Fabrication, Structure, and Thermal Properties of Mg–Cu Alloys as High Temperature PCM for Thermal Energy Storage
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Preparation
2.2. Analysis Methods
3. Results
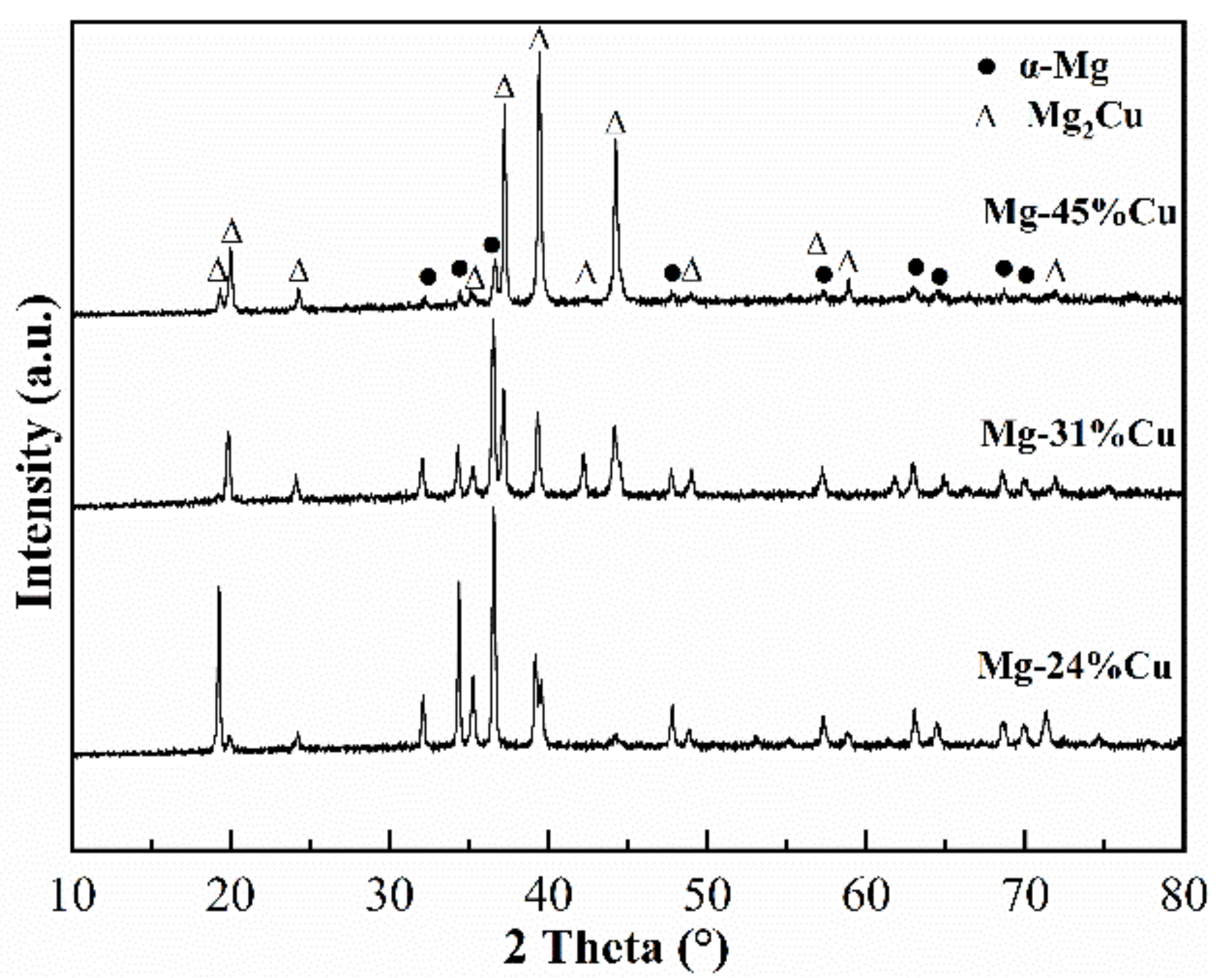
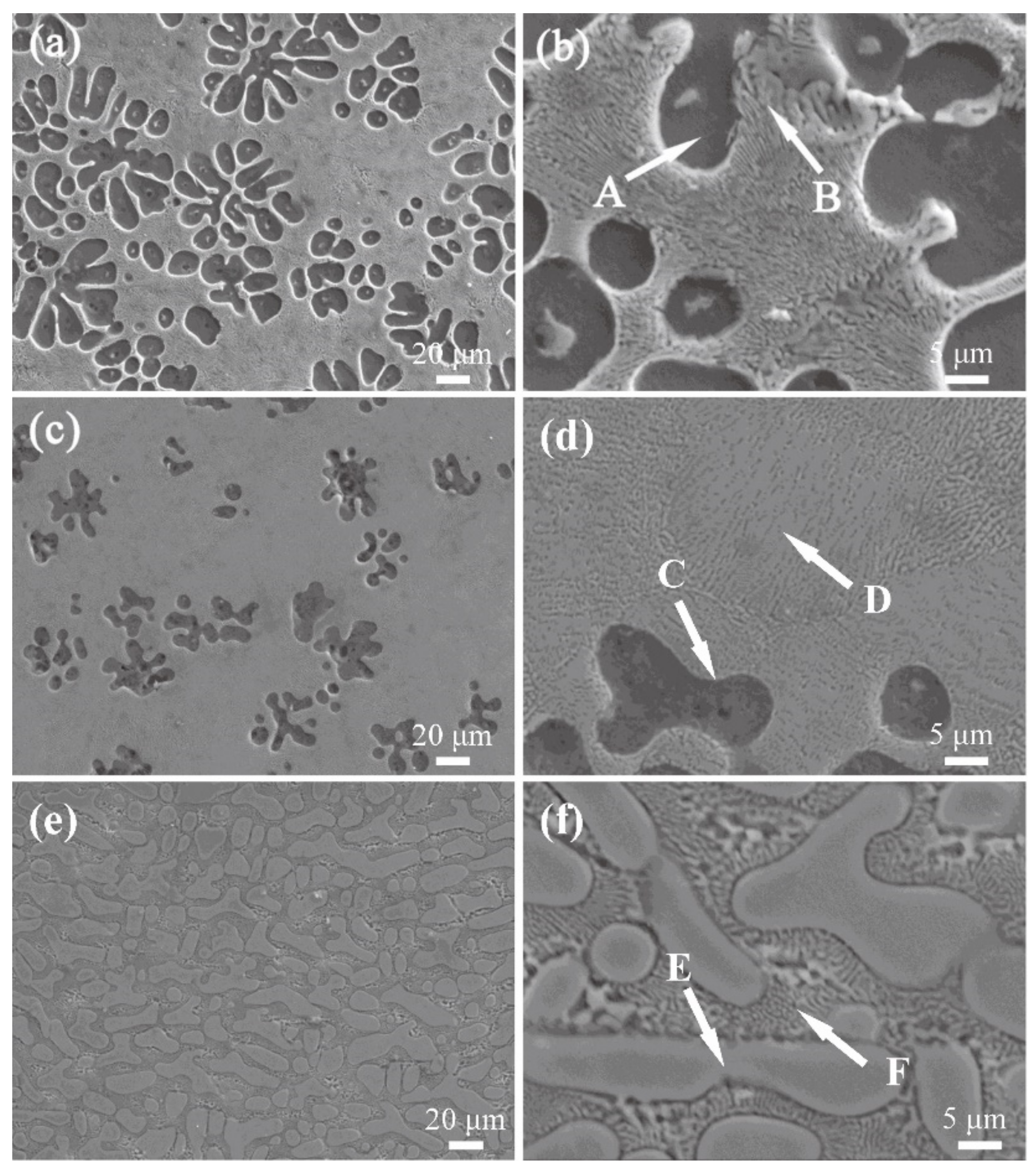
3.1. Structural Analysis
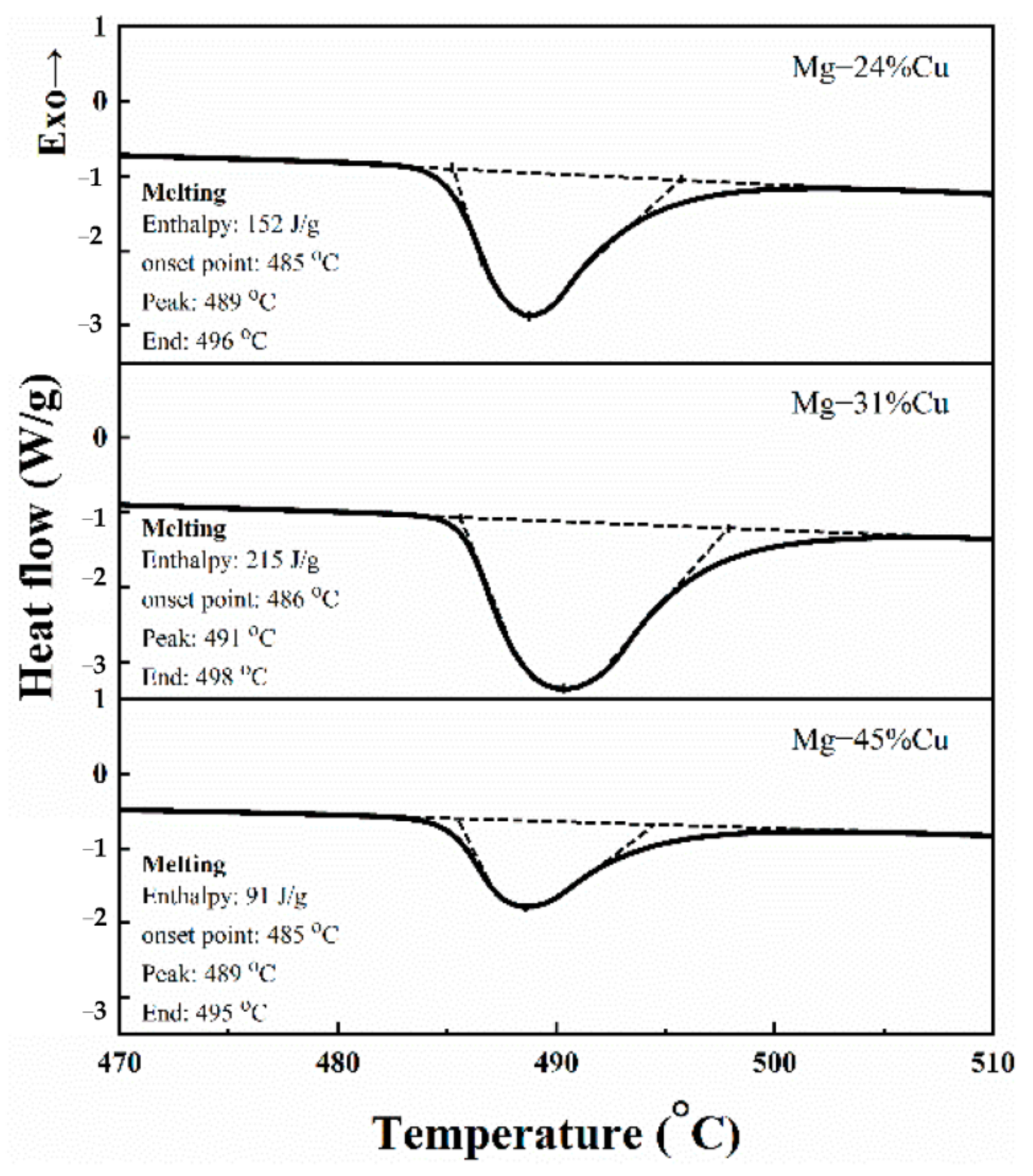
3.2. Phase Change Temperatures and Enthalpies
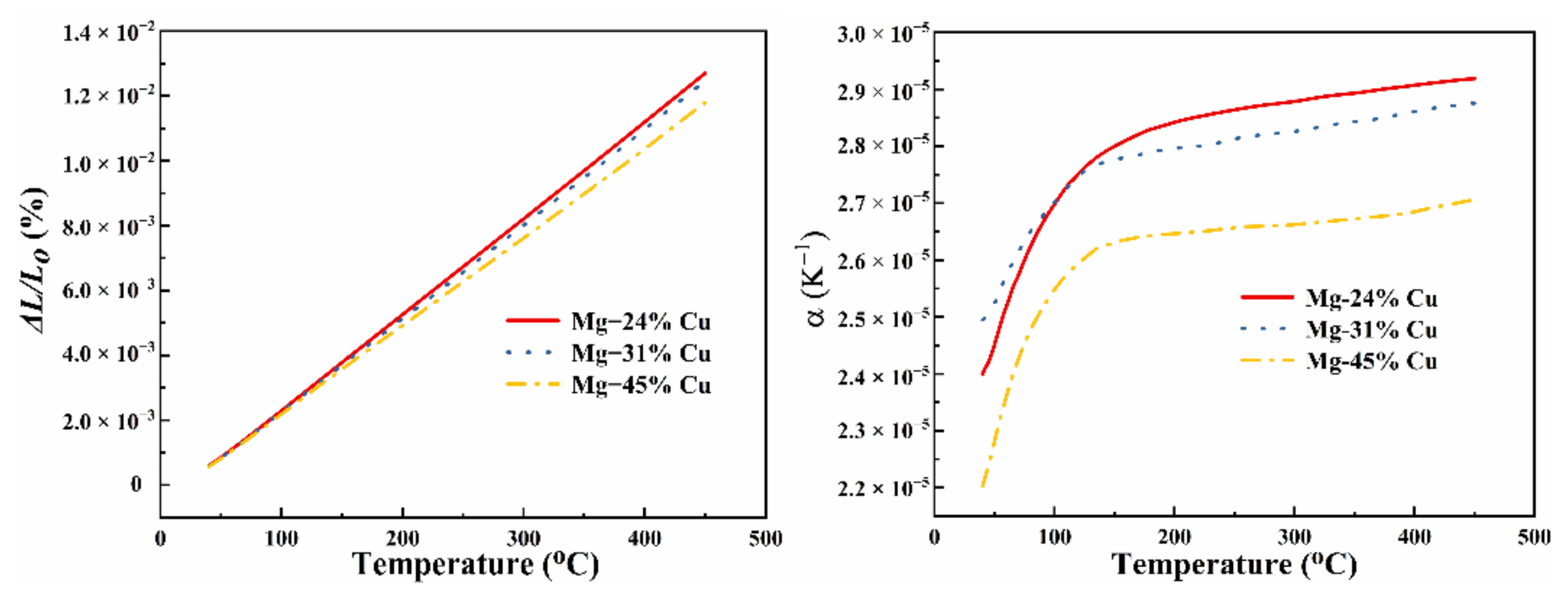
3.3. Thermal Expansion
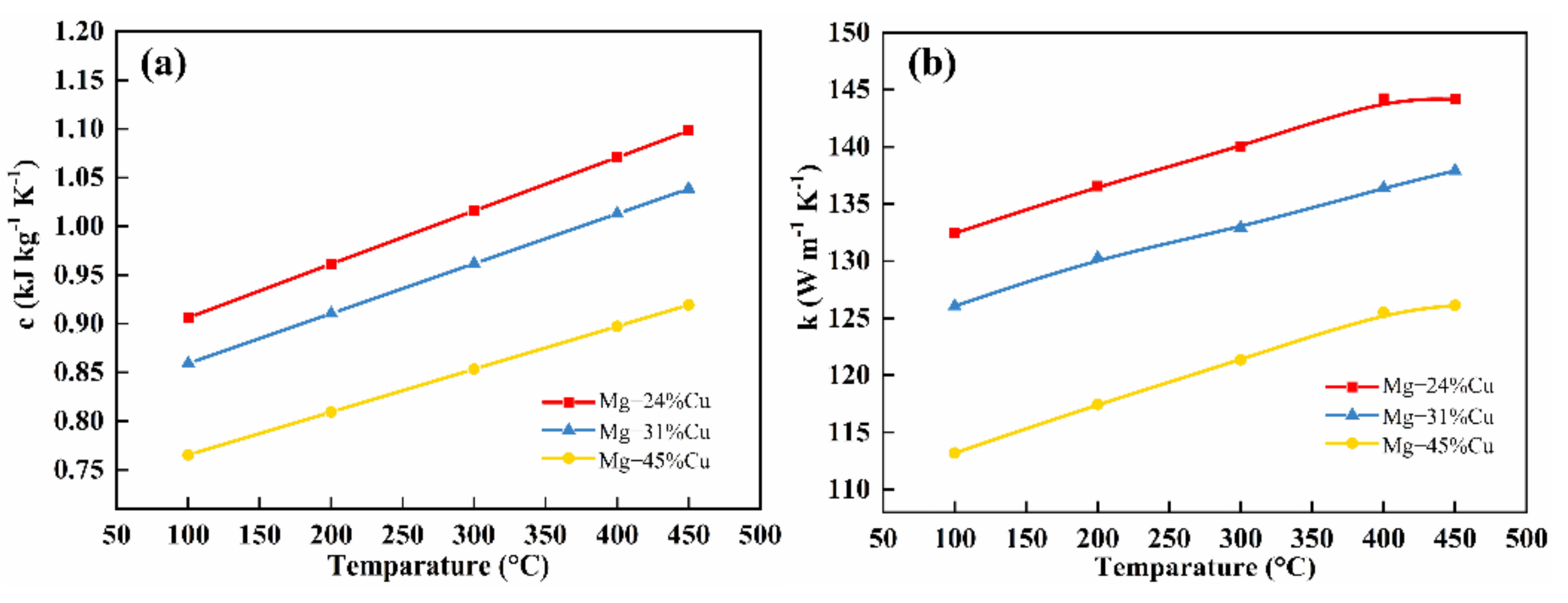
3.4. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medrano, M.; Gil, A.; Martorell, I.; Potau, X.; Cabeza, L.F. State of the art on high-temperature thermal energy storage for power generation. Part 2—Case studies. Renew. Sustain. Energy Rev. 2010, 14, 56–72. [Google Scholar] [CrossRef]
- Maruoka, N.; Akiyama, T. Exergy recovery from steelmaking off-gas by latent heat storage for methanol production. Energy 2006, 31, 1632–1642. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Wang, R. Numerical heat transfer analysis of the packed bed latent heat storage system based on an effective packed bed model. Energy 2010, 35, 2022–2032. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; pp. 6–9. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Hoshi, A.; Mills, D.R.; Bittar, A.; Saitoh, T.S. Screening of high melting point phase change materials (PCM) in solar thermal concentrating technology based on CLFR. Sol. Energy 2005, 79, 332–339. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high tem-perature phase change thermal storage systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Birchenall, C.E.; Riechman, A.F. Heat storage in eutectic alloys. Metall. Mater. Trans. A 1980, 11, 1415–1420. [Google Scholar] [CrossRef]
- Khare, S.; Dell’Amico, M.; Knight, C.; McGarry, S. Selection of materials for high temperature latent heat energy storage. Sol. Energy Mater. Sol. Cells 2012, 117, 20–27. [Google Scholar] [CrossRef]
- Sugo, H.; Kisi, E.; Cuskelly, D. Miscibility gap alloys with inverse microstructures and high thermal conductivity for high energy density thermal storage applications. Appl. Therm. Eng. 2013, 51, 1345–1350. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhang, Y.; Di, H.; Jiang, Y. Experimental research on a kind of novel high temperature phase change storage heater. Energy Convers. Manag. 2006, 47, 2211–2222. [Google Scholar] [CrossRef]
- He, Q.; Zhang, W. A study on latent heat storage exchangers with the high-temperature phase-change material. Int. J. Energy Res. 2001, 25, 331–341. [Google Scholar] [CrossRef]
- Kotzé, J.P.; von Backström, T.; Erens, P.J. High Temperature Thermal Energy Storage Utilizing Metallic Phase Change Materials and Metallic Heat Transfer Fluids. J. Sol. Energy Eng. 2013, 135, 035001. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, H.; Wang, H.; Kim, S.; Esfarjani, K.; Ren, Z.; Chen, G. Metallic Composites Phase-Change Materials for High-Temperature Thermal Energy Storage. In Proceedings of the ASME 2013 7th International Conference on Energy Sustainability, Engineering and Technology. American Society of Mechanical Engineers, Minneapolis, MN, USA, 14–19 July 2013. [Google Scholar]
- Sun, J.; Zhang, R.; Liu, Z.; Lu, G. Thermal reliability test of Al–34%Mg–6%Zn alloy as latent heat storage material and corrosion of metal with respect to thermal cycling. Energy Convers. Manag. 2007, 48, 619–624. [Google Scholar] [CrossRef]
- Wang, D.; Shi, Z.; Zou, L.-J. A liquid aluminum corrosion resistance surface on steel substrate. Appl. Surf. Sci. 2003, 214, 304–311. [Google Scholar]
- Villars, P.; Prince, A.; Okamoto, H. Handbook of Ternary Alloy Phase Diagram, Volume 10; ASM International: Geauga County, OH, USA, 1995; pp. 9665–9672. [Google Scholar]
- Fang, D.; Cheng, X.; Li, Y.; Sun, Z. Microstructure and thermal characteristics of Mg–Sn alloys as phase change materials for thermal energy storage. RSC Adv. 2016, 6, 96327–96333. [Google Scholar] [CrossRef]
- Zhang, C.M.; Hui, X.; Yao, K.F.; Li, Z.G.; Chen, L. Formation of high strength Mg–Cu–Zn–Y alloys. Mater. Sci. Eng. A 2008, 491, 470–475. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kawamura, Y. Thermal diffusivity and thermal conductivity of Mg–Zn–rare earth element alloys with long-period stacking ordered phase. Scr. Mater. 2009, 60, 264–267. [Google Scholar] [CrossRef]
- Blanco-Rodríguez, P.; Rodríguez-Aseguinolaza, J.; Risueño, E.; Tello, M. Thermophysical characterization of Mg–51% Zn eutectic metal alloy: A phase change material for thermal energy storage in direct steam generation applications. Energy 2014, 72, 414–420. [Google Scholar] [CrossRef]
- Rodríguez-Aseguinolaza, J.; Blanco-Rodríguez, P.; Risueño, E.; Tello, M.J.; Doppiu, S. Thermodynamic study of the eutectic Mg49–Zn51 alloy used for thermal energy storage. J. Therm. Anal. Calorim. 2014, 117, 93–99. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Mostafa, A.; Medraj, M. Essential Magnesium Alloys Binary Phase Diagrams and Their Thermochemical Data. J. Mater. 2014, 2014, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhang, K.; Zhang, X.; Li, X.; Li, T.; Li, Y.; Ma, M.; Shi, G. Thermal characteristics of Mg–Zn–Mn alloys with high specific strength and high thermal conductivity. J. Alloy. Compd. 2013, 578, 32–36. [Google Scholar] [CrossRef]
- Knacke, B. Thermochemical Properties of Inorganic Substances; Springer: Berlin/Heidelberg, Germany, 1973. [Google Scholar]
- Fang, D.; Sun, Z.; Li, Y.; Cheng, X. Preparation, microstructure and thermal properties of MgBi alloys as phase change materials for thermal energy storage. Appl. Therm. Eng. 2016, 92, 187–193. [Google Scholar] [CrossRef]
- El Karim, Y.; Grosu, Y.; Faik, A.; Lbibb, R. Investigation of magnesium-copper eutectic alloys with high thermal conductivity as a new PCM for latent heat thermal energy storage at intermediate-high temperature. J. Energy Storage 2019, 26, 100974. [Google Scholar] [CrossRef]

| Samples | Compounds | Composition (wt.%) | ||
|---|---|---|---|---|
| Mg | Cu | O | ||
| M-1 | Mg-24%Cu | 74.91 | 24.37 | 0.72 |
| M-2 | Mg-31%Cu | 67.99 | 31.15 | 0.84 |
| M-3 | Mg-45%Cu | 54.50 | 45.10 | 0.40 |
| Phase | A | B | C | D | E | F |
|---|---|---|---|---|---|---|
| Mg | 98.38 | 52.90 | 91.95 | 61.80 | 44.09 | 50.72 |
| Cu | 1.23 | 46.10 | 3.50 | 36.68 | 54.63 | 46.53 |
| O | 0.39 | 1.00 | 4.55 | 1.51 | 1.28 | 2.75 |
| Closest phase | α-Mg | α-Mg + Mg2Cu | α-Mg | α-Mg + Mg2Cu | Mg2Cu | Mg2Cu + α-Mg |
| Samples | Compounds | Area of Non-Eutectic Phase (%) | Area of Eutectic Phase (%) | Enthalpy of Phase Transition (J·g−1) |
|---|---|---|---|---|
| M-1 | Mg-24%Cu | 40.627 | 59.373 | 152.0 |
| M-2 | Mg-31%Cu | 14.404 | 85.596 | 215.0 |
| M-3 | Mg-45%Cu | 63.943 | 35.040 | 91.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zou, L.; Cheng, X.; Zhu, J.; Li, Y.; Zhou, W. Fabrication, Structure, and Thermal Properties of Mg–Cu Alloys as High Temperature PCM for Thermal Energy Storage. Materials 2021, 14, 4246. https://doi.org/10.3390/ma14154246
Sun Z, Zou L, Cheng X, Zhu J, Li Y, Zhou W. Fabrication, Structure, and Thermal Properties of Mg–Cu Alloys as High Temperature PCM for Thermal Energy Storage. Materials. 2021; 14(15):4246. https://doi.org/10.3390/ma14154246
Chicago/Turabian StyleSun, Zheng, Liyi Zou, Xiaomin Cheng, Jiaoqun Zhu, Yuanyuan Li, and Weibing Zhou. 2021. "Fabrication, Structure, and Thermal Properties of Mg–Cu Alloys as High Temperature PCM for Thermal Energy Storage" Materials 14, no. 15: 4246. https://doi.org/10.3390/ma14154246
APA StyleSun, Z., Zou, L., Cheng, X., Zhu, J., Li, Y., & Zhou, W. (2021). Fabrication, Structure, and Thermal Properties of Mg–Cu Alloys as High Temperature PCM for Thermal Energy Storage. Materials, 14(15), 4246. https://doi.org/10.3390/ma14154246








