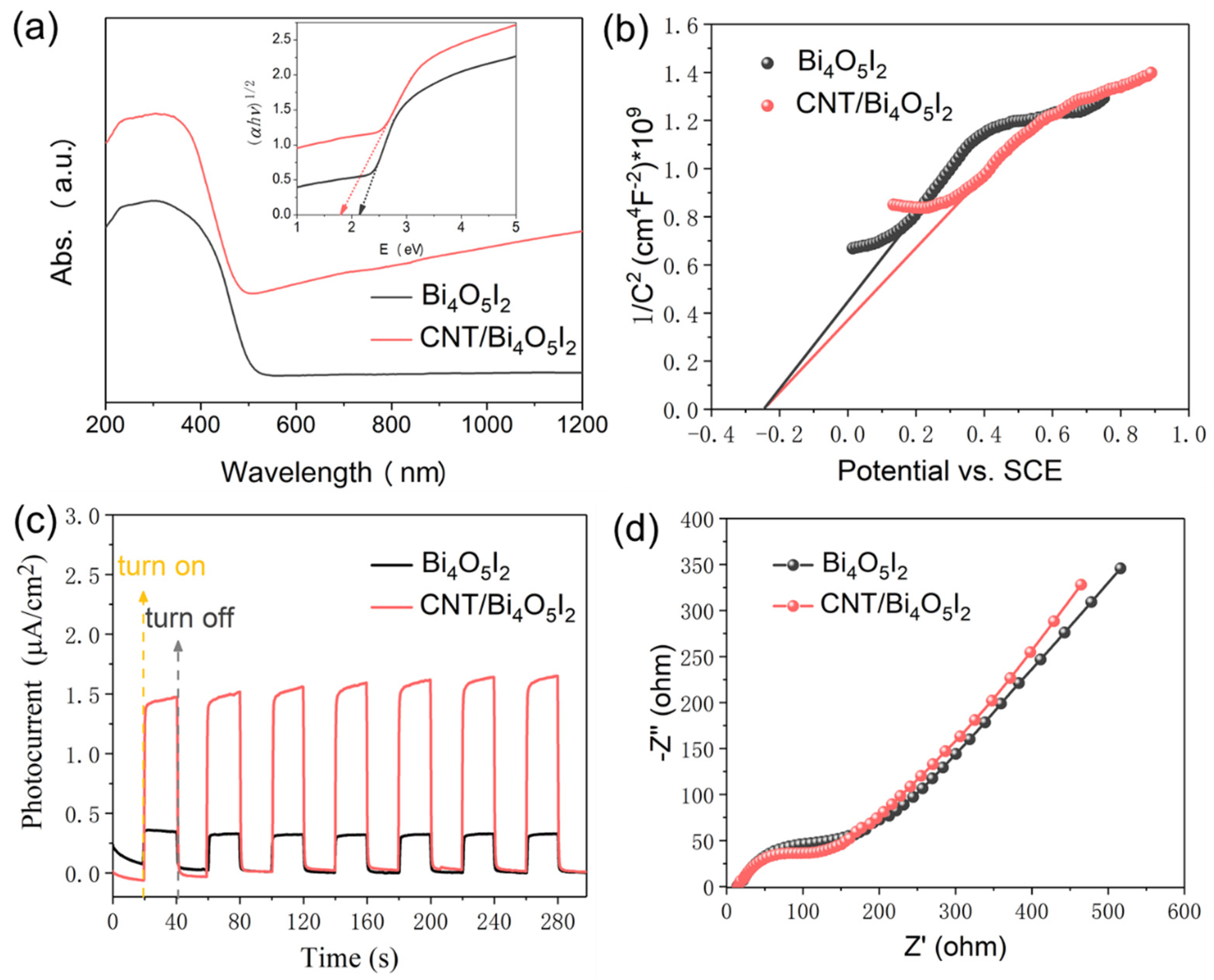
3.2. Piezo-and Piezophoto-Catalytic Performances
3.3. Catalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, L.; Sun, S.; Chen, Y.; Wang, P.; Wang, J.; Zhang, X.; Zou, J.-J.; Wang, Z.L. Advances in piezo-phototronic effect enhanced photocatalysis and photoelectrocatalysis. Adv. Energy Mater. 2020, 10, 2000214. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Z.; Zhao, J.; Zhang, Z.; Li, X.; Zhang, J. Recent advances of ferro-, piezo-, and pyroelectric nanomaterials for catalytic applications. ACS Appl. Nano Mater. 2020, 3, 1063–1079. [Google Scholar] [CrossRef]
- Wang, K.; Shao, D.; Zhang, L.; Zhou, Y.; Wang, H.; Wang, W. Efficient piezo-catalytic hydrogen peroxide production from water and oxygen over graphitic carbon nitride. J. Mater. Chem. A 2019, 7, 20383–20389. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, M.; Kumar, A.; Powar, S.; Vaish, R. Rapid bacterial disinfection using low frequency piezocatalysis effect. J. Ind. Eng. Chem. 2019, 77, 355–364. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, J.; Qin, N.; Lin, E.; Kang, Z.; Bao, D. Sm-doped Pb(Mg1/3Nb2/3)O3-xPbTiO3 piezocatalyst: Exploring the relationship between piezoelectric property and piezocatalytic activity. Appl. Mater. Today 2019, 17, 183–192. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Z.L. Piezotronics and piezo-phototronics for adaptive electronics and optoelectronics. Nat. Rev. Mater. 2016, 1, 16031. [Google Scholar] [CrossRef]
- Guo, L.; Zhong, C.; Cao, J.; Hao, Y.; Lei, M.; Bi, K.; Sun, Q.; Wang, Z.L. Enhanced photocatalytic H2 evolution by plasmonic and piezotronic effects based on periodic Al/BaTiO3 heterostructures. Nano Energy 2019, 62, 513–520. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Fan, S.; Chen, X.; Qin, M.; Long, D.; Tadé, M.O.; Liu, S. Impact of oxygen vacancy occupancy on piezo-catalytic activity of BaTiO3 nanobelt. Appl. Catal. B Environ. 2020, 279, 119340. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Z.; Zhang, J.; Li, S.; Zhao, Z.; Zhu, L.; Liu, W.; Lin, Y.; Liu, H.; Zhang, Z. Enhanced catalytic performance by multi-field coupling in KNbO3 nanostructures: Piezo-photocatalytic and ferro-photoelectrochemical effects. Nano Energy 2019, 58, 695–705. [Google Scholar] [CrossRef]
