Carbon Nanotubes for Improved Performances of Endodontic Sealer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of CHX/AgNPs Loaded CNTs and Incorporation into Endodontic Sealer
2.2. FTIR Spectroscopy
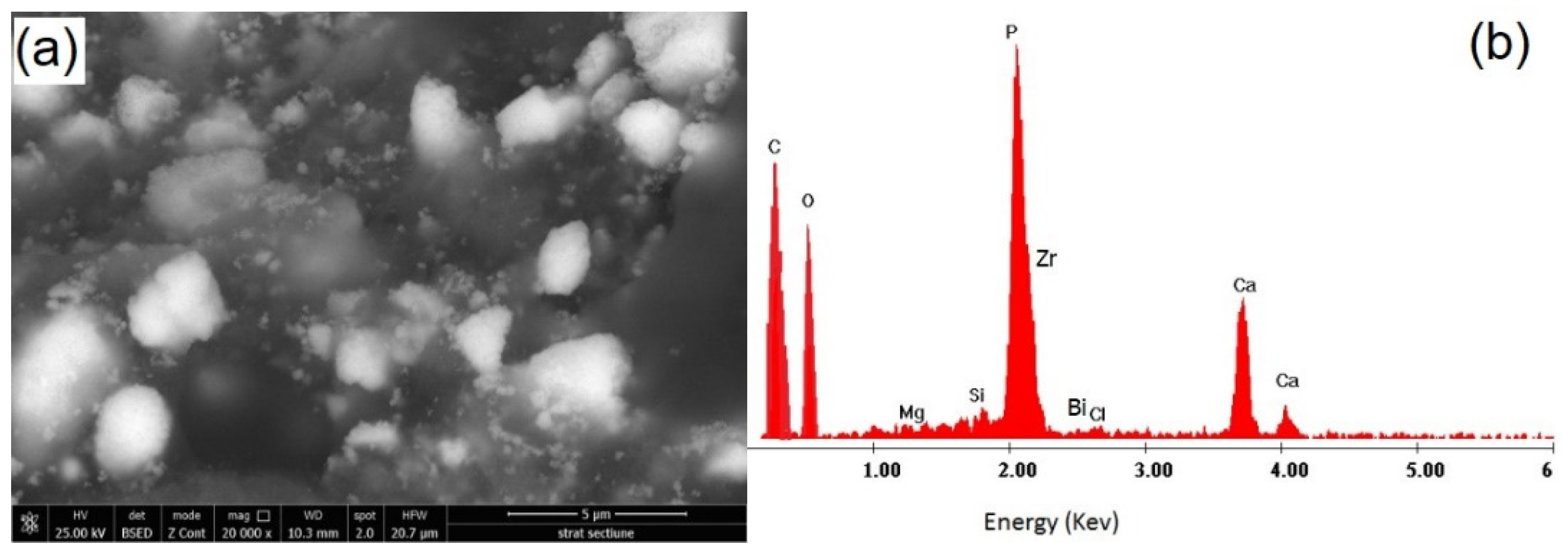
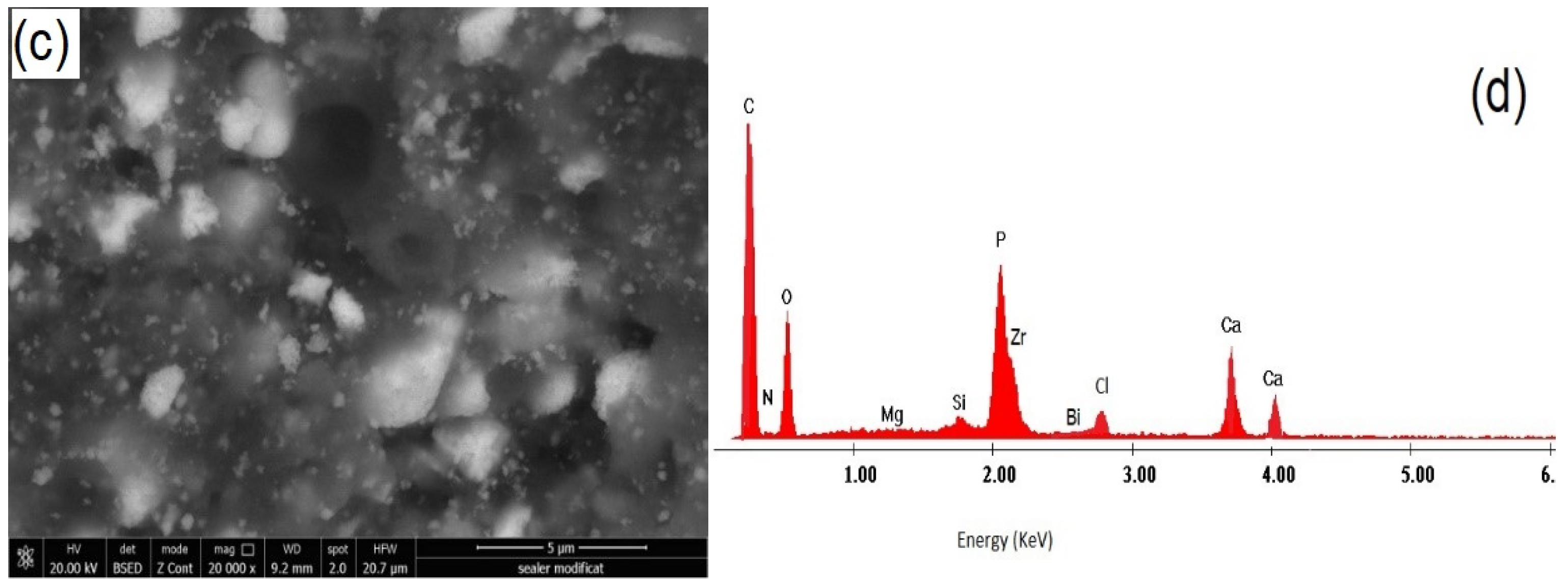
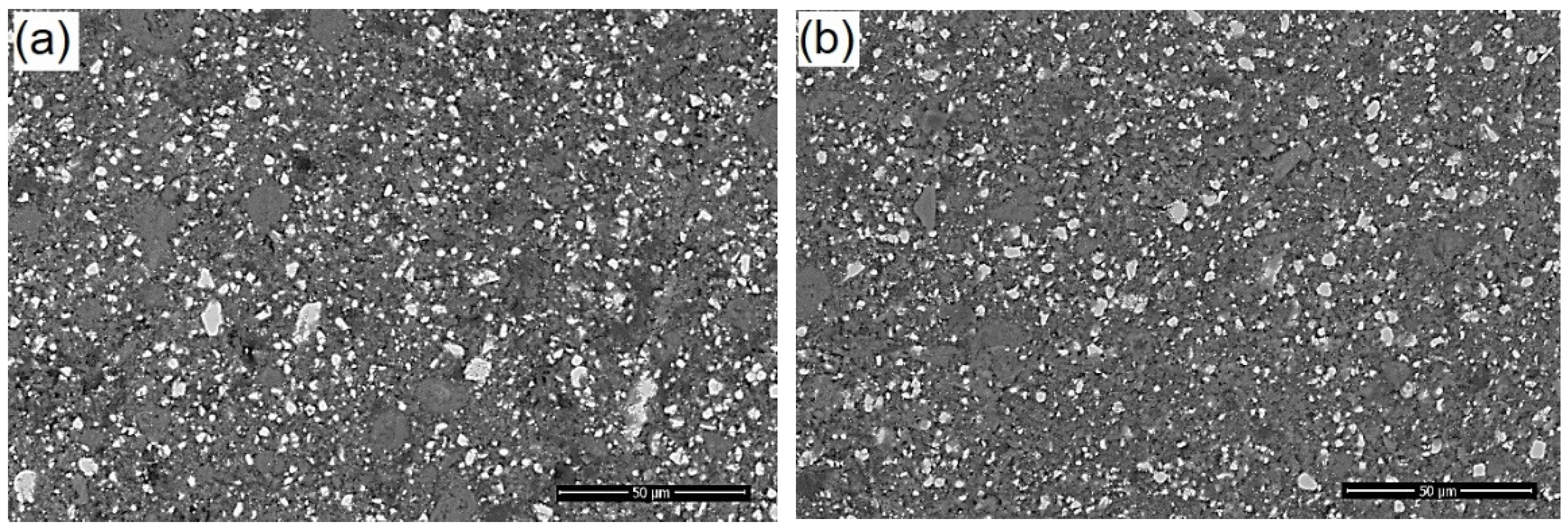
2.3. SEM Examination (Mixture AgNPs/CHX/CNTs, Commercial and Nanomodified Sealer)
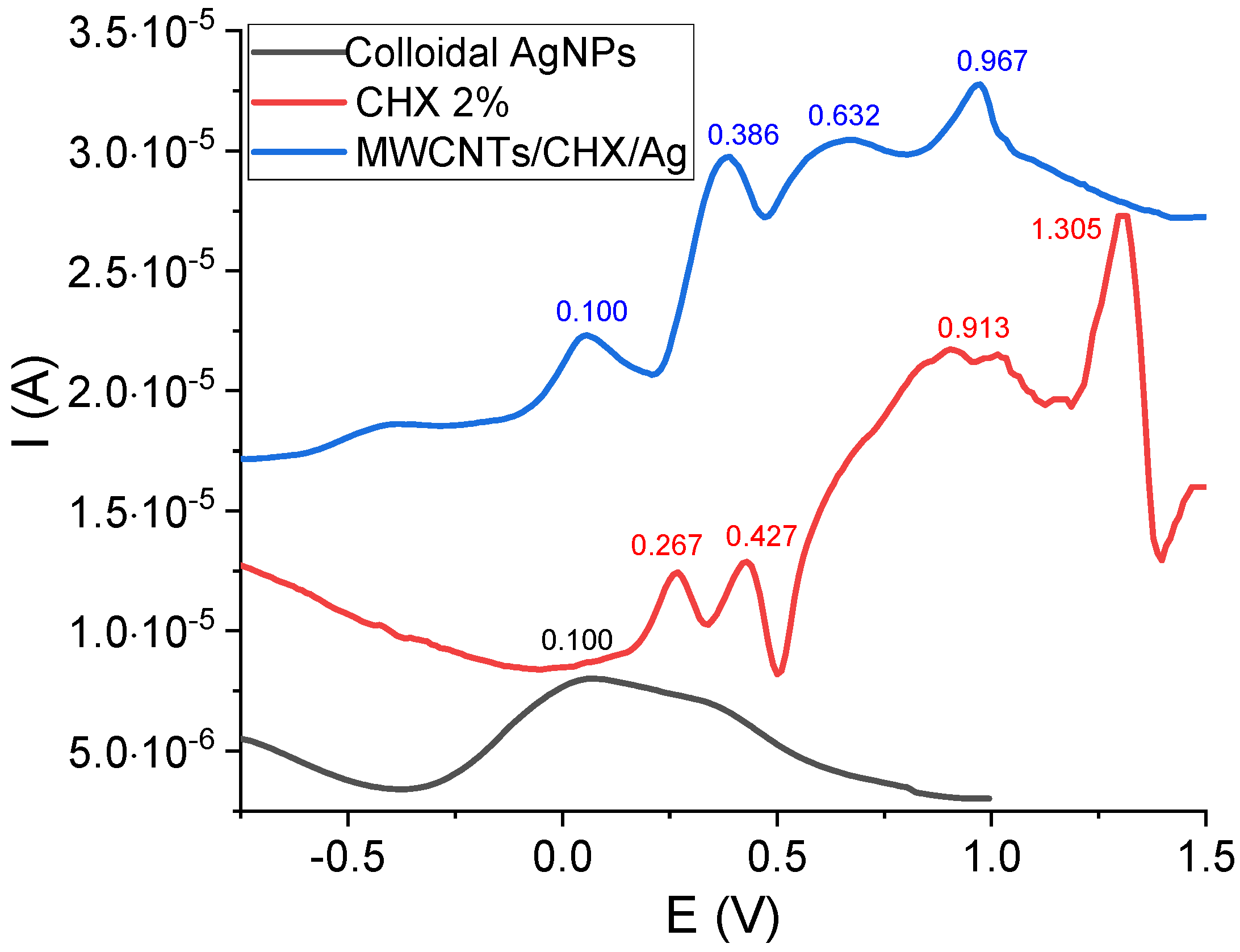
2.4. Electrochemical Measurements
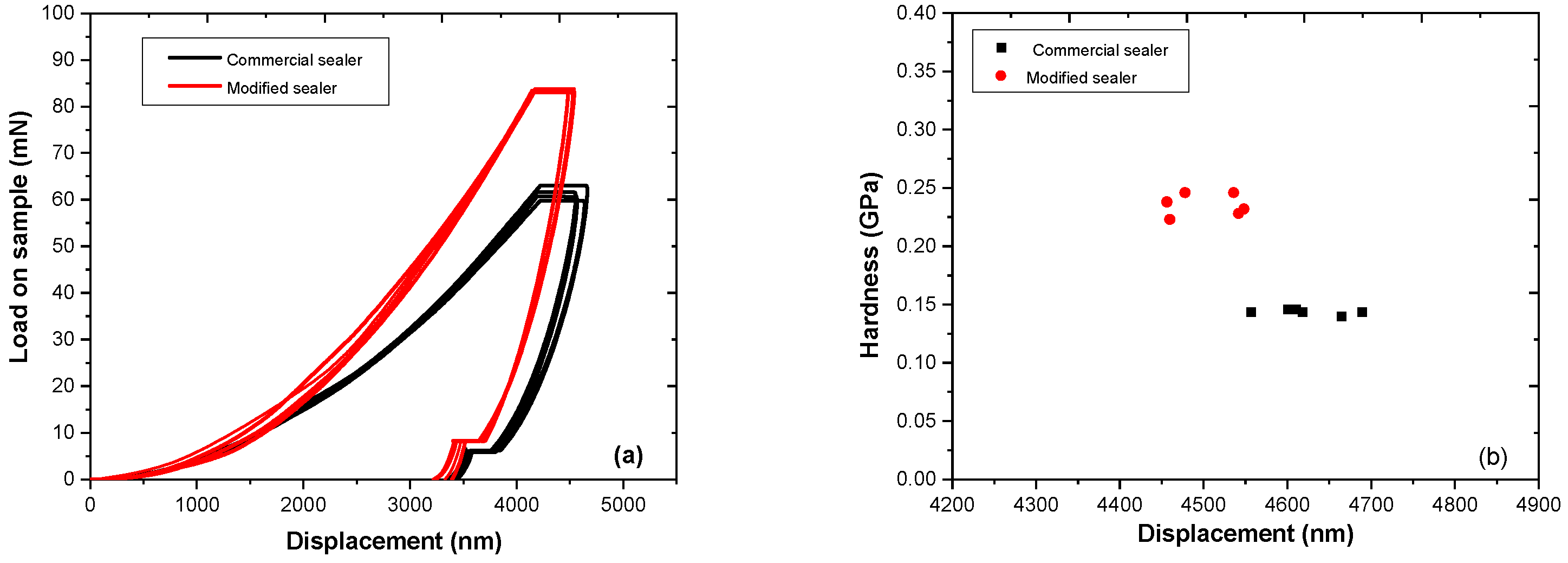
2.5. Nanoindentation Measurements
2.6. Thermogravimetrical Analysis (TGA)
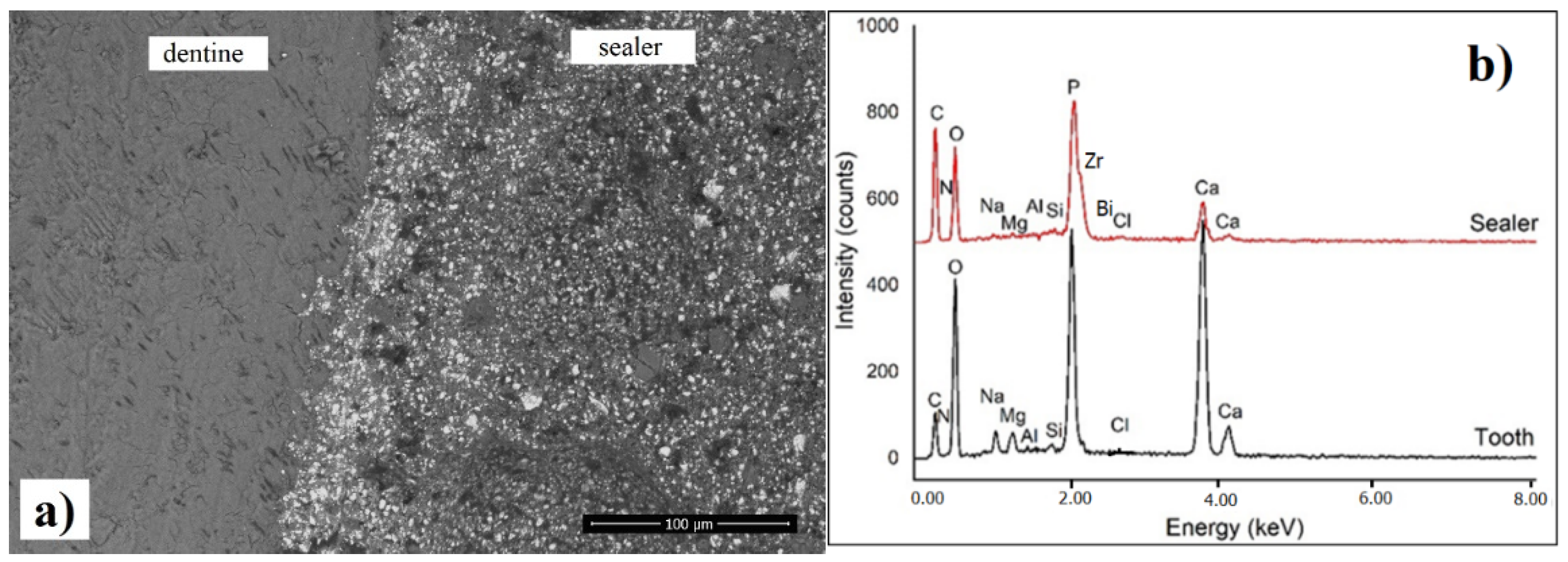
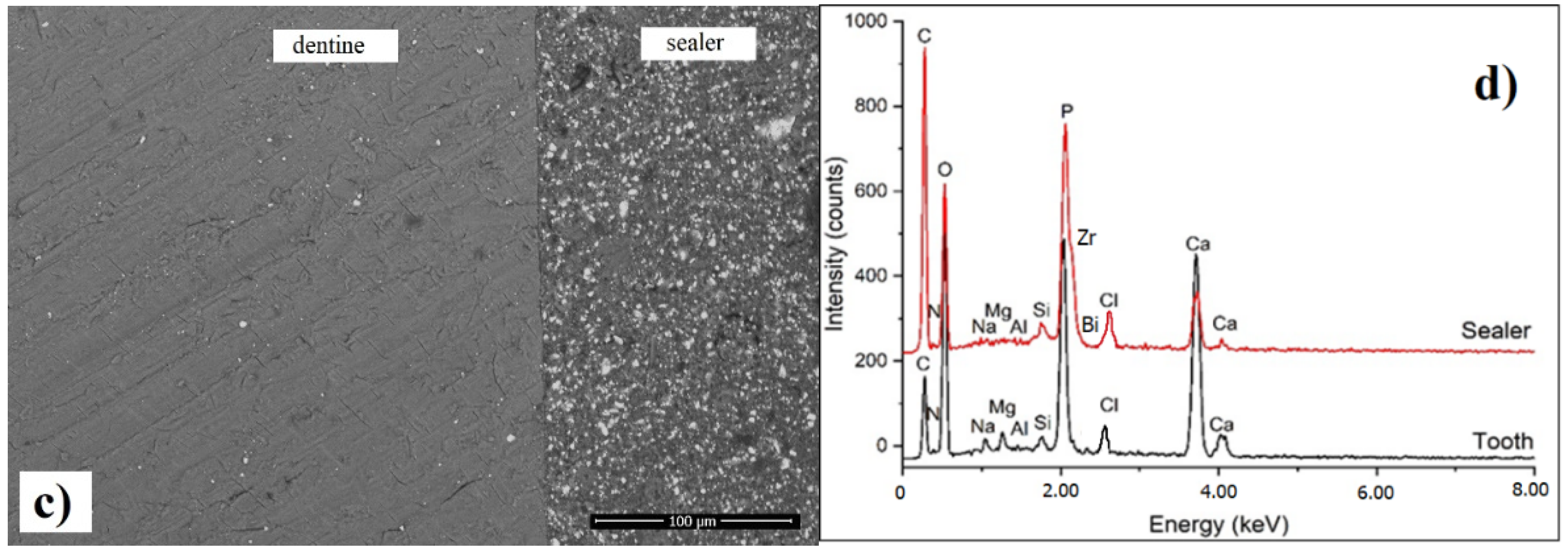
2.7. SEM Investigation of the Interfacial Adaptation to Root Canal Dentine
