Macroporosity Control by Phase Separation in Sol-Gel Derived Monoliths and Microspheres
Abstract
1. Introduction and Motivation
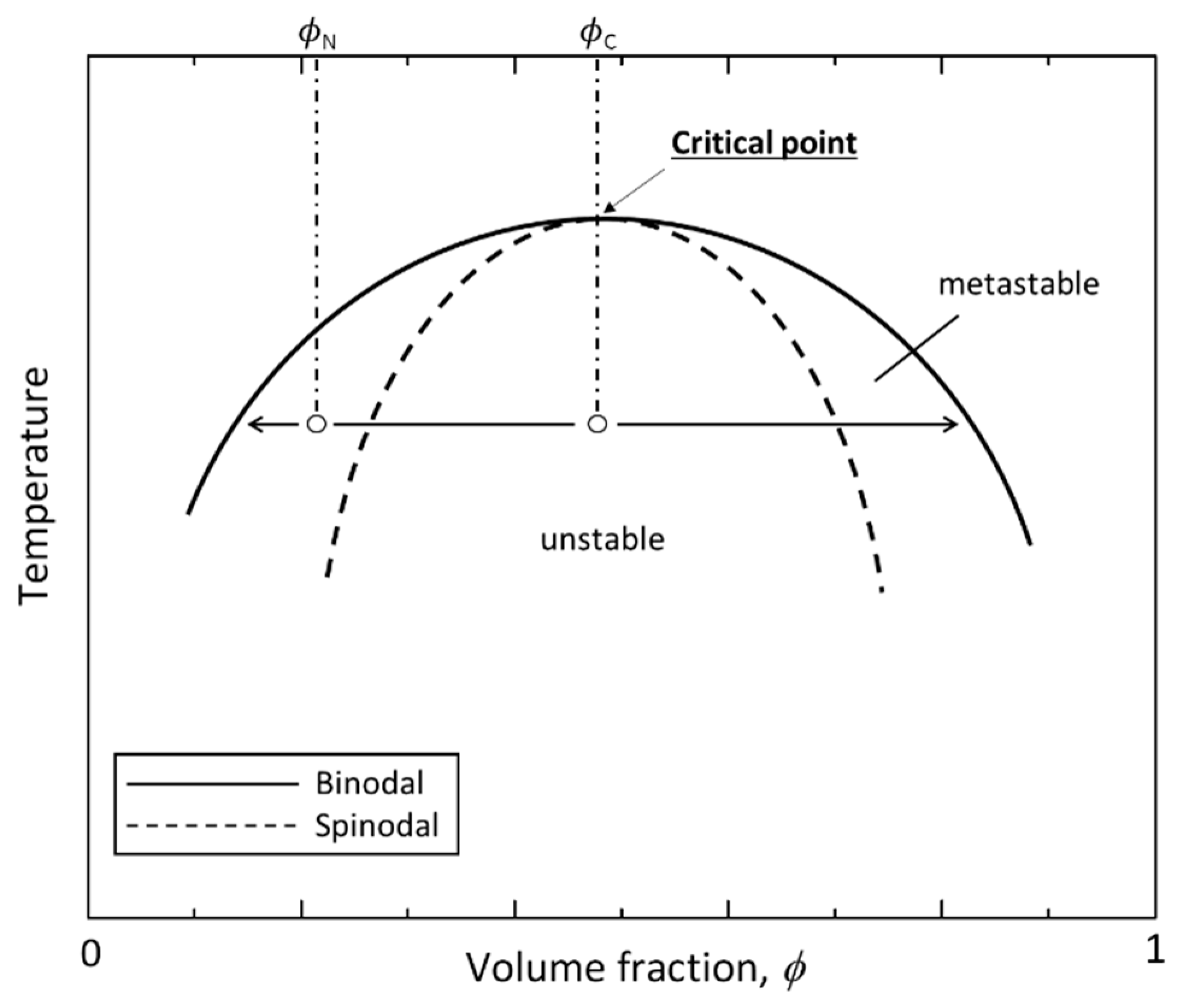
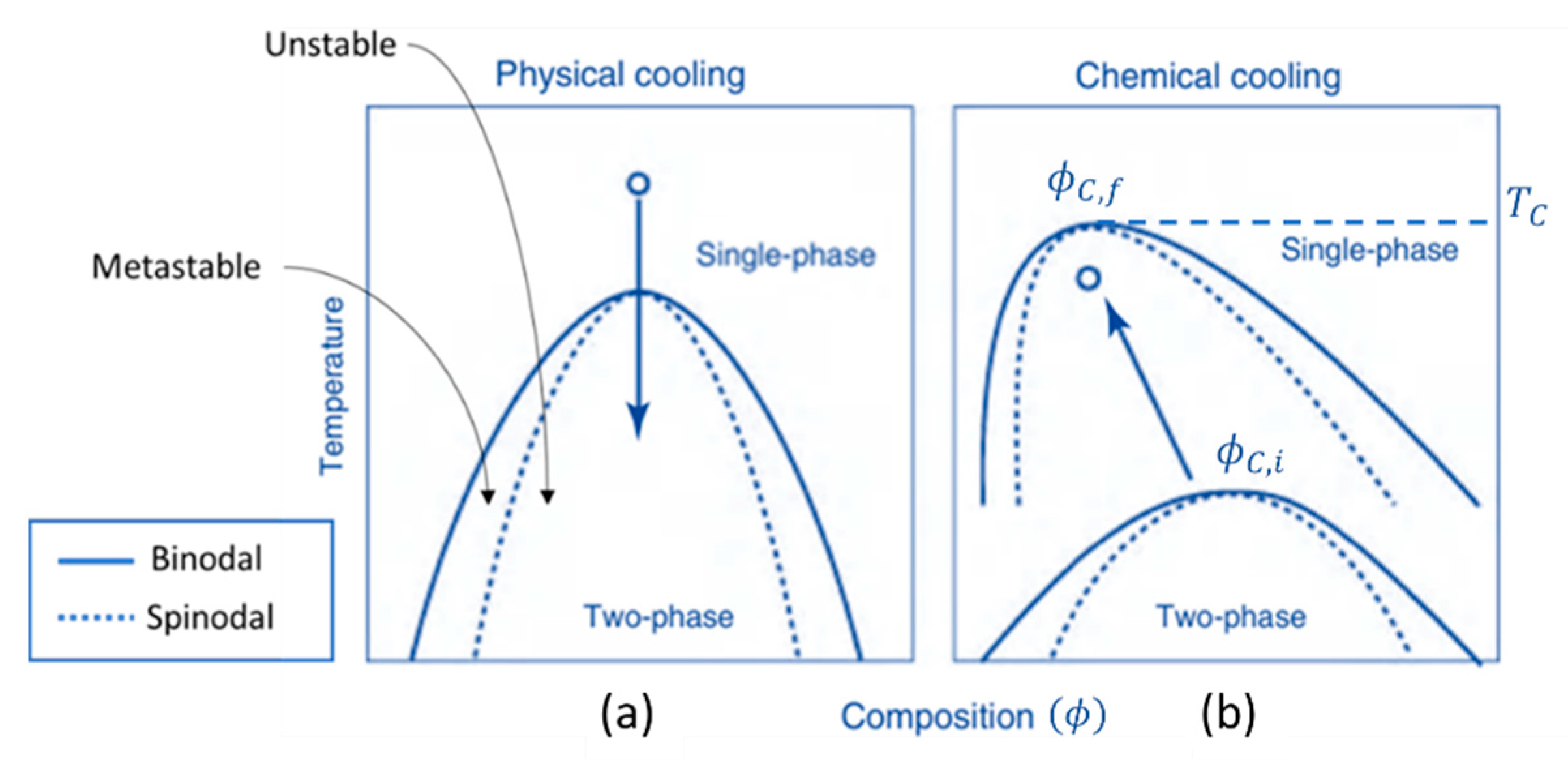
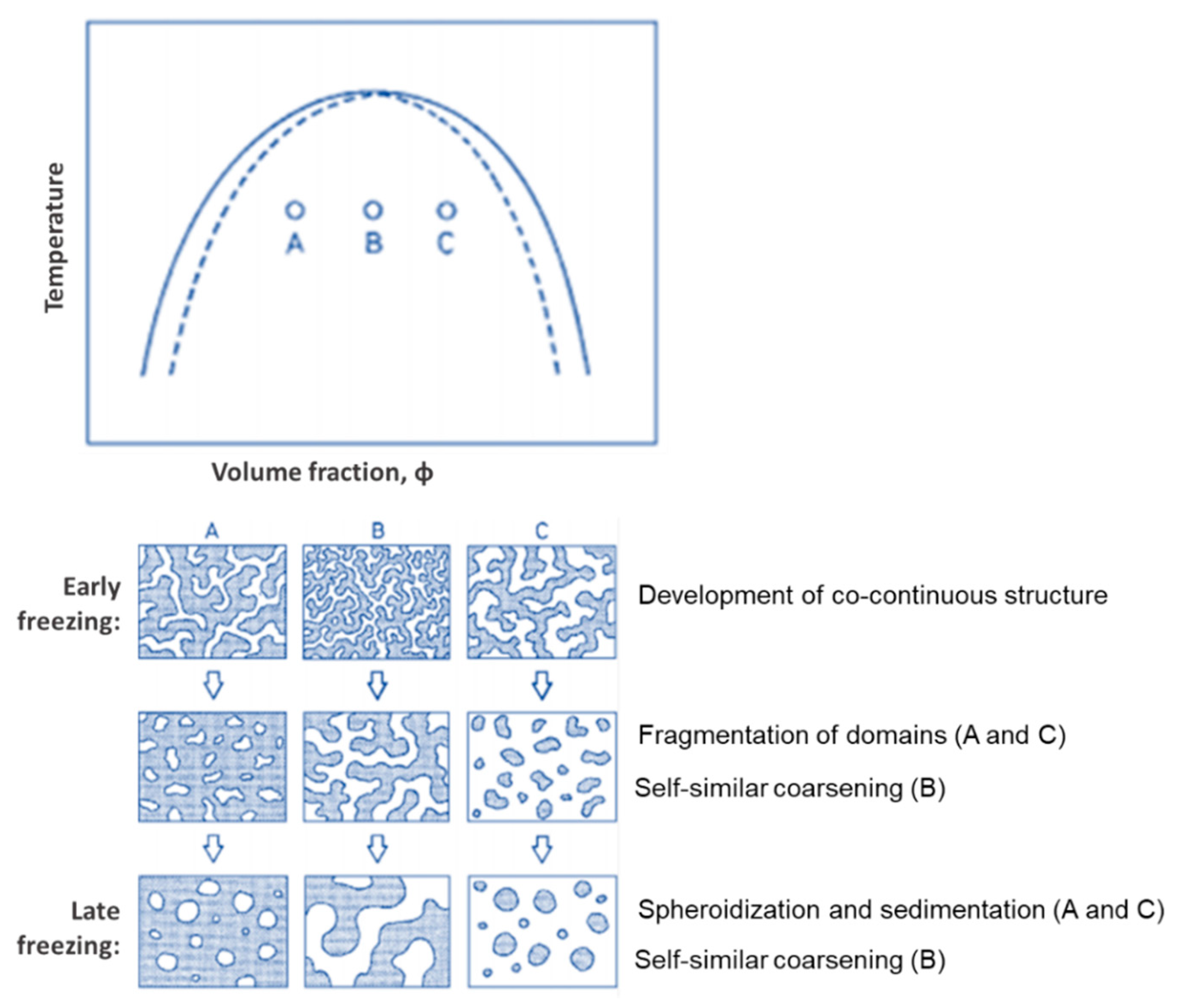
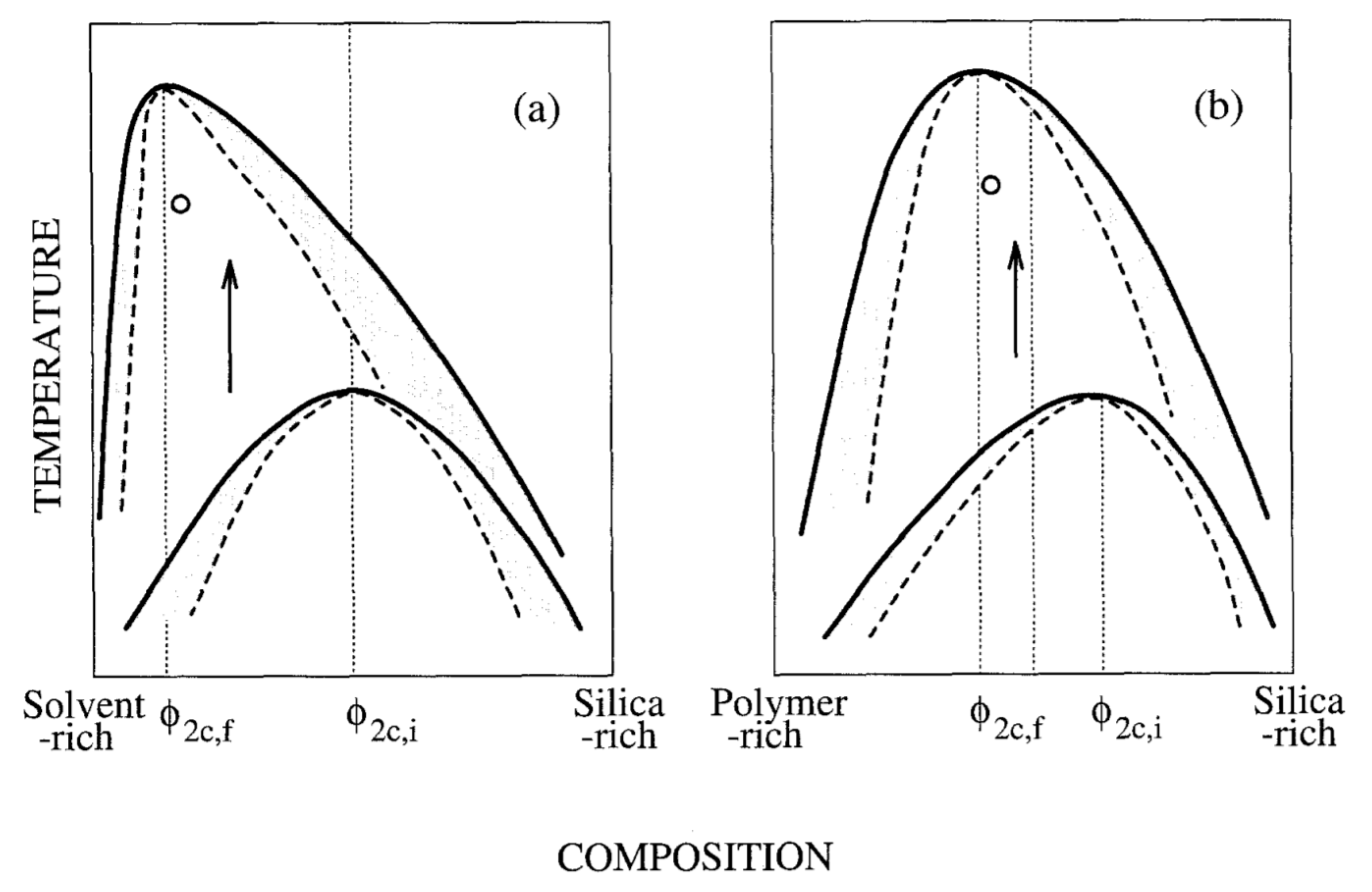
2. Background on Phase Separation
3. Macroporous Silica (SiO2) Monoliths
3.1. Pioneering Works
3.2. Further Developments on Inorganic and Inorganic-Organic SiO2 Monoliths
4. Macroporous SiO2-Based Multicomponent Oxide Monoliths
5. Macroporous Non-Siliceous Single Oxide, Multi-Oxide, and Non-Oxide Monoliths
5.1. Titania Macroporous Monoliths
5.1.1. Formamide-Based Systems
5.1.2. Chelated Systems
5.2. Zirconia and Alumina Macroporous Monoliths
5.3. Other Non-Siliceous Macroporous Monolithic Systems
6. Macroporous Microspheres
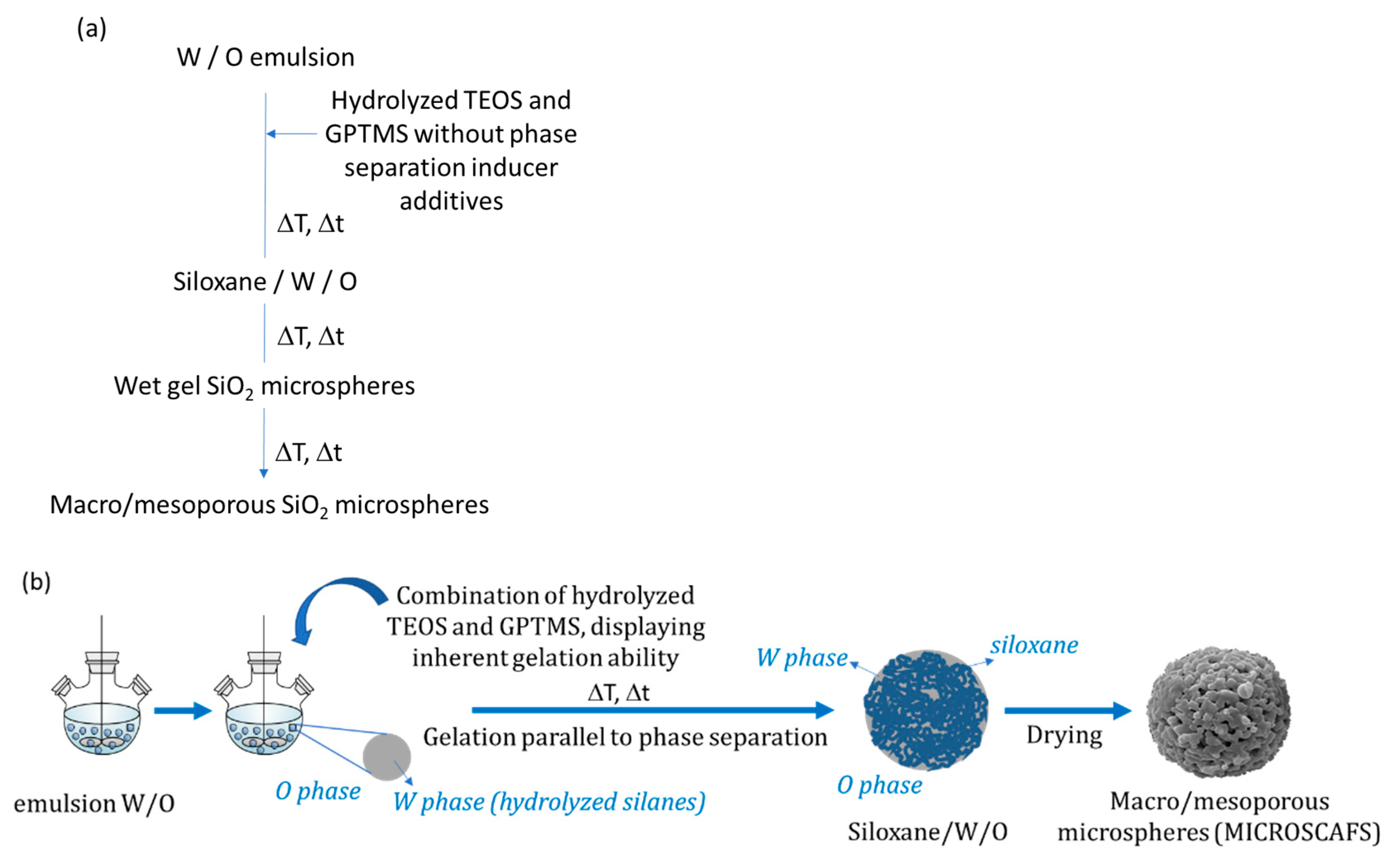
6.1. Macroporous Microspheres by Sol-Gel/Phase Separation
6.1.1. Multicavities/Incontinuous Inner Macroporosity
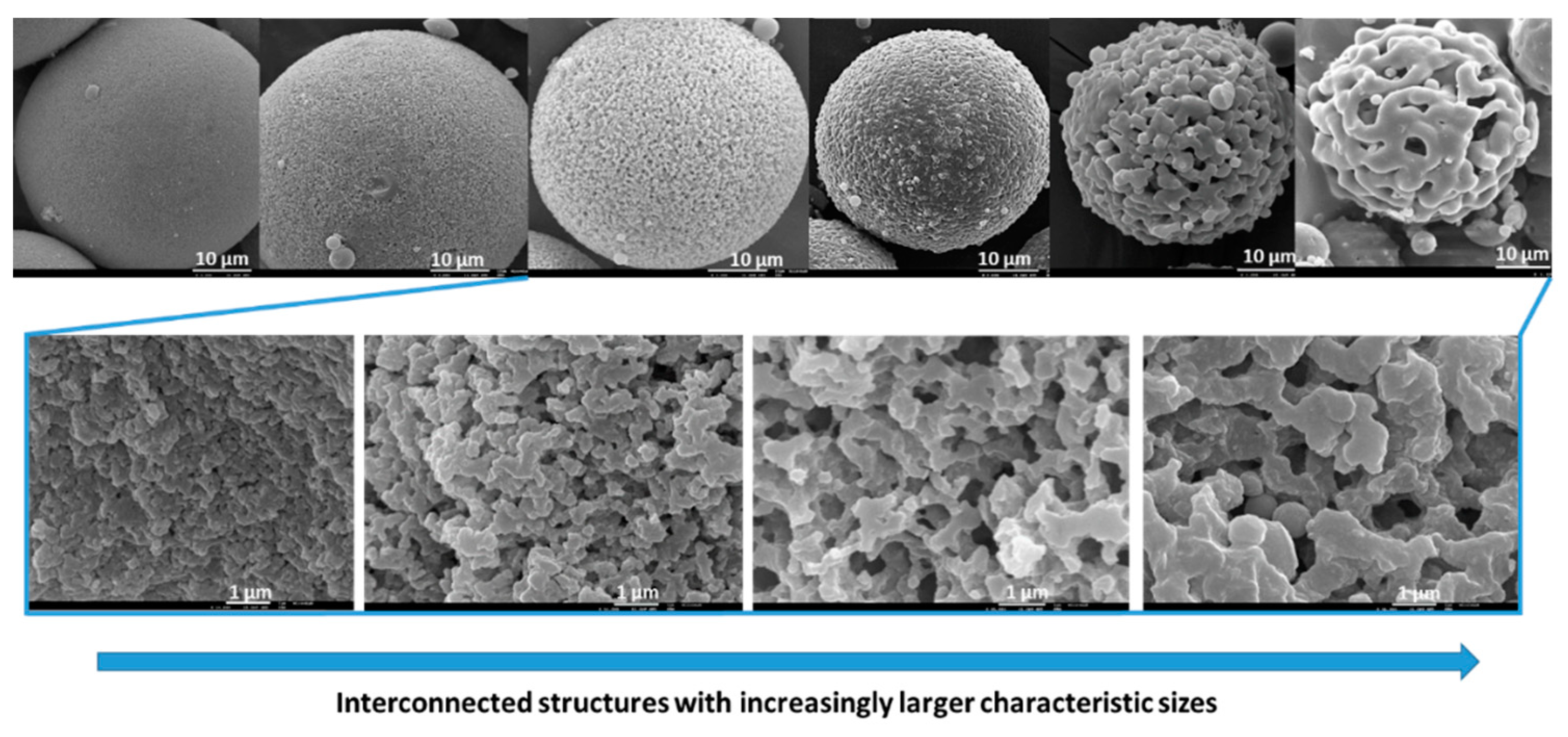
6.1.2. Interconnected Macroporosity
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Acac | Acetylacetone |
| ATR | Attenuated total reflection |
| BTME | 1,2-Bis(trimethoxysilyl)ethane |
| BTMH | bis(trimethoxysilyl)hexane |
| BzOH | Benzyl alcohol |
| CTAB | n-hexadecyltrimethylammonium bromide |
| CTAC | n-hexadecyltrimethylammonium chloride |
| Decalin | Decahydronaphthalene |
| DEG | Diethylene glycol |
| DI | Deionized |
| DMDMS | Dimethoxydimethylsilane |
| DMF | N,N-dimethylformamide |
| DTA | Differential thermal analysis |
| EDA | Ethylenediamine |
| EG | Ethylene glycol |
| EGDMA | Ethylene glycol dimethacrylate |
| EGMT | (Ethylene glycol)-modified titanate |
| EO | Ethylene oxide |
| EtAcAc | Ethyl acetoacetate |
| FTIR | Fourier Transform Infrared |
| GMA | Glycidyl methacrylate |
| GPTMS | (3-glycidyloxypropyl) trimethoxysilane |
| HDTMS | Hexadecyltrimethoxysilane |
| HLB | Hydrophilic-lipophilic balance |
| HPAA | Poly(acrylic acid) |
| LCST | Lower critical solution temperature |
| MOFs | Metal–organic frameworks |
| MTES | Methyltriethoxysilane |
| MTMS | Methyltrimethoxysilane |
| NaPSS | Poly(sodium-4-styrene sulfonate) |
| NMF | N-methylformamide |
| NMR | Nuclear magnetic resonance |
| nOTES | octyltriethoxysilane |
| NPs | nanoparticles |
| O | oil phase |
| O/W | oil-in-water |
| OP-10 | alkylphenol ethoxylate surfactant |
| P123 | triblock copolymer surfactant |
| PAAm | poly(acrylamide) |
| PEG | Polyethylene glycol |
| PEO | Poly(ethylene oxide) |
| PhTMS | Phenyltrimethoxysilane |
| PMSQ | Polymethylsilsesquioxane |
| PO | Propilene Oxide |
| PPG | Polypropylene glycol |
| PS | Polystyrene |
| PVP | Poly(vinylpyrrolidone) |
| RE | Rare earth |
| RF | Resorcinol–formaldehyde |
| RT | Room temperature |
| SAXS | Small-angle x-ray scattering |
| SDS | Sodium dodecyl sulfate |
| TBOT | Titanium tetrabutoxide |
| TEA | Triethylamine |
| TEG | Triethylene glycol |
| TEOS | Tetraethyl orthosilicate |
| tg | gelation time |
| TG | Thermogravimetry |
| TiPOT | Titanium isopropoxide |
| TMB | Trimethyl benzene |
| TMOS | Tetramethoxysilane |
| TOA | Trioctylamine |
| TPZR | zirconium tetra-2-propoxide |
| UCST | Upper critical solution temperature |
| VTMS | Vinyltrimethoxysilane |
| W | water phase |
| W/O | water-in-oil |
| XRD | X-ray diffraction |
| YAG | Yttrium aluminum garnet |
| YZA | yttria-stabilized zirconia |
References
- Feinle, A.; Elsaesser, M.S.; Hüsing, N. Sol-gel synthesis of monolithic materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3377–3399. [Google Scholar] [CrossRef] [PubMed]
- Vale, M.; Loureiro, M.V.; Ferreira, M.J.; Marques, A.C. Silica-based microspheres with interconnected macroporosity by phase separation. J. Sol-Gel Sci. Technol. 2020, 95, 746–759. [Google Scholar] [CrossRef]
- Marques, A.C.; Vale, M.; Vicente, D.; Schreck, M.; Tervoort, E.; Niederberger, M. Porous Silica Microspheres with Immobilized Titania Nanoparticles for In-Flow Solar-Driven Purification of Wastewater. Glob. Chall. 2021, 5, 2000116. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.; Fu, Z.; Su, B.L. Hierarchically structured porous materials: Synthesis strategies and applications in energy storage. Natl. Sci. Rev. 2020, 7, 1667–1701. [Google Scholar] [CrossRef]
- Huang, H.B.; Yang, Y.; Chen, L.H.; Wang, Y.; Huang, S.Z.; Tao, J.W.; Ma, X.T.; Hasan, T.; Li, Y.; Xu, Y.; et al. Hierarchical TiO2/C nanocomposite monoliths with a robust scaffolding architecture, mesopore-macropore network and TiO2-C heterostructure for high-performance lithium ion batteries. Nanoscale 2016, 8, 10928–10937. [Google Scholar] [CrossRef]
- Lu, X.; Hasegawa, G.; Kanamori, K.; Nakanishi, K. Hierarchically porous monoliths prepared via sol–gel process accompanied by spinodal decomposition. J. Sol-Gel Sci. Technol. 2020, 95, 530–550. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent advances and future perspectives of sol-gel derived porous bioactive glasses: A review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef]
- Siggia, E.D. Late stages of spinodal decomposition in binary mixtures. Phys. Rev. A 1979, 20, 595–605. [Google Scholar] [CrossRef]
- Koch, S.W.; Desai, R.C.; Abraham, F.F. Dynamics of phase separation in two-dimensional fluids: Spinodal decomposition. Phys. Rev. A 1983, 27, 2152–2167. [Google Scholar] [CrossRef]
- Shimizu, R.; Tanaka, H. A novel coarsening mechanism of droplets in immiscible fluid mixtures. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Tateno, M.; Tanaka, H. Power-law coarsening in network-forming phase separation governed by mechanical relaxation. Nat. Commun. 2021, 12, 912. [Google Scholar] [CrossRef]
- Cahn, J.W. Phase separation by spinodal decomposition in isotropic systems. J. Chem. Phys. 1965, 42, 93–99. [Google Scholar] [CrossRef]
- Huggins, M.L. Solutions of long chain compounds. J. Chem. Phys. 1941, 9, 440. [Google Scholar] [CrossRef]
- Huggins, M.L. Some properties of solutions of long-chain compounds. J. Phys. Chem. 1942, 46, 151–158. [Google Scholar] [CrossRef]
- Flory, P.J. Themodynamics of high polymer solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Huggins, M.L. The Viscosity of Dilute Solutions of Long-Chain Molecules. IV. Dependence on Concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics, 1st ed.; Oxford University Press: Kettering, OH, USA, 2003; ISBN 019852059X. [Google Scholar]
- Nakanishi, K. Pore Structure Control of Silica Gels Based on Phase Separation. J. Porous Mater. 1997, 4, 67–112. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N. Phase Separation in Gelling Silica–Organic Polymer Solution: Systems Containing Poly(sodium styrenesulfonate). J. Am. Ceram. Soc. 1991, 74, 2518–2530. [Google Scholar] [CrossRef]
- Kaji, H.; Nakanishi, K.; Soga, N. Polymerization-induced phase separation in silica sol-gel systems containing formamide. J. Sol-Gel Sci. Technol. 1993, 1, 35–46. [Google Scholar] [CrossRef]
- Schaefer, D.W.; Keefer, K.D. Structure of Soluble Silicates. MRS Proc. 1984, 32. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N.; Matsuoka, H.; Ise, N. Small-Angle X-ray Scattering Study of Gelling Silica–Organic Polymer Solution: Systems Containing Poly(Sodium Styrenesulfonate). J. Am. Ceram. Soc. 1992, 75, 971–975. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N. Phase separation in silica sol-gel system containing polyacrylic acid I. Gel formaation behavior and effect of solvent composition. J. Non-Cryst. Solids 1992, 139, 1–13. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N. Phase separation in silica sol-gel system containing polyacrylic acid II. Effects of molecular weight and temperature. J. Non-Cryst. Solids 1992, 139, 14–24. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N. Phase separation in silica sol-gel system containing polyacrylic acid. III. Effect of catalytic condition. J. Non-Cryst. Solids 1992, 142, 36–44. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N. Phase separation in silica sol-gel system containing polyacrylic acid. IV. Effect of chemical additives. J. Non-Cryst. Solids 1992, 142, 45–54. [Google Scholar] [CrossRef]
- Hasegawa, G.; Yano, T.; Akamatsu, H.; Hayashi, K.; Nakanishi, K. Variation of meso- and macroporous morphologies in resorcinol–formaldehyde (RF) gels tailored via a sol–gel process combined with soft-templating and phase separation. J. Sol-Gel Sci. Technol. 2020, 95, 801–812. [Google Scholar] [CrossRef]
- Nakanishi, K.; Nagakane, T.; Soga, N. Designing Double Pore Structure in Alkoxy-Derived Silica Incorporated with Nonionic Surfactant. J. Porous Mater. 1998, 5, 103–110. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yamasaki, Y.; Kaji, H.; Soga, N.; Inoue, T.; Nemoto, N. Phase separation kinetics in silica sol-gel system containing polyethylene oxide. I. Initial stage—Code: C7. J. Sol-Gel Sci. Technol. 1994, 2, 227–231. [Google Scholar] [CrossRef]
- Nakanishi, K.; Komura, H.; Takahashi, R.; Soga, N. Phase Separation in Silica Sol–Gel System Containing Poly(ethylene oxide). I. Phase Relation and Gel Morphology. Bull. Chem. Soc. Jpn. 1994, 67, 1327–1335. [Google Scholar] [CrossRef]
- Nakanishi, K.; Soga, N. Phase Separation in Silica Sol-Gel System Containing Poly(ethylene oxide) II. Effects of Molecular Weight and Temperature. Bull. Chem. Soc. Jpn. 1997, 70, 587–592. [Google Scholar] [CrossRef]
- Meinusch, R.; Hormann, K.; Hakim, R.; Tallarek, U.; Smarsly, B.M. Synthesis and morphological characterization of phenyl-modified macroporous-mesoporous hybrid silica monoliths. RSC Adv. 2015, 5, 20283–20294. [Google Scholar] [CrossRef]
- Sato, Y.; Nakanishi, K.; Hirao, K.; Jinnai, H.; Shibayama, M.; Melnichenko, Y.B.; Wignall, G.D. Formation of ordered macropores and templated nanopores in silica sol-gel system incorporated with EO-PO-EO triblock copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 117–122. [Google Scholar] [CrossRef]
- Nakanishi, K.; Amatani, T.; Yano, S.; Kodaira, T. Multiscale templating of siloxane gels via polymerization-induced phase separation. Chem. Mater. 2008, 20, 1108–1115. [Google Scholar] [CrossRef]
- Nakanishi, K.; Sato, Y.; Ruyat, Y.; Hirao, K. Supramolecular templating of mesopores in phase-separating silica Sol-Gels incorporated with cationic surfactant. J. Sol-Gel Sci. Technol. 2003, 26, 567–570. [Google Scholar] [CrossRef]
- Smått, J.H.; Schunk, S.; Lindén, M. Versatile double-templating synthesis route to silica monoliths exhibiting a multimodal hierarchical porosity. Chem. Mater. 2003, 15, 2354–2361. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yamato, T.; Hirao, K. Phase separation in alkylene-bridged polysilsesquioxane sol-gel systems. MRS Proc. 2002, 726, 291–296. [Google Scholar] [CrossRef]
- Itagaki, A.; Nakanishi, K.; Hirao, K. Phase separation in sol-gel system containing mixture of 3- and 4-functional alkoxysilanes. J. Sol-Gel Sci. Technol. 2003, 26, 153–156. [Google Scholar] [CrossRef]
- Dong, H.; Brennan, J.D. Controlling the morphology of methylsilsesquioxane monoliths using a two-step processing method. Chem. Mater. 2006, 18, 541–546. [Google Scholar] [CrossRef]
- Kanamori, K.; Kodera, Y.; Hayase, G.; Nakanishi, K.; Hanada, T. Transition from transparent aerogels to hierarchically porous monoliths in polymethylsilsesquioxane sol-gel system. J. Colloid Interface Sci. 2011, 357, 336–344. [Google Scholar] [CrossRef]
- Tao, S.; Wang, Y.; An, Y. Superwetting monolithic SiO2 with hierarchical structure for oil removal. J. Mater. Chem. 2011, 21, 11901–11907. [Google Scholar] [CrossRef]
- Kurahashi, M.; Kanamori, K.; Takeda, K.; Kaji, H.; Nakanishi, K. Role of block copolymer surfactant on the pore formation in methylsilsesquioxane aerogel systems. RSC Adv. 2012, 2, 7166–7173. [Google Scholar] [CrossRef]
- Huesing, N.; Raab, C.; Torma, V.; Roig, A.; Peterlik, H. Periodically mesostructured silica monoliths from diol-modified silanes. Chem. Mater. 2003, 15, 2690–2692. [Google Scholar] [CrossRef]
- Brandhuber, D.; Torma, V.; Raab, C.; Peterlik, H.; Kulak, A.; Hüsing, N. Glycol-modified silanes in the synthesis of mesoscopically organized silica monoliths with hierarchical porosity. Chem. Mater. 2005, 17, 4262–4271. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, J.; Wang, P.; Yang, J.; Yang, Q. Inorganic salt aided synthesis of monolithic silica with meso/macro hierarchical structure. Microporous Mesoporous Mater. 2009, 123, 63–70. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Highly reusable visible light active hierarchical porous WO3/SiO2 monolith in centimeter length scale for enhanced photocatalytic degradation of toxic pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Nakanishi, K.; Motowaki, S.; Soga, N. Preparation of SiO2-TiO2 Gels with Controlled Pore Structure via Sol-Gel Route. Bull. Inst. Chem. Res. Kyoto Univ. 1992, 70, 144–151. [Google Scholar]
- Ruzimuradov, O.; Nurmanov, S.; Kodani, Y.; Takahashi, R.; Yamada, I. Morphology and dispersion control of titania-silica monolith with macro-meso pore system. J. Sol-Gel Sci. Technol. 2012, 64, 684–693. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Z.; Gao, H.; Xie, Z. Synthesis and characterization of hierarchical titania-silica monolith. Catal. Today 2013, 216, 90–94. [Google Scholar] [CrossRef]
- Takahashi, R.; Nakanishi, K.; Soga, N. Phase Separation Process of Polymer—Incorporated Silica-Zirconia Sol-Gel System. J. Sol-Gel Sci. Technol. 1997, 8, 71–76. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Yabuki, M. Silica-alumina catalyst with bimodal pore structure prepared by phase separation in sol-gel process. J. Catal. 2001, 200, 197–202. [Google Scholar] [CrossRef]
- Murai, S.; Fujita, K.; Nakanishi, K.; Hirao, K. Morphology control of phase-separation-induced alumina-silica macroporous gels for rare-earth-doped scattering media. J. Phys. Chem. B 2004, 108, 16670–16676. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Du, W.; Dong, C.; Li, L. Preparation and characterization of bimodal porous alumina-silica and its application to removal of basic nitrogen compounds from light oil. J. Mater. Chem. 2007, 17, 2233–2240. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Q.; Liu, Z.; Gao, H.; Xie, Z. Controllable synthesis of aluminosilica monoliths with hierarchical pore structure and their catalytic performance. Microporous Mesoporous Mater. 2010, 127, 213–218. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.; Nakanishi, K.; Kanamori, K.; Zhu, Y.; Yang, H. Preparation of mullite monoliths with well-defined macropores and mesostructured skeletons via the sol-gel process accompanied by phase separation. J. Eur. Ceram. Soc. 2013, 33, 1967–1974. [Google Scholar] [CrossRef]
- Nakamura, N.; Takahashi, R.; Sato, S.; Sodesawa, T.; Yoshida, S. Ni/SiO2 catalyst with hierarchical pore structare prepared by phase separation in sol-gel process. Phys. Chem. Chem. Phys. 2000, 2, 4983–4990. [Google Scholar] [CrossRef]
- Zheng, M.; Zhao, T.; Xu, W.; Li, F.; Yang, Y. Preparation and characterization of CuO/SiO2 and NiO/SiO2 with bimodal pore structure by sol-gel method. J. Sol-Gel Sci. Technol. 2006, 39, 151–157. [Google Scholar] [CrossRef]
- Marques, A.C.; Almeida, R.M.; Thiema, A.; Wang, S.; Falk, M.M.; Jain, H. Sol-gel-derived glass scaffold with high pore interconnectivity and enhanced bioactivity. J. Mater. Res. 2009, 24, 3495–3502. [Google Scholar] [CrossRef]
- Marques, A.C.; Jain, H.; Almeida, R.M. Sol-gel derived nano/macroporous scaffolds. Phys. Chem. Glas. Eur. J. Glass Sci. Technol. Part B 2007, 48, 65–68. [Google Scholar]
- Guo, X.; Nakanishi, K.; Kanamori, K.; Zhu, Y.; Yang, H. Preparation of macroporous cordierite monoliths via the sol-gel process accompanied by phase separation. J. Eur. Ceram. Soc. 2014, 34, 817–823. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, T.; Li, Z.; Ma, Z.; Wang, J.; Li, F. Sol-gel synthesis of macro-mesoporous Al2O3-SiO2-TiO2 monoliths via phase separation route. Ceram. Int. 2016, 42, 15926–15932. [Google Scholar] [CrossRef]
- Takahashi, R.; Nakanishi, K.; Soga, N. Morphology Control of Macroporous Silica-Zirconia Gel Based on Phase Separation. J. Ceram. Soc. Jpn. 1998, 106, 772–777. [Google Scholar] [CrossRef][Green Version]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Suzuki, K.; Tafu, M.; Nakanishi, K.; Soga, N. Phase Separation in Sol-Gel Process of Alkoxide-Derived Silica-Zirconia in the Presence of Polyethylene Oxide. J. Am. Ceram. Soc. 2001, 84, 1968–1976. [Google Scholar] [CrossRef]
- Murai, S.; Fujita, K.; Nakanishi, K.; Hirao, K. Direct observation of the spatial distribution of samarium ions in alumina-silica macroporous monoliths by laser scanning confocal microscopy. J. Alloys Compd. 2006, 408–412, 831–834. [Google Scholar] [CrossRef]
- Marques, A.C.; Jain, H.; Kiely, C.; Song, K.; Kiely, C.J.; Almeida, R.M. Nano/macroporous monolithic scaffolds prepared by the sol-gel method. J. Sol-Gel Sci. Technol. 2009, 51, 42–47. [Google Scholar] [CrossRef]
- Fujita, K.; Konishi, J.; Nakanishi, K.; Hirao, K. Strong light scattering in macroporous TiO2 monoliths induced by phase separation. Appl. Phys. Lett. 2004, 85, 5595–5597. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K. Phase-separation-induced titania monoliths with well-defined macropores and mesostructured framework from colloid-derived sol-gel systems. Chem. Mater. 2006, 18, 864–866. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K. Monolithic TiO2 with controlled multiscale porosity via a template-free sol-gel process accompanied by phase separation. Chem. Mater. 2006, 18, 6069–6074. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Nakanishi, K.; Hanada, T. Facile Preparation of Hierarchically Porous TiO2 Monoliths. J. Am. Ceram. Soc. 2010, 93, 3110–3115. [Google Scholar] [CrossRef]
- Hasegawa, G.; Morisato, K.; Kanamori, K.; Nakanishi, K. New hierarchically porous titania monoliths for chromatographic separation media. J. Sep. Sci. 2011, 34, 3004–3010. [Google Scholar] [CrossRef]
- Konishi, J.; Fujita, K.; Nakanishi, K.; Hirao, K.; Morisato, K.; Miyazaki, S.; Ohira, M. Sol-gel synthesis of macro-mesoporous titania monoliths and their applications to chromatographic separation media for organophosphate compounds. J. Chromatogr. A 2009, 1216, 7375–7383. [Google Scholar] [CrossRef]
- Li, W.; Guo, X.; Zhu, Y.; Hui, Y.; Kanamori, K.; Nakanishi, K. Sol-gel synthesis of macroporous TiO2 from ionic precursors via phase separation route. J. Sol-Gel Sci. Technol. 2013, 67, 639–645. [Google Scholar] [CrossRef]
- Backlund, S.; Smått, J.; Rosenholm, J.B.; Lindén, M. Template-Free Sol-Gel Synthesis of Hierarchically Macro- and Mesoporous Monolithic TiO2. J. Dispers. Sci. Technol. 2007, 28, 115–119. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Nakanishi, K.; Hanada, T. Facile preparation of transparent monolithic titania gels utilizing a chelating ligand and mineral salts. J. Sol-Gel Sci. Technol. 2010, 53, 59–66. [Google Scholar] [CrossRef][Green Version]
- Konishi, J.; Fujita, K.; Oiwa, S.; Nakanishi, K.; Hirao, K. Crystalline ZrO2 monoliths with well-defined macropores and mesostructured skeletons prepared by combining the alkoxy-derived sol-gel process accompanied by phase separation and the solvothermal process. Chem. Mater. 2008, 20, 2165–2173. [Google Scholar] [CrossRef]
- Tokudome, Y.; Fujita, K.; Nakanishi, K.; Miura, K.; Hirao, K. Synthesis of monolithic Al2O3 with well-defined macropores and mesostructured skeletons via the sol-gel process accompanied by phase separation. Chem. Mater. 2007, 19, 3393–3398. [Google Scholar] [CrossRef]
- Wu, L.A.; Jiang, X.; Wu, S.; Yao, R.; Qiao, X.; Fan, X. Synthesis of monolithic zirconia with macroporous bicontinuous structure via epoxide-driven sol-gel process accompanied by phase separation. J. Sol-Gel Sci. Technol. 2014, 69, 1–8. [Google Scholar] [CrossRef]
- Guo, X.; Song, J.; Lvlin, Y.; Nakanishi, K.; Kanamori, K.; Yang, H. Preparation of macroporous zirconia monoliths from ionic precursors via an epoxide-mediated sol-gel process accompanied by phase separation. Sci. Technol. Adv. Mater. 2015, 16. [Google Scholar] [CrossRef]
- Guo, X.; Song, J.; Ren, J.; Yang, F.; Kanamori, K.; Nakanishi, K. Facile preparation of well-defined macroporous yttria-stabilized zirconia monoliths via sol–gel process accompanied by phase separation. J. Porous Mater. 2016, 23, 867–875. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Song, J.; Yang, H. Sol–gel synthesis of macroporous barium zirconate monoliths from ionic precursors via a phase separation route. J. Phys. Chem. Solids 2017, 102, 105–109. [Google Scholar] [CrossRef]
- Kido, Y.; Hasegawa, G.; Kanamori, K.; Nakanishi, K. Porous chromium-based ceramic monoliths: Oxides (Cr2O3), nitrides (CrN), and carbides (Cr3C2). J. Mater. Chem. A 2014, 2, 745–752. [Google Scholar] [CrossRef]
- Kido, Y.; Nakanishi, K.; Miyasaka, A.; Kanamori, K. Synthesis of monolithic hierarchically porous iron-based xerogels from iron(III) salts via an epoxide-mediated sol-gel process. Chem. Mater. 2012, 24, 2071–2077. [Google Scholar] [CrossRef]
- Lu, X.; Kanamori, K.; Nakanishi, K. Synthesis of hierarchically porous MgO monoliths with continuous structure via sol–gel process accompanied by phase separation. J. Sol-Gel Sci. Technol. 2019, 89, 29–36. [Google Scholar] [CrossRef]
- Guo, X.; Ding, L.; Ren, J.; Yang, H. Preparation of macroporous Li2ZrO3 monoliths via an epoxide-mediated sol–gel process accompanied by phase separation. J. Porous Mater. 2017, 24, 1319–1326. [Google Scholar] [CrossRef]
- Herwig, J.; Titus, J.; Kullmann, J.; Wilde, N.; Hahn, T.; Glaser, R.; Enke, D. Hierarchically structured porous spinels via an epoxide-mediated sol−gel process accompanied by polymerization-induced phase separation. ACS Omega 2018, 3, 1201–1212. [Google Scholar] [CrossRef]
- Yin, P.; Lei, W.; Yang, H.; Guo, X. Preparation of Porous ZnAl2O4 Spinel Monoliths Through Sol-Gel Route Accompanied with Phase Separation. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2018, 47, 75–78. [Google Scholar]
- Wang, S.; Li, W.; Wang, S.; Jiang, J.; Chen, Z. Synthesis of well-defined hierarchical porous La2Zr2O7 monoliths via non-alkoxide sol-gel process accompanied by phase separation. Microporous Mesoporous Mater. 2016, 221, 32–39. [Google Scholar] [CrossRef]
- Guo, X.; Yin, P.; Lei, W.; Yang, H.; Kanamori, K.; Nakanishi, K. Synthesis and characterization of monolithic ZnAl2O4 spinel with well-defined hierarchical pore structures via a sol-gel route. J. Alloys Compd. 2017, 727, 763–770. [Google Scholar] [CrossRef]
- Sun, M.; Zhao, T.; Ma, Z.; Li, Z. Facile preparation of macro-mesoporous zirconium titanate monoliths via a sol-gel reaction accompanied by phase separation. J. Mater. Res. 2019, 34, 4066–4075. [Google Scholar] [CrossRef]
- Lu, X.; Kanamori, K.; Nakanishi, K. Preparation of zinc oxide with a three-dimensionally interconnected macroporous structure via a sol-gel method accompanied by phase separation. New J. Chem. 2019, 43, 11720–11726. [Google Scholar] [CrossRef]
- Gash, A.E.; Satcher, J.H.; Simpson, R.L. Monolithic nickel(II)-based aerogels using an organic epoxide: The importance of the counterion. J. Non-Cryst. Solids 2004, 350, 145–151. [Google Scholar] [CrossRef]
- Lu, X.; Kanamori, K.; Nakanishi, K. Hierarchically porous monoliths based on low-valence transition metal (Cu, Co, Mn) oxides: Gelation and phase separation. Natl. Sci. Rev. 2020, 7, 1656–1666. [Google Scholar] [CrossRef]
- Li, M.; Zhou, N.; Luo, X.; Zhang, G.; Xie, Z.; Xu, L.; Liu, P. Macroporous MgO monoliths prepared by sol–gel process with phase separation. Ceram. Int. 2016, 42, 16368–16373. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y.; Guo, X.; Li, D.; Yang, H. Preparation and graft modification of hierarchically porous ferriferrous oxide for heavy metal ions adsorption. J. Sol-Gel Sci. Technol. 2020, 96, 360–369. [Google Scholar] [CrossRef]
- Kido, Y.; Nakanishi, K.; Kanamori, K. Sol-gel synthesis of zinc ferrite-based xerogel monoliths with well-defined macropores. RSC Adv. 2013, 3, 3661–3666. [Google Scholar] [CrossRef]
- Liu, F.; Feng, D.; Yang, H.; Guo, X. Preparation of macroporous transition metal hydroxide monoliths via a sol-gel process accompanied by phase separation. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Guo, X.; Nakanishi, K.; Kanamori, K.; Yang, H. Preparation of a hierarchically porous AlPO4 monolith via an epoxide-mediated sol-gel process accompanied by phase separation. Sci. Technol. Adv. Mater. 2013, 14, 045007. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kanamori, K.; Moitra, N.; Kadono, K.; Ohi, S.; Shimobayashi, N.; Nakanishi, K. Metal zirconium phosphate macroporous monoliths: Versatile synthesis, thermal expansion and mechanical properties. Microporous Mesoporous Mater. 2016, 225, 122–127. [Google Scholar] [CrossRef]
- Ruzimuradov, O.; Hasegawa, G.; Kanamori, K.; Nakanishi, K. Preparation of hierarchically porous nanocrystalline CaTiO3, SrTiO3 and BaTiO3 perovskite monoliths. J. Am. Ceram. Soc. 2011, 94, 3335–3339. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, W.; Cai, X.; Liu, S.; Yang, H. Preparation of monolithic aluminium titanate with well-defined macropores via a sol-gel process accompanied by phase separation. Mater. Des. 2015, 83, 314–319. [Google Scholar] [CrossRef]
- Lu, X.; Kanamori, K.; Hasegawa, G.; Nakanishi, K. Preparation of hierarchically porous spinel CoMn2O4 monoliths via sol–gel process accompanied by phase separation. J. Am. Ceram. Soc. 2020, 104, 2449–2459. [Google Scholar] [CrossRef]
- Zhu, W.; Yang, H.; Nakanishi, K.; Kanamori, K.; Guo, X. Sol-gel synthesis of nanocrystal-constructed hierarchically porous TiO2 based composites for lithium ion batteries. RSC Adv. 2015, 5, 24803–24813. [Google Scholar] [CrossRef]
- Hasegawa, G.; Kanamori, K.; Kiyomura, T.; Kurata, H.; Abe, T.; Nakanishi, K. Hierarchically Porous Carbon Monoliths Comprising Ordered Mesoporous Nanorod Assemblies for High-Voltage Aqueous Supercapacitors. Chem. Mater. 2016, 28, 3944–3950. [Google Scholar] [CrossRef]
- Schoiber, J.; Koczwara, C.; Rumswinkel, S.; Whitmore, L.; Prehal, C.; Putz, F.; Elsaesser, M.S.; Paris, O.; Hüsing, N. A Facile One-Pot Synthesis of Hierarchically Organized Carbon/TiO2 Monoliths with Ordered Mesopores. ChemPlusChem 2021, 86, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Kanamori, K.; Nakanishi, K. Self-Assembly of Metal–Organic Frameworks into Monolithic Materials with Highly Controlled Trimodal Pore Structures. Angew. Chem. Int. Ed. 2019, 58, 19047–19053. [Google Scholar] [CrossRef]
- Kato, K.; Tsuzuki, A.; Torii, Y.; Taoda, H.; Kato, T.; Butsugan, Y. Morphology of thin anatase coatings prepared from alkoxide solutions containing organic polymer, affecting the photocatalytic decomposition of aqueous acetic acid. J. Mater. Sci. 1995, 30, 837–841. [Google Scholar] [CrossRef]
- Kajihara, K.; Yao, T. Macroporous morphology of the titania films prepared by a sol-gel dip-coating method from the system containing poly(ethylene glycol): Effects of molecular weight and dipping temperature. J. Sol-Gel Sci. Technol. 2000, 19, 219–222. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Tao, A.; Song, M.; Wang, B.; Niu, J.; Yu, F.; Wu, Y. Synthesis of a bicontinuous structured SrTiO3 porous film with significant photocatalytic activity by controlling phase separation process. J. Sol-Gel Sci. Technol. 2020, 94, 288–297. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Nigorikawa, S.; Sakaue, T.; Fujiwara, K.; Tokita, M. Multiple patterns of polymer gels in microspheres due to the interplay among phase separation, wetting, and gelation. Proc. Natl. Acad. Sci. USA 2014, 111, 15894–15899. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Shi, Z. guo Waxberry-like hierarchically porous ethyl-bridged hybrid silica microsphere: A substrate for enzyme catalysis and high-performance liquid chromatography. J. Chromatogr. A 2019, 1587, 79–87. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Q.; Ding, X.; Shen, Q.; Wu, C.; Zhang, L.; Yang, H. Synthesis and application of several sol–gel-derived materials via sol–gel process combining with other technologies: A review. J. Sol-Gel Sci. Technol. 2016, 79, 328–358. [Google Scholar] [CrossRef]
- Shi, Z.G.; Feng, Y.Q. Synthesis and characterization of hierarchically porous silica microspheres with penetrable macropores and tunable mesopores. Microporous Mesoporous Mater. 2008, 116, 701–704. [Google Scholar] [CrossRef]
- Guo, T.; Wang, C.; Dong, L.; Lü, J.; Liang, T. Effect of Al2O3 on the Process Performance of ZrO2 Microspheres. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 841–846. [Google Scholar] [CrossRef]
- Smått, J.H.; Schüwer, N.; Järn, M.; Lindner, W.; Lindén, M. Synthesis of micrometer sized mesoporous metal oxide spheres by nanocasting. Microporous Mesoporous Mater. 2008, 112, 308–318. [Google Scholar] [CrossRef]
- Luan, Y.; Xue, Y.W.; Shi, Z.G. Synthesis of hierarchically macro/meso/microporous carbon spheres and its application in fast rechargeable electric double layer capacitor. Mater. Lett. 2012, 88, 30–32. [Google Scholar] [CrossRef]
- Lu, L.; Teng, F.; SenTapas; Qi, D.; Wang, L.; Zhang, J. Synthesis of visible-light driven CrxOy-TiO2 binary photocatalyst based on hierarchical macro-mesoporous silica. Appl. Catal. B Environ. 2015, 163, 9–15. [Google Scholar] [CrossRef]
- Grosso, D.; Soler Illia, G.J.D.A.A.; Crepaldi, E.L.; Charleux, B.; Sanchez, C. Nanocrystalline transition-metal oxide spheres with controlled multi-scale porosity. Adv. Funct. Mater. 2003, 13, 37–42. [Google Scholar] [CrossRef]
- Shen, J.; Yang, H.; Shen, Q.; Feng, Y. Synthesis of 3D micro- and nano-hierarchical structure InVO4 porous microspheres with improved visible-light photocatalytic activities. J. Mater. Sci. 2013, 48, 7574–7580. [Google Scholar] [CrossRef]
- Shen, Q.; You, Z.; Yu, Y.; Qin, T.; Su, Y.; Wang, H.; Wu, C.; Zhang, F.; Yang, H. A Carbon Quantum Dots/Porous InVO4 Microsphere Composite with Enhanced Photocatalytic Activity. Eur. J. Inorg. Chem. 2018, 2018, 1080–1086. [Google Scholar] [CrossRef]
- He, J.; Yang, C.; Xiong, X.; Jiang, B. Preparation and characterization of monodisperse porous silica microspheres with controllable morphology and structure. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2889–2897. [Google Scholar] [CrossRef]
- Teng, C.; He, J.; Zhu, L.; Ren, L.; Chen, J.; Hong, M.; Wang, Y. Fabrication and Characterization of Monodisperse Magnetic Porous Nickel Microspheres as Novel Catalysts. Nanoscale Res. Lett. 2015, 5, 8952. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Ren, L.; Wang, Y.; Teng, C.; Hong, M.; Zhao, J.; Jiang, B. Fabrication of monodisperse porous zirconia microspheres and their phosphorylation for friedel-crafts alkylation of indoles. ACS Appl. Mater. Interfaces 2014, 6, 2718–2725. [Google Scholar] [CrossRef]
- Mori, H.; Yamashita, H.; Nakamura, K.; Maekawa, T. Synthesis of Spherical Porous Silica Particles. J. Ceram. Soc. Jpn. 1993, 101, 1180–1183. [Google Scholar] [CrossRef][Green Version]
- Yang, H.; Xie, Y.; Hao, G.; Cai, W.; Guo, X. Preparation of porous alumina microspheres via an oil-in-water emulsion method accompanied by a sol-gel process. New J. Chem. 2016, 40, 589–595. [Google Scholar] [CrossRef]
- Cai, W.W.; Yang, H.; Guo, X.Z. A facile one-step route to synthesize titania hollow microspheres with incontinuous multicavities. Chin. Chem. Lett. 2014, 25, 441–446. [Google Scholar] [CrossRef]
- Guo, X.; Hao, G.; Xie, Y.; Cai, W.; Yang, H. Preparation of porous zirconia microspheres via emulsion method combined with phase separation. J. Sol-Gel Sci. Technol. 2015, 76, 651–657. [Google Scholar] [CrossRef]
- Yang, L.M.; Wang, Y.J.; Sun, Y.W.; Luo, G.S.; Dai, Y.Y. Synthesis of micrometer-sized hard silica spheres with uniform mesopore size and textural pores. J. Colloid Interface Sci. 2006, 299, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.X.; Shi, Z.G.; Chen, F.; Feng, Y.Q.; Guo, Q.Z. Synthesis of penetrable macroporous silica spheres for high-performance liquid chromatography. J. Chromatogr. A 2009, 1216, 7388–7393. [Google Scholar] [CrossRef]
- Long, T.; Guo, Q.Z.; Xu, L.Y. Fast separation of sulfanilamides using macroporous silica spheres as the separation media. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1391–1398. [Google Scholar] [CrossRef]
- Yin, C.R.; Ma, L.Y.; Huang, J.G.; Xu, L.; Shi, Z.G. Fast profiling ecotoxicity and skin permeability of benzophenone ultraviolet filters using biopartitioning micellar chromatography based on penetrable silica spheres. Anal. Chim. Acta 2013, 804, 321–327. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Zhao, J.; Liao, H.; Xu, L.; Shi, Z.G. Penetrable silica microspheres for immobilization of bovine serum albumin and their application to the study of the interaction between imatinib mesylate and protein by frontal affinity chromatography. Anal. Bioanal. Chem. 2016, 408, 805–814. [Google Scholar] [CrossRef]
- Qiao, T.; Ma, L.Y.; Wang, X.; Shi, Z.G. The flow-through silica as the matrix to immobilize gold nanoparticles for HPLC applications. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 107–113. [Google Scholar] [CrossRef]
- Mao, X.J.; Li, J.; Liu, D.; Qiao, T.; Ma, L.; Sun, X.; Xu, L.; Shi, Z.G. Flow-through silica: A potential matrix for fast chromatographic enantioseparation with high enantioselectivity. Talanta 2018, 178, 583–587. [Google Scholar] [CrossRef]
- Shi, Z.G.; Guo, Q.Z.; Liu, Y.T.; Xiao, Y.X.; Xu, L. Drug delivery devices based on macroporous silica spheres. Mater. Chem. Phys. 2011, 126, 826–831. [Google Scholar] [CrossRef]
- Xu, L.Y.; Huang, Y.Y.; Long, T.; Shi, Z.G. Synthesis of macroporous silica spheres for drug delivery. Mater. Manuf. Process. 2013, 28, 626–630. [Google Scholar] [CrossRef]
- Ataei-Germi, T.; Nematollahzadeh, A. Bimodal porous silica microspheres decorated with polydopamine nano-particles for the adsorption of methylene blue in fixed-bed columns. J. Colloid Interface Sci. 2016, 470, 172–182. [Google Scholar] [CrossRef]
- Guo, Q.; Hu, L.; Fan, C.; Zhang, Y.; Zhang, Q. Perforative silica microsphere-modified phenolphthalein-based poly(arylene ether sulfone) composites: Tensile and thermal properties. Iran. Polym. J. Engl. Ed. 2018, 27, 611–619. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Xue, K.; Zhao, Y.; Guo, X. Facile Synthesis and Chiral Separation of Chiral Mesoporous Silica Microspheres. J. Chromatogr. Sci. 2017, 55, 736–741. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Qiao, X.; Wan, J.; Wu, L.A.; Chen, B.; Fan, X. Facile synthesis of monodisperse YAG:Ce3+ microspheres with high quantum yield via an epoxide-driven sol-gel route. J. Mater. Chem. C 2017, 5, 8952–8957. [Google Scholar] [CrossRef]
- Loureiro, M.V.; Vale, M.; De Schrijver, A.; Bordado, J.C.; Silva, E.; Marques, A.C. Hybrid custom-tailored sol-gel derived microscaffold for biocides immobilization. Microporous Mesoporous Mater. 2018, 261, 252–258. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Li, G.; Zhao, X.; Yu, L.; Zou, C.; Xu, Y. Electrospun TiO2-SiO2 fibres with hierarchical pores from phase separation. CrystEngComm 2017, 19, 2673–2680. [Google Scholar] [CrossRef]



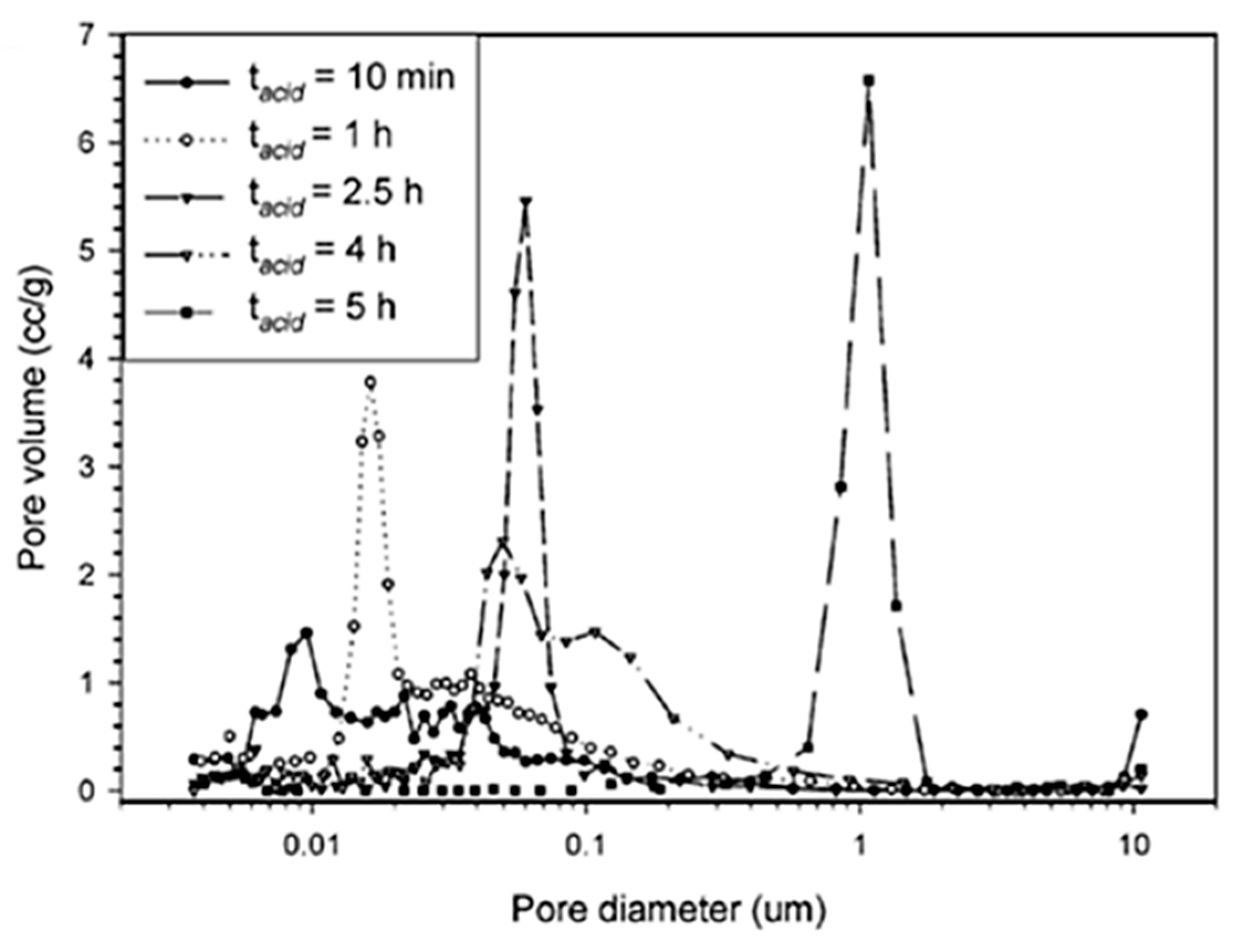
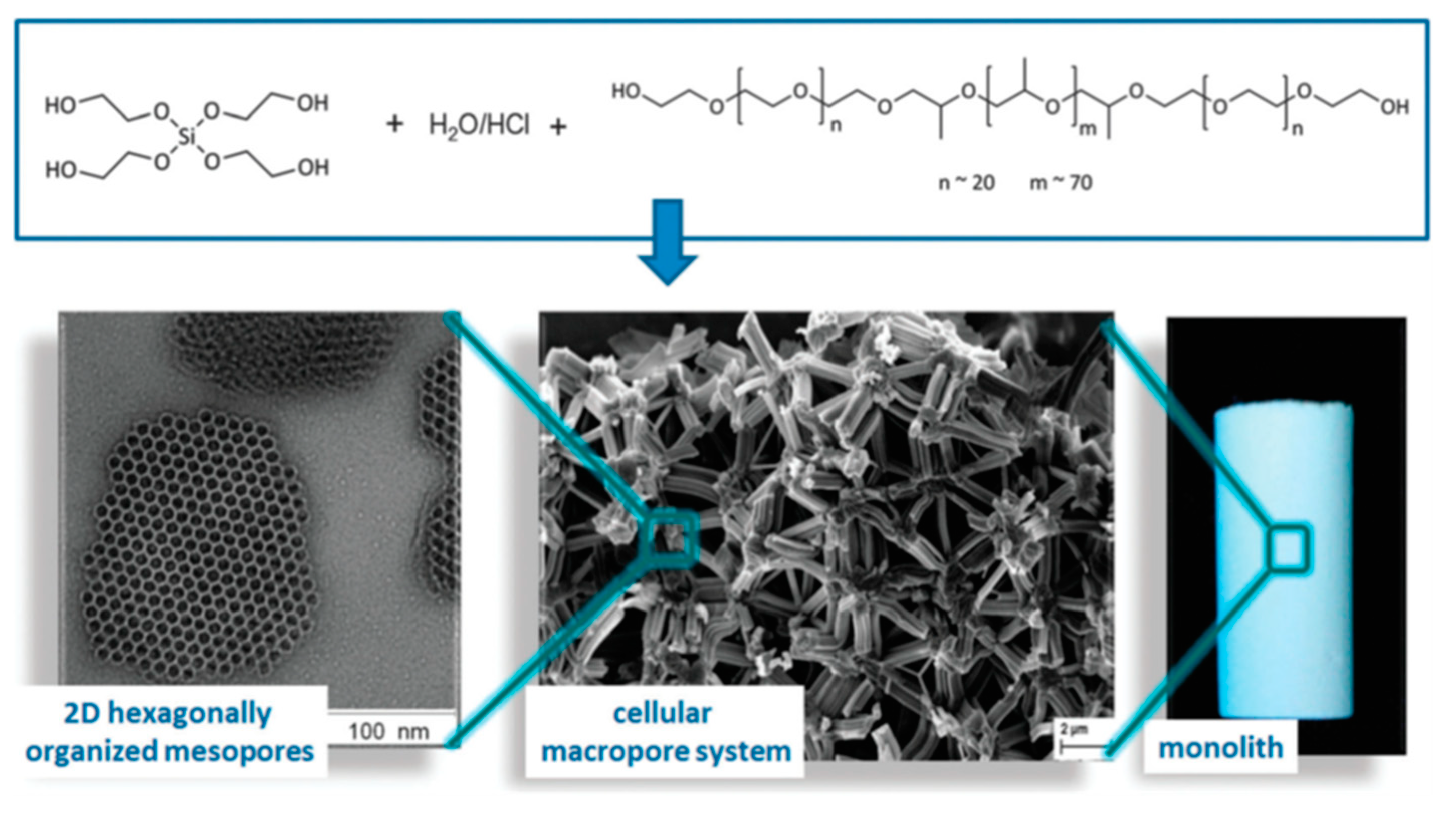
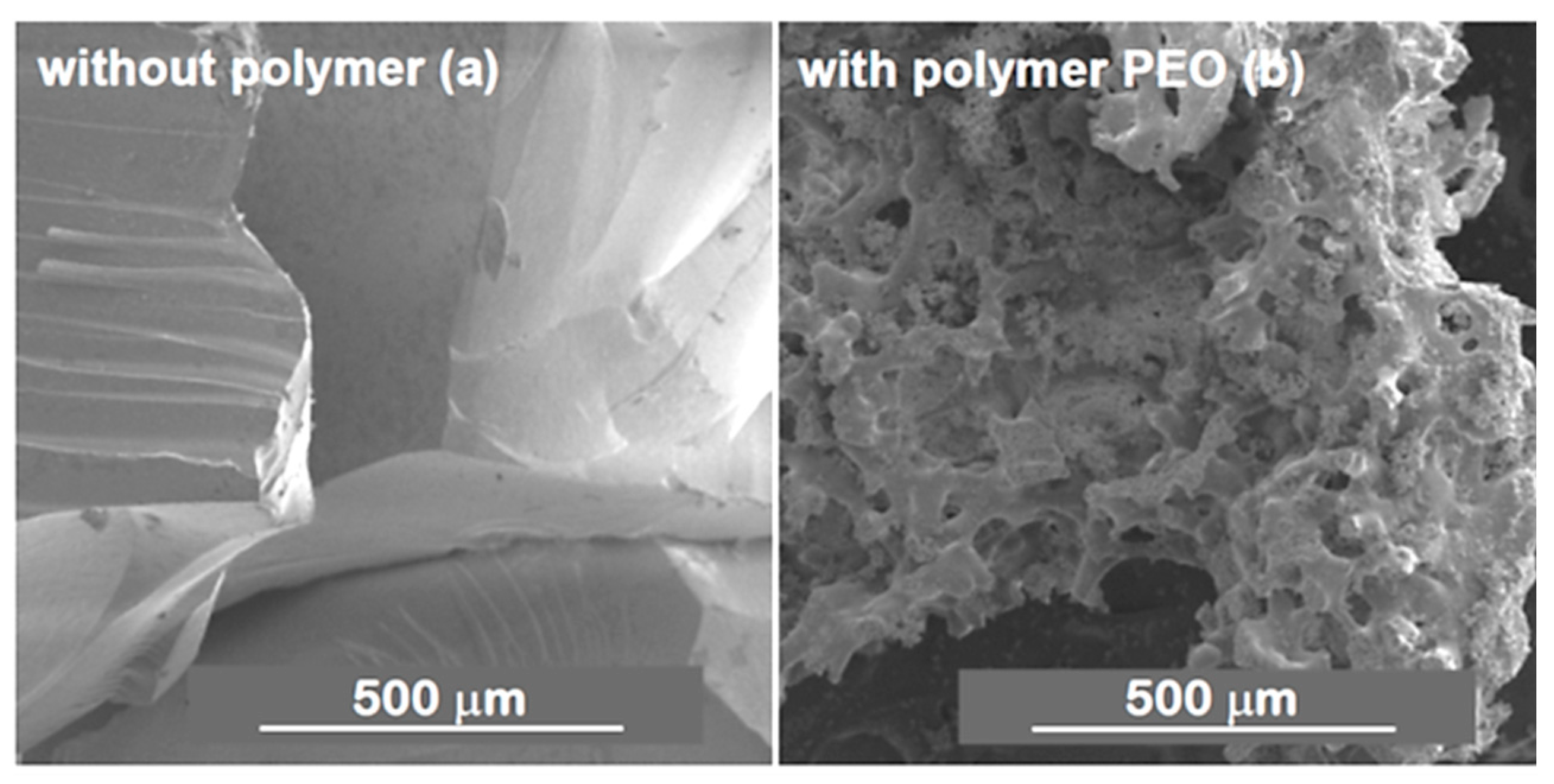


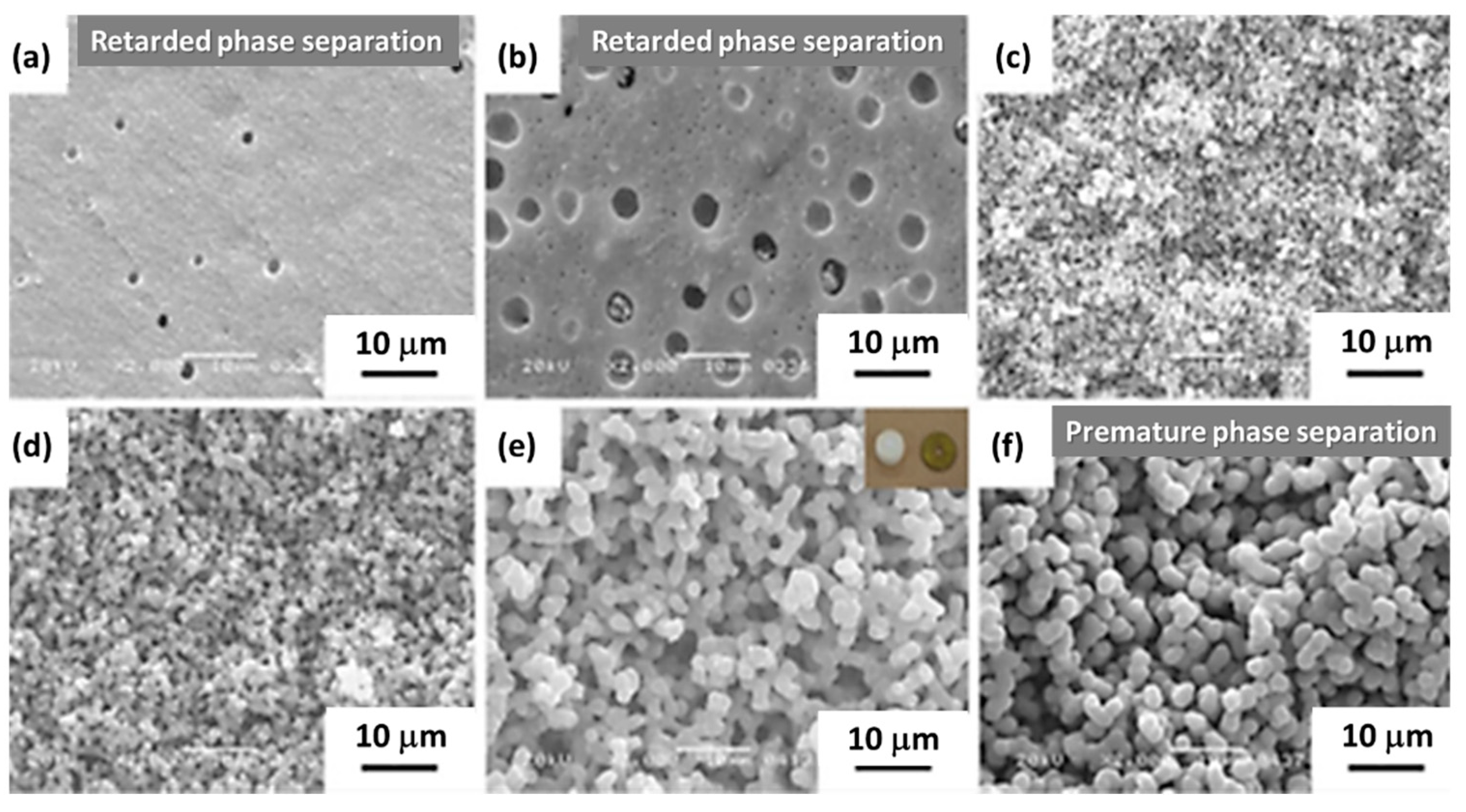
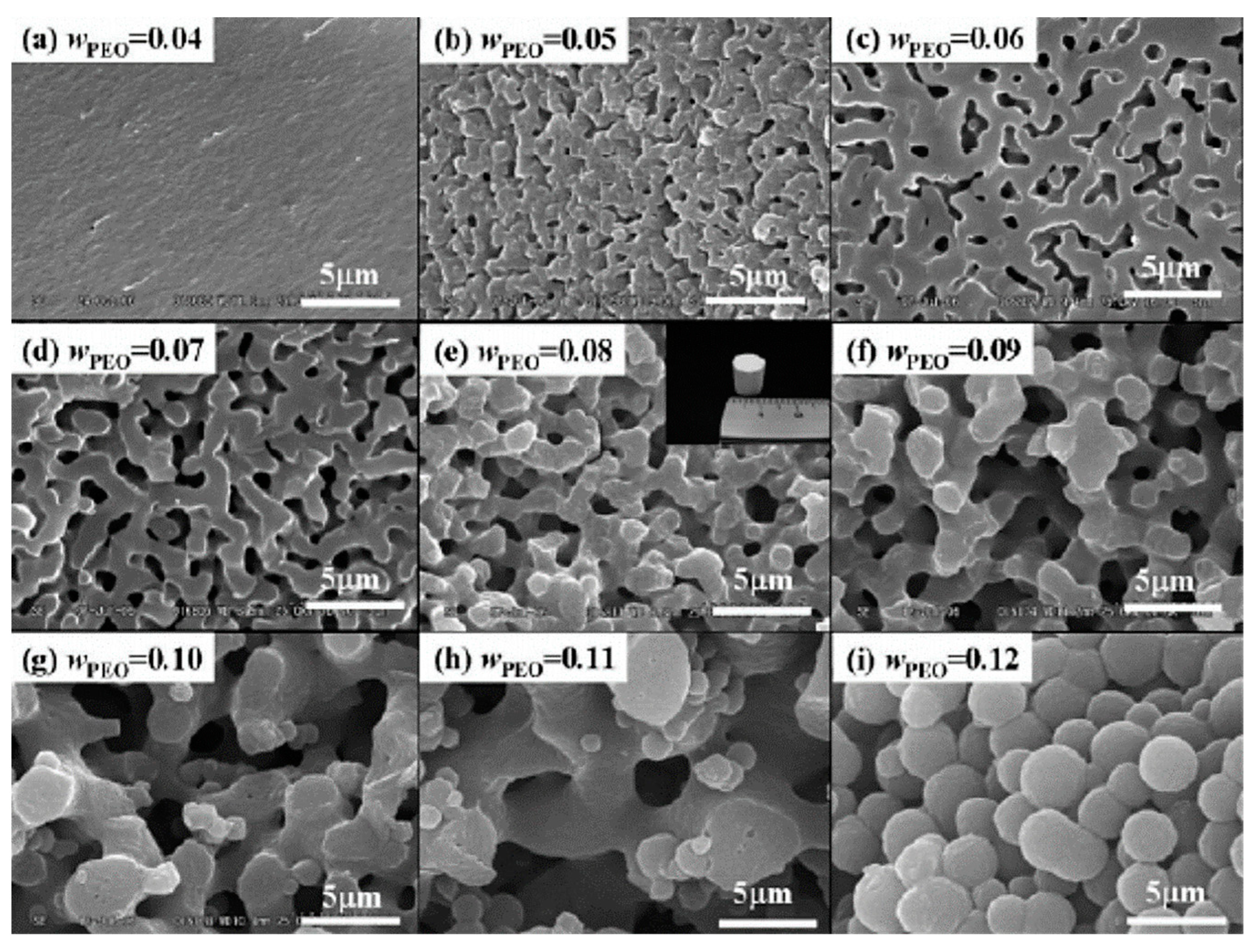

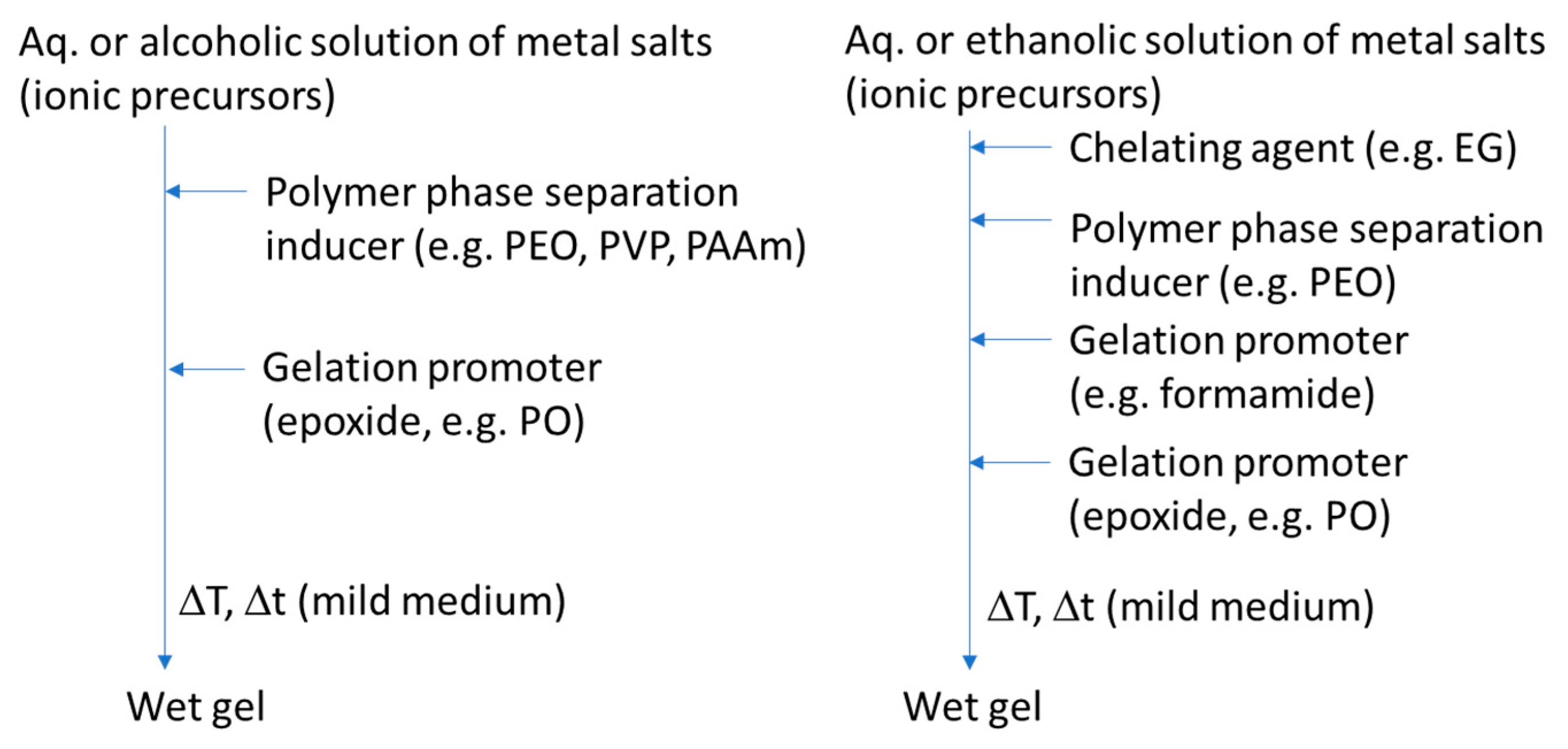
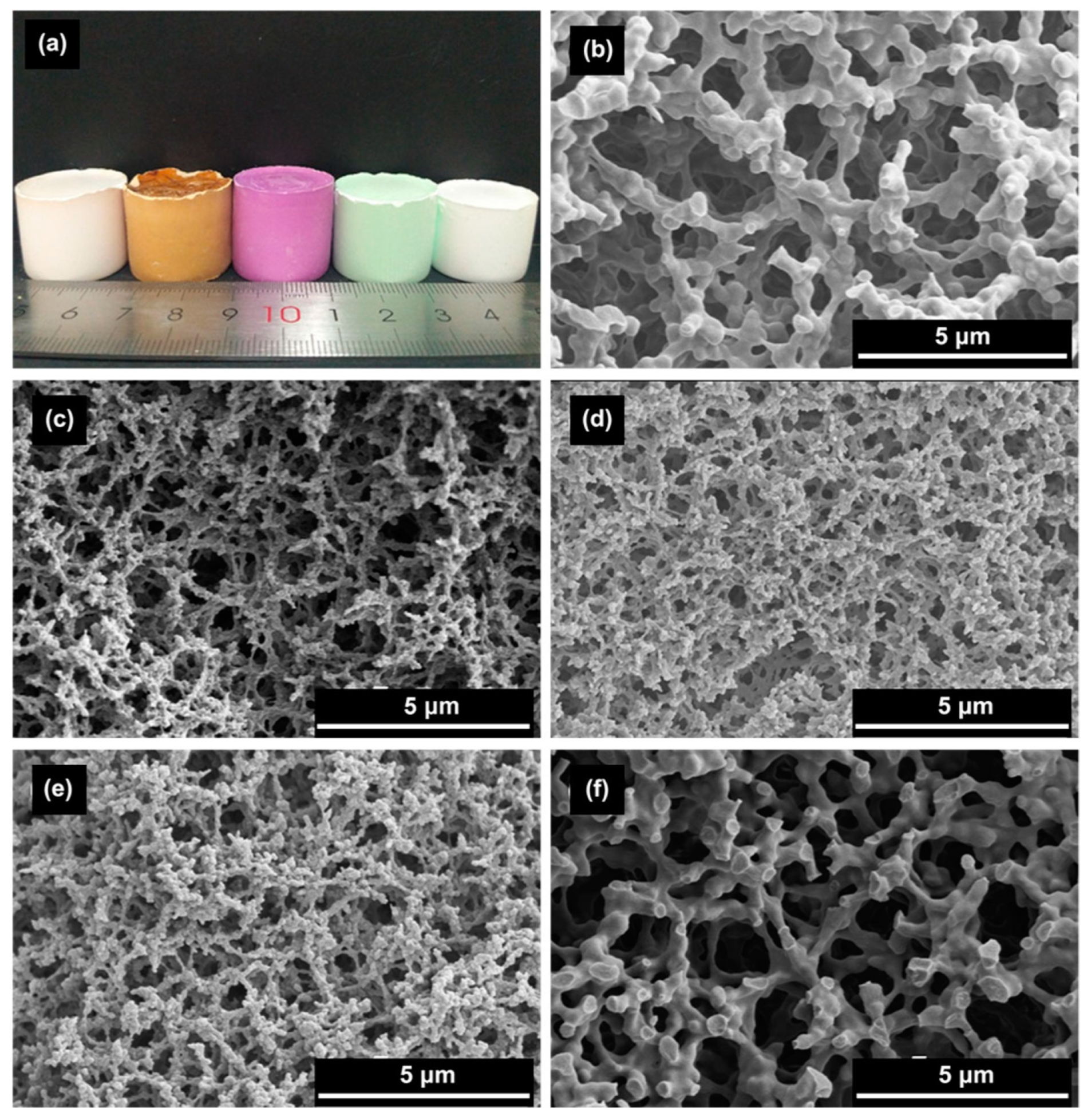
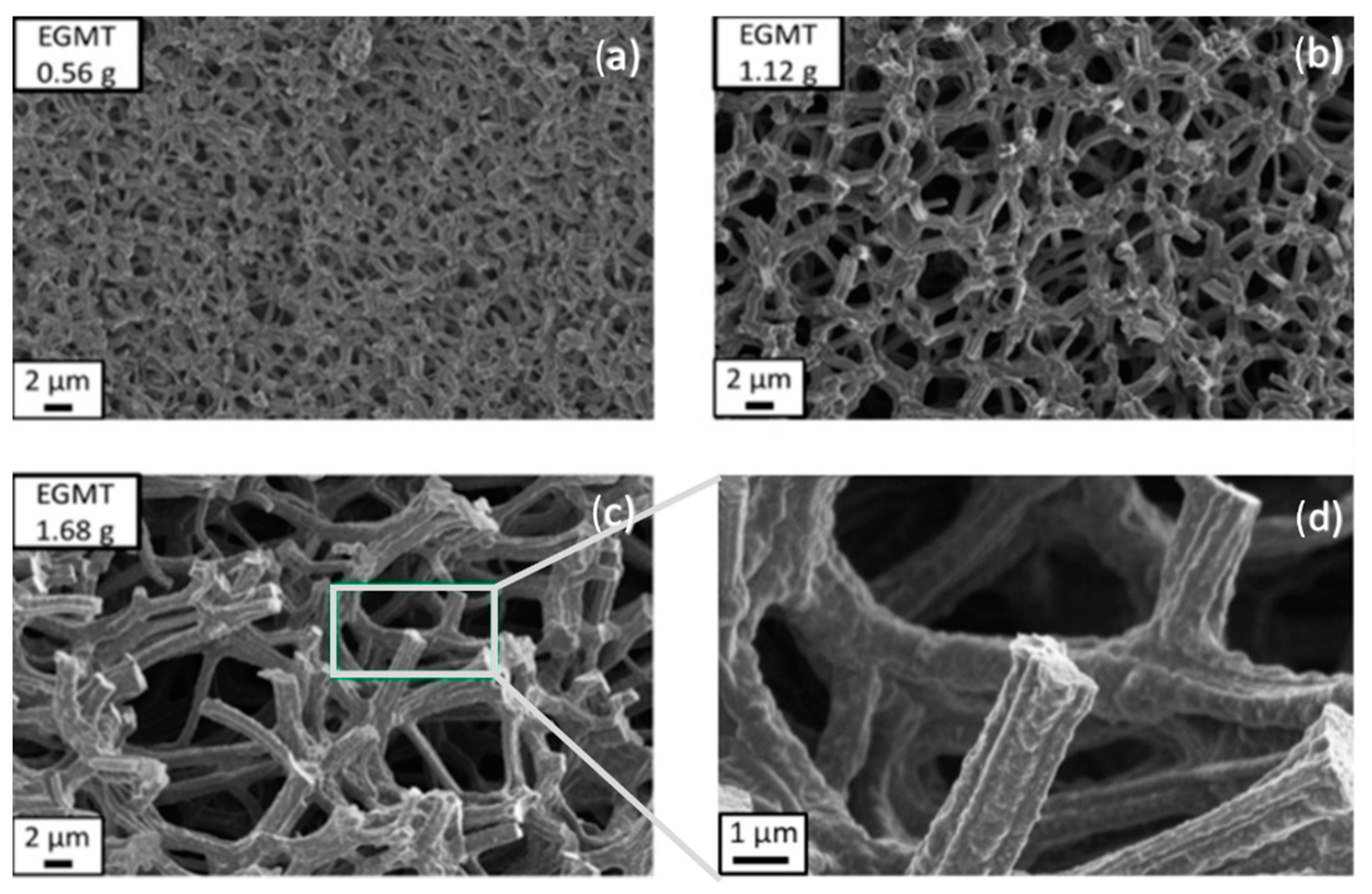
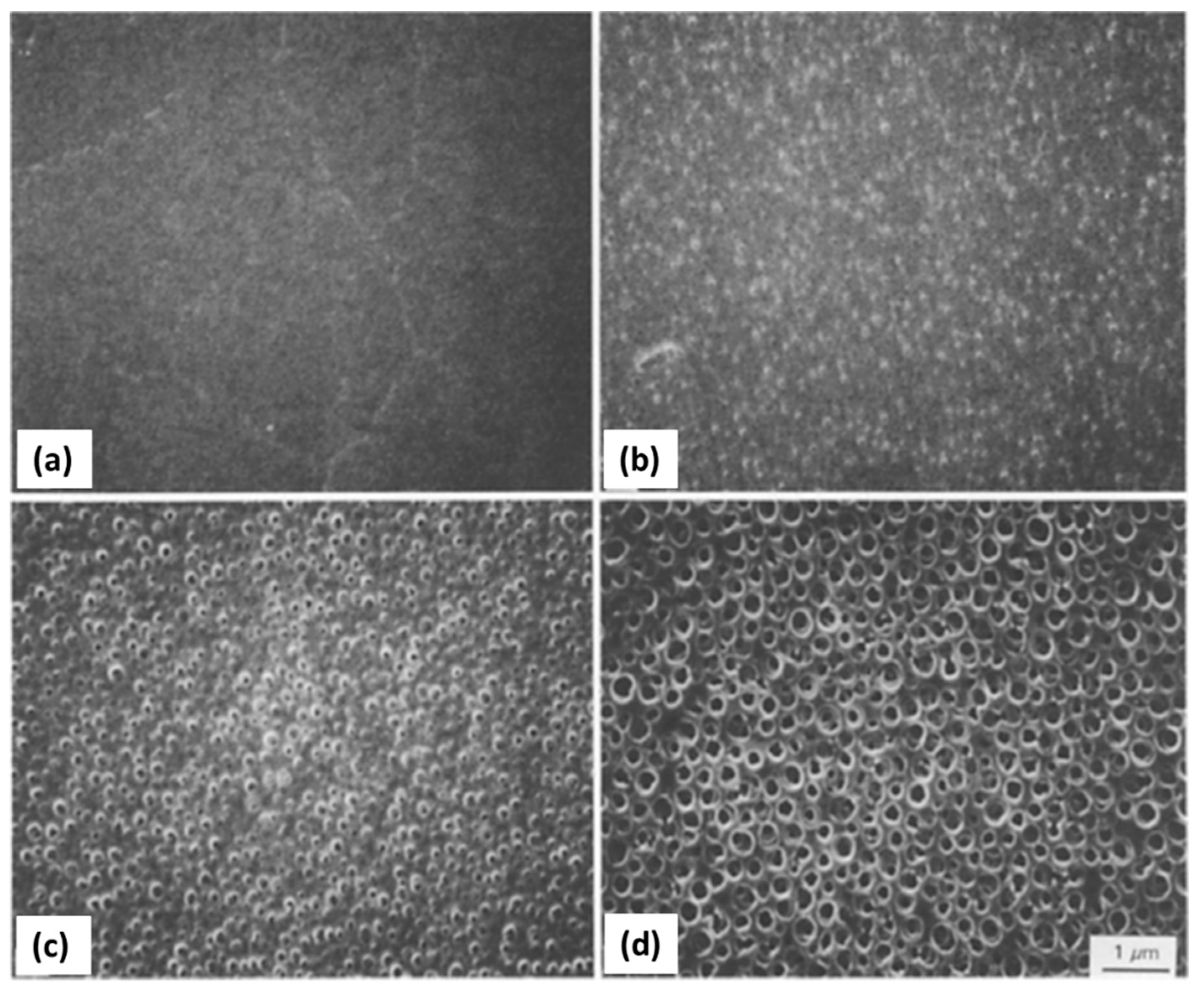
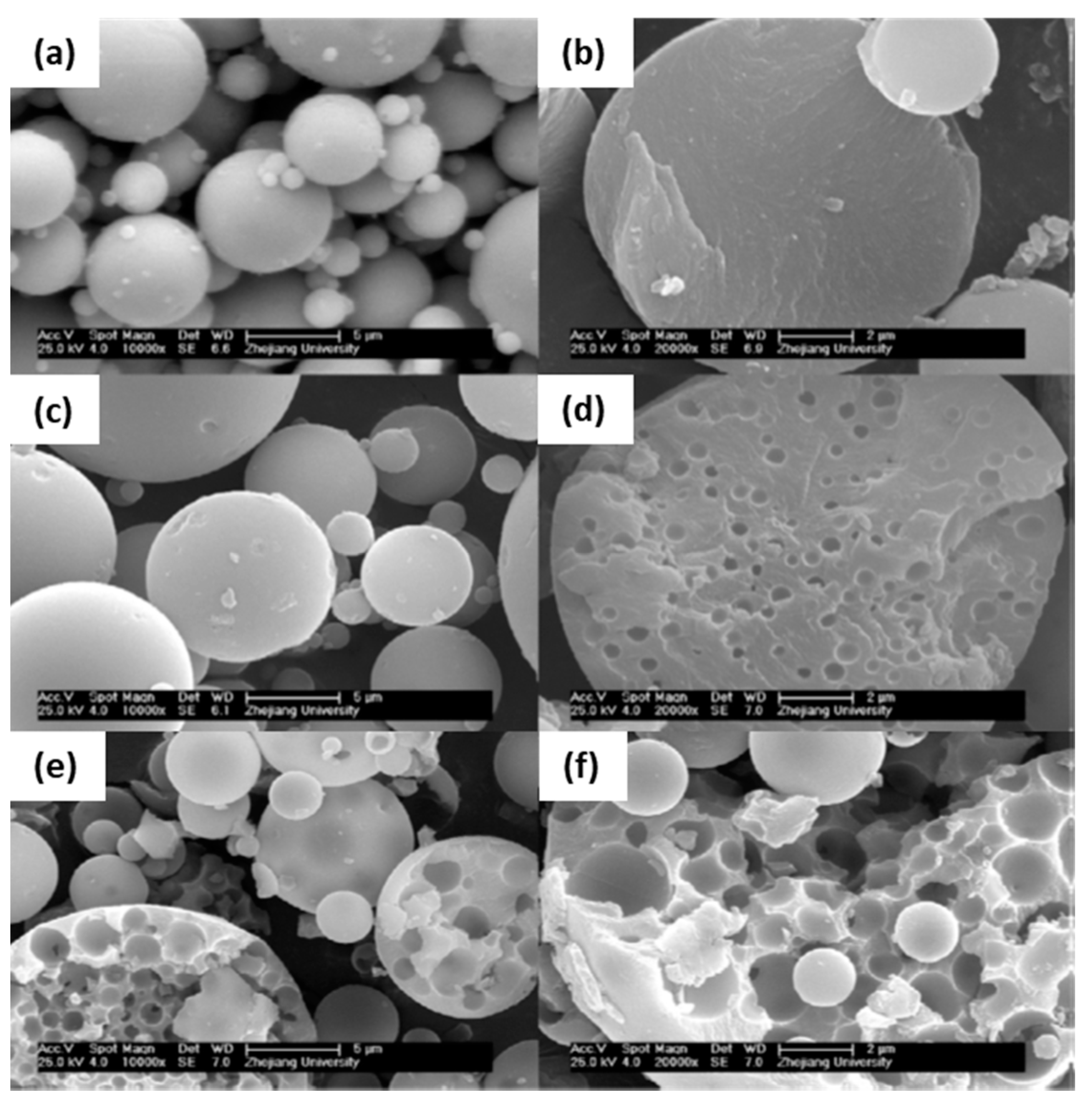



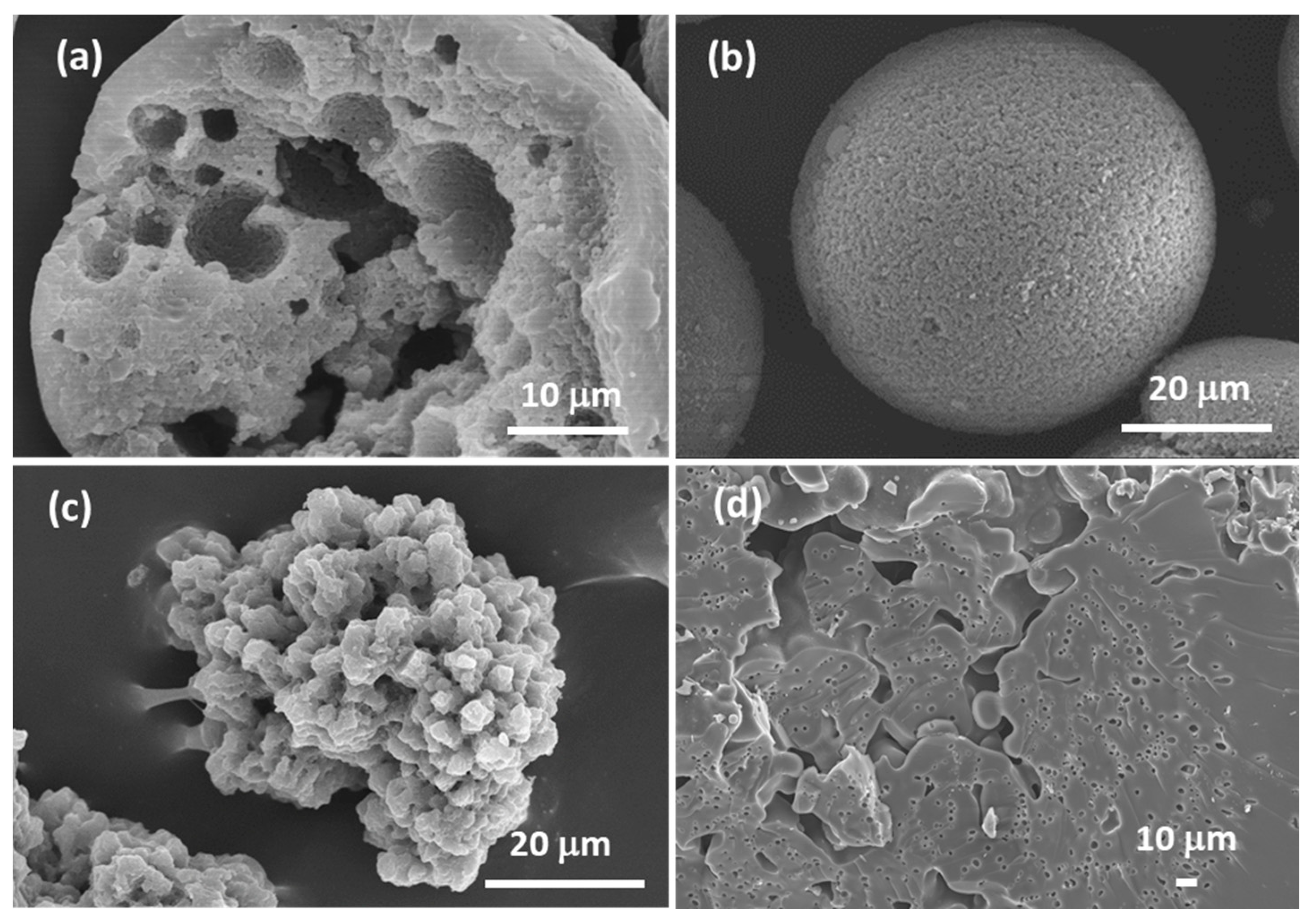
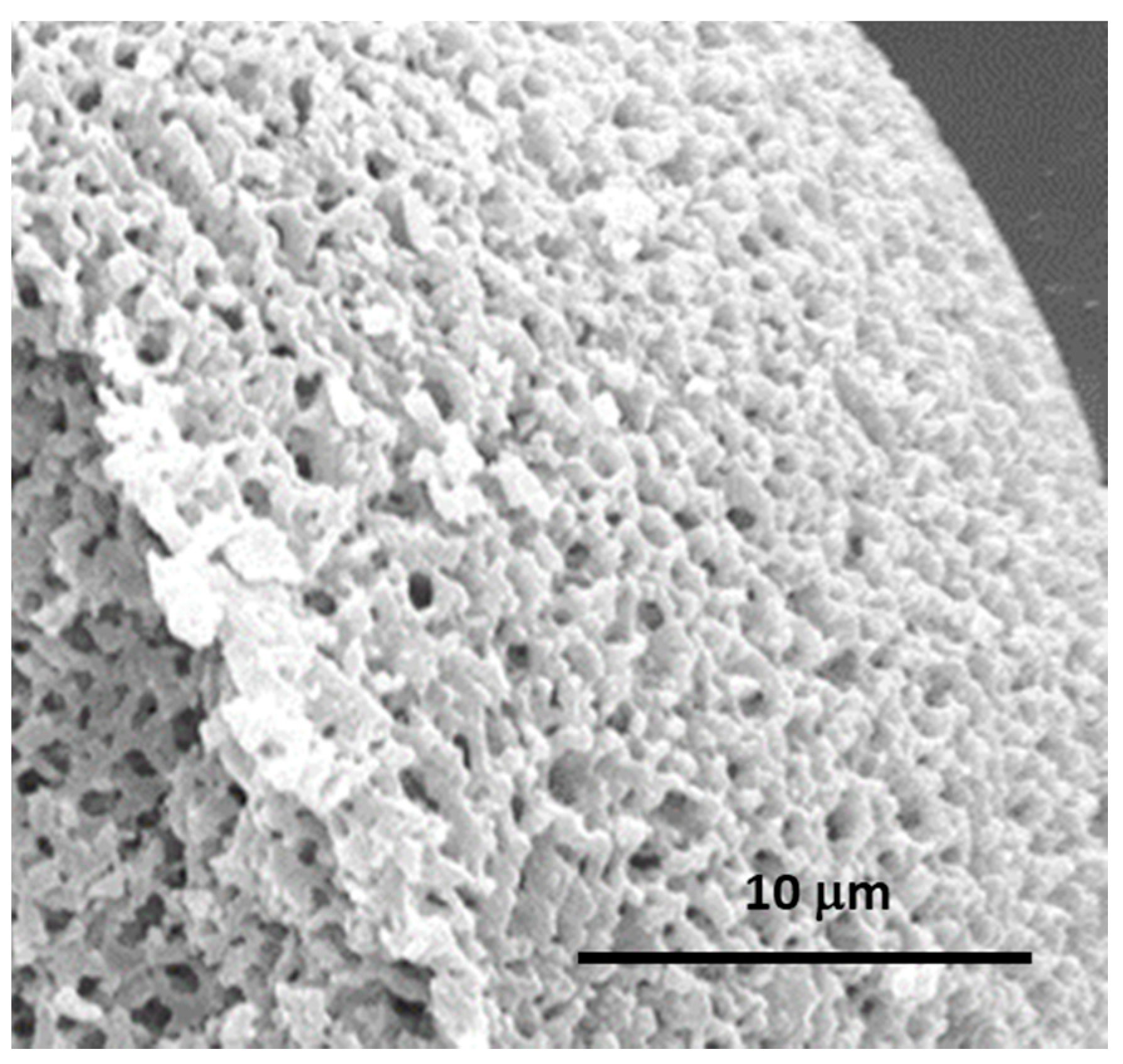
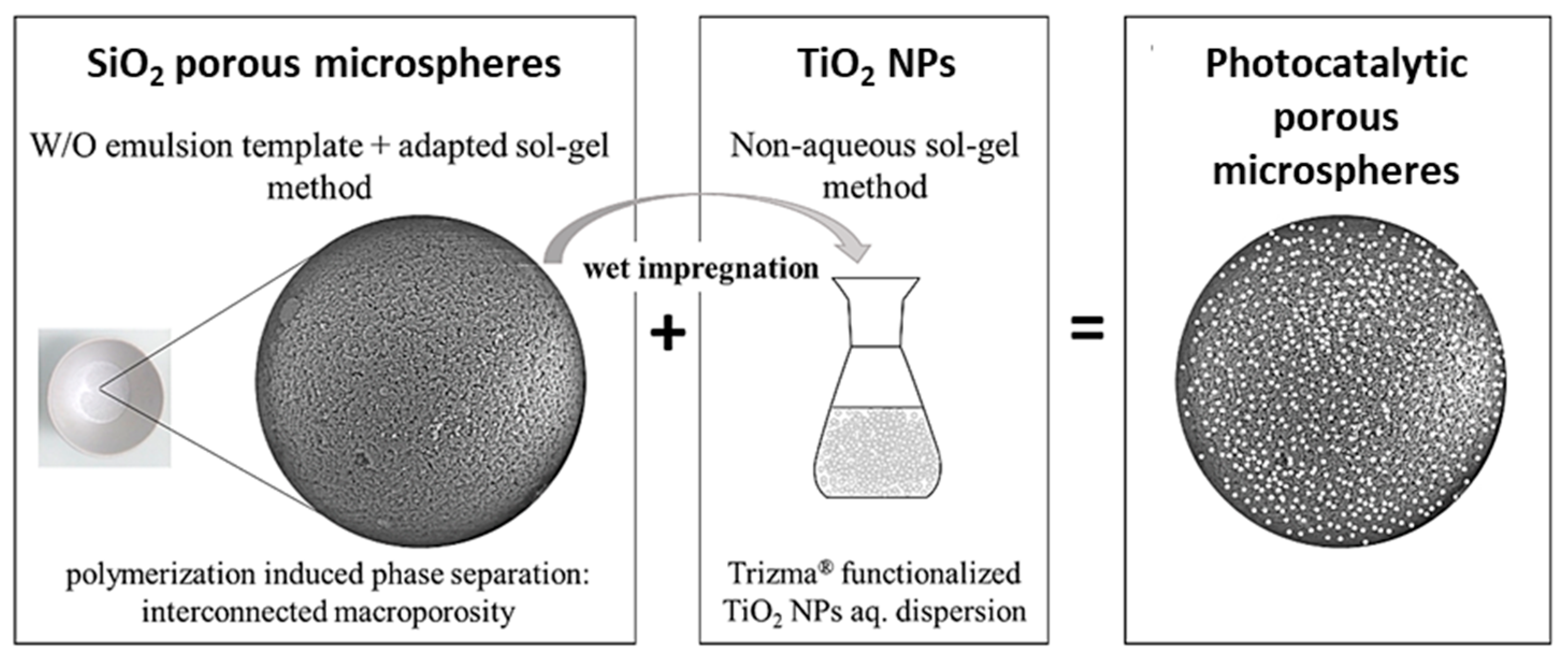
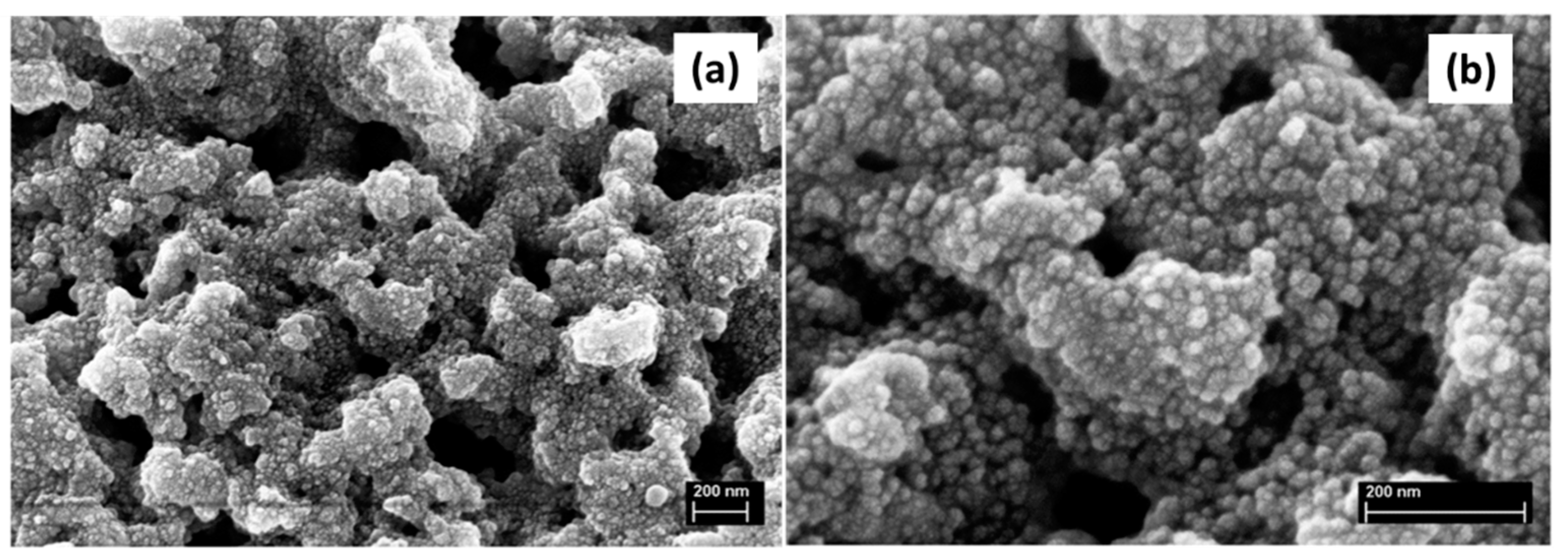
| Synthesis Strategy | Authors, Year Reference |
|---|---|
| Acidic sol-gel synthesis using a nonionic triblock copolymer (P123) as a phase separation inducer. Precursor: TMOS | Nakanishi et al., 1998 [28] |
| Acidic sol-gel synthesis without polymeric phase separation inducer additive, with methanol and water as common solvents. Precursor: bis(trimethoxysilyl)hexane (BTMH) | Nakanishi et al., 2002 [37] |
| Formamide-mediated sol-gel reaction. Acidic sol-gel synthesis without polymeric phase separation inducer additive, with methanol and water as common solvents, and formamide as additive polar solvent to promote gelation and phase separation. Precursor: TMOS and VTMS | Itagaki et al., 2003 [38] |
| Double-templating synthesis route: PEO as a phase separation inducer in combination with an ionic (CTAB) or nonionic surfactant (P123) as structure-directing agents at the nanometer range. Precursor: TEOS | Smått et al., 2003 [36] |
| Ethylene glycol-modified silane (precursor) in the presence of P123, for long-range ordering of the mesopores. | Hüsing et al., 2003 [43] |
| Propane-1,2-diol and glycerol-modified silane (precursor) in the presence of P123, for long-range ordering of the mesopores. | Hüsing et al., 2005 [44] |
| Acid/base two-step processing method to obtain macroporous PMSQ monoliths. No use of phase separation inducers. Precursor: MTMS | Dong et al., 2006 [39] |
| Acidic sol-gel synthesis using triblock copolymers (P123 and F127), and TMB as pore expander. Precursor: TMOS or bis(trimethoxysilyl)ethane (BTME) | Nakanishi et al., 2008 [34] |
| Weak acidic sol-gel synthesis using P123, and inorganic salts (NaCl, NaNO3 or Na2SO4) for ordered mesostructured and greater silica cross-linking. Precursor: TMOS | Zhong et al., 2009 [45] |
| Acid/base two-step processing method, using triblock copolymers (F127), to obtain macro/mesoporous PMSQ monoliths. Precursor: MTMS | Kanamori et al., 2011 [40] |
| Varied nonionic PEO-b-PPO-b-PEO triblock copolymers with different Mw and PO/EO ratios tested as phase separation inducers (PMSQ monoliths). Precursor: MTMS | Kurahashi et al., 2012 [42] |
| Composition | Synthesis Strategy | Authors, Year Reference |
|---|---|---|
| SiO2-TiO2 | Acidic sol-gel synthesis using a phase separation inducer (HPAA). Precursors: TMOS and TBOT | Nakanishi et al., 1992 [47] |
| SiO2-TiO2 | Acidic sol-gel synthesis using a phase separation inducer (PEG, 20,000 Mw) and two-step hydrolysis, or acac-complexation route. Precursors: TEOS and Ti salts and alkoxides | Ruzimuradov et al., 2012 [48] |
| SiO2-TiO2 | Double-templating synthesis route: a phase separation inducer (PEG, 10,000 Mw), nonionic surfactant (P123), together with NH4F for further Ti incorporation in the framework. Precursors: TMOS and TiPOT | Yang et al., 2013 [49] |
| SiO2-ZrO2 | Acidic sol-gel synthesis adding a phase separation inducer (PEO, 100,000 Mw) and specific alcohols to increase the domain size. Precursors: TMOS and zirconium tetra-2-propoxide (TPZR) | Takahashi et al., 1997 [50] |
| SiO2-Al2O3 | Acidic sol-gel synthesis adding a phase separation inducer (PEO, 100,000 Mw). Precursors: TEOS and aluminum nitrate | Takahashi et al., 2001 [51] |
| SiO2-Al2O3 | Acidic sol-gel synthesis adding a phase separation inducer (PEO, 10,000 Mw). Precursors: TMOS and aluminium sec-butoxide | Morai et al., 2004 [52] |
| SiO2-Al2O3 | Double-templating synthesis route: a phase separation inducer (PEO, 100,000 Mw) and C16EO10 as the structure-directing agent. Precursors: TMOS and aluminum nitrate, or aluminum isopropoxide | Wu et al., 2007 [53] |
| SiO2-Al2O3 | Double-templating synthesis route: aphase separation inducer (PEO, 10,000 Mw) and P123 as the structure-directing agent. Precursors: TMOS and aluminum nitrate | Yang et al., 2010 [54] |
| SiO2-Al2O3 (mullite) | Epoxide (propylene oxide, PO)-mediated sol-gel reaction + a phase separation inducer (PEO, 100,000 Mw) Precursors: TMOS and aluminum chloride | Guo et al., 2013 [55] |
| Ni/SiO2 | Acidic sol-gel synthesis adding a phase separation inducer (PEO, 100,000 Mw). Precursors: TEOS and nickel nitrate | Nakamura et al., 2000 [56] |
| CuO/SiO2, NiO/SiO2 | Acidic sol-gel synthesis adding a phase separation inducer (PEO, 10,000 Mw). Precursors: TMOS and nickel/copper nitrate | Zheng et al., 2006 [57] |
| SiO2-CaO | Acidic sol-gel synthesis adding a phase separation inducer (PEO, 100,000 Mw). Precursors: TMOS and calcium nitrate tetrahydrate (Ca(NO3)2·4H2O) | Marques et al., 2009 [58] |
| SiO2-CaO-P2O5 | Acidic sol-gel synthesis, using two strategies: (i) PEO (100,000 Mw) as a phase separation inducer and urea; (ii) P123 as a phase separation inducer and 1,3,5-trimethylbenzene (TMB) as a pore expander (micelle-swelling agent). Precursors: TMOS, calcium nitrate tetrahydrate (Ca (NO3)2·4H2O), and triethyl orthophosphate | Marques et al., 2007; [59] |
| MgO–Al2O3–SiO2 (cordierite) | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PAAm). Precursors: TMOS, magnesium chloride and aluminum chloride | Guo et al., 2014 [60] |
| Al2O3 -SiO2-TiO2 | Formamide-mediated sol-gel reaction + a phase separation inducer (PEO). Precursors: TEOS, TiPOT, aluminum nitrate nonahydrate | Sun et al., 2016 [61] |
| Composition | Synthesis Strategy | Authors, Year Reference |
|---|---|---|
| TiO2 | Formamide-mediated sol-gel reaction (acidic medium) + a phase separation inducer (PEO, 300,000 and 100,000 Mw). Precursor: colloidal anatase-type TiO2 (7 nm size particles, aqueous dispersion, pH 1.7) | Fujita et al., 2004 and 2006 [66,67] |
| Formamide-mediated sol-gel reaction (strongly acidic medium, HCl) + low temperatures. Precursor: TiPOT | Konishi et al., 2006 [68] | |
| Nearly neutral sol-gel synthesis using a chelating agent (EtAcAc) + mineral salt (NH4NO3) + a phase separation inducer (PEO, 10,000 Mw). Precursor: TiPOT | Hasegawa et al., 2010 [69] | |
| NMF-mediated sol-gel reaction (strongly acidic medium, HCl) + low temperatures + a phase separation inducer (PEO, 10,000 Mw). Precursor: TiPOT | Konishi et al., 2009 [71] | |
| Reaction rate controlling additives: strongly acidic sol-gel synthesis (HCl) + a chelating agent (acetic acid). Precursor: TiPOT | Backlund et al., 2007 [73] | |
| Formamide-mediated sol-gel reaction (acidic medium) + a phase separation inducer (PVP, 10,000 Mw) + EG as a chelating agent. Precursor: TiOSO4 | Li et al., 2013 [72] | |
| ZrO2 | NMF-mediated sol-gel reaction (strongly acidic medium, HNO3) + low temperatures + a phase separation inducer (PEO, 35,000 Mw). Precursor: Zirconium isopropoxide | Konishi et al., 2008 [75] |
| Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PEO, 1,000,000 Mw) Precursors: Zirconium oxychloride octahydrate (ZrOCl2·8H2O) | Guo et al., 2015 [78] | |
| Magnesia and yttria stabilized ZrO2 | Epoxide (PO)- and NMF-mediated sol-gel reaction + a phase separation inducer (PEO, 1,000,000 Mw) Precursors: anydrous zirconium chloride (ZrCl4) and MgCl2·6H2O or YCl3·6H2O | Wu et al., 2014 [77] |
| Yttria stabilized ZrO2 (YZA) | Epoxide (PO)- and formamide-mediated sol-gel reaction + a phase separation inducer (PEO, 300,000 Mw) + EG as a chelating agent Precursors: Zirconium oxychloride (ZrOCl2·8H2O) and yttrium chloride (YCl3·6H2O) | Guo et al., 2016 [79] |
| Al2O3 | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PEO, 1,000,000 Mw) Precursor: AlCl3·6H2O | Tokodume et al., 2007 [76] |
| Cr2O3 | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (HPAA) + urea. H.T under air atmosphere. CrN-C and Cr3C2-C composites will form if H.T. is under N2 atmosphere. Precursor: chromium chloride hexahydrate CrCl3·6H2O | Kido et al., 2014 [81] |
| MgO | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PVP, 100,000 Mw). Precursor: Magnesium chloride hexahydrate (MgCl2·6H2O) | Li et al., 2016 [93] |
| Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PVP, 10,000 Mw) + 1,3,5-benzenetricarboxylic acid to preserve the fine crystallite size. Precursor: Magnesium chloride hexahydrate (MgCl2·6H2O) | Lu et al., 2019 [83] | |
| ZnO | Epoxide (PO)-mediated sol-gel reaction + citric acid to coordinate to Zn cations and promote phase separation. 1,2-epoxybutane was also tested and found to have lower solubility and compatibility than PO, enhancing phase separation. Precursor: Zn(NO3)2·6H2O | Lu et al., 2019 [90] |
| CoO2, CuO2, MnO2 | Epoxide (epichlorohydrin)-mediated sol-gel reaction (acidic medium) + a phase separation inducer (PEO 100,000–600,000 Mw and/or PVP 40,000 Mw). Precursors: metal bromides (MBr2, with M = Cu,Co,Mn), which transform into brominated metal alkoxides, by reaction with epichlorohydrin | Lu et al., 2020 [92] |
| Fe2O3 | Epoxide (PO and trimethylene oxide)-mediated sol-gel reaction + a phase separation inducer (PAAm, 10,000 Mw). Precursor: Iron(III) chloride hexahydrate (FeCl3·6H2O) | Kido et al., 2012 [82] |
| Fe3O4 | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PAAm, 10,000 Mw). Precursor: Iron(II) chloride tetrahydrate (FeCl2·4H2O) | Wang et al., 2020 [94] |
| Composition | Synthesis Strategy | Authors, Year Reference |
|---|---|---|
| Aluminum phosphate AlPO4 | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PEO, 10,000 Mw). Precursor: AlCl3·6H2O and H3PO4 | Li et al., 2013 [97] |
| Metal zirconium phosphates (MZr2(PO4)3) | Acidic sol-gel synthesis adding a phase separation inducer (PEO, 35,000 Mw and PAAm, 10,000 Mw). Precursors: ZrOCl2·8H2O, H3PO4, and several metal chlorides | Zu et al., 2016 [98] |
| Lithium zirconate Li2ZrO3 | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PEO, 1,000,000 Mw). Precursors: ZrOCl2·8H2O and lithium acetate dehydrate (LiOAc·2H2O) | Guo et al., 2017, [84] |
| Barium zirconate BaZrO3 | Epoxide (PO)- and formamide-mediated sol-gel reaction + a phase separation inducer (PEO, 300,000 Mw) + EG as a chelating agent Precursors: Zirconium oxychloride (ZrOCl2·8H2O) and barium chloride dihydrate (BaCl2·2H2O) | Guo et al., 2017 [80] |
| Zirconium titanate ZrTiO4 | N-methyl formamide-mediated sol-gel reaction (strongly acidic medium, HNO3, ice cooling) + low temperatures + a phase separation inducer (PEO, 100,000 Mw). Precursors: ZrOCl2·8H2O and TiPOT | Sun et al., 2019 [89] |
| CaTiO3, SrTiO3 and BaTiO3 perovskite | Impregnation of preformed macroporous TiO2 monoliths, with alkaline-earth metal ions, in urea solution. Nearly neutral sol-gel synthesis using a chelating agent (EtAcAc) + mineral salt (NH4Cl) + a phase separation inducer (PEO, 10,000 Mw). Precursors: TiPOT, CaCl2·2H2O, SrCl2·6H2O, and BaCl2·2H2O | Ruzimuradov et al., 2011 [99] |
| La2Zr2O7 | Epoxide (PO)- and formamide-mediated sol-gel reaction + a phase separation inducer (PEO, 300,000 Mw). Precursors: Zr(NO3)4·5H2O, ZrOCl2·8H2O, La(NO3)3·6H2O, and LaCl3·6H2O | Wang et al., 2016 [87] |
| Aluminum titanate Al2TiO5 | Formamide-mediated sol-gel reaction + a phase separation inducer (PEO, 100,000 Mw) + a chelating agent (citric acid) and ice cooling. Precursors: AlCl3·6H2O and titanium tetrabutoxide (Ti(OBu)4) | Guo et al., 2015 [100] |
| ZnFe2O4 spinel | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PAAm, 10,000 Mw). Precursors: FeCl3·6H2O and ZnCl2 (Zn/Fe, R = 0.50 in molar ratio) (low-valence elements) | Kido et al., 2013 [95] |
| NiAl2O4 and CoAl2O4 spinel | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PEO, 900,000 Mw). Precursors: AlCl3·6H2O, NiCl2· 6H2O, or CoCl2·6H2O (low-valence elements). | Herwig et al., 2018 [85] |
| ZnAl2O4 spinel | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (PEO, 1,000,000 Mw). Precursors: ZnCl2 and AlCl3·6H2O (low-valence elements) | Guo et al., 2017 [88] |
| CoMn2O4 spinel | Epoxide (epichlorohydrin)-mediated sol-gel reaction (acidic medium) + a phase separation inducer (PEO 400,000 Mw and/or PVP 10000, 40000, and 360,000 Mw). Precursors: metal bromides (MBr2, with M=Co, Mn), which transform into brominated metal alkoxides, by reaction with epichlorohydrin (low-valence elements) | Lu et al., 2020 [101] |
| Transition metal hydroxides, ZnOH, CuOH, MnOH and binary compositions | Epoxide (PO)-mediated sol-gel reaction + a phase separation inducer (HPAA, 100000Mw). Precursors: ZnCl2, CoCl2·6H2O, NiCl2·6H2O, MnCl2·4H2O, and FeCl2·4H2O | Liu et al., 2020 [96] |
| Carbon (C)/TiO2 | Formamide-mediated sol-gel reaction + a phase separation inducer and C source (PVP, 10,000 Mw) + ethylene glycol, solvent, and a chelating agent. Precursor: TiOSO4·xH2O | Zhu et al., 2015 [102] |
| Resorcinol formaldehyde (RF) and C | Acidic sol-gel synthesis, using surfactant F127 as a phase separation inducer and pore directing agent, TMB and benzyl alcohol BzOH (cosurfactant) as micelles´ swelling agents, and TEG as a compatible solvent with RF/F127 oligomers to suppress excess phase separation. Precursors: resorcinol (and formaldehyde) | Hasegawa et al., 2016 [103] |
| RF and C | Acidic sol-gel synthesis, using surfactant F127, TMB, BzOH, TEG, and inorganic salt KCl. Precursors: resorcinol (and formaldehyde) | Hasegawa et al., 2020 [27] |
| RF/TiO2 and C/TiO2 | Acidic sol-gel synthesis, using surfactant F127, TMB, BzOH, and TEG. Precursors: resorcinol (formaldehyde) and EGMT | Schoiber et al., 2021 [104] |
| Metal organic frameworks (MOFs) | Sol-gel synthesis and self-assembly-induced phase separation, using PPG (1000 Mn), solvent DMF, and acetic acid as a mediator for reorganization of the microstructure Precursors: ZrOCl2·8H2O and 2-aminoterephthalic acid (BDC-NH2) as an organic linker | Hara et al., 2019 [105] |
| Characteristics of the Microspheres | Synthesis Strategy/Applications | Author, Year Reference | |
|---|---|---|---|
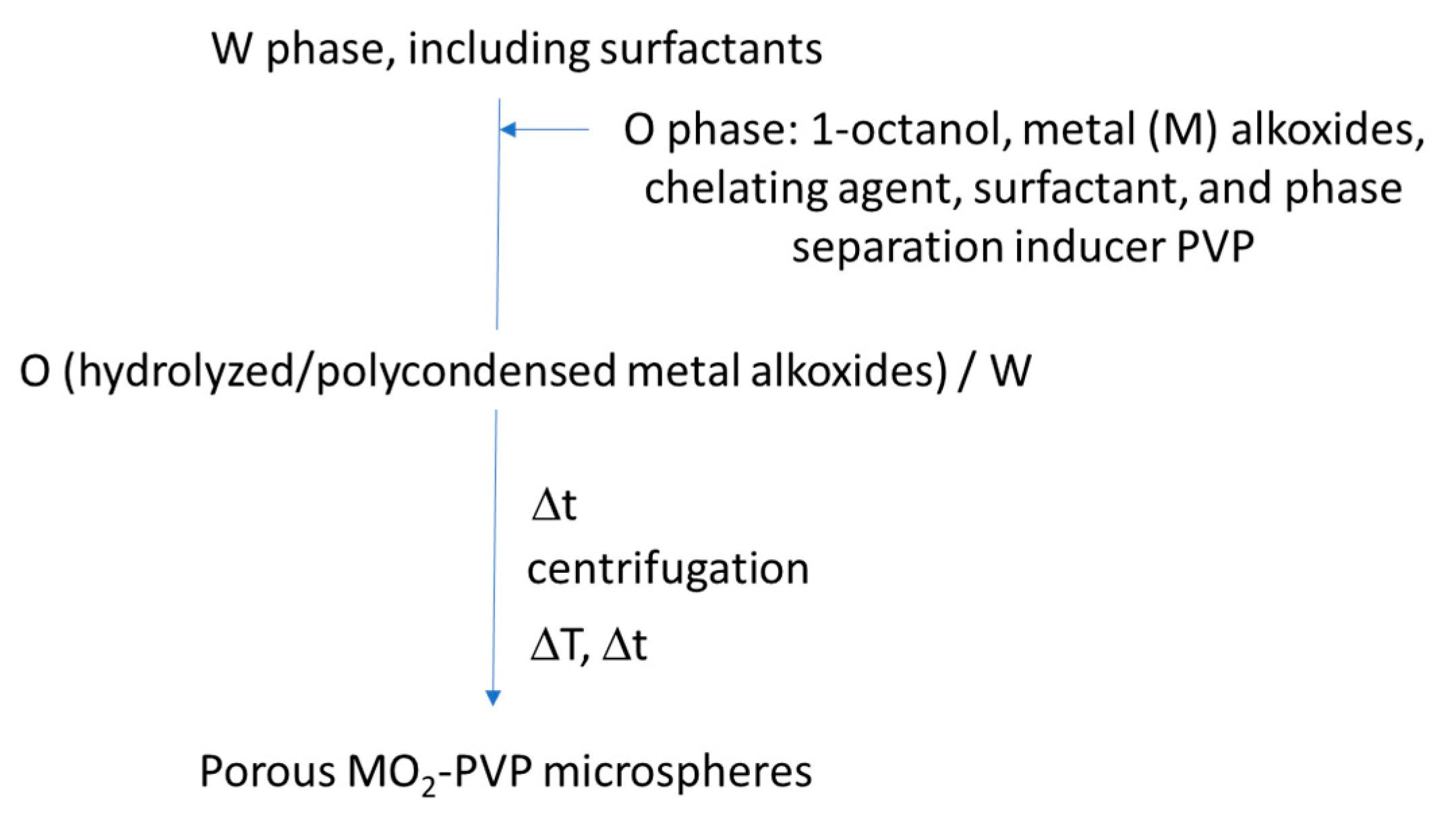
| Incontinuous multicavities | TiO2, ZrO2 and Al2O3 hollow microspheres with incontinuous multicavities | (1) O/W emulsion (O phase: metal alkoxide, EtAcAc, 1-octanol, Span 80, PVP; W phase: DI water and surfactants SDS and OP-10) (2) nucleation growth phase separation (3) nearly neutral sol-gel synthesis using a chelating agent (EtAcAc) + a phase separation inducer (PVP 30,000 Mw) and Span 80 as a phase separation and spherical morphology stabilizer. Precursor: TBOT, or zirconium propoxide, or aluminum tri-sec-butoxide Applications: liquid chromatography and heat insulation | Cai et al., 2014 [125] |
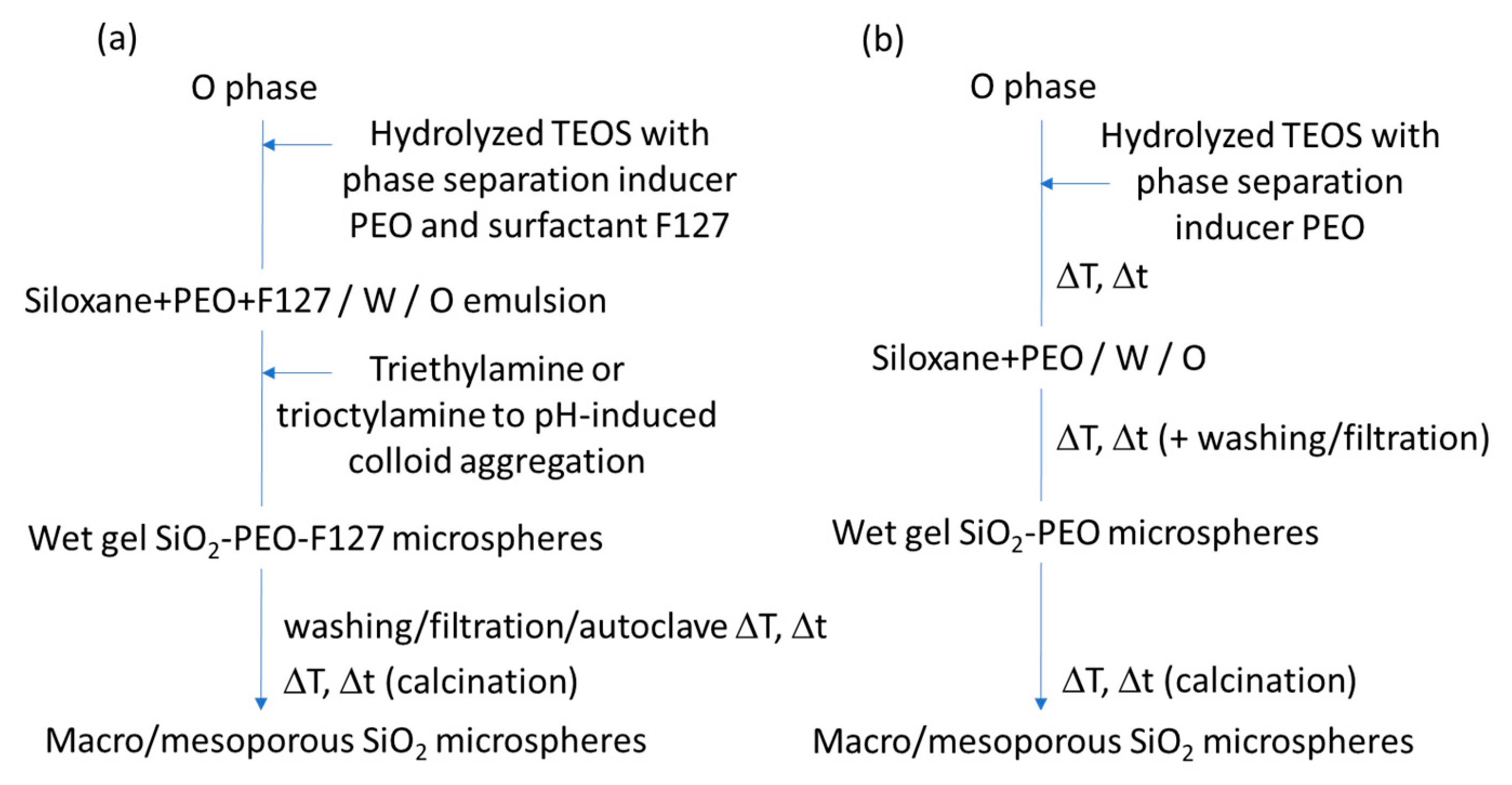
| Macro/ mesoporosity | Inorganic SiO2 microspheres | (1) W/O emulsion (W phase: hydrolyzed silane solution using a molar ratio H2O/precursor ~ 11, F127 as surfactant and PEO 20000Mw as macropore former); O phase: paraffin oil and Span 80 (2) pH induced rapid colloid aggregation method, by addition of triethylamine (TEA): sol-gel transition in parallel with phase separation. (3) calcination Precursors: TEOS No specific application tested | Yang et al., 2006 [127] |
| Inorganic SiO2 microspheres | (1) W/O emulsion (W phase: hydrolyzed silane solution using a molar ratio H2O/precursor ~18, and PEO 100,000 Mw); O phase: paraffin oil and Span 80 (2) growth of siloxane oligomers: sol-gel transition in parallel with phase separation (3) burning of PEO leads to mesopores and fluid phase evaporation leads to macropores. (4) calcination Precursors: TEOS Applications: SiO2 porous microspheres covalently bonded with octadecyl tested for liquid chromatography (fast separation) | Shi et al., 2008 [112] | |
| Waxberry-like and ethyl-bridged hybrid SiO2 microspheres | (1) W/O emulsion (W phase: hydrolysed silanes solution using a molar ratio H2O/precursors ~ 25 and PEO 10,000 Mw); O phase: petroleum ether, Triton X-100 and Span 80 (2) growth of siloxane oligomers: sol-gel transition in parallel with phase separation (3) burning of PEO leads to mesopores and fluid phase evaporation leads to macropores Precursors: TEOS and BTME Applications: Alkali resistant carrier with fast mass transfer property; tested for catalysis and liquid chromatography | Li et al., 2019 [110] | |
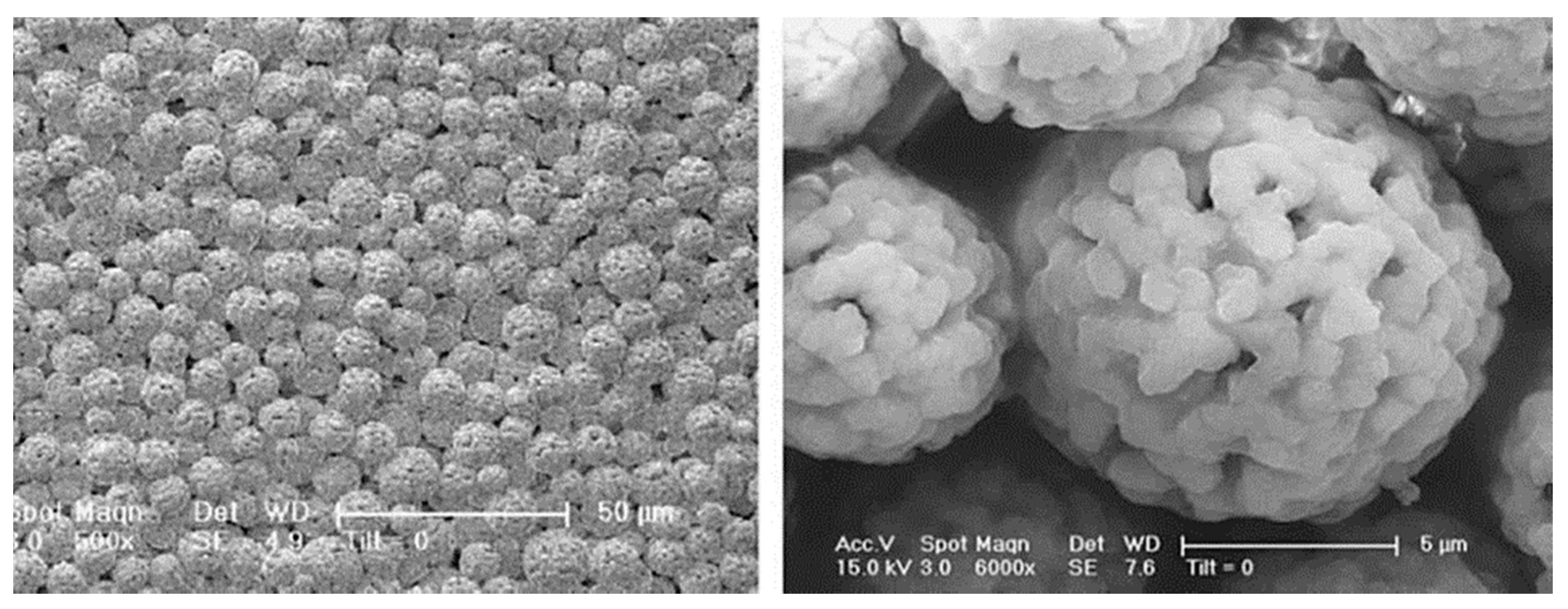
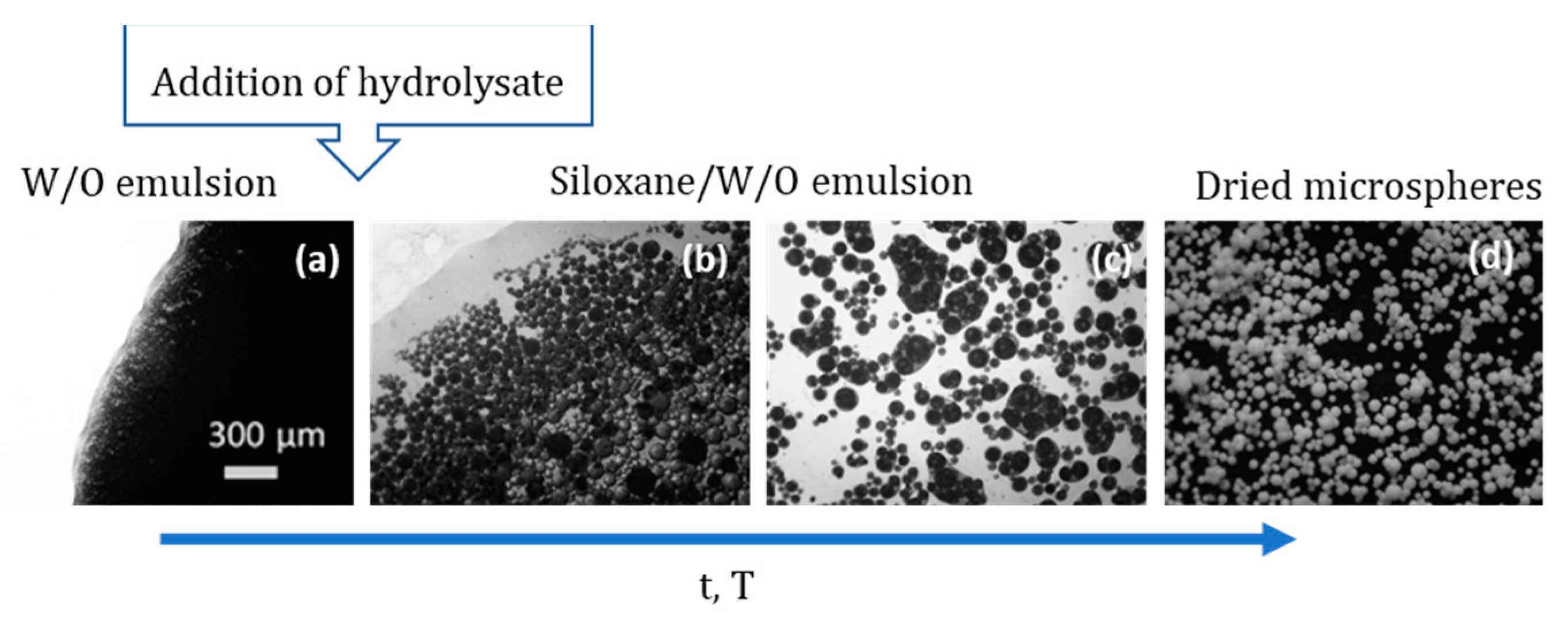
| Hybrid, epoxy functionalized and inorganic SiO2 microspheres | (1) W/O emulsion (W phase: water; O phase: decahydronaphthalene and Span 80) (2) addition of the hydrolysed silanes solution (with molar ratio H2O/precursors = 4.7) to the W phase (3) growth of silica-epoxy oligomers; sol-gel transition in parallel with phase separation inside the emulsion droplets; no need for templates or a phase separation inducer additives, no need for calcination to obtain meso and macroporosity. Study of synthesis parameters [2]. Precursors: TEOS and GPTMS (1/0.89 molar ratio) Applications: (i) Biocide Econea® immobilization within the porous microspheres (grafting) for antifouling applications [140]; (ii) TiO2 NPs immobilization within the porous microspheres for solar light driven photocatalysis, using a continuous flow reactor [3] | Loureiro et al., 2018 [140] Vale et al., 2020 [2] Marques et al., 2021 [3] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.C.; Vale, M. Macroporosity Control by Phase Separation in Sol-Gel Derived Monoliths and Microspheres. Materials 2021, 14, 4247. https://doi.org/10.3390/ma14154247
Marques AC, Vale M. Macroporosity Control by Phase Separation in Sol-Gel Derived Monoliths and Microspheres. Materials. 2021; 14(15):4247. https://doi.org/10.3390/ma14154247
Chicago/Turabian StyleMarques, Ana C., and Mário Vale. 2021. "Macroporosity Control by Phase Separation in Sol-Gel Derived Monoliths and Microspheres" Materials 14, no. 15: 4247. https://doi.org/10.3390/ma14154247
APA StyleMarques, A. C., & Vale, M. (2021). Macroporosity Control by Phase Separation in Sol-Gel Derived Monoliths and Microspheres. Materials, 14(15), 4247. https://doi.org/10.3390/ma14154247







