Influence of Liquid-to-Solid and Alkaline Activator (Sodium Silicate to Sodium Hydroxide) Ratios on Fresh and Hardened Properties of Alkali-Activated Palm Oil Fuel Ash Geopolymer
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
2.2. Mix Design and Procedure for Alkali-Activated POFA Paste
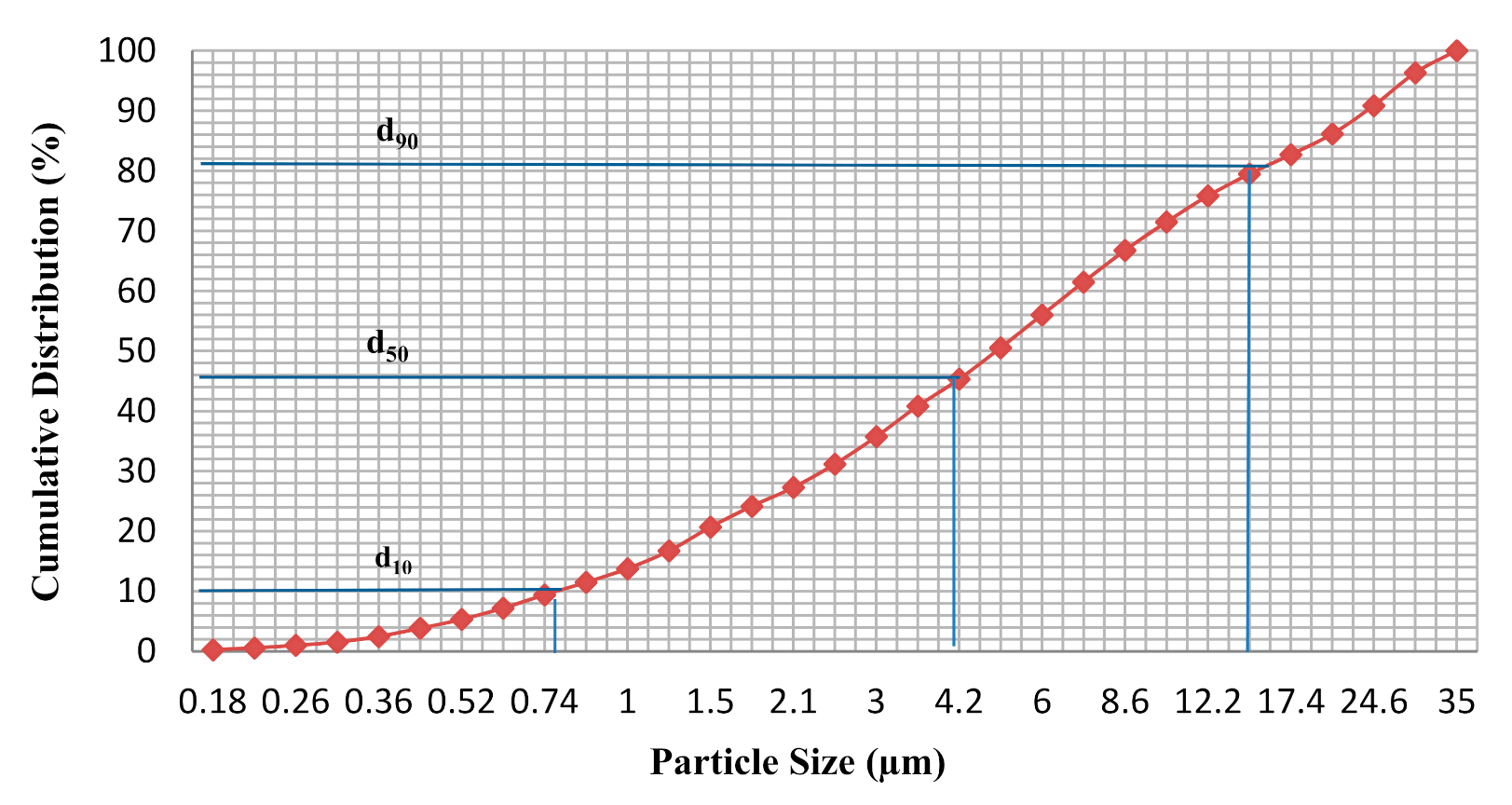
2.3. Testing Methods
3. Results and Discussion
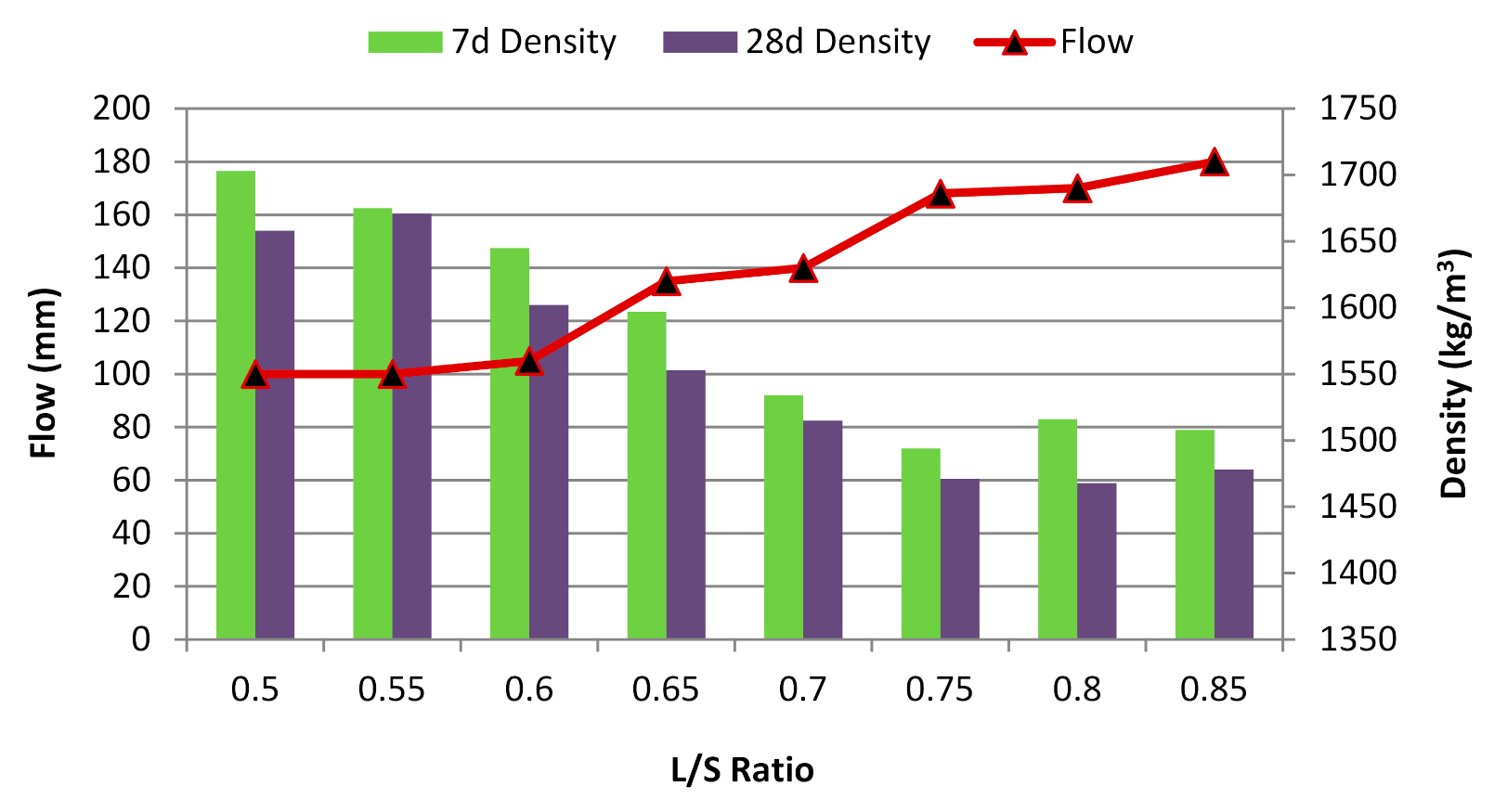
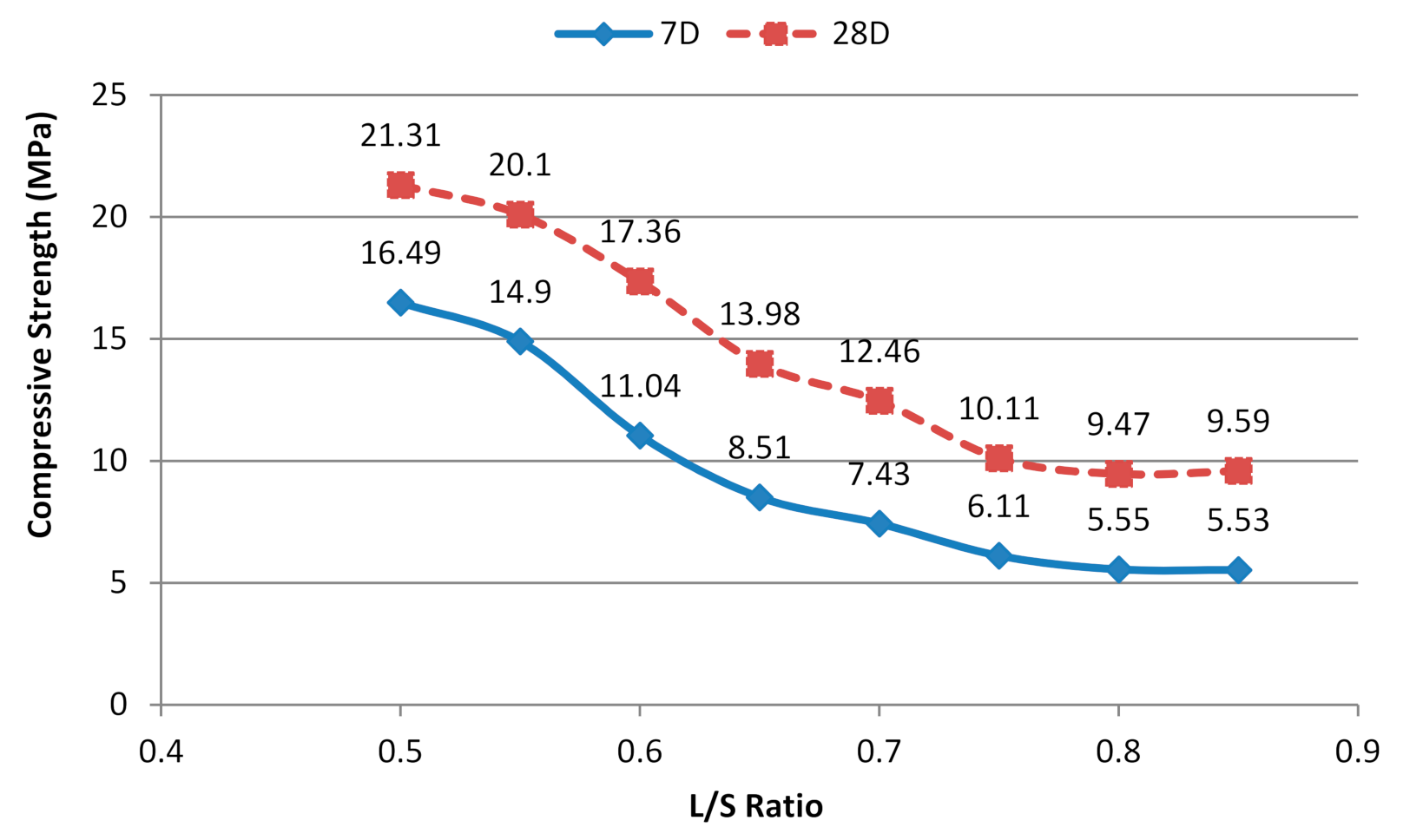
3.1. Effect of L/S Ratio
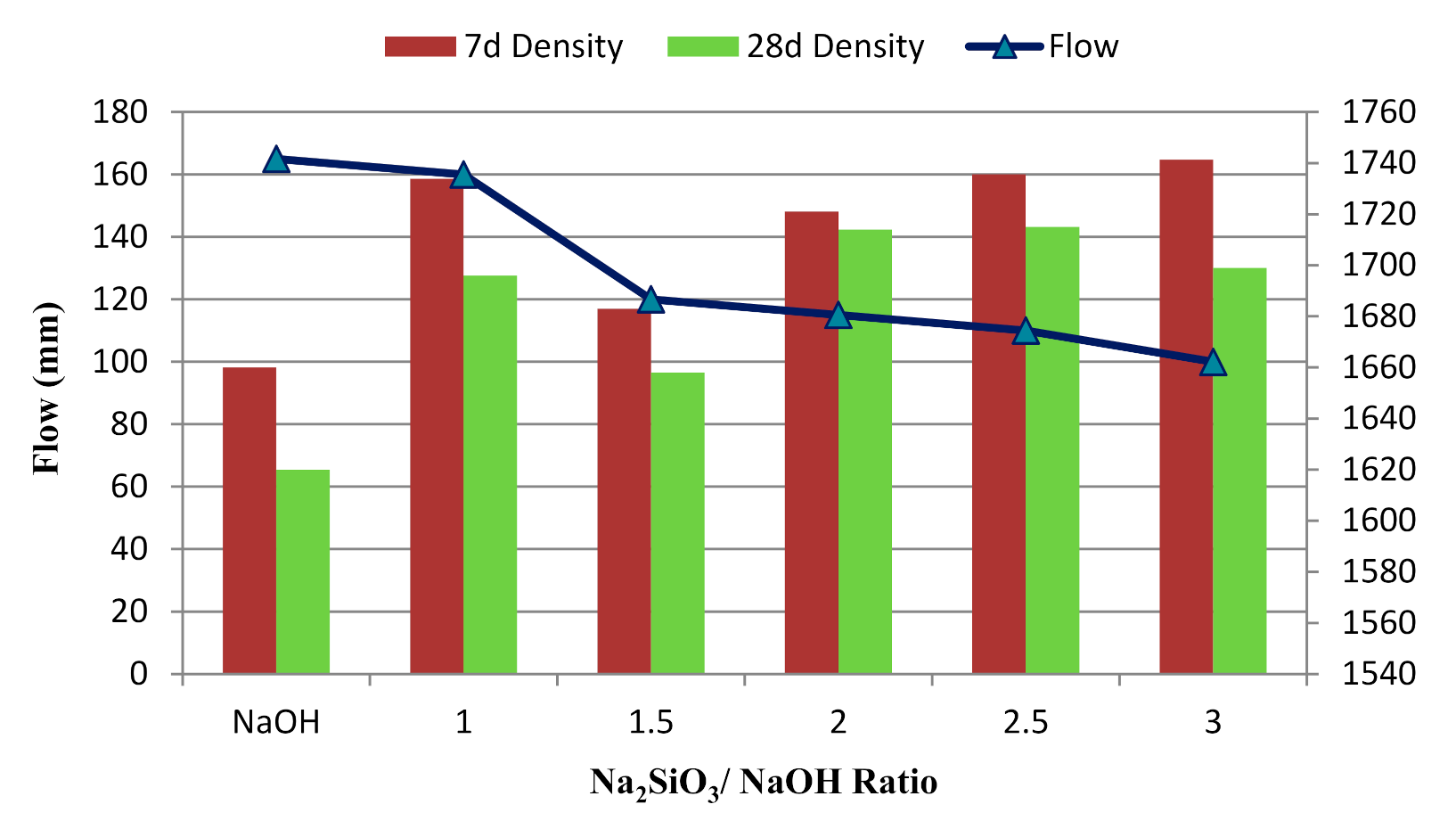
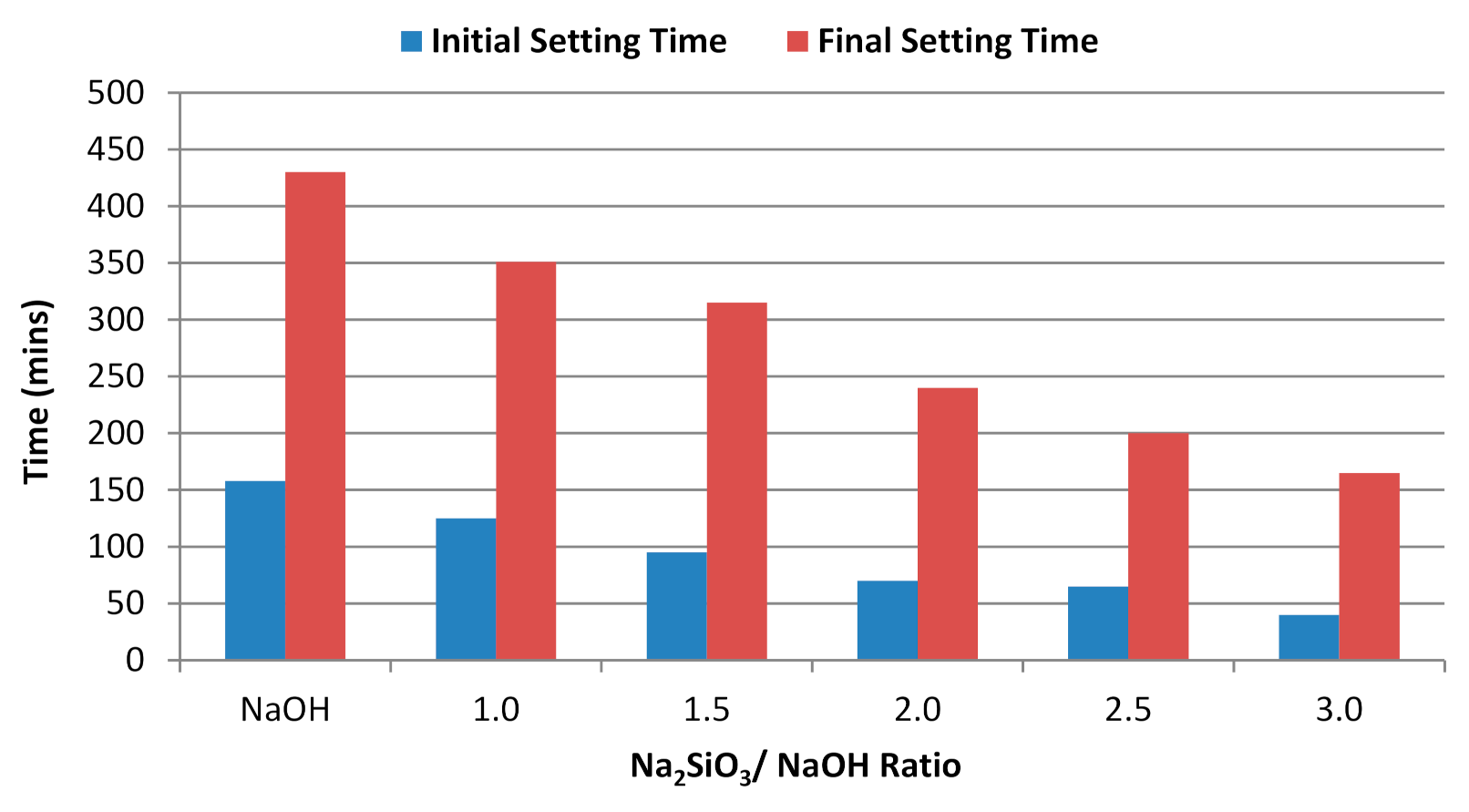
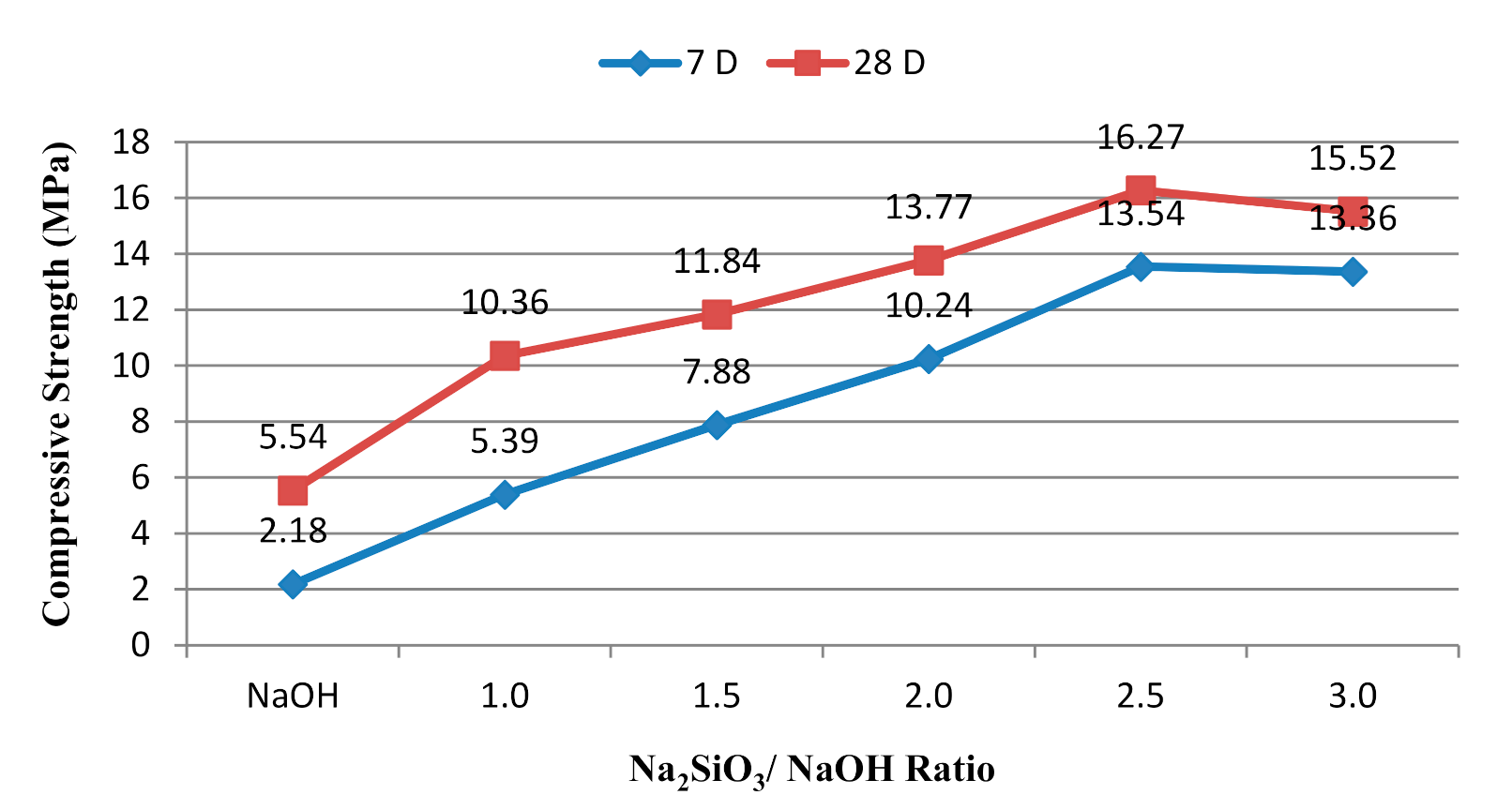
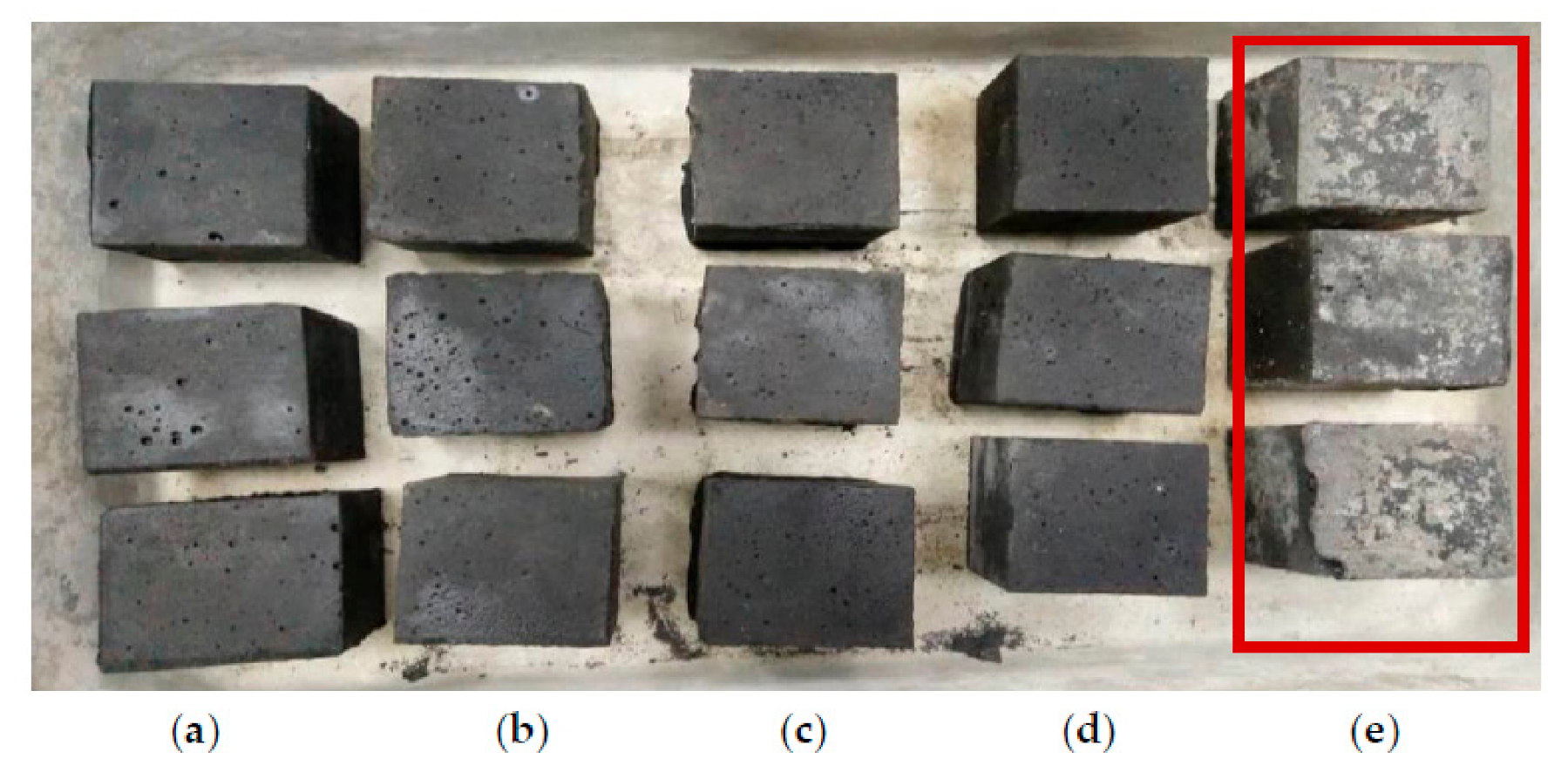
3.2. Effect of Alkaline Activator Ratio
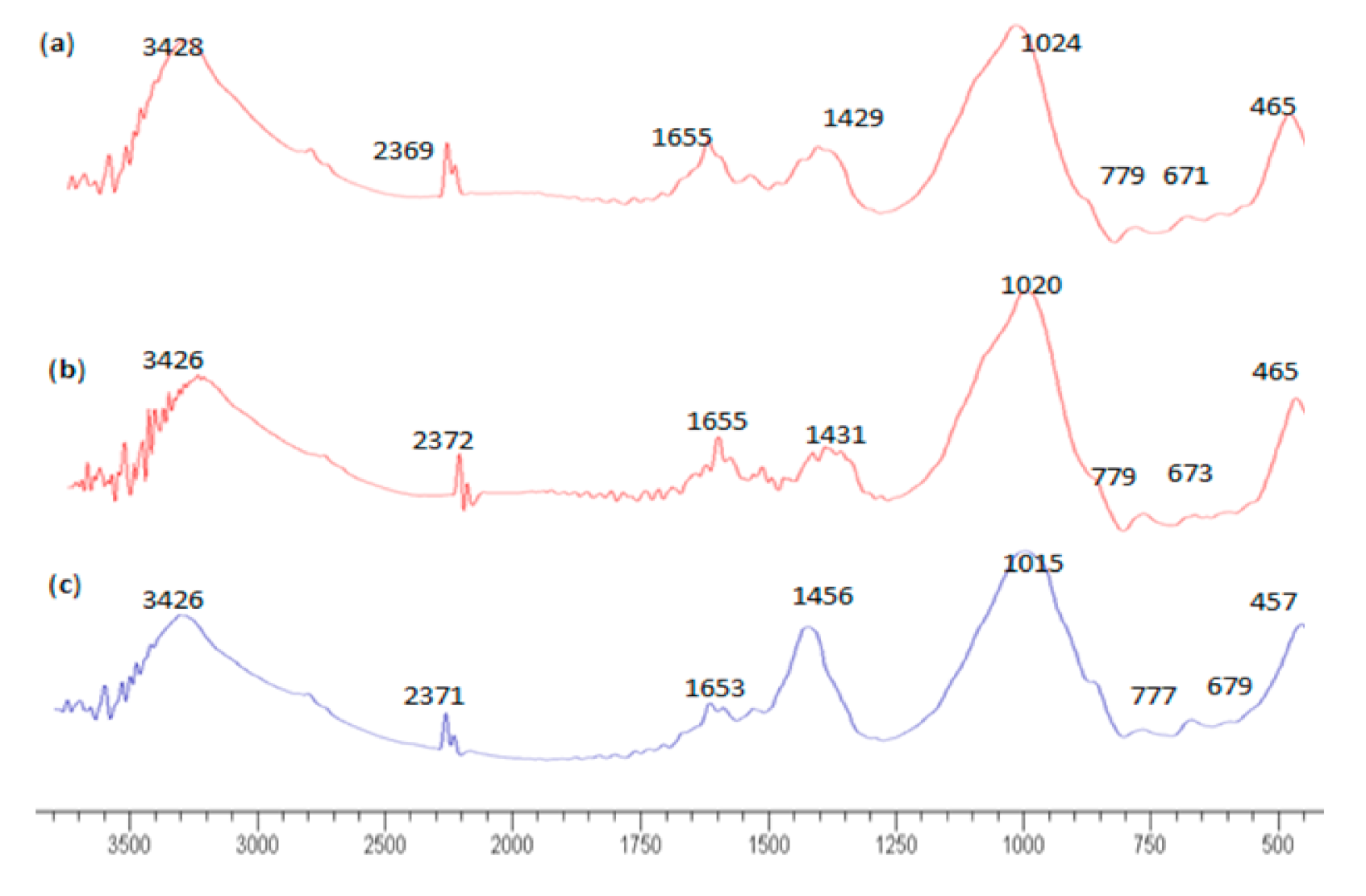
3.3. FTIR Analysis
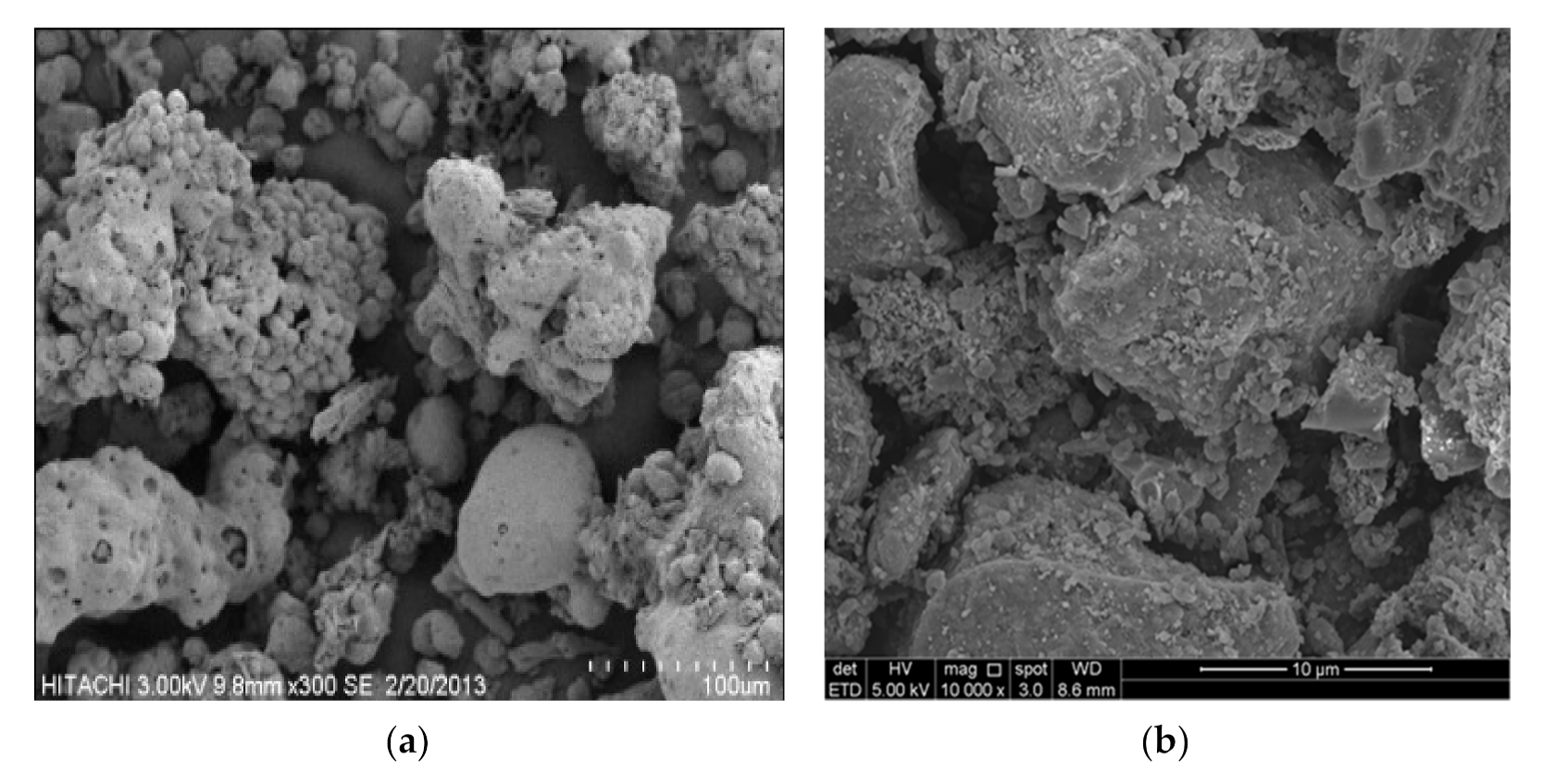
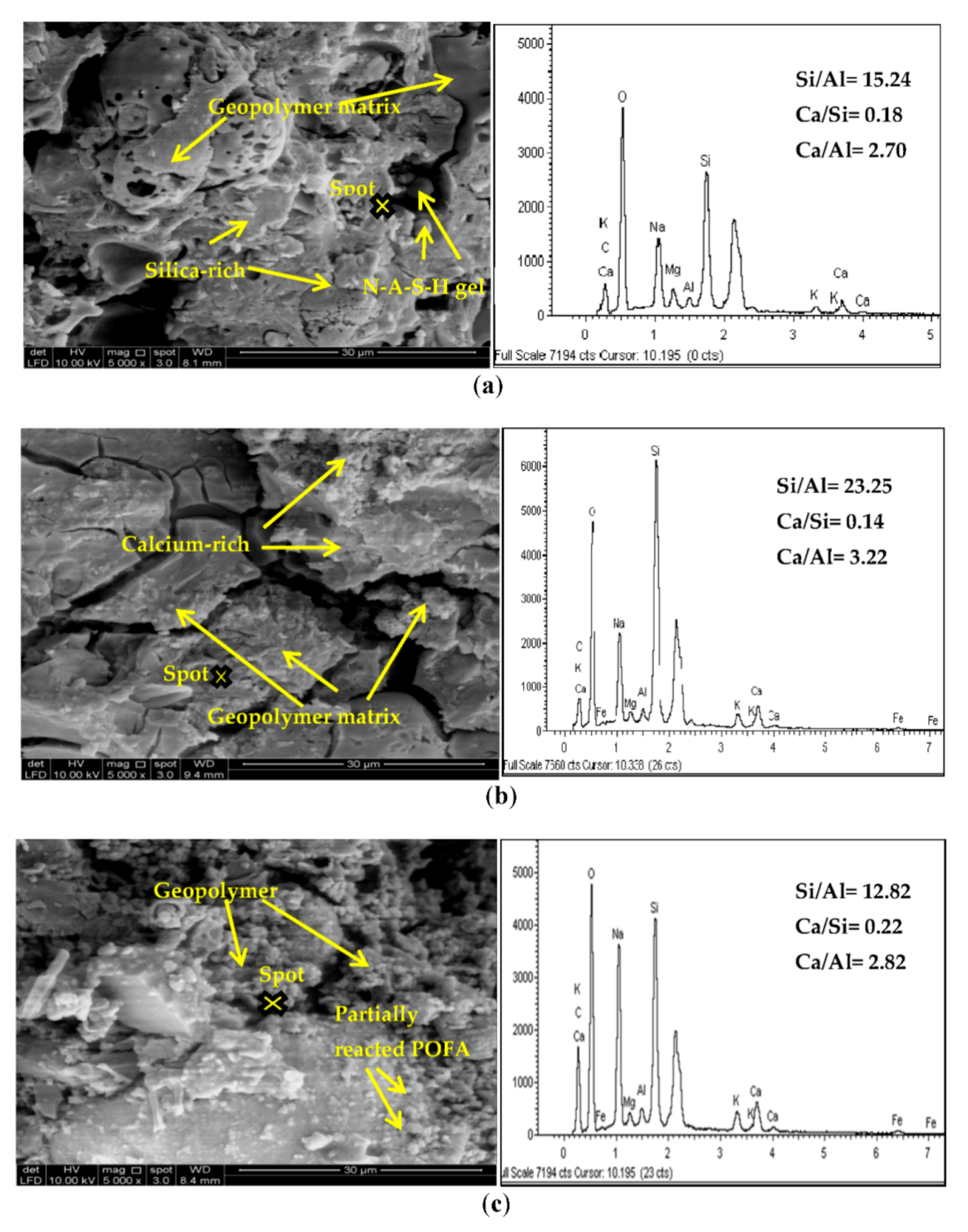
3.4. Morphological Analysis by SEM and EDX
4. Conclusions
- The L/S and alkaline activator ratios are the most important parameters that influence the physical and compressive performance of alkali-activated POFA geopolymer paste.