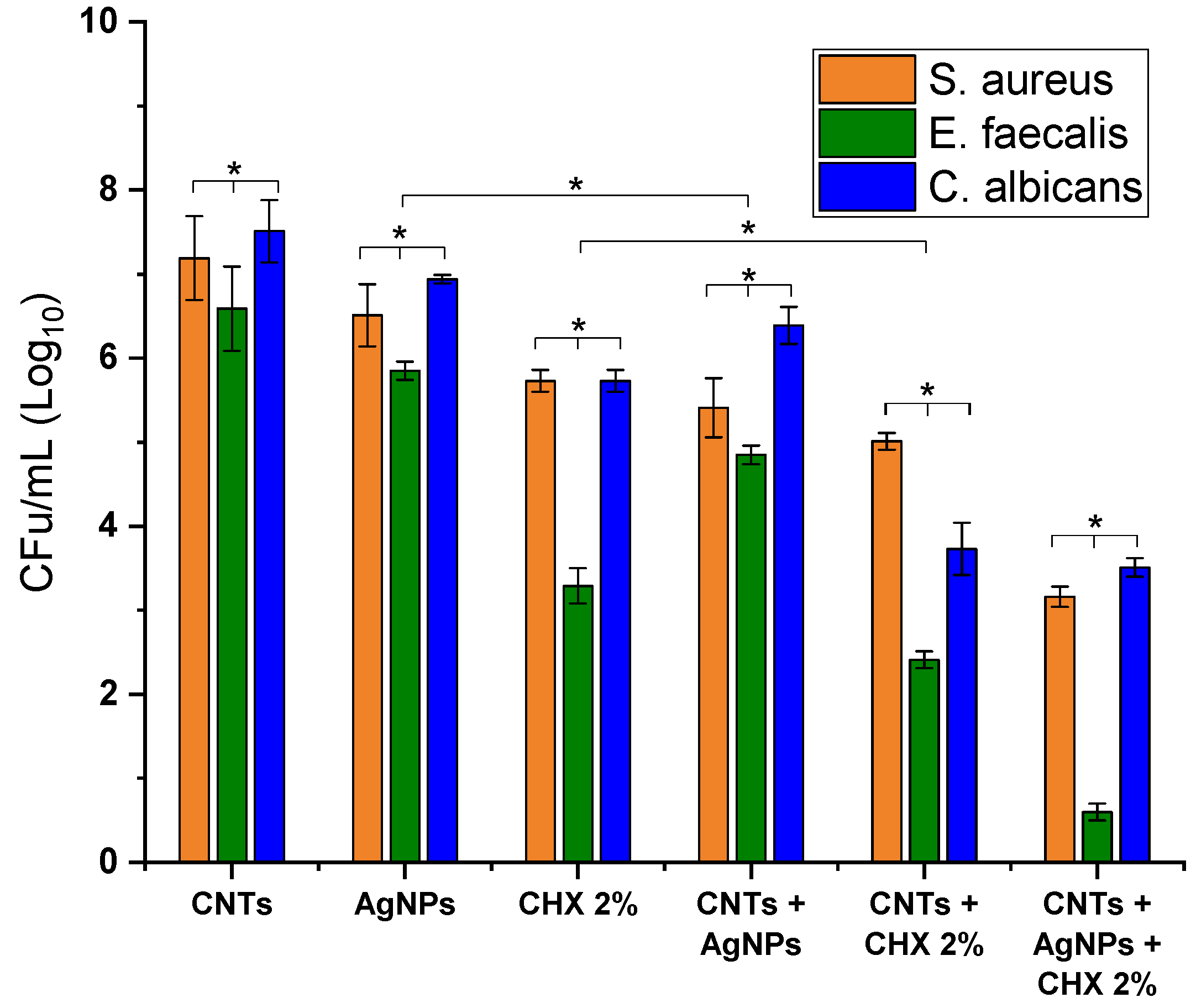
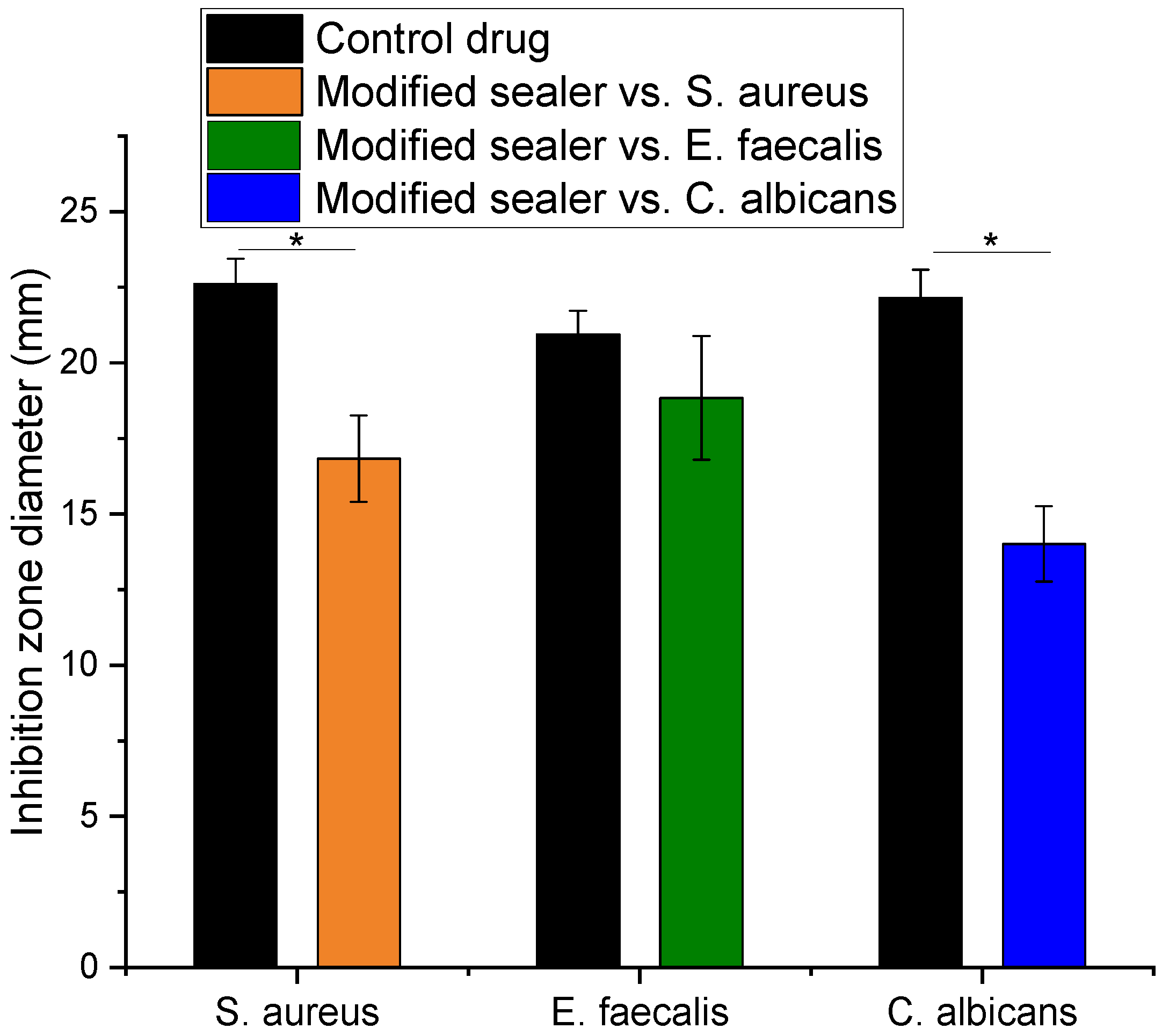
2.8. Antimicrobial and Antifungal Efficacy
3. Results
3.1. FTIR Spectroscopy
3.2. Electrochemical Measurements
3.3. SEM Investigation of Modified Root Canal Sealer
3.4. Nanoindentation Measurements
3.5. Thermal Analysis
3.6. SEM Investigation of Interfacial Adaptation to Root Canal Dentine
3.7. Antimicrobial and Antifungal Effect of the Modified Sealer
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chogle, S.M.A.; Kinaia, B.M.; Goodis, H.E. Scope of Nanotechnology in Endodontics. In Nanobiomaterials in Clinical Dentistry, 1st ed.; Subramani, K., Ahmed, W., Hartsfield, J.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 432–445. [Google Scholar]
