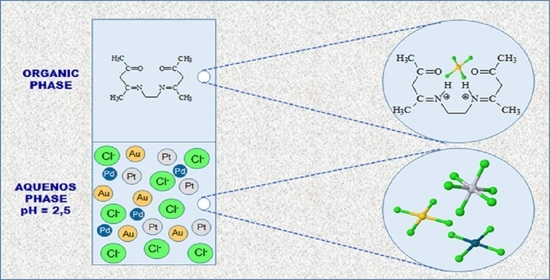
Separation and Recovery of Gold(III), Palladium(II) and Platinum(IV) by Solvent Extraction Using a New β-Diketone Derivative from Acidic Solutions
Abstract
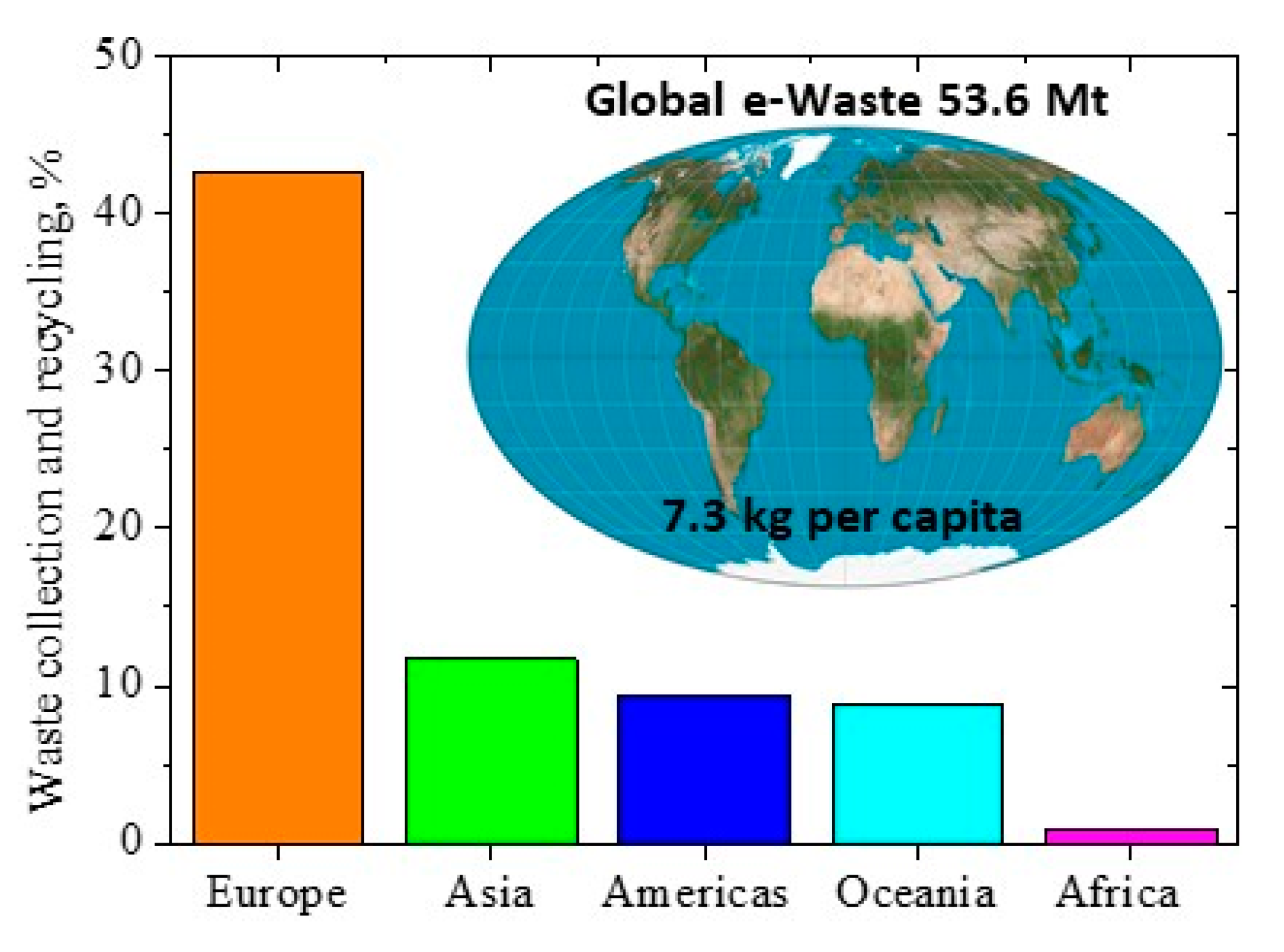
:1. Introduction
2. Materials and Methods
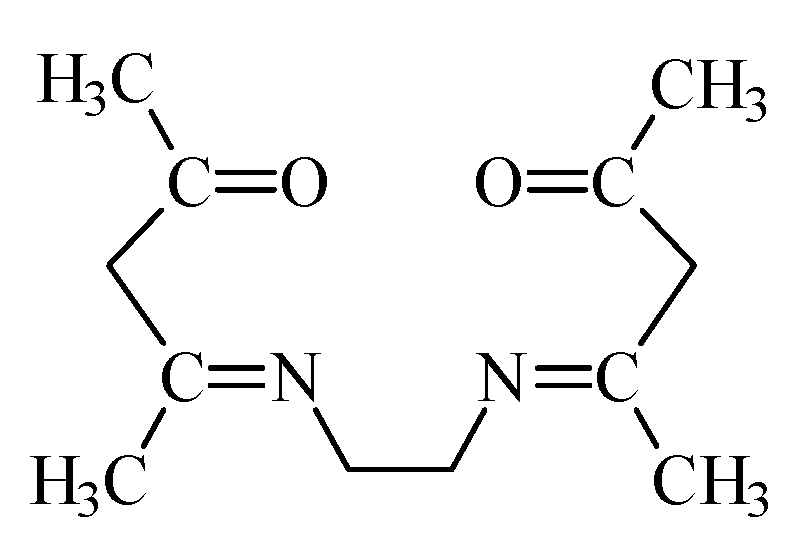
2.1. Reagents
2.2. Extraction and Stripping
3. Results and Discussion
3.1. Extraction from One-Component System
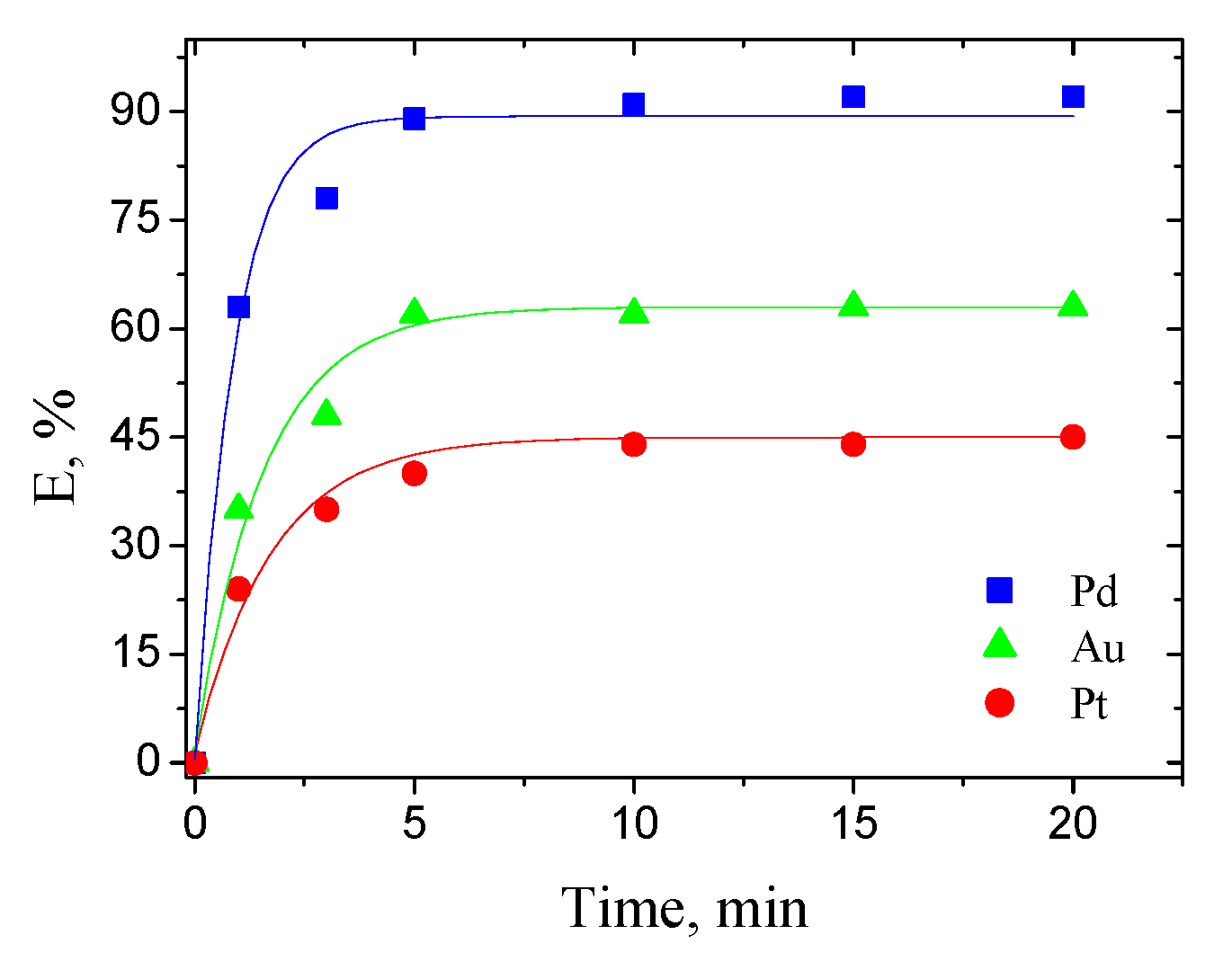
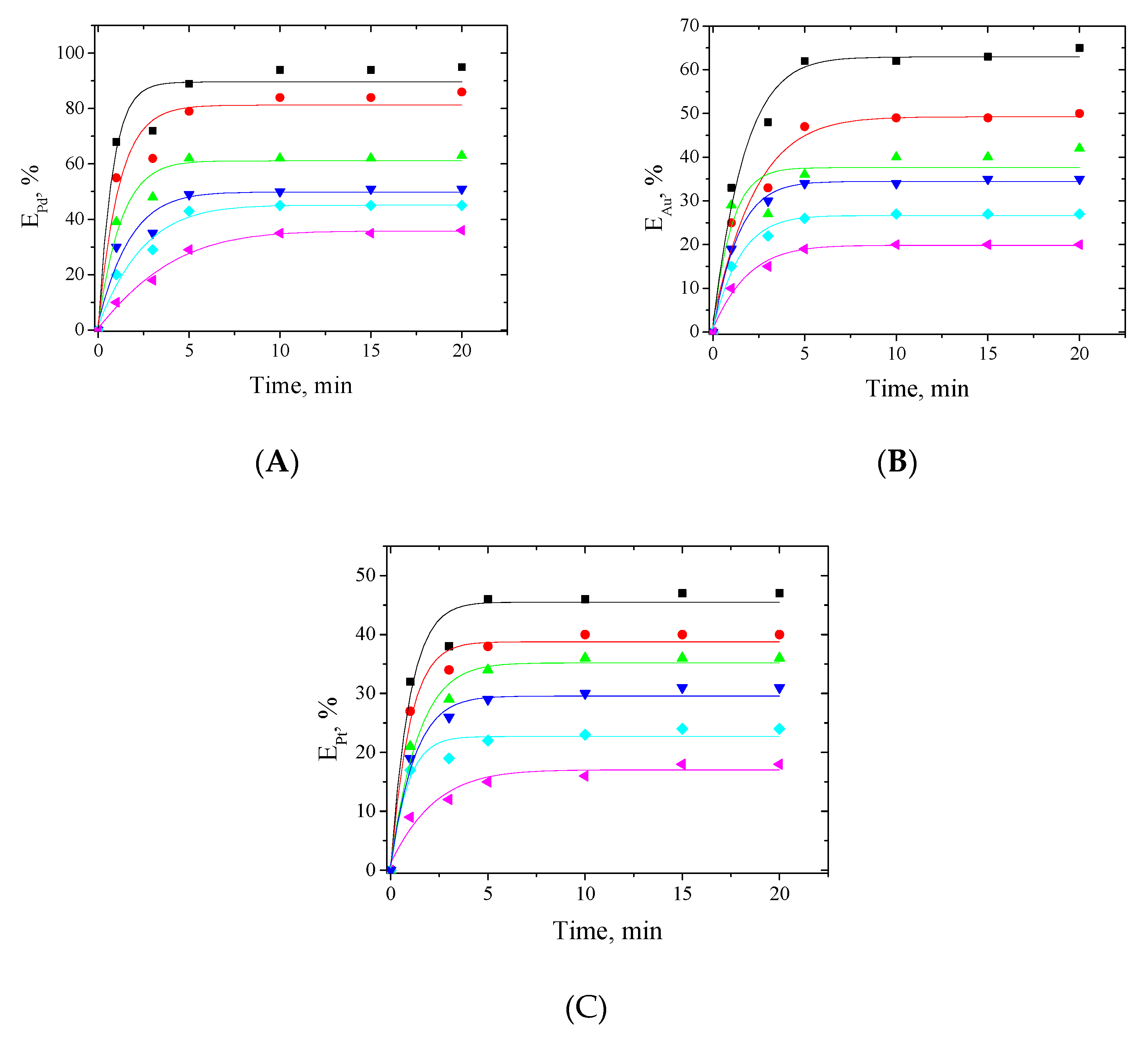
3.1.1. Effect of Extraction Time
3.1.2. Effect of HCl Concentration
3.1.3. Equilibrium of Pd(II), Au(III), and Pt(IV) Extraction
[PtCl6]2− + [(LH2)2+2Cl−] = [(LH2) PtCl6] + 2Cl−
[AuCl4]− + [(LH2)2+2Cl−] = [(LH2) (AuCl4)2] + 2Cl−
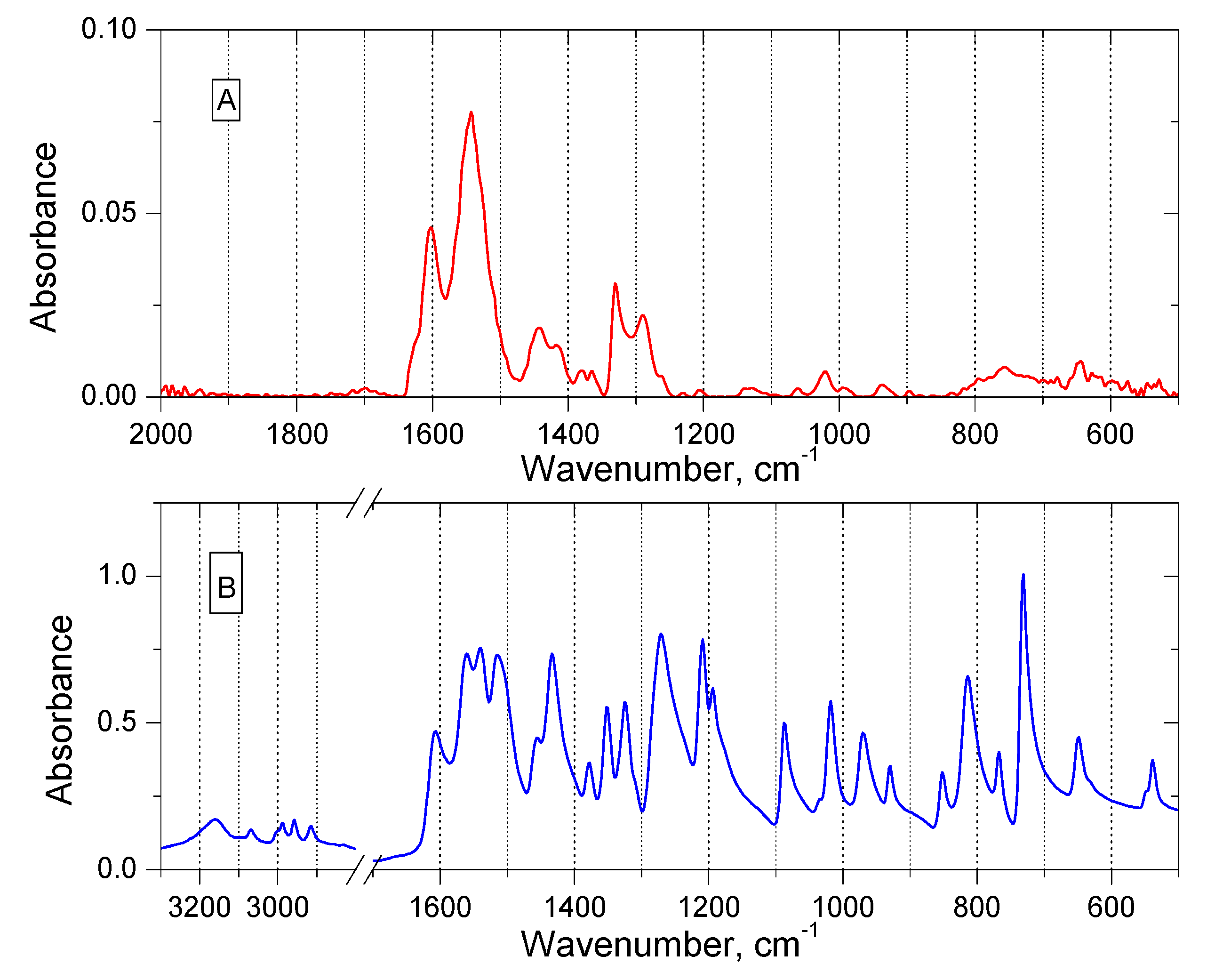
3.1.4. FT-IR Analysis of the Methylene Chloride Phase
3.2. Extraction from Three-Component System
Influence of the Diluent on the Extraction Efficiency
3.3. Stripping Experiments
3.4. Recovery of Pd(II), Au(III), and Pt(IV) from Model Waste Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Debnath, B.; Chowdhury, R.; Ghosh, S.K. Sustainability of metal recovery from E-waste. Front. Environ. Sci. Eng. 2018, 12, 2. Available online: http://hdl.handle.net/20.500.11794/24666 (accessed on 14 April 2021). [CrossRef]
- Perkins, D.N.; Brune Drisse, M.N.; Nxele, T.; Sly, P.D. E-waste: A global hazard. Ann. Glob. Health 2014, 80, 286–295. [Google Scholar] [CrossRef] [PubMed]
- BASF Catalysts—Metal Prices. Available online: https://apps.catalysts.basf.com/apps/eibprices/mp/ (accessed on 19 April 2021).
- Reck, B.K.; Graedel, T.E. Challenges in metal recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.P.; Zinnerman, J.B.; Anastas, P.T.; Plata, D.L. A strategy for material supply chain sustainability: Enabling a circular economy in the electronics industry through green engineering. ACS Sustain. Chem. Eng. 2016, 4, 5879–5888. [Google Scholar] [CrossRef]
- Hagelüken, C.; Corti, C.W. Recycling of gold from electronics: Cost-effective use through ‘Design for Recycling’. Gold Bull. 2010, 43, 209–220. [Google Scholar] [CrossRef] [Green Version]
- Hall, W.J.; Williams, P.T. Separation and recovery of materials from scrap printed circuit boards. Resour. Conserv. Recycl. 2007, 51, 691–709. [Google Scholar] [CrossRef] [Green Version]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-waste Monitor 2020: Quantities, Flows and the Circular Economy Potential. United Nations University (UNU)/United Nations Institute For Training And Research (UNITAR)—Co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Bonn/Geneva/Rotterdam. Available online: https://collections.unu.edu/view/UNU:7737 (accessed on 30 June 2021).
- Lu, Y.; Xu, Z. Precious metals recovery from waste printed circuit boards: A review for current status and perspective. Resour. Conserv. Recycl. 2016, 113, 28–39. [Google Scholar] [CrossRef]
- Willer, J.; Fornalczyk, A. Electronic scraps as a source of precious metals. Przemysl Chem. 2012, 91, 517–522. [Google Scholar]
- E-waste Guide Info. E-waste in the EU: Facts and Figures. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20201208STO93325/e-waste-in-the-eu-facts-and-figures-infographic (accessed on 10 June 2021).
- Sanito, R.C.; You, S.-J.; Wang, j.-F. Application of plasma technology for treating e-waste: A review. J. Environ. Manag. 2021, 188, 112380. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Aziz, A.A.; Monir, M.U.; Teo, S.H.; Hasan, M. Advances in sustainable approaches to recover metals from e-waste—A review. J. Clean. Product. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Andrews, D.; Raychaudhuri, A.; Frias, C. Environmentally sound technologies for recycling secondary lead. J. Power Sources 2000, 88, 124–129. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Xiao, Y.; Sietsma, J.; Jin, W.; Agterhuis, H.; Yang, Y. Toward sustainability for recovery of critical metals from electronic waste: The hydrochemistry processes. ACS Sustain. Chem. Eng. 2017, 5, 21–40. [Google Scholar] [CrossRef]
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Free, M.L. Hydrometallurgy: Fundamentals and Applications; Wiley & Sons Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kejun, L.; Yen, W.T.; Shibayama, A.; Miyazaki, T.; Fujita, T. Gold extraction from thiosulfate solution using trioctylmethylammonium chloride. Hydrometallurgy 2004, 73, 41–53. [Google Scholar] [CrossRef]
- Fontana, D.; Pietrantonio, M.; Pucciarmati, S.; Torelli, N.G.; Bonomi, C. Palladium recovery from monolithic ceramic capacitors by leaching, solvent extraction and reduction. J. Mater. Cycles Waste Manag. 2017, 20. [Google Scholar] [CrossRef]
- Oshima, T.; Iwao, S.; Matsuo, N.; Ohe, K. Extraction behavior of precious metals in hydrochloric-acid media using a novel amine extractant bearing a furan group. Solv. Extr. Res. Dev. Jpn. 2019, 26, 69–80. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sonu, C.H.; Lee, M.S. Separation of platinum(IV) and palladium(II) from concentrated hydrochloric acid solutions by mixtures of amines with neutral extractants. J. Ind. Eng. Chem. 2015, 32, 238–245. [Google Scholar] [CrossRef]
- Wei, W.; Cho, C.W.; Kim, S.; Song, M.H.; Kwame Bediako, J.; Yun, Y.S. Selective recovery of Au(III), Pt(IV), and Pd(II) from aqueous solutions by liquid-liquid extraction using ionic liquid Aliquat-336. J. Mol. Liq. 2016, 216, 18–24. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Recycling copper and gold from e-waste by a two-stage leaching and solvent extraction process. Sep. Purif. Technol. 2021, 263, 118400. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wieczorek, D. Extraction and separation of palladium (II), platinum (IV), gold (III) and rhodium (III) using piperidine-based extractants. Hydrometallurgy 2018, 175, 359–366. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Wisniewski, M.; Borowiak-Resterna, A.; Cieszynska, A.; Sastre, A.M. Selective extraction of palladium(II) from hydrochloric acid solutions with pyridine carboxamides and ACORGA®CLX50. Sep. Purif. Technol. 2007, 53, 337–341. [Google Scholar] [CrossRef]
- Wiecka, Z.; Rzelewska-Piekut, M.; Wojciechowska, I.; Wieszczycka, K.; Regel-Rosocka, M. Recovery of Palladium(II) and Platinum(IV) in Novel Extraction Systems. Materials 2021, 14, 285. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.V. PGM ore processing: LIX reagents for palladium extraction & platinum stripping from Alamine 336 using NaOH-NaCl. Miner. Eng. 2019, 138, 119–124. [Google Scholar] [CrossRef]
- Truong, H.T.; Lee, M.S.; Senanayake, G. Separation of Pt(IV), Rh(III) and Fe(III) in acid chloride leach solutions of glass scraps by solvent extraction with various extractants. Hydrometallurgy 2018, 175, 232–239. [Google Scholar] [CrossRef]
- Grad, O.A.; Ciopec, M.; Negrea, A.; Duteanu, N.; Negrea, P.; Vodă, R. Evaluation of performance of functionalized amberlite XAD7 with dibenzo-18-crown ether-6 for palladium recovery. Materials 2021, 14, 1003. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, F.J.; Cobo, A. Solvent extraction with LIX 973N for selective separation of copper and nickel. J. Chem. Technol. Biotechnol. 1999, 74, 467–471. [Google Scholar] [CrossRef]
- Dziwinski, E.J.; Szymanowski, J. Composition of copper extractant LIX 54–100. Solv. Ext. Ion Exch. 1996, 14, 219–226. [Google Scholar] [CrossRef]
- Xie, F.; Lu, D.; Yang, H.; Dreisinger, D. Solvent extraction of silver and gold from alkaline cyanide solution with LIX 7950. Miner. Process. Extr. Metal. Rev. 2014, 35, 229–238. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Ulewicz, M. Separation of Zn(II), Cr(III), and Ni(II) ions using the polymer inclusion membranes containing acetylacetone derivative as the carrier. Membranes 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Pyszka, I.; Radzyminska-Lenarcik, E. New polymer inclusion membranes in the separation of nonferrous metal ion from aqueous solutions. Membranes 2020, 10, 385. [Google Scholar] [CrossRef]
- Takeuchi, T.; Böttcher, A.; Quezada, C.M.; Meade, T.J.; Gray, H.B. Inhibition of thermolysin and human-thrombin by cobalt(III) Schiff base complexes. Bioorg. Med. Chem. 1999, 7, 815–819. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of methods of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Pirogov, A.V.; Havel, J. Determination of platinum, palladium, osmium, iridium, rhodium and gold as chloro complexes by capillary zone electrophoresis. J. Chromatogr. A 1997, 772, 347–355. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kumar, R.; Kim, J.S.; Park, H.K.; Yoon, Y.S. Liquid–liquid extraction/separation of platinum (IV) and rhodium (III) from acidic chloride solutions using tri-iso-octylamine. J. Hazard. Mater. 2009, 168, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Swain, B.; Jeong, J.; Kim, S.; Lee, J. Separation of platinum and palladium from chloride solution by solvent extraction using Alamine 300. Hydrometallurgy 2010, 104, 1–7. [Google Scholar] [CrossRef]
- Zhang, A.; Wanyan, G.; Kumagai, M. Association behavior of 4-acylpyrazolone derivative and tertiary amine of high molecular weight in antagonistic synergistic extraction of palladium. J. Solut. Chem. 2004, 33, 1017–1028. [Google Scholar] [CrossRef]
