Nano-Porous-Silicon Powder as an Environmental Friend
Abstract
:1. Introduction
2. Materials and Methods
2.1. NPS Powder Production and Characterization
2.2. Positron Annihilation Lifetime Measurements
2.3. Doppler Broadening Measurements
2.4. Dye Decolorization Using the Batch Procedure
3. Results and Discussion
3.1. NPS Powder Characterization
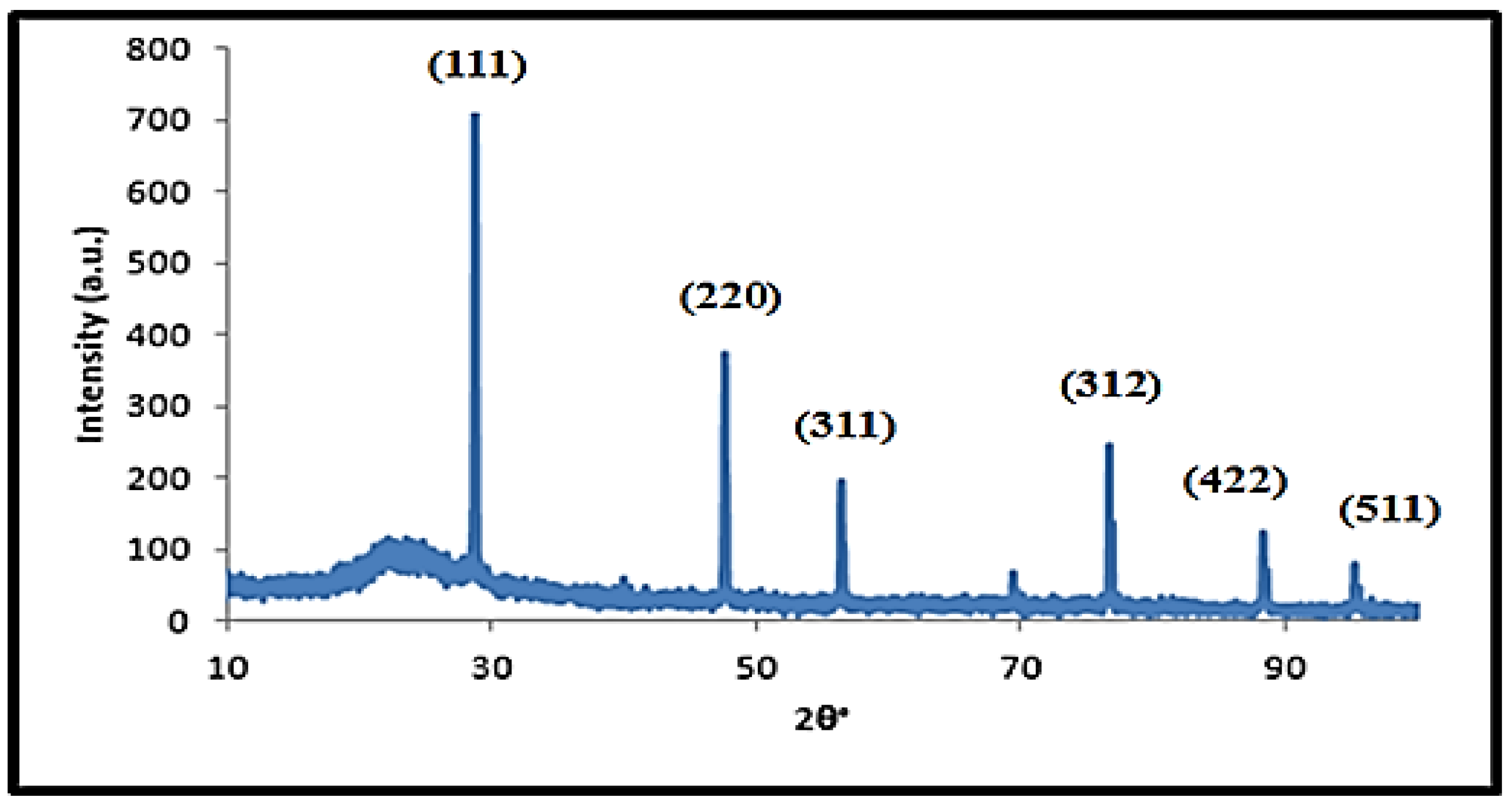
3.1.1. X-ray Diffraction Analyses
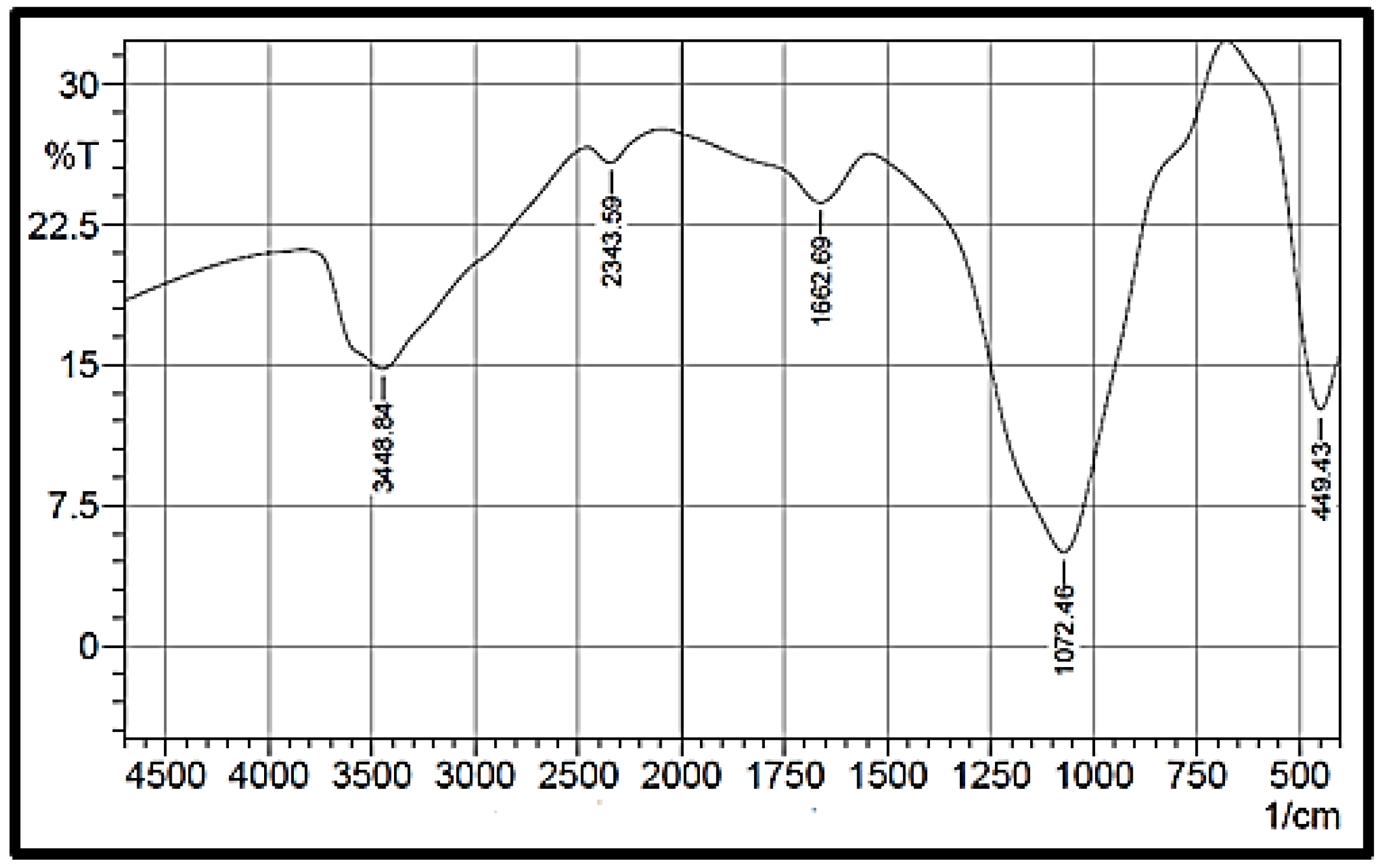
3.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
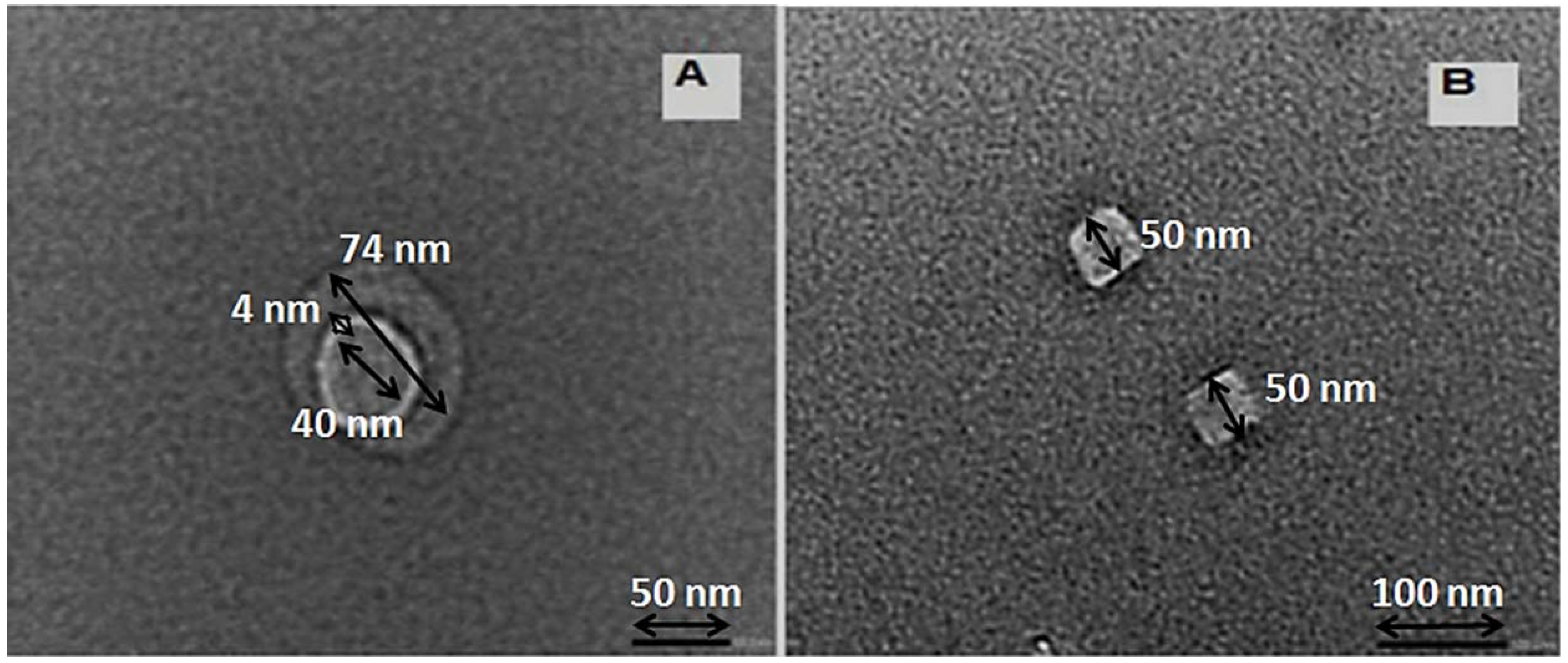
3.1.3. Transmission Electron Microscopic Analyses (TEM)
3.2. Positron Annihilation Lifetime (PAL) Parameters
3.3. Doppler Broadening Spectroscopy Measurements
3.4. Basic Dyes Decolourization Process onto the Synthesized NPS Powder Using a Batch Adsorption Technique
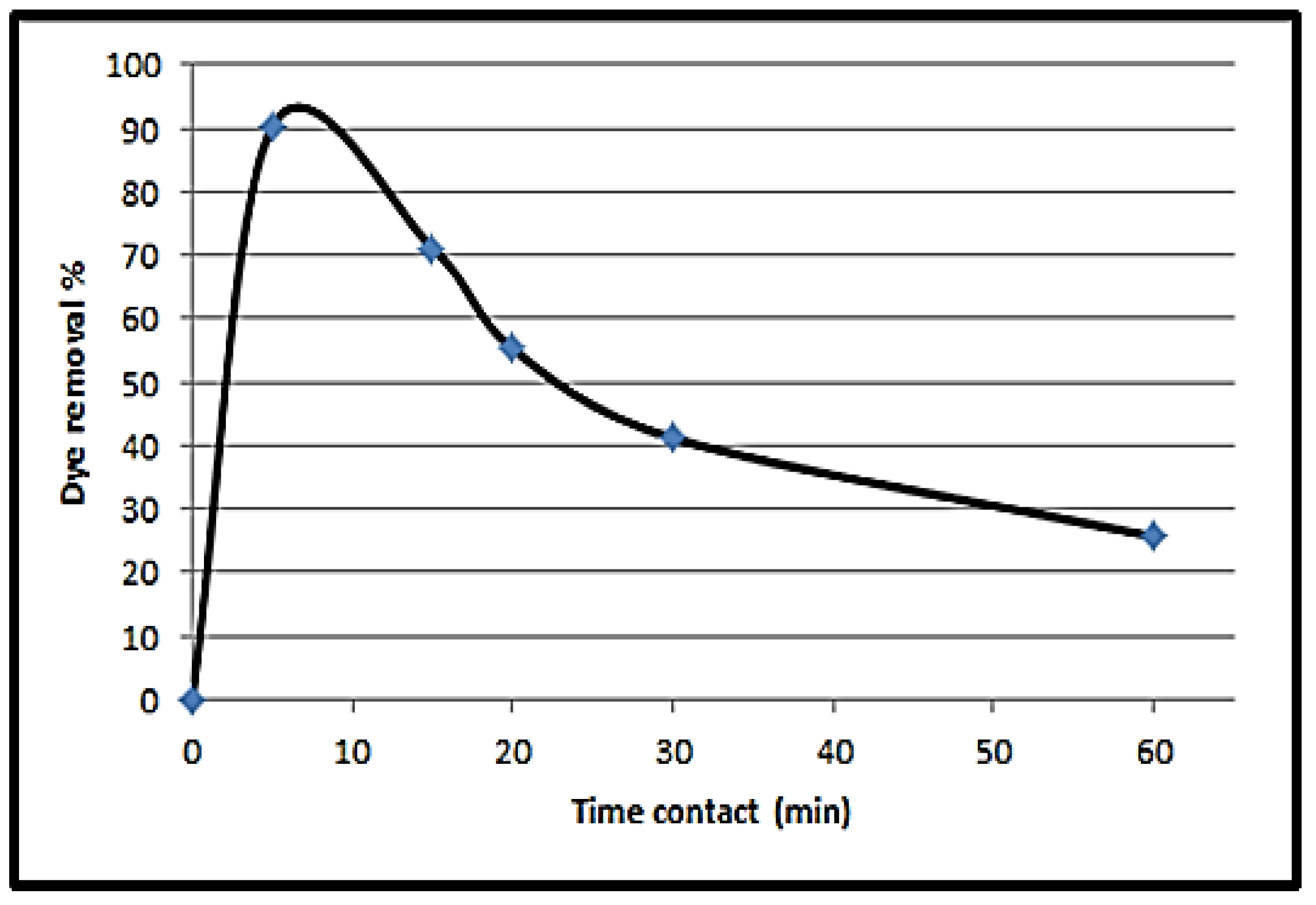
3.4.1. Effect of Contact Time
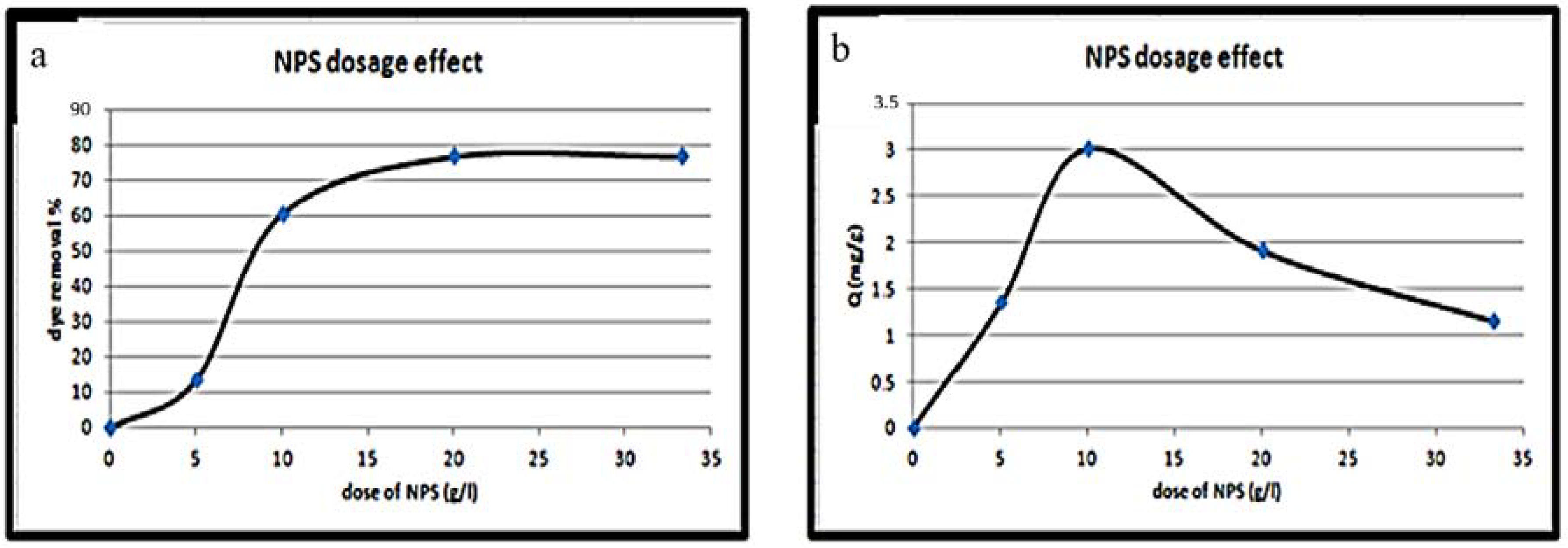
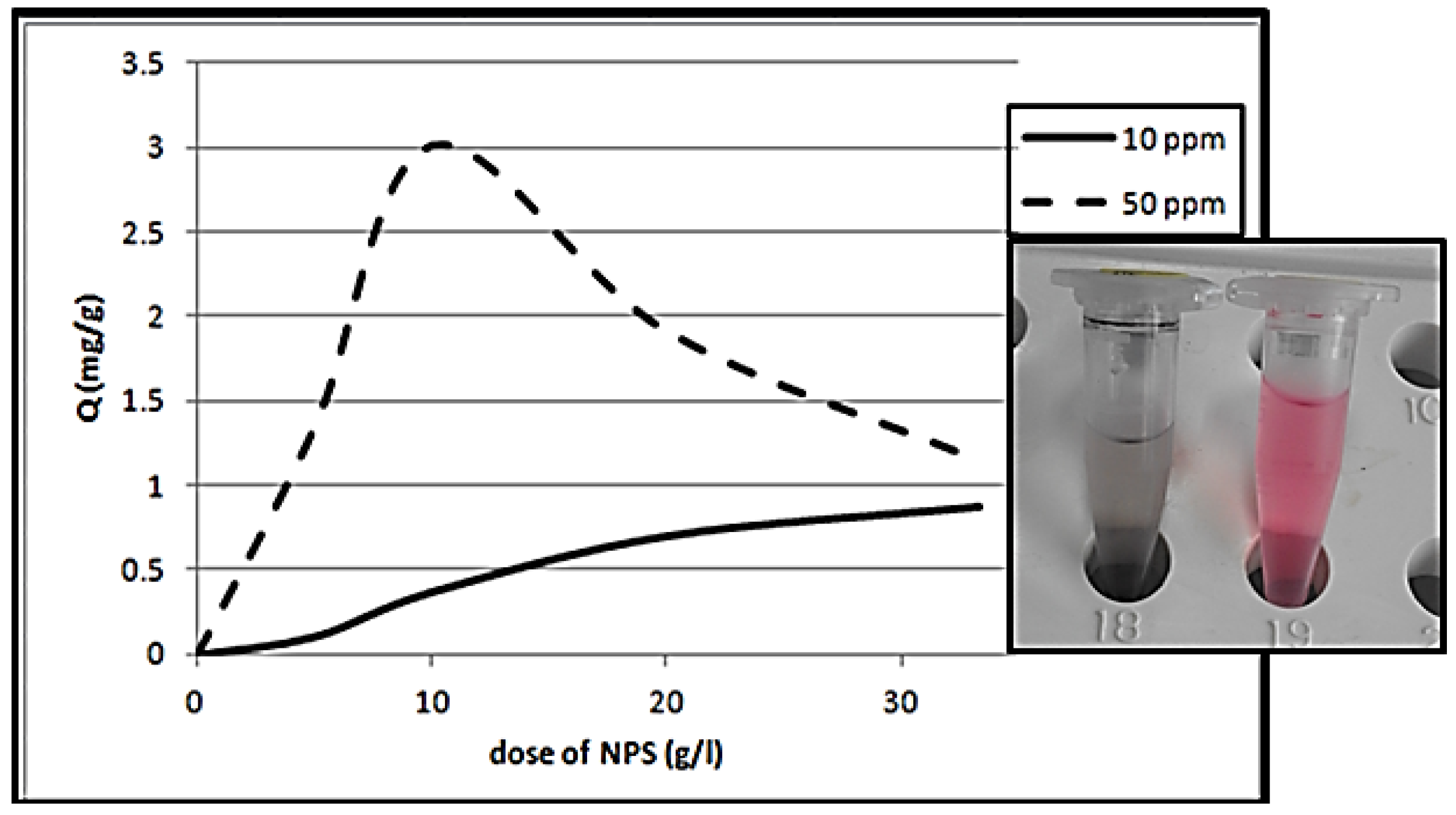
3.4.2. Effect of NPS Powder Dosage
3.4.3. Initial Dye Concentration Impact
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiménez, J.J.; Algarra, M.; Guimarães, V.; Bobos, I.; Rodríguez-Castellón, E. The application of functionalized pillared porous phosphate heterostructures for the removal of textile dyes from wastewater. Materials 2017, 10, 1111. [Google Scholar] [CrossRef] [Green Version]
- Anjaneyulu, Y.; Chary, N.S.; Raj, D.S.S. Decolourization of industrial effluents—Available methods and emerging technologies—A review. Rev. Environ. Sci. Bio/Technol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Grégorio, C. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar]