- Amiri, O.; Salar, K.; Othman, P.; Rasul, T.; Faiq, D.; Saadat, M. Purification of wastewater by the piezo-catalyst effect of PbTiO3 nanostructures under ultrasonic vibration. J. Hazard. Mater. 2020, 394, 122514. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, S.; Li, C.; Yan, F.; Bai, H.; Shen, B.; Zeng, H.; Zhai, J. Piezophototronic effect in enhancing charge carrier separation and transfer in ZnO/BaTiO3 heterostructures for high-efficiency catalytic oxidation. Nano Energy 2019, 66, 104127. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, H.; Zou, Y.; Dong, X.; Jia, Y.; Wang, F. Synergetic photocatalysis/piezocatalysis of bismuth oxybromide for degradation of organic pollutants. J. Alloy. Compd. 2019, 809, 151840. [Google Scholar] [CrossRef]
- Meng, F.; Ma, W.; Wang, Y.; Zhu, Z.; Chen, Z.; Lu, G. A tribo-positive Fe@MoS2 piezocatalyst for the durable degradation of tetracycline: Degradation mechanism and toxicity assessment. Environ. Sci. Nano 2020, 7, 1704–1718. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Z.; Yu, D.; Zhao, J.-Z.; Su, Y.; Liu, Y.; Lin, Y.; Liu, W.; Xu, H.; Zhang, Z. Few-layer transition metal dichalcogenides (MoS2, WS2, and WSe2) for water splitting and degradation of organic pollutants: Understanding the piezocatalytic effect. Nano Energy 2019, 66, 104083. [Google Scholar] [CrossRef]
- Hu, C.; Huang, H.; Chen, F.; Zhang, Y.; Yu, H.; Ma, T. Coupling Piezocatalysis and Photocatalysis in Bi4NbO8X (X = Cl, Br) Polar Single Crystals. Adv. Funct. Mater. 2020, 30, 1908168. [Google Scholar] [CrossRef]
- Kang, Z.; Qin, N.; Lin, E.; Wu, J.; Yuan, B.; Bao, D. Effect of Bi2WO6 nanosheets on the ultrasonic degradation of organic dyes: Roles of adsorption and piezocatalysis. J. Clean. Prod. 2020, 261, 121125. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E.; Chang, Y.T.; Chang, C.-K. Piezo-catalytic effect on the enhancement of the ultra-high degradation activity in the dark by single- and few-layers MoS2 nanoflowers. Adv. Mater. 2016, 28, 3718–3725. [Google Scholar] [CrossRef]
- You, H.; Wu, Z.; Zhang, L.; Ying, Y.; Liu, Y.; Fei, L.; Chen, X.; Jia, Y.; Wang, Y.; Wang, F.; et al. Harvesting the vibration energy of BiFeO3 nanosheets for hydrogen evolution. Angew. Chem. Int. Ed. 2019, 58, 11779–11784. [Google Scholar] [CrossRef]
- Ning, X.; Hao, A.; Cao, Y.; Hu, J.; Xie, J.; Jia, D. Effective promoting piezocatalytic property of zinc oxide for degradation of organic pollutants and insight into piezocatalytic mechanism. J. Colloid Interface Sci. 2020, 577, 290–299. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, M.; Vaish, R. Exploring the piezocatalytic dye degradation capability of lithium niobate. Adv. Powder Technol. 2020, 31, 1771–1775. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Lin, E.Z.; Kang, Z.H.; Bao, D.H. Enhancement of piezocatalytic activity at the ferro-paraelectric phase transition of Ba1-xSrxTiO3 nanopowders. Mater. Today Energy 2021, 21, 100732. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Lin, E.; Yuan, B.; Kang, Z.; Bao, D. Synthesis of Bi4Ti3O12 decussated nanoplates with enhanced piezocatalytic activity. Nanoscale 2019, 11, 21128–21136. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Su, Y.; Zhang, B.; Zhao, Z.; Li, S.; Zhang, Y.; Li, P.; Xu, M.; Ren, R. Few-layer MoS2 nanosheet-coated KNbO3 nanowire heterostructures: Piezo-photocatalytic effect enhanced hydrogen production and organic pollutant degradation. Nanoscale 2019, 11, 7690–7700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Liu, Z.; Geng, X.; Song, W.; Lu, J.; Xie, B.; Ke, S.; Shu, L. Ultrasonic vibration driven piezocatalytic activity of lead-free K0.5Na0.5NbO3 materials. Ceram. Int. 2019, 45, 22486–22492. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, J.; Qin, N.; Lin, E.; Bao, D. Enhanced piezocatalytic performance of (Ba, Sr) TiO3 nanowires to degrade organic pollutants. Acs Appl. Nano Mater. 2018, 1, 5119–5127. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Dai, B.; Fang, J.; Yu, Y.; Sun, M.; Huang, H.; Lu, C.; Kou, J.; Zhao, Y.; Xu, Z. Construction of infrared-light-responsive photoinduced carriers driver for enhanced photocatalytic hydrogen evolution. Adv. Mater. 2020, 32, 1906361. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.A.; Fu, Y.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Construction of CDs/CdS photocatalysts for stable and efficient hydrogen production in water and seawater. Appl. Catal. B Environ. 2019, 242, 178–185. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, L.; Zhang, Y.; Sun, H. Significantly enhanced piezo-photocatalytic capability in BaTiO3 nanowires for degrading organic dye. J. Mater. 2020, 6, 256–262. [Google Scholar] [CrossRef]
- Huang, X.; Lei, R.; Yuan, J.; Gao, F.; Jiang, C.; Feng, W.; Zhuang, J.; Liu, P. Insight into the piezo-photo coupling effect of PbTiO3/CdS composites for piezo-photocatalytic hydrogen production. Appl. Catal. B Environ. 2021, 282, 119586. [Google Scholar] [CrossRef]
- Yin, R.; Li, Y.; Zhong, K.; Yao, H.; Zhang, Y.; Lai, K. Multifunctional property exploration: Bi4O5I2 with high visible light photocatalytic performance and a large nonlinear optical effect. RSC Adv. 2019, 9, 4539–4544. [Google Scholar] [CrossRef] [Green Version]
- Schlange, A.; Dos Santos, A.R.; Kunz, U.; Turek, T. Continuous preparation of carbon-nanotube-supported platinum catalysts in a flow reactor directly heated by electric current. Beilstein J. Org. Chem. 2011, 7, 1412–1420. [Google Scholar] [CrossRef]
- Petit, C.; Burress, J.; Bandosz, T.J. The synthesis and characterization of copper-based metal–organic framework/graphite oxide composites. Carbon 2011, 49, 563–572. [Google Scholar] [CrossRef]
- Xia, J.; Ji, M.; Di, J.; Wang, B.; Yin, S.; He, M.; Zhang, Q.; Li, H. Improved photocatalytic activity of few-layer Bi4O5I2 nanosheets induced by efficient charge separation and lower valence position. J. Alloy. Compd. 2017, 695, 922–930. [Google Scholar] [CrossRef]
- Lin, W.; Yu, X.; Zhu, Y.; Zhang, Y. Graphene Oxide/BiOCl Nanocomposite Films as Efficient Visible Light Photocatalysts. Front. Chem. 2018, 6, 274. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Jie, H.X.; Meng, Y.; Chen, Z.G. N-CQDs accelerating surface charge transfer of Bi4O5I2 hollow nanotubes with broad spectrum photocatalytic activity. Appl. Catal. B Environ. 2018, 237, 1033–1043. [Google Scholar]
- Sapkota, K.P.; Islam, M.A.; Hanif, M.A.; Akter, J.; Hahn, J.R. Hierarchical Nanocauliflower Chemical Assembly Composed of Copper Oxide and Single-Walled Carbon Nanotubes for Enhanced Photocatalytic Dye Degradation. Nanomaterials 2021, 11, 696. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yu, D.; Liu, Y.; Liu, X.; Shi, Y. Boosting Piezo/Photo-Induced Charge Transfer of CNT/Bi4O5I2 Catalyst for Efficient Ultrasound-Assisted Degradation of Rhodamine B. Materials 2021, 14, 4449. https://doi.org/10.3390/ma14164449
Wang Y, Yu D, Liu Y, Liu X, Shi Y. Boosting Piezo/Photo-Induced Charge Transfer of CNT/Bi4O5I2 Catalyst for Efficient Ultrasound-Assisted Degradation of Rhodamine B. Materials. 2021; 14(16):4449. https://doi.org/10.3390/ma14164449
Chicago/Turabian StyleWang, Yang, Dongfang Yu, Yue Liu, Xin Liu, and Yue Shi. 2021. "Boosting Piezo/Photo-Induced Charge Transfer of CNT/Bi4O5I2 Catalyst for Efficient Ultrasound-Assisted Degradation of Rhodamine B" Materials 14, no. 16: 4449. https://doi.org/10.3390/ma14164449
APA StyleWang, Y., Yu, D., Liu, Y., Liu, X., & Shi, Y. (2021). Boosting Piezo/Photo-Induced Charge Transfer of CNT/Bi4O5I2 Catalyst for Efficient Ultrasound-Assisted Degradation of Rhodamine B. Materials, 14(16), 4449. https://doi.org/10.3390/ma14164449