- Marcus, Y. The properties of organic liquids that are relevant to their use as solvating solvents. Chem. Soc. Rev. 1993, 22, 409. [Google Scholar] [CrossRef]
- Rydberg, J.; Cox, M.; Musakis, C.; Chopin, G.R. Principles and Practices of Solvent Extraction, 2nd ed.; M. Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- Gutmann, V. Empirical parameters for donor and acceptor properties of solvents. Electrochim. Acta 1976, 21, 661–670. [Google Scholar] [CrossRef]


| Type | Content, % | Content, % × 10−3 | ||||
|---|---|---|---|---|---|---|
| Fe | Al | Cu | Ag | Au | Pd | |
| CD | 7 | 5 | 18 | 280 | 20 | 10 |
| Mobile phone | 7 | 3 | 13 | 900 | 200 | 80 |
| DVD player | 62 | 2 | 5 | 115 | 15 | 4 |
| Calculator | 4 | 5 | 3 | 260 | 50 | 5 |
| Name | Fe | Al | Cu | Pb | Zn | Sn | Ni |
|---|---|---|---|---|---|---|---|
| Concentration wt, % | 20.47 | 14.17 | 6.93 | 6.30 | 2.20 | 1.01 | 0.85 |
| Name | Ag | Ti | Ta | Co | Sb | Cd | As | Au | Se | Ge | Ga | Pd | Pt | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt, % × 10−3 | 18.9 | 15.7 | 15.7 | 15.7 | 9.4 | 9.4 | 6.3 | 1.6 | 1.6 | 1.6 | 1.3 | 0.3 | 0.2 | 0.2 |
| Metal Ion | Dilutent | E, % | DM | Separation Coefficient Pd/Au Pd/Pt | |
|---|---|---|---|---|---|
| Pd(II) | toluene | 54 | 1.2 | 2.4 | 12.0 |
| Au(III) | 35 | 0.5 | |||
| Pt(IV) | 12 | 0.1 | |||
| Pd(II) | chloroform | 87 | 6.7 | 5.2 | 13.4 |
| Au(III) | 56 | 1.3 | |||
| Pt(IV) | 31 | 0.5 | |||
| Pd(II) | methylene chloride | 95 | 10.1 | 7.2 | 12.6 |
| Au(III) | 59 | 1.4 | |||
| Pt(IV) | 44 | 0.8 | |||
| Pd(II) | 2-ethylhexanol | 93 | 13.3 | 8.3 | 19.0 |
| Au(III) | 62 | 1.6 | |||
| Pt(IV) | 40 | 0.7 | |||
| Stripping Solution | Stripping Percent, % | ||
|---|---|---|---|
| Pd | Au | Pt | |
| Water | 0 | 0 | 0 |
| 0.5 M ammonia aq. | 100 | 0 | 0 |
| 0.5 M NH4SCN | 96 | 10 | 63 |
| 5 M HCl | 70 | 2 | 32 |
| 5 M HNO3 | 45 | 0 | 99 |
| 0.1 M thiourea in 0.1 M HCl | 100 | 100 | 20 |
| 0.1 M thiourea in 1.0M HCl | 100 | 100 | 55 |
| Metals | Pd(II) | Pt(IV) | Au(III) |
|---|---|---|---|
| Extraction E, % | 82 | 2 | 60 |
| Stripping percent, % | 90 | - | 94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzyminska-Lenarcik, E.; Pyszka, I.; Kosciuszko, A. Separation and Recovery of Gold(III), Palladium(II) and Platinum(IV) by Solvent Extraction Using a New β-Diketone Derivative from Acidic Solutions. Materials 2021, 14, 4436. https://doi.org/10.3390/ma14164436
Radzyminska-Lenarcik E, Pyszka I, Kosciuszko A. Separation and Recovery of Gold(III), Palladium(II) and Platinum(IV) by Solvent Extraction Using a New β-Diketone Derivative from Acidic Solutions. Materials. 2021; 14(16):4436. https://doi.org/10.3390/ma14164436
Chicago/Turabian StyleRadzyminska-Lenarcik, Elzbieta, Ilona Pyszka, and Artur Kosciuszko. 2021. "Separation and Recovery of Gold(III), Palladium(II) and Platinum(IV) by Solvent Extraction Using a New β-Diketone Derivative from Acidic Solutions" Materials 14, no. 16: 4436. https://doi.org/10.3390/ma14164436
APA StyleRadzyminska-Lenarcik, E., Pyszka, I., & Kosciuszko, A. (2021). Separation and Recovery of Gold(III), Palladium(II) and Platinum(IV) by Solvent Extraction Using a New β-Diketone Derivative from Acidic Solutions. Materials, 14(16), 4436. https://doi.org/10.3390/ma14164436