- The, S.J.; Lai, C.W. Carbon Nanotubes for Dental Implants in Applications of Nanocomposite Materials in Dentistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–105. [Google Scholar] [CrossRef]
- Alenazy, M.S.; Mosadomi, H.A.; Al-Nazhan, S.; Rayan, M.R. Clinical considerations of nanobiomaterials in endodontics. A systematic review. Saudi Endod. J. 2018, 8, 163–169. [Google Scholar] [CrossRef]
- Gomes, B.P.; Pinheiro, E.T.; Gade-Neto, C.R.; Sousa, E.L.R.; Ferraz, C.C.R.; Zaia, A.A.; Teixeria, F.B.; Souza-Filho, F.J. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 2004, 19, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, Z.; Abbott, P.V. The properties and applications of chlorhexidine in endodontics. Int. Endodod. J. 2009, 42, 288–302. [Google Scholar] [CrossRef]
- Sirtes, G.; Waltimo, T.; Schaetzle, M.; Zehnder, M. The effects of temperature on sodium hypochlorite short term stability, pulp dissolution capacity and antimicrobial efficacy. J. Endod. 2005, 31, 669–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, B.; Souza, S.F.C.; Ferraz, C.C.R.; Teixeira, F.B.; Zaia, A.A.; Valdrighi, L.; Souza-Filho, F.J. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int. Endod. J. 2003, 36, 267–275. [Google Scholar] [CrossRef]
- Kandaswamy, D.; Venkateshbabu, N. Root canal irrigants. J. Conserv. Dent. 2010, 13, 256–264. [Google Scholar] [CrossRef]
- Chang, Y.E.; Shin, D.H. Effect of chlorhexidine application methods on microtensile bond strength to dentine in class I cavities Oper. Dent. 2010, 35, 618–623. [Google Scholar]