- Abd El-Latif, M.M.; Ibrahim, A.M.; El-Kady, M.F. Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. J. Am. Sci. 2010, 6, 267–283. [Google Scholar]
- Kay, G.M. The adsorption of dyestuffs from aqueous solution using activated carbon: Analytical solution for batch adsorption based on external mass transfer. Chem. Eng. J. 1983, 27, 187–196. [Google Scholar]
- Kay, G.M. Analytical solution using a pore diffusion model for a pseudo irreversible isotherm for the adsorption of basic dye on silica. AIChE. J. 1984, 30, 692–697. [Google Scholar]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (Chitosan zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids. Surf. B Biointerfaces 2010, 80, 86–93. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Newman, A.P.; Al-Daffaee, H.; Phull, S.; Cresswell, N.; York, S. Deposition of anatase on the surface of activated carbon. Surf. Coat. Technol. 2004, 187, 284–292. [Google Scholar] [CrossRef]
- Kashyout, A.H.; Soliman, H.M.A.; Nabil, M.; Bishara, A.A. Fabrication of congo red/oxidized porous silicon (CR/OPS) pH-sensors. Mater. Sci. Appl. 2013, 4, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Pastor, E.; Balaguer, M.; Bychto, L.; Salonen, J.; Lehto, V.P.; Matveeva, E.; Chirvony, V. Porous silicon for photosensitized formation of singlet oxygen in water and in simulated body fluid: Two methods of modification by undecylenic acid. J. Nanosci. Nanotechnol. 2008, 8, 1–7. [Google Scholar] [CrossRef]