- At different L/S ratios, the alkali-activated POFA geopolymer’s flowability was inversely proportional to the compressive strength due to the excess liquid content, where it provides extra solution to react with the POFA for hydration.
- For the Na2SiO3/NaOH ratio, the alkali-activated POFA geopolymer’s flowability was directly proportional to compressive strength. A suitable alkaline activator ratio will provide an alkaline environment and inhibit polymerisation. However, when the ratio of alkaline activator mixture exceeds three, it retards the reaction. This is due to the excessive silicate ion content in the geopolymerisation reaction. Moreover, the formation of efflorescence obviously affects the performance of the alkali-activated POFA geopolymer, which occurs as result of a relatively high proportion of NaOH in the activator.
- In the early stages, the alkali-activated POFA geopolymer paste that was cured at room temperature had a lower strength than samples cured under general geopolymer curing conditions. However, this paste still reached a comparable strength later on in the experiment. This alkali-activated POFA geopolymer can attain binder characteristics for use as construction materials, such as cement replacement.
- C-S-H binding gel and sodium aluminosilicate hydrates are dominant in the POFA activated by alkaline.
- Through observation by SEM and EDX imagery, we found that the compressive strength determined the performance of the samples made by using different mixtures. There was a large difference between Si/Al ratios with difference activator ratios defined from EDX, which concluded that high Si/Al ratios lead to a highly crystalline matrix. However, FTIR spectra indicated the existence of a C-S-H formation.
- The activator POFA has properties that give it the potential to be used as a sustainable building material. Its cementitious characteristic as a binder would provide the ideal outcome for the construction industry.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chindaprasirt, P.; Rattanasak, U. Fire-resistant geopolymer bricks synthesized from high-calcium fly ash with outdoor heat exposure. Clean. Technol. Environ. Policy 2018, 20, 1097–1103. [Google Scholar] [CrossRef]
- Liew, Y.; Kamarudin, H.; Al Bakri, A.M.; Binhussain, M.; Luqman, M.; Nizar, I. Influence of solids-to-liquid and activator ratios on calcined kaolin cement powder. Phys. Procedia 2011, 22, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Witjes, S.; Lozano, R. Towards a more Circular Economy: Proposing a framework linking sustainable public procurement and sustainable business models. Resour. Conserv. Recycl. 2016, 112, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Ez-zaki, H.; Bellotto, M.; Valentini, L.; Garbin, E.; Artioli, G. Influence of cellulose nanofibrils on the rheology, microstructure and strength of alkali activated ground granulated blast-furnace slag: A comparison with ordinary Portland cement. Mater. Struct. 2021, 54, 1–18. [Google Scholar] [CrossRef]
- Morla, P.; Gupta, R.; Azarsa, P.; Sharma, A. Corrosion Evaluation of Geopolymer Concrete Made with Fly Ash and Bottom Ash. Sustainability 2021, 13, 398. [Google Scholar] [CrossRef]
- Vitola, L.; Pundiene, I.; Pranckeviciene, J.; Bajare, D. The Impact of the Amount of Water Used in Activation Solution and the Initial Temperature of Paste on the Rheological Behaviour and Structural Evolution of Metakaolin-Based Geopolymer Pastes. Sustainability 2020, 12, 8216. [Google Scholar] [CrossRef]
- Razak, R.; Abdullah, M.; Hussin, K.; Ismail, K.; Hardjito, D.; Yahya, Z. Optimization of NaOH molarity, LUSI mud/alkaline activator, and Na2SiO3/NaOH ratio to produce lightweight aggregate-based geopolymer. Int. J. Mol. Sci. 2015, 16, 11629–11647. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Zhang, Z.; Zhu, H.; Chen, Y. Geopolymerization process of alkali–metakaolinite characterized by isothermal calorimetry. Thermochim. Acta 2009, 493, 49–54. [Google Scholar] [CrossRef]
- Salih, M.A.; Farzadnia, N.; Ali, A.A.A.; Demirboga, R. Effect of different curing temperatures on alkali activated palm oil fuel ash paste. Constr. Build. Mater. 2015, 94, 116–125. [Google Scholar] [CrossRef]
- ASTM C-109/C109M. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). In Annual Book of ASTM Standards Section; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Salih, M.A.; Ali, A.A.A.; Farzadnia, N. Characterization of mechanical and microstructural properties of palm oil fuel ash geopolymer cement paste. Constr. Build. Mater. 2014, 65, 592–603. [Google Scholar] [CrossRef]
- Memon, F.A.; Nuruddin, M.F.; Khan, S.; Shafiq, N.; Ayub, T. Effect of sodium hydroxide concentration on fresh properties and compressive strength of self-compacting geopolymer concrete. J. Eng. Sci. Technol. 2013, 8, 44–56. [Google Scholar]
- Awaluddin, A.; Ahmad, A.; Olivia, M. Parametric study on the compressive strength geopolymer paving block. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012018. [Google Scholar] [CrossRef]
- Hairi, S.N.M.; Jameson, G.N.; Rogers, J.J.; MacKenzie, K.J. Synthesis and properties of inorganic polymers (geopolymers) derived from Bayer process residue (red mud) and bauxite. J. Mater. Sci. 2015, 50, 7713–7724. [Google Scholar] [CrossRef]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patino, C.L. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Kastiukas, G.; Zhou, X. Effects of waste glass on alkali-activated tungsten mining waste: Composition and mechanical properties. Mater. Struct. 2017, 50, 194. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Van Deventer, J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, G.; Jeyasehar, C.; Kandasamy, S. Fly ash Based Geopolymer Concrete-A State of the Art Review. J. Eng. Sci. Technol. Rev. 2013, 6, 25–32. [Google Scholar] [CrossRef]
- Swanepoel, J.; Strydom, C. Utilisation of fly ash in a geopolymeric material. Appl. Geochem. 2002, 17, 1143–1148. [Google Scholar] [CrossRef]
- Hussin, M.W.; Ismail, M.A.; Budiea, A.; Muthusamy, K. Durability of high strength concrete containing palm oil fuel ash of different fineness. Malays. J. Civ. Eng. 2009, 21, 180–194. [Google Scholar]
- Zeyad, A.M.; Johari, M.A.M.; Tayeh, B.A.; Yusuf, M.O. Pozzolanic reactivity of ultrafine palm oil fuel ash waste on strength and durability performances of high strength concrete. J. Clean. Prod. 2017, 144, 511–522. [Google Scholar] [CrossRef]
- Salih, M.A.; Farzadnia, N.; Ali, A.A.A.; Demirboga, R. Development of high strength alkali activated binder using palm oil fuel ash and GGBS at ambient temperature. Constr. Build. Mater. 2015, 93, 289–300. [Google Scholar] [CrossRef]
- Hussin, M.W.; Muthusamy, K.; Zakaria, F. Effect of mixing constituent toward engineering properties of POFA cement-based aerated concrete. J. Mater. Civ. Eng. 2010, 22, 287–295. [Google Scholar] [CrossRef]
- Pourakbar, S.; Asadi, A.; Huat, B.B.; Fasihnikoutalab, M.H. Stabilization of clayey soil using ultrafine palm oil fuel ash (POFA) and cement. Transp. Geotech. 2015, 3, 24–35. [Google Scholar] [CrossRef]
- Karim, M.; Hossain, M.; Khan, M.; Zain, M.; Jamil, M.; Lai, F. On the utilization of pozzolanic wastes as an alternative resource of cement. Materials 2014, 7, 7809–7827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.Y.J.; Chua, C.P.; Alengaram, U.J.; Jumaat, M.Z. Utilization of palm oil fuel ash as binder in lightweight oil palm shell geopolymer concrete. Adv. Mater. Sci. Eng. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Amri, A.; Hendri, Y.B.; Malindo, E.; Rahman, M.M. Graphene Nanosheets (GNs) Addition on the Palm Oil Fuel Ash (POFA) Based Geopolymer with KOH Activator. J. Phys. Conf. Ser. 2019, 012101. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, N.; Behnia, A.; Alsubari, B.; Birgani, P.M.; Jumaat, M.Z. Durability and mechanical properties of self-compacting concrete incorporating palm oil fuel ash. J. Clean. Prod. 2016, 112, 723–730. [Google Scholar] [CrossRef]
- Eldagal, O.E.A.; Elmukhtar, O. Study on the Behaviour of High Strength Palm Oil Fuel Ash (POFA) Concrete; Universiti Teknologi Malaysia: Johor, Malaysia, 2008. [Google Scholar]
- Baltakys, K.; Jauberthie, R.; Siauciunas, R.; Kaminskas, R. Influence of modification of SiO2 on the formation of calcium silicate hydrate. Mater. Sci. 2007, 25, 663–670. [Google Scholar]
- Dulaimi, A.; Al Nageim, H.; Ruddock, F.; Seton, L. High performance cold asphalt concrete mixture for binder course using alkali-activated binary blended cementitious filler. Constr. Build. Mater. 2017, 141, 160–170. [Google Scholar] [CrossRef]
- Kwek, S.Y.; Awang, H. Utilisation of recycled silt from water treatment and palm oil fuel ash as geopolymer artificial lightweight aggregate. Sustainability 2021, 13, 6091. [Google Scholar] [CrossRef]
- Liu, M.Y.J.; Alengaram, U.J.; Santhanam, M.; Jumaat, M.Z.; Mo, K.H. Microstructural investigations of palm oil fuel ash and fly ash based binders in lightweight aggregate foamed geopolymer concrete. Constr. Build. Mater. 2016, 120, 112–122. [Google Scholar] [CrossRef]
- Huseien, G.F.; Hamzah, H.K.; Sam, A.R.M.; Khalid, N.H.A.; Shah, K.W.; Deogrescu, D.P. Alkali-activated mortars blended with glass bottle waste nano powder: Environmental benefit and sustainability. J. Clean. Prod. 2020, 243, 118636. [Google Scholar] [CrossRef]
- Alehyen, S.; Achouri, M.; Taibi, M. Characterization, microstructure and properties of fly ash-based geopolymer. J. Mater. Environ. Sci. 2017, 8, 1783–1796. [Google Scholar]
- Ranjbar, N.; Mehrali, M.; Alengaram, U.J.; Metselaar, H.S.C.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr. Build. Mater. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, J.; Kaur, M. Compressive strength of rice husk ash based geopolymer: The effect of alkaline activator. Constr. Build. Mater. 2018, 169, 188–192. [Google Scholar] [CrossRef]
- Singh, G.B.; Subramaniam, K.V. Evaluation of sodium content and sodium hydroxide molarity on compressive strength of alkali activated low-calcium fly ash. Cem. Concr. Compos. 2017, 81, 122–132. [Google Scholar] [CrossRef]
- Naganathan, S.; Jamali, S.; Silvadanan, S.; Chung, T.Y.; Nicolasselvam, M.F. Use of Bottom Ash and Fly Ash in Masonry Mortar. Constr. Build. Mater. 2016, 1. [Google Scholar] [CrossRef]
- ASTM C. 230. Standard Specification for Flow Table for Use in Tests of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Sena da Fonseca, B.; Castela, A.; Neves, R.; Duarte, R.; Galhano, C.; Montemor, M. Saving Raw Materials for Cement Manufacture and Reusing an Untreated Waste from the Petrochemical Industry. Resources 2018, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- ASTM C. 191. Test Method for Time of Setting of Hydraulic Cement by Vicat Needle. In 1995 Annual Book of ASTM Standards Section; ASTM International: West Conshohocken, PA, USA, 2004; Volume 4. [Google Scholar]
- Nazari, A.; Bagheri, A.; Riahi, S. Properties of geopolymer with seeded fly ash and rice husk bark ash. Mater. Sci. Eng. A 2011, 528, 7395–7401. [Google Scholar] [CrossRef]
- Zhang, M.; El-Korchi, T.; Zhang, G.; Liang, J.; Tao, M. Synthesis factors affecting mechanical properties, microstructure, and chemical composition of red mud–fly ash based geopolymers. Fuel 2014, 134, 315–325. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Lulu Enterprises Inc.: Morrisville, NC, USA, 2009; p. 574. [Google Scholar]
- Nadziri, N.; Ismail, I.; Hamdan, S. Binding gel characterization of alkali-activated binders based on palm oil fuel ash (POFA) and fly ash. J. Sustain. Cem. Based Mater. 2018, 7, 1–14. [Google Scholar] [CrossRef]
- Herbert Sinduja, J.; Sakthieswaran, N.; Shiny Brintha, G. Review on geopolymer concerte with different additives. Int. J. Eng. Res. 2015, 1, 21–31. [Google Scholar]
- Villa, C.; Pecina, E.T.; Torres, R.; Gómez, L. Geopolymer synthesis using alkaline activation of natural zeolite. Constr. Build. Mater. 2010, 24, 2084–2090. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Yahya, Z.; Abdul Razak, R. Fly ash-based geopolymer lightweight concrete using foaming agent. Int. J. Mol. Sci. 2012, 13, 7186–7198. [Google Scholar] [CrossRef]
- Ayeni, O. Performance of a Nigerian Metakaolin-Based Geopolymer as a Sustainable Building Material. Ph.D. Thesis, African University of Science and Technology, Garki, Auja, Nigeria, 2017. [Google Scholar]
- Škvára, F.; Šmilauer, V.; Hlaváček, P.; Kopecký, L.; Cilova, Z. A weak alkali bond in (N, K)–A–S–H gels: Evidence from leaching and modeling. Ceram. Silik. 2012, 56, 374–382. [Google Scholar]
- ASTM C. 267. Standard test methods for chemical resistance of mortars, grouts, and monolithic surfacings and polymer concretes. In Annual Book of ASTM Standards Section; ASTM International: West Conshohocken, PA, USA, 1997; Volume 04.05. [Google Scholar]
- Tchakouté, H.K.; Rüscher, C.H.; Kamseu, E.; Djobo, J.N.; Leonelli, C. The influence of gibbsite in kaolin and the formation of berlinite on the properties of metakaolin-phosphate-based geopolymer cements. Mater. Chem. Phys. 2017, 199, 280–288. [Google Scholar] [CrossRef]
- Amri, A.; Fathurrahman, G.; Najib, A.A.; Awaltanova, E. Composites of palm oil fuel ash (POFA) based geopolymer and graphene oxide: Structural and compressive strength. IOP Conf. Ser. Mater. Sci. Eng. 2018, 012063. [Google Scholar] [CrossRef] [Green Version]
- Hwang, C.L.; Huynh, T.P. Effect of alkali-activator and rice husk ash content on strength development of fly ash and residual rice husk ash-based geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Shubbar, A.A.; Jafer, H.; Dulaimi, A.; Hashim, K.; Atherton, W.; Sadique, M. The development of a low carbon binder produced from the ternary blending of cement, ground granulated blast furnace slag and high calcium fly ash: An experimental and statistical approach. Constr. Build. Mater. 2018, 187, 1051–1060. [Google Scholar] [CrossRef]
- Salih, M.A.; Ali, A.A.A.; Demirboga, R.; Al Bakri, M. Properties of fresh palm oil fuel ash based geopolymer material. Adv. Environ. Biol. 2013, 7, 3572–3580. [Google Scholar]
- Wen, J.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Yang, R. The complexation of rhizosphere and nonrhizosphere soil organic matter with chromium: Using elemental analysis combined with FTIR spectroscopy. Ecotoxicol. Environ. Saf. 2018, 154, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Runyut, D.A.; Robert, S.; Ismail, I.; Ahmadi, R.; Abdul Samat, N.A.S.B. Microstructure and Mechanical Characterization of Alkali-Activated Palm Oil Fuel Ash. J. Mater. Civ. Eng. 2018, 30, 04018119. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Maurina, S.; Conci, A.; Salviati, A.; Carturan, G.; Cocco, G. Bauxite ‘red mud’in the ceramic industry. Part 2: Production of clay-based ceramics. J. Eur. Ceram. Soc. 2000, 20, 245–252. [Google Scholar] [CrossRef]
- Kourti, I.; Amutha Rani, D.; Boccaccini, A.; Cheeseman, C. Geopolymers from DC plasma–treated air pollution control residues, metakaolin, and granulated blast furnace slag. J. Mater. Civ. Eng. 2010, 23, 735–740. [Google Scholar] [CrossRef]
- Jamo, H.; Noh, M.Z.; Ahmad, Z.A. Structural Analysis and Surface Morphology of a Treated Palm Oil Fuel Ash. In Proceedings of the Seminar Kebangsaan Aplikasi Sains dan Matematik (SKASM’13), Batu Pahat, Malaysia, 27–28 November 2013; pp. 65–70. [Google Scholar]
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Qureshi, M.N.; Ghosh, S. Effect of Si/Al ratio on engineering properties of alkali-activated GGBS pastes. Green Mater. 2014, 2, 123–131. [Google Scholar] [CrossRef]

| Mix | POFA (g) | Alkaline (g) | L/S |
|---|---|---|---|
| 1 | 900 | 450 | 0.50 |
| 2 | 900 | 495 | 0.55 |
| 3 | 900 | 540 | 0.60 |
| 4 | 900 | 585 | 0.65 |
| 5 | 900 | 630 | 0.70 |
| 6 | 900 | 675 | 0.75 |
| 7 | 900 | 720 | 0.80 |
| 8 | 900 | 765 | 0.85 |
| Mix | POFA(g) | Alkaline (g) | L/S | Na2SiO3/NaOH |
|---|---|---|---|---|
| 1 | 900 | 540 | 0.6 | 1.0 |
| 2 | 900 | 585 | 0.6 | 1.5 |
| 3 | 900 | 630 | 0.6 | 2.0 |
| 4 | 900 | 675 | 0.6 | 2.5 |
| 5 | 900 | 720 | 0.6 | 3.0 |
| FTIR Peak, cm−1 | Functional Group Assigned | ||
|---|---|---|---|
| Na2SiO3/NaOH = 1.0 | Na2SiO3/NaOH = 2.5 | NaOH | |
| 3428 | 3426 | 3426 | Stretching vibration O-H |
| 1655 | 1655 | 1653 | Bending vibration H-O-H |
| 1429 | 1431 | 1456 | Asymmetric stretching vibration O-C-O |
| 1024 | 1020 | 1015 | Asymmetric stretching vibration Si-O-T |
| 779 | 779 | 777 | Bending symmetric stretching vibration Si-O-Si |
| 671 | 673 | 679 | Bending symmetric Si-O of SiO4 |
| 465 | 465 | 457 | Bending vibration Si-O-Si and O-Si-O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwek, S.Y.; Awang, H.; Cheah, C.B. Influence of Liquid-to-Solid and Alkaline Activator (Sodium Silicate to Sodium Hydroxide) Ratios on Fresh and Hardened Properties of Alkali-Activated Palm Oil Fuel Ash Geopolymer. Materials 2021, 14, 4253. https://doi.org/10.3390/ma14154253
Kwek SY, Awang H, Cheah CB. Influence of Liquid-to-Solid and Alkaline Activator (Sodium Silicate to Sodium Hydroxide) Ratios on Fresh and Hardened Properties of Alkali-Activated Palm Oil Fuel Ash Geopolymer. Materials. 2021; 14(15):4253. https://doi.org/10.3390/ma14154253
Chicago/Turabian StyleKwek, Shi Ying, Hanizam Awang, and Chee Ban Cheah. 2021. "Influence of Liquid-to-Solid and Alkaline Activator (Sodium Silicate to Sodium Hydroxide) Ratios on Fresh and Hardened Properties of Alkali-Activated Palm Oil Fuel Ash Geopolymer" Materials 14, no. 15: 4253. https://doi.org/10.3390/ma14154253
APA StyleKwek, S. Y., Awang, H., & Cheah, C. B. (2021). Influence of Liquid-to-Solid and Alkaline Activator (Sodium Silicate to Sodium Hydroxide) Ratios on Fresh and Hardened Properties of Alkali-Activated Palm Oil Fuel Ash Geopolymer. Materials, 14(15), 4253. https://doi.org/10.3390/ma14154253