- Dionysopoulos, D. Effect of digluconate chlorhexidine on bond strength between dentinal adhesive systems and dentin: A systematic review. J. Conserv. Dent. 2016, 19, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbizam, J.V.; Trope, M.; Tanomaru-Filho, M.; Teixeira, E.C.; Teixeira, F.B. Bond strength of different endodontic sealers to dentin: Pushout test. J. Appl. Oral Sci. 2011, 19, 644–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marciano, M.A.; Guimaraes, B.M.; Ordinola-Zapata, R.; Bramante, C.M.; Cavenago, B.C.; Garcia, R.B.; Bernardineli, N.; Andrade, F.B.; Moraes, I.G.; Duarte, M.A. Physical properties and interfacial adaptation of three epoxy resin-based sealers. J. Endod. 2011, 37, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Grossman, L.I. Endodontic Practice, 10th ed.; Henry Kimpton: Philadelphia, PA, USA, 1981; p. 297. [Google Scholar]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-based root canal sealers: A review. Int. J. Biomater. 2016. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Kwak, S.W.; Ha, J.H.; Lee, W.; Kim, H.C. Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg. Chem. Appl. 2017. [Google Scholar] [CrossRef] [Green Version]
- Marin-Bauza, G.A.; Silva-Sousa, Y.T.C.; da Cunha, S.A.; Rached-Junior, F.J.A.; Bonetti-Filho, I.; Sousa-Neto, M.D.; Miranda, C.E.S. Physicochemical properties of endodontic sealers of different bases, J. Appl. Oral Sci. 2012, 20, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.A.; Paula, A.B.; Marto, C.M.; Coelho, A.; Paulo, S.; Martinho, J.P.; Carrilho, E.; Ferreira, M.M. Biocompatibility