- Gidley, D.W.; Peng, H.G.; Vallery, R.S. Positron annihilation as a method to characterize porous materials. Ann. Rev. Mater. Res. 2006, 36, 49–79. [Google Scholar] [CrossRef] [Green Version]
- Eldrup, M.; Lightbody, D.; Sherwood, J.N. The temperature dependence of positron lifetimes in solid pivalic acid. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Tao, S.J.J. Positronium annihilation in molecular substances. Chem. Phys. 1972, 56, 5499–5510. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ujihira, Y. Application of positron annihilation to the characterization of zeolites. J. Phys. Chem. 1982, 86, 4446–4450. [Google Scholar] [CrossRef]
- Ito, K.; Nakanishi, H.; Ujihira, Y. Extension of the equation for the annihilation lifetime of ortho-positronium at a cavity larger than 1 nm in radius. J. Phys. Chem. B 1999, 103, 4555–4558. [Google Scholar] [CrossRef]
- Dutta, D.; Ganguly, B.; Gangopadhyay, D.; Mukherjee, T.; Dutta-Roy, B.J. General trends of positronium pick-off annihilation in molecular substances. Phys. Condens. Matter. 2002, 14, 7539–7549. [Google Scholar] [CrossRef]
- Mackenzie, I.K.; Eady, I.A.; Gingerich, R.R. The interaction between positrons and dislocations in copper and in an aluminum alloy. Phys. Lett. A 1970, 33, 279–280. [Google Scholar] [CrossRef]
- Dutta, D.; Pujari, P.K.; Sudarshan, K.; Sharma, S.K. Effect of confinement on the phase transition of benzene in nanoporous silica: A positron annihilation study. J. Phys. Chem. C 2008, 112, 19055–19060. [Google Scholar] [CrossRef]
- Grafutin, V.I.; Zaluzhnyi, A.G.; Kalugin, V.V.; Ilyukhina, O.V.; Myasishcheva, G.G.; Prokop’ev, E.P.; Timoshenkov, S.P.; Funtikov, Y.V.; Khmelevskii, N.O. On the feasibility of investigation of some defect and porous systems by means of positron annihilation spectroscopy. High Energy Chem. 2008, 42, 478–484. [Google Scholar] [CrossRef]
- Biasini, M.; Ferro, G.; Monge, M.A.; Franci, G.D.; Ferrara, V.L. Study of the structure of porous silicon via positron annihilation experiments. J. Phys. Condens. Matter. 2000, 12, 5961–5970. [Google Scholar] [CrossRef]
- Dannefaer, S.; Kerr, D.; Craigen, D.; Bretagnon, T.; Taliercio, T.; Foucaran, A. A positron annihilation investigation of porous silicon. J. Appl. Phys. 1996, 79, 9110–9117. [Google Scholar] [CrossRef]
- Itoh, Y.; Murakami, H.; Kinoshita, A. Positron annihilation in porous silicon. Appl. Phys. Lett. 1993, 63, 2798–2799. [Google Scholar] [CrossRef]
- Surowiec, Z.; Wiertel, M.; Zaleski, R.; Budzyński, M.; Goworek, J. Positron annihilation study of iron oxide nanoparticles in mesoporous silica MCM-41 template. Nukleonika 2010, 55, 91–96. [Google Scholar]
- Williams, J.F.; Guagliardo, P.; Sudarshan, K.; Ranganathaiah, C.; Koutsantonis, G.; Hondow, N.; Samarin, S. Positron annihilation studies of mesoporous silica MCM-41. J. Phys Conf. Ser. 2013, 443, 012063–012066. [Google Scholar] [CrossRef]
- Ismail, A.M.; Mahmoud, K.R.; Abd-El Salam, M.H. Electrical conductivity and positron annihilation characteristics of ternary silicone rubber/carbon black/TiB2 nanocomposites. Polym. Test. 2015, 48, 37–43. [Google Scholar] [CrossRef]
- El-meniawi, M.A.H.; Mahmoud, K.R.; Megahed, M. Positron annihilation spectroscopy and mechanical properties studies for epoxy matrices reinforced with different nanoparticles. J. Polym. Res. 2016, 23, 181–192. [Google Scholar] [CrossRef]
- Mahmoud, K.R.; Khodair, A.I.; Shaban, S.Y. Positron annihilation lifetime studies of changes in free volume on some biorelevant nitrogen heterocyclic compounds and their S-glycosylation. Appl. Radiat. Isotop. 2015, 105, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.R.; Refat, M.S.; Sharshar, T.; Adam, A.M.A.; Manaaa, E.S.A. Synthesis of amino acid iodine charge transfer complexes in situ methanolic medium: Chemical and physical investigations. J. Mol. Liq. 2016, 222, 1061–1067. [Google Scholar] [CrossRef]
- Shaban, S.Y.; Mahmoud, K.R.; Sharshar, T. Positron annihilation studies of bio-related N2S2-tetradentate ligands and their zinc complexes. Rad. Phys. Chem. 2013, 83, 12–15. [Google Scholar] [CrossRef]
- Mahmoud, K.R.; Al-Sigeny, S.; Sharshar, T.; El-Hamshary, H. Positron annihilation study on free volume of amino acid modified, starch-grafted acrylamide copolymer. Radiat. Phys. Chem. 2006, 75, 590–595. [Google Scholar] [CrossRef]
- Nabil, M.; Mahmoud, K.R.; Nomeir, R.; El-Maghraby, E.M.; Motaweh, H.A. 3D Porous Silicon (Nanorods Array, Nanosheets, and Nanoclusters) Production. Egypt. J. Chem. 2020, 63, 1269–1278. [Google Scholar] [CrossRef]
- Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl. Instrum. Meth. A 1996, 374, 235–244. [Google Scholar] [CrossRef]
- Mc-Gonigle, E.A.; Liggat, J.J.; Pethrick, R.A.; Jenkins, S.D.; Daly, G.H.; Hayward, D. Permeability of N2, Ar, He, O2 and CO2 through biaxially oriented polyester films dependence on free volume. Polymer 2001, 42, 2413–2426. [Google Scholar] [CrossRef]
- Porto, A.O.; Silva, G.G.; Magalhães, W.F. Free volume-size dependence on temperature and average molecular-weight in poly (ethyleneoxide) determined by positron annihilation life time spectroscopy. J. Polym. Sci. 1999, 37, 219–226. [Google Scholar] [CrossRef]
- Jean, Y.C.; Hong, X.; Liu, J.; Huang, C.M.; Cao, H.; Chung, C.Y.; Dai, G.H.; Cheng, K.L.; Hsinjin, Y. High sensitivity of positron annihilation lifetime to time and pressure effects in gas-exposed polymers. J. Radioanal. Nucl. Chem. 2005, 210, 513–518. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of copper (II), chromium (III), nickel (II) and lead (II) ions from aqueous solutions by meranti sawdust. J. Hazard. Mater. 2009, 170, 969–977. [Google Scholar] [CrossRef]
- Nabil, M.; Motaweh, H.A. Enhanced thermal stability of promising nano-porous silicon powder. Adv. Nanopart. 2016, 5, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Kashyout, A.H.; Soliman, H.M.A.; Nabil, M.; Bishara, A.A. Fabrication of nano-porous silicon using alkali etching process. Mater. Lett. 2013, 100, 184–187. [Google Scholar] [CrossRef]
- Dannefaer, S.; Wiebe, C.; Kerr, D. Positron annihilation investigation of porous silicon heat treated to 1000 °C. J. Appl. Phys. 1998, 84, 6559–6564. [Google Scholar] [CrossRef]
- Itoh, Y.; Murakami, H.; Kinoshita, A. Characterization of porous silicon by positron annihilation. J. Phys. IV Coll. 1993, 3, 193–195. [Google Scholar] [CrossRef]
- Idris, M.N.; Ahmad, Z.A.; Ahmad, M.A. Adsorption equilibrium of malachite green dye onto rubber seed coat based activated carbon. Int. J. Basic Appl. Sci. 2011, 11, 38–43. [Google Scholar]
- Mezenner, N.Y.; Bensmaili, A. Biosorption behavior of basic red 46 and violet 3 by dead pleurotus mutilus from single- and multicomponent systems. Chem. Eng. J. 2009, 147, 87–98. [Google Scholar] [CrossRef]
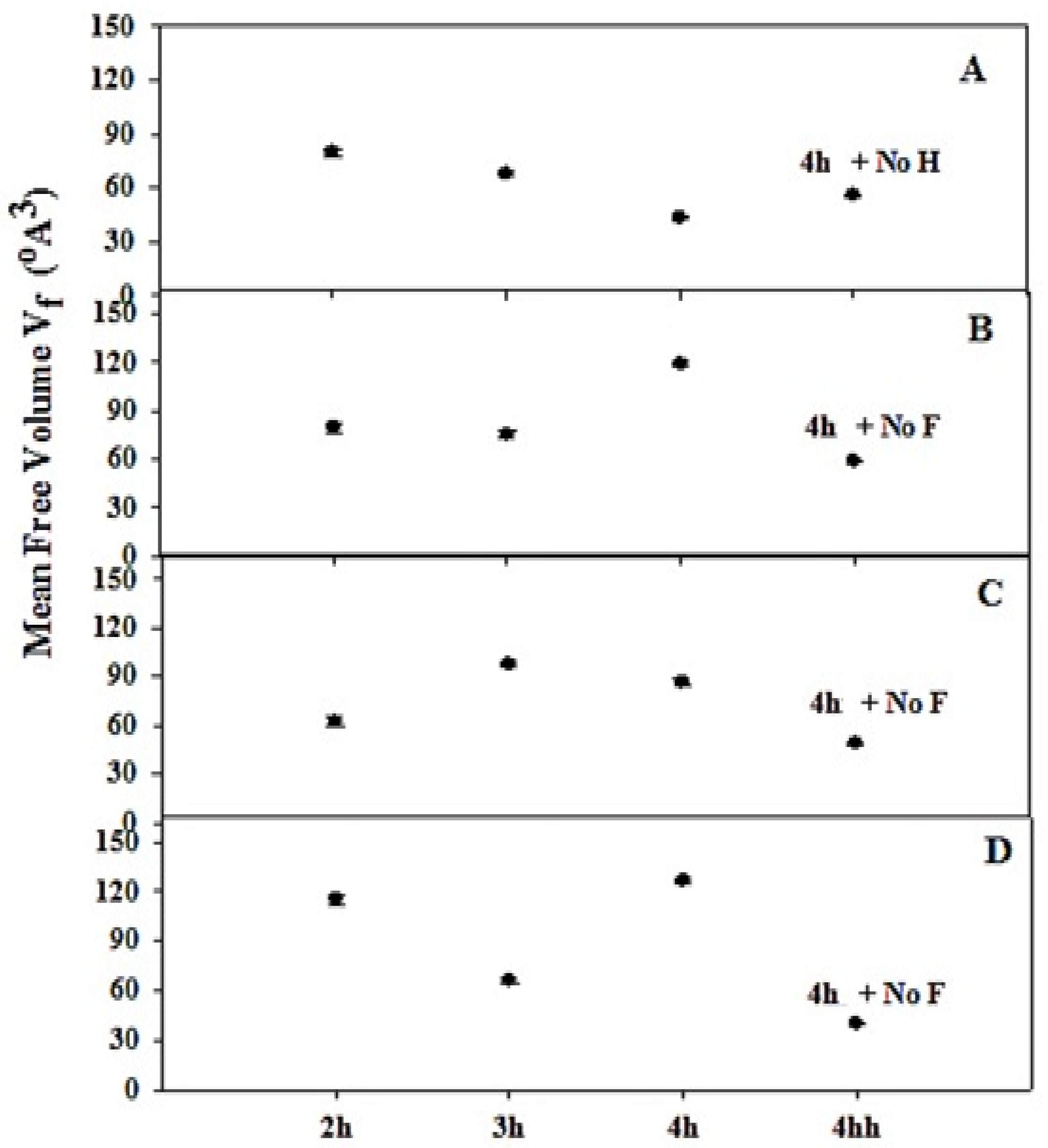
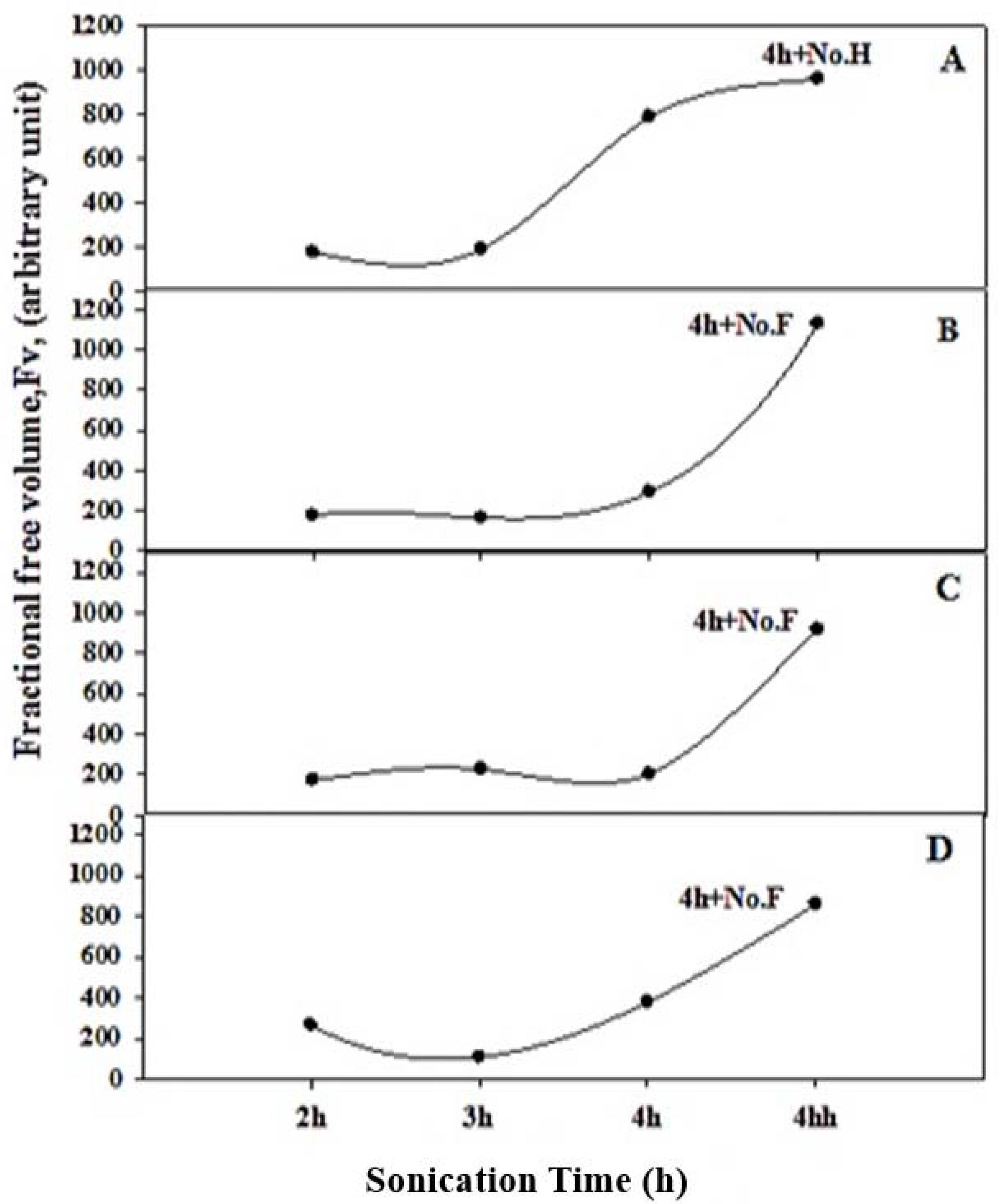
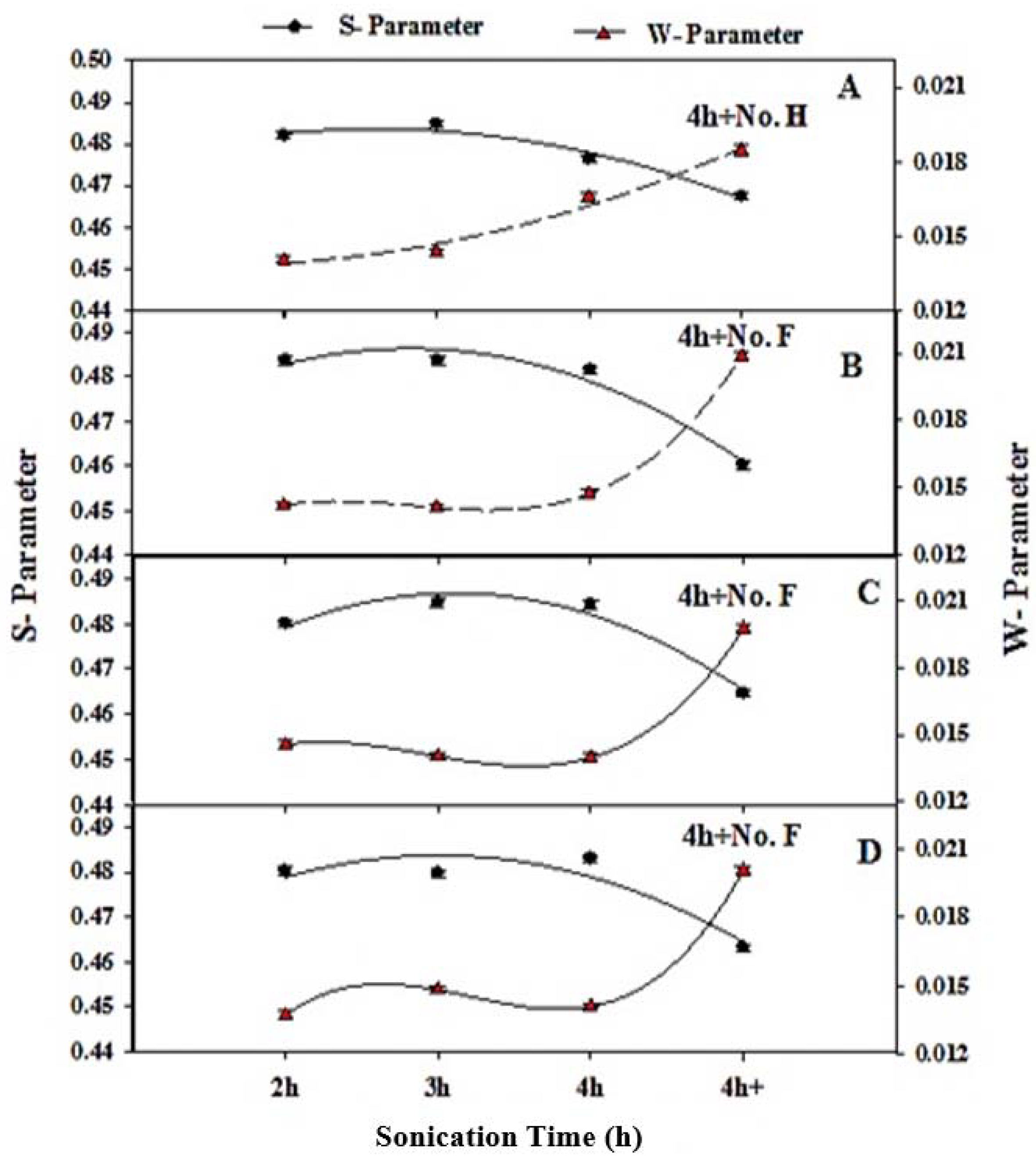
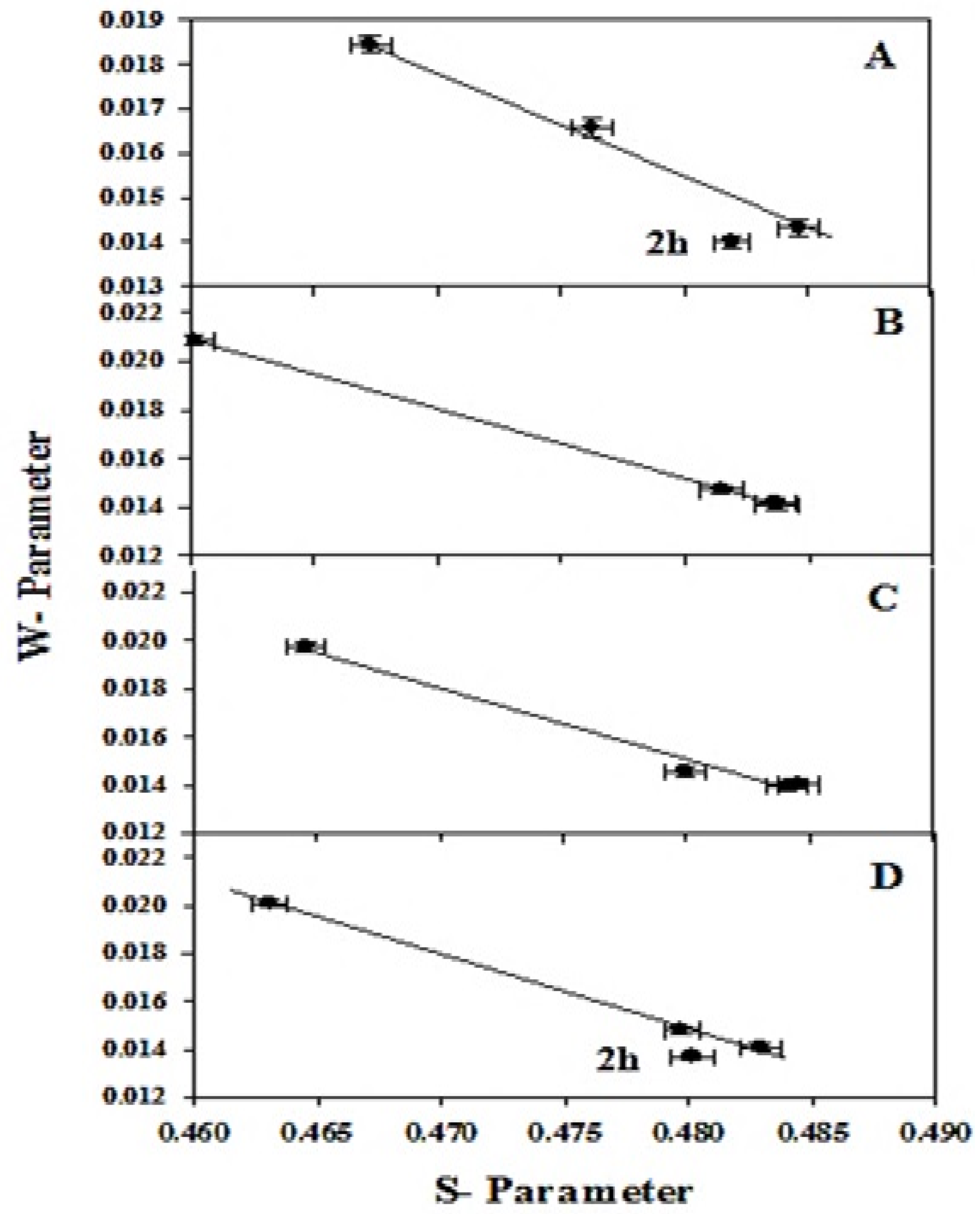
| Sample Composition | Sonication Time (h) | τ2 (ns) | τ3 (ns) | I1 (%) | I2 (%) | I3 (%) |
|---|---|---|---|---|---|---|
| 7 g Si Powder + 6 wt.% KOH | 2 | 0.359 ± 0.004 | 1.780 ± 0.055 | 57.22 ± 0.50 | 40.56 ± 0.50 | 2.21 ± 0.10 |
| 3 | 0.378 ± 0.002 | 1.648 ± 0.019 | 68.20 ± 0.17 | 29.00 ± 0.17 | 2.79 ± 0.043 | |
| 4 | 0.449 ± 0.004 | 1.334 ± 0.012 | 41.33 ± 0.19 | 39.61 ± 0.20 | 18.3 ± 0.59 | |
| 4 + No. H * | 0.008 ± 0.456 | 1.510 ± 0.013 | 40.01 ± 0.94 | 42.79 ± 0.86 | 17.2 ± 0.42 | |
| 7 g Si Powder + 4.5 wt.% KOH | 2 | 0.351 ± 0.004 | 1.78 ± 0.055 | 57.2 ± 0.50 | 40.6 ± 0.50 | 2.21 ± 0.10 |
| 3 | 0.369 ± 0.006 | 1.73 ± 0.043 | 63.3 ± 0.93 | 34.5 ± 0.93 | 2.18 ± 0.10 | |
| 4 | 0.372 ± 0.002 | 2.19 ± 0.026 | 55.8 ± 0.53 | 41.7 ± 0.53 | 2.45 ± 0.03 | |
| 4 + No. F * | 0.470 ± 0.009 | 1.54 ± 0.014 | 36.6 ± 0.83 | 44.2 ± 0.70 | 19.3 ± 0.43 | |
| 7 g Si Powder + 3 wt.% KOH | 2 | 0.376 ± 0.009 | 1.58 ± 0.080 | 61.0 ± 1.40 | 36.0 ± 1.5 | 2.79 ± 0.20 |
| 3 | 0.358 ± 0.004 | 1.97 ± 0.038 | 58.9 ± 1.00 | 38.8 ± 1.0 | 2.32 ± 0.08 | |
| 4 | 0.373 ± 0.007 | 1.86 ± 0.050 | 64.4 ± 1.00 | 33.3 ± 1.0 | 2.33 ± 0.11 | |
| 4 + No. F * | 0.497 ± 0.011 | 1.42 ± 0.015 | 43.9 ± 0.88 | 37.1 ± 0.8 | 18.9 ± 0.50 | |
| 5 g Si Powder + 3 wt.% KOH | 2 | 0.351 ± 0.003 | 2.15 ± 0.05 | 57.7 ± 0.95 | 40.06 ± 0.95 | 2.28 ± 0.07 |
| 3 | 0.353 ± 0.004 | 1.63 ± 0.05 | 62.7 ± 0.48 | 35.66 ± 0.48 | 1.63 ± 0.07 | |
| 4 | 0.382 ± 0.015 | 2.26 ± 0.026 | 64.4 ± 0.42 | 32.59 ± 0.42 | 2.99 ± 0.04 | |
| 4 + No. F * | 0.481 ± 0.014 | 1.31 ± 0.014 | 41.0 ± 0.89 | 37.49 ± 0.91 | 21.47 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabil, M.; Mahmoud, K.R.; Nomier, R.; El-Maghraby, E.-M.; Motaweh, H. Nano-Porous-Silicon Powder as an Environmental Friend. Materials 2021, 14, 4252. https://doi.org/10.3390/ma14154252
Nabil M, Mahmoud KR, Nomier R, El-Maghraby E-M, Motaweh H. Nano-Porous-Silicon Powder as an Environmental Friend. Materials. 2021; 14(15):4252. https://doi.org/10.3390/ma14154252
Chicago/Turabian StyleNabil, Marwa, Kamal Reyad Mahmoud, Raghda Nomier, El-Maghraby El-Maghraby, and Hussien Motaweh. 2021. "Nano-Porous-Silicon Powder as an Environmental Friend" Materials 14, no. 15: 4252. https://doi.org/10.3390/ma14154252
APA StyleNabil, M., Mahmoud, K. R., Nomier, R., El-Maghraby, E.-M., & Motaweh, H. (2021). Nano-Porous-Silicon Powder as an Environmental Friend. Materials, 14(15), 4252. https://doi.org/10.3390/ma14154252






