Various Simulated Body Fluids Lead to Significant Differences in Collagen Tissue Engineering Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collagen Scaffolds
2.2. Media Exposure
2.3. Compression Test
2.4. Mass Loss
2.5. Scanning Electron Microscopy and Energy-Dispersive Spectrometry
2.6. Micro-CT Analysis
2.7. Infrared Spectroscopy
2.8. X-ray Diffraction Analysis
2.9. Statistical Evaluation
3. Results
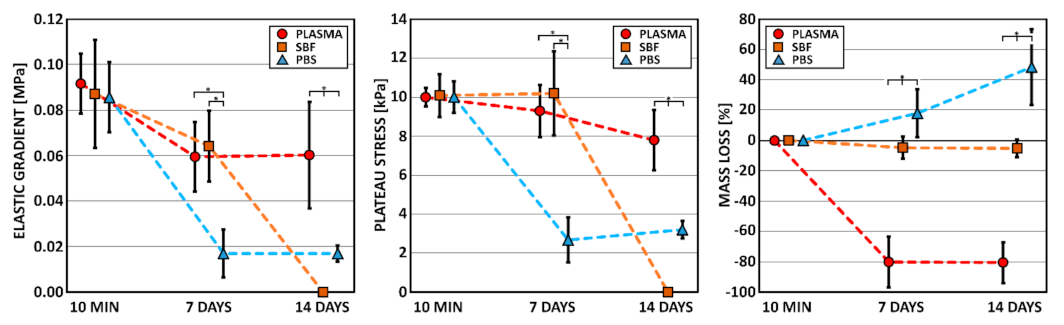
3.1. Compression Test
3.2. Mass Loss
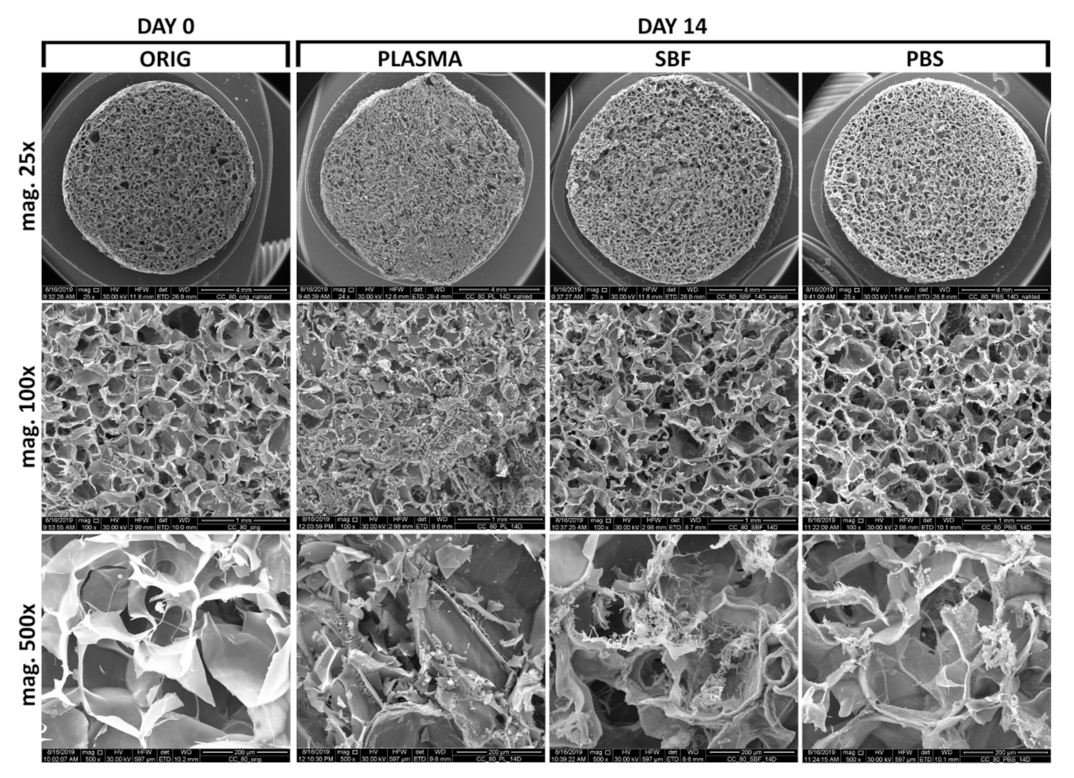
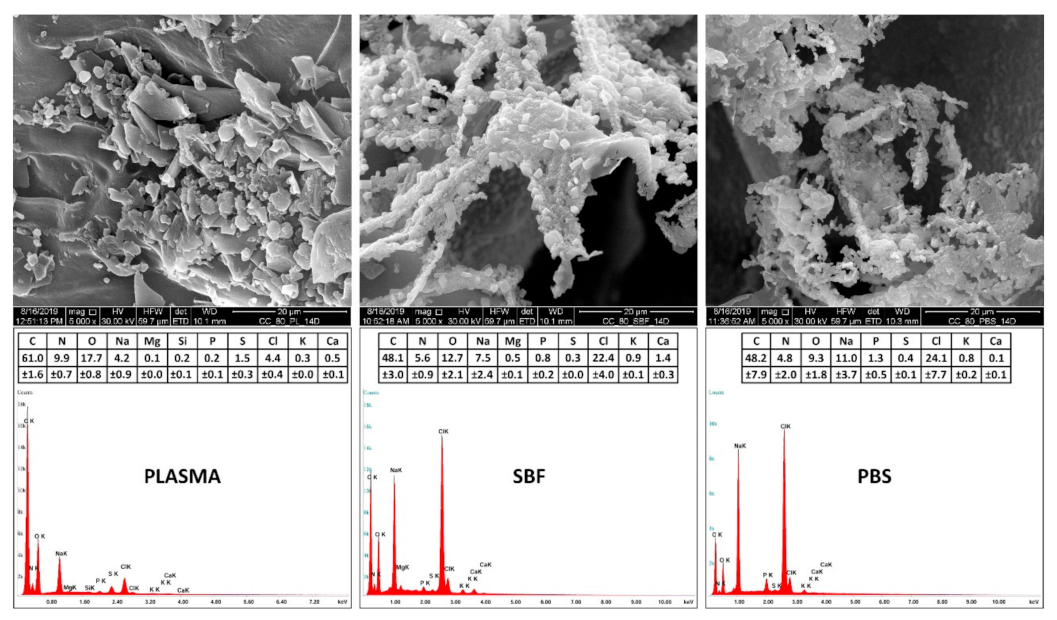
3.3. Scanning Electron Microscopy and Energy-Dispersive Spectrometry
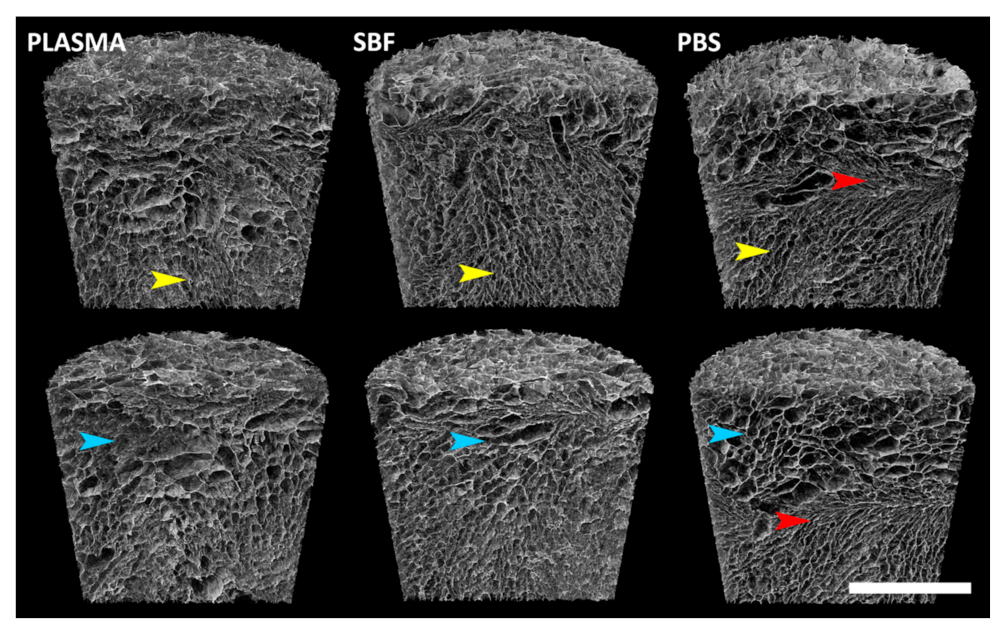
3.4. Micro-CT Analysis
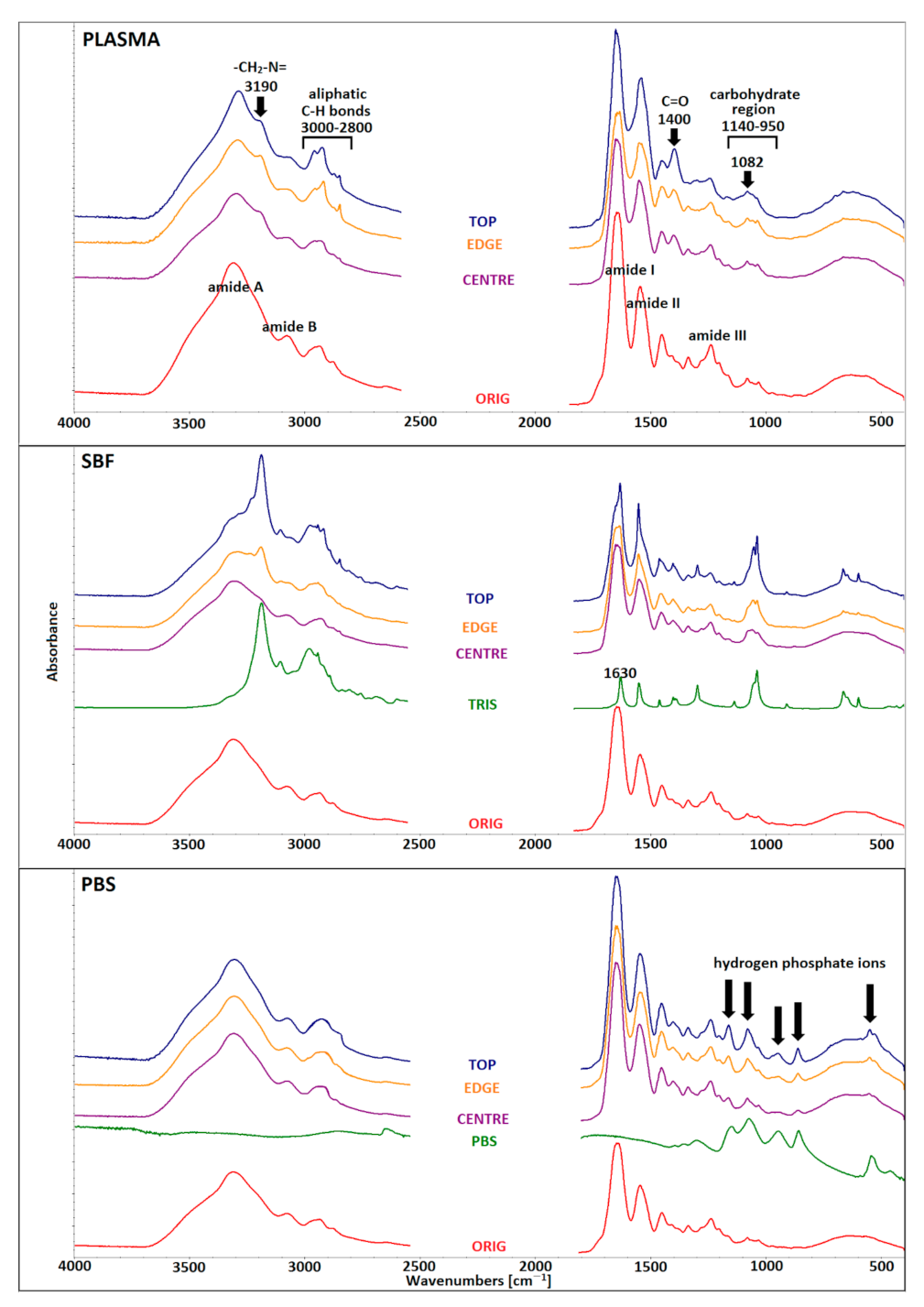
3.5. Infrared Spectroscopy
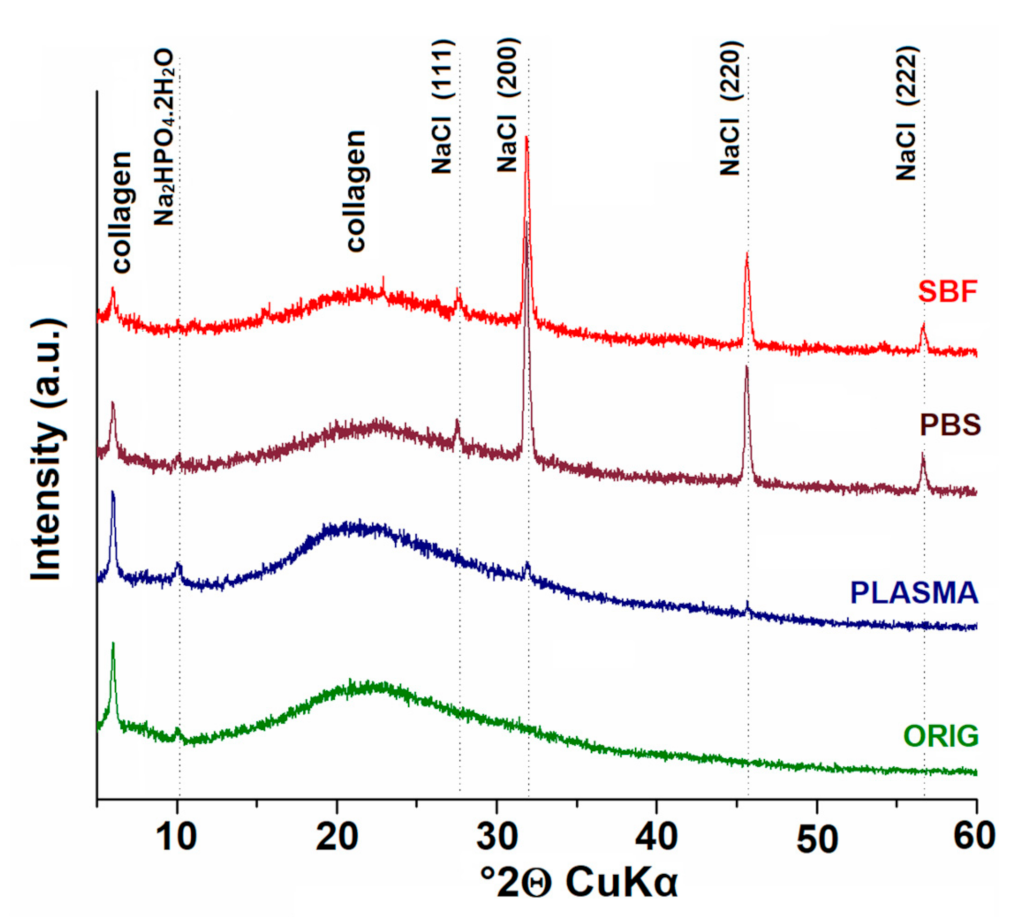
3.6. X-ray Diffraction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Yu, X.; Lin, T.; Peng, J.; Wang, A.; Zhang, Z.; Zhang, Y.; Liu, S.; Zhao, M. Effect of PCL concentration on PCL/CaSiO3 porous composite scaffolds for bone engineering. Ceram. Int. 2020, 46, 13082–13087. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, C.; Zhang, P.; Zhao, K.; Wu, Z. Degradation behaviour of non-sintered graphene/barium titanate/magnesium phosphate cement bio-piezoelectric composites. Ceram. Int. 2020, 46, 12626–12636. [Google Scholar] [CrossRef]
- Kareem, M.M.; Tanner, K.E. Optimising micro-hydroxyapatite reinforced poly (lactide acid) electrospun scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 1–13. [Google Scholar] [CrossRef]
- Chen, Z.; Kang, L.; Meng, Q.-Y.; Liu, H.; Wang, Z.; Guo, Z.; Cui, F.-Z. Degradability of injectable calcium sulfate/mineralized collagen-based bone repair material and its effect on bone tissue regeneration. Mater. Sci. Eng. C 2014, 45, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Ma, Q.; Su, T.; Wang, Z. Preparation of porous materials by selective enzymatic degradation: Effect of in vitro degradation and in vivo compatibility. Sci. Rep. 2020, 10, 7031. [Google Scholar] [CrossRef]
- Rynkevic, R.; Martins, P.; Fernandes, A.; Vange, J.; Gallego, M.R.; Wach, R.A.; Mes, T.; Bosman, A.W.; Deprest, J. In vitro simulation of in vivo degradation and cyclic loading of novel degradable electrospun meshes for prolapse repair. Polym. Test. 2019, 78, 105957. [Google Scholar] [CrossRef]
- Rynkevic, R.; Martins, P.; Pereira, F.; Ramião, N.; Fernandes, A.A. In vitro study of the mechanical performance of hernia mesh under cyclic loading. J. Mater. Sci. Mater. Electron. 2017, 28, 176. [Google Scholar] [CrossRef]
- Zhen, Z.; Xi, T.-F.; Zheng, Y. A review on in vitro corrosion performance test of biodegradable metallic materials. Trans. Nonferr. Met. Soc. China 2013, 23, 2283–2293. [Google Scholar] [CrossRef]
- Tas, A.C. The use of physiological solutions or media in calcium phosphate synthesis and processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef]
- Győri, E.; Fábián, I.; Lázár, I. Effect of the Chemical Composition of Simulated Body Fluids on Aerogel-Based Bioactive Composites. J. Compos. Sci. 2017, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Hong, S.I. Apatite deposition and collagen coating effects in Ti-Al-V and Ti-Al-Nb alloys. Phys. Met. Met. 2014, 115, 1307–1312. [Google Scholar] [CrossRef]
- Su, C.-Y.; Zhou, Q.; Zou, C.-H. Surface Deposition on Titania in a Physiological Solution with Ultraviolet Irradiation in Situ and Effect of Heat Treatment. Coatings 2019, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, L.; Yang, X.; Li, Z.; Sun, X.; Lin, M.; Yang, G.; Gou, Z. Micronutrients-incorporated calcium phosphate particles with protective effect on osteoporotic bone tissue. J. Nutr. Health Aging 2013, 17, 426–433. [Google Scholar] [CrossRef]
- Chen, F.; Cao, X.; Yu, J.; Su, H.; Wei, S.; Hong, H.; Liu, C. Quaternary Ammonium Groups Modified Starch Microspheres for Instant Hemorrhage Control. Colloids Surf. B Biointerfaces 2017, 159, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cao, X.; Chen, X.; Wei, J.; Liu, C. Calcium-modified microporous starch with potent hemostatic efficiency and excellent degradability for hemorrhage control. J. Mater. Chem. B 2015, 3, 4017–4026. [Google Scholar] [CrossRef]
- Eagle, H. Amino Acid Metabolism in Mammalian Cell Cultures. Science 1959, 130, 432–437. [Google Scholar] [CrossRef]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Pazarçeviren, A.E.; Tezcaner, A.; Evis, Z. Historical development of simulated body fluids used in biomedical applications: A review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J. Role of the Short Distance Order in Glass Reactivity. Materials 2018, 11, 415. [Google Scholar] [CrossRef] [Green Version]
- Šupová, M.; Suchý, T.; Sucharda, Z.; Filová, E.; Kinderen, J.N.L.M.; Steinerová, M.; Bačáková, L.; Martynková, G.S. The comprehensive in vitro evaluation of eight different calcium phosphates: Significant parameters for cell behavior. J. Am. Ceram. Soc. 2018, 102, 2882–2904. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C. Biomimetic Collagen/Hydroxyapatite Composite Scaffolds: Fabrication and Characterizations. J. Bionic Eng. 2014, 11, 600–609. [Google Scholar] [CrossRef]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “False” cytotoxicity of ions-adsorbing hydroxyapatite—Corrected method of cytotoxicity evaluation for ceramics of high specific surface area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef]
- Zhao, W.; Lemaître, J.; Bowen, P. A comparative study of simulated body fluids in the presence of proteins. Acta Biomater. 2017, 53, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almora-Barrios, N.; De Leeuw, N.H. Molecular Dynamics Simulation of the Early Stages of Nucleation of Hydroxyapatite at a Collagen Template. Cryst. Growth Des. 2012, 12, 756–763. [Google Scholar] [CrossRef]
- Suchy, T.; Šupová, M.; Sauerová, P.; Verdánová, M.; Sucharda, Z.; Rýglová, Š.; Žaloudková, M.; Sedlacek, R.; Kalbacova, M.H. The effects of different cross-linking conditions on collagen-based nanocomposite scaffolds—an in vitro evaluation using mesenchymal stem cells. Biomed. Mater. 2015, 10, 065008. [Google Scholar] [CrossRef] [PubMed]
- Suchý, T.; Šupová, M.; Bartoš, M.; Sedláček, R.; Piola, M.; Soncini, M.; Fiore, G.B.; Sauerova, P.; Kalbacova, M.H. Dry versus hydrated collagen scaffolds: Are dry states representative of hydrated states? J. Mater. Sci. Mater. Med. 2018, 29, 20. [Google Scholar] [CrossRef]
- Jiřík, M.; Bartoš, M.; Tomášek, P.; Malečková, A.; Kural, T.; Horakova, J.; Lukáš, D.; Suchý, T.; Kochová, P.; Kalbacova, M.H.; et al. Generating standardized image data for testing and calibrating quantification of volumes, surfaces, lengths, and object counts in fibrous and porous materials using X-ray microtomography. Microsc. Res. Tech. 2018, 81, 551–568. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef]
- Prystupa, D.; Donald, A. Infrared study of gelatin conformations in the gel and sol states. Polym. Gels Netw. 1996, 4, 87–110. [Google Scholar] [CrossRef]
- Jackson, M.; Choo, L.-P.; Watson, P.H.; Halliday, W.C.; Mantsch, H.H. Beware of connective tissue proteins: Assignment and implications of collagen absorptions in infrared spectra of human tissues. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 1995, 1270, 1–6. [Google Scholar] [CrossRef]
- Pielesz, A. Temperature-dependent FTIR spectra of collagen and protective effect of partially hydrolysed fucoidan. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 287–293. [Google Scholar] [CrossRef]
- Rabotyagova, O.S.; Cebe, P.; Kaplan, D.L. Collagen structural hierarchy and susceptibility to degradation by ultraviolet radiation. Mater. Sci. Eng. C 2008, 28, 1420–1429. [Google Scholar] [CrossRef] [Green Version]
- Usoltsev, D.; Sitnikova, V.; Kajava, A.; Uspenskaya, M. FTIR Spectroscopy Study of the Secondary Structure Changes in Human Serum Albumin and Trypsin under Neutral Salts. Biomolecules 2020, 10, 606. [Google Scholar] [CrossRef] [Green Version]
- Susi, H.; Byler, D.M. Resolution-enhanced fourier transform infrared spectroscopy of enzymes. Methods Enzymol. 1986, 130, 290–311. [Google Scholar] [CrossRef] [PubMed]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR microscopic imaging of collagen and proteoglycan in bovine carti-lage. Biopolymers 2001, 62, 1–8. [Google Scholar] [CrossRef]
- Rieppo, L.; Saarakkala, S.; Närhi, T.; Helminen, H.; Jurvelin, J.; Rieppo, J. Application of second derivative spectroscopy for increasing molecular specificity of fourier transform infrared spectroscopic imaging of articular cartilage. Osteoarthr. Cartil. 2012, 20, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Guilbert, M.; Said, G.; Happillon, T.; Untereiner, V.; Garnotel, R.; Jeannesson, P.; Sockalingum, G.D. Probing non-enzymatic glycation of type I collagen: A novel approach using Raman and infrared biophotonic methods. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3525–3531. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Guille, M.-M.; Besseau, L.; Chopin, C.; Durand, P.; Herbage, D. Structural aspects of fish skin collagen which forms ordered arrays via liquid crystalline states. Biomaterials 2000, 21, 899–906. [Google Scholar] [CrossRef]
- Ellis, D.; McGavin, S. The structure of collagen—An X-ray study. J. Ultrastruct. Res. 1970, 32, 191–211. [Google Scholar] [CrossRef]
- Słota, D.; Głąb, M.; Tyliszczak, B.; Douglas, T.; Rudnicka, K.; Miernik, K.; Urbaniak, M.M.; Rusek-Wala, P.; Sobczak-Kupiec, A. Composites Based on Hydroxyapatite and Whey Protein Isolate for Applications in Bone Regeneration. Materials 2021, 14, 2317. [Google Scholar] [CrossRef]
- Tan, R.; Feng, Q.; She, Z.; Wang, M.; Jin, H.; Li, J.; Yu, X. In vitro and in vivo degradation of an injectable bone repair composite. Polym. Degrad. Stab. 2010, 95, 1736–1742. [Google Scholar] [CrossRef]
- Grover, C.N.; Cameron, R.; Best, S.M. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 10, 62–74. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Hussain, F.S.J.; Rasad, M.S.B.A.; Yusoff, M. In vitro degradation study of novel HEC/PVA/collagen nanofibrous scaffold for skin tissue engineering applications. Polym. Degrad. Stab. 2014, 110, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, T.; Aravinthan, A.; Sharmila, J.; Kim, N.S.; Kim, J.-H. Collagen/chitosan porous bone tissue engineering composite scaffold incorporated with Ginseng compound K. Carbohydr. Polym. 2016, 152, 566–574. [Google Scholar] [CrossRef]
- Li, X.; Feng, Q.; Cui, F. In vitro degradation of porous nano-hydroxyapatite/collagen/PLLA scaffold reinforced by chitin fibres. Mater. Sci. Eng. C 2006, 26, 716–720. [Google Scholar] [CrossRef]
- Al-Munajjed, A.A.; Plunkett, N.A.; Gleeson, J.P.; Weber, T.; Jungreuthmayer, C.; Levingstone, T.; Hammer, J.; O’Brien, F.J. Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Lickorish, D.; Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V.; Howlett, C.R. Collagen-hydroxyapatite composite prepared by biomimetic process. J. Biomed. Mater. Res. 2003, 68, 19–27. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Kiryukhantsev-Korneev, P.V.; Gudz, K.Y.; Konopatsky, A.S.; Polčak, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Shtansky, D.V.; Manakhov, A.M. Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds. Nanomaterials 2019, 9, 1769. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Li, H.; Xu, Z.; Li, K.; Cao, S.; Jiang, G. Selective preparation and characterization of nano-hydroxyapatite/collagen coatings with three-dimensional network structure. Surf. Coat. Technol. 2017, 322, 227–237. [Google Scholar] [CrossRef]
- Ficai, A.; Andronescu, E.; Voicu, G.; Albu, M.G.; Ilie, A. Biomimetically synthesis of collagen/hydroxyapatite composite ma-terials. Mater. Plast. 2010, 47, 205–208. [Google Scholar]
- Bartoš, M.; Suchý, T.; Foltán, R. Note on the use of different approaches to determine the pore sizes of tissue engineering scaffolds: What do we measure? Biomed. Eng. Online 2018, 17, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]

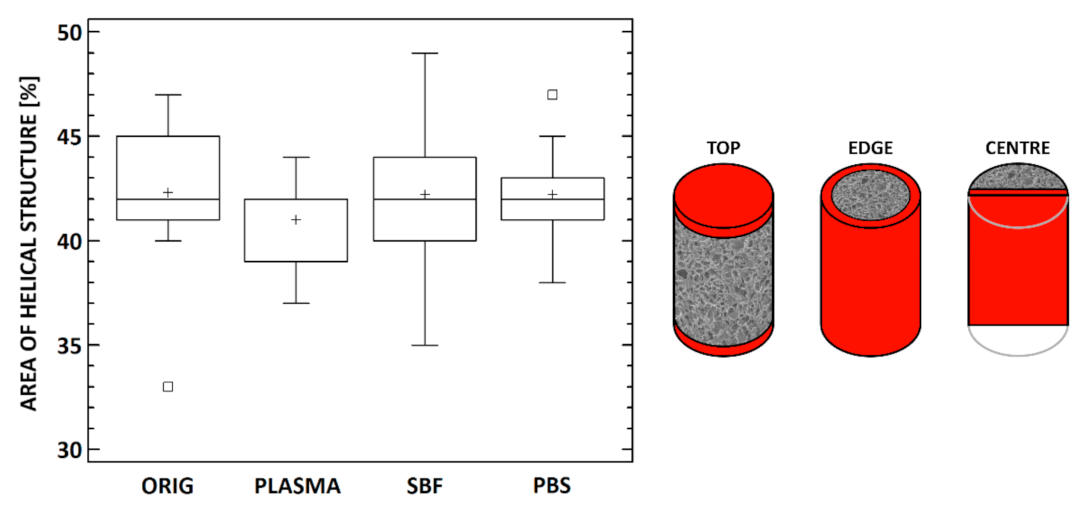
| Parameter | Original | Plasma | SBF | PBS |
|---|---|---|---|---|
| Percent object volume [%] | 17.41 ± 0.67 | 19.52 ± 2.62 | 20.28 ± 3.45 | 19.35 ± 0.89 |
| Object surface density [mm−1] | 40.89 ± 2.51 | 43.84 ± 5.68 | 43.60 ± 7.14 | 43.58 ± 5.25 |
| Structure thickness [mm] | 0.016 ± 0.001 | 0.017 ± 0.001 | 0.017 ± 0.001 | 0.017 ± 0.001 |
| Structure separation [mm] | 0.073 ± 0.010 | 0.072 ± 0.015 | 0.073 ± 0.019 | 0.077 ± 0.025 |
| Open porosity [%] | 82.59 ± 0.67 | 80.48 ± 2.62 | 79.72 ± 3.45 | 80.65 ± 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchý, T.; Bartoš, M.; Sedláček, R.; Šupová, M.; Žaloudková, M.; Martynková, G.S.; Foltán, R. Various Simulated Body Fluids Lead to Significant Differences in Collagen Tissue Engineering Scaffolds. Materials 2021, 14, 4388. https://doi.org/10.3390/ma14164388
Suchý T, Bartoš M, Sedláček R, Šupová M, Žaloudková M, Martynková GS, Foltán R. Various Simulated Body Fluids Lead to Significant Differences in Collagen Tissue Engineering Scaffolds. Materials. 2021; 14(16):4388. https://doi.org/10.3390/ma14164388
Chicago/Turabian StyleSuchý, Tomáš, Martin Bartoš, Radek Sedláček, Monika Šupová, Margit Žaloudková, Gražyna Simha Martynková, and René Foltán. 2021. "Various Simulated Body Fluids Lead to Significant Differences in Collagen Tissue Engineering Scaffolds" Materials 14, no. 16: 4388. https://doi.org/10.3390/ma14164388
APA StyleSuchý, T., Bartoš, M., Sedláček, R., Šupová, M., Žaloudková, M., Martynková, G. S., & Foltán, R. (2021). Various Simulated Body Fluids Lead to Significant Differences in Collagen Tissue Engineering Scaffolds. Materials, 14(16), 4388. https://doi.org/10.3390/ma14164388