of root canal sealers: A systematic review of in vitro and in vivo studies. Materials 2019, 12, 4113. [Google Scholar] [CrossRef] [Green Version]
- Cavalu, S.; Simon, V. Microstructure and bioactivity of acrylic bone cements for prosthetic surgery. J. Optoelectron. Adv. Mater. 2006, 8, 1520–1523. [Google Scholar]
- Fritea, L.; Bănică, F.; Costea, T.O.; Moldovan, L.; Iovan, C.; Cavalu, S. A gold nanoparticles—Graphene based electrochemical sensor for sensitive determination of nitrazepam. J. Electroanal. Chem. 2018, 830–831, 63–71. [Google Scholar] [CrossRef]
- Fritea, L.; Tertis, M.; Sandulescu, R.; Cristea, C. Enzymes-graphene platforms for electrochemical biosensors design with biomedical applications. Methods Enzymol. 2018, 609, 293–333. [Google Scholar]
- Cavalu, S.; Antoniac, I.; Fritea, L.; Mohan, A.; Semenescu, A.; Laslo, V.; Vicas, S. Surface modifications of the titanium mesh for cranioplasty using selenium nanoparticles coating. J. Adhes. Sci. Technol. 2018, 32, 2509–2522. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Nanomaterials for healthcare biosensing applications. Sensors 2019, 19, 5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalu, S.; Ratiu, C.; Ponta, O.; Simon, V.; Rugina, D.; Miclaus, V. Improving osseointegration of alumina/zirconia ceramic implants by fluoride surface treatment. Dig. J. Nanomater. Biostruct. 2014, 9, 797–808. [Google Scholar]
- Tabassum, S.; Khan, F.R. Failure of endodontic treatment: The usual suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef]
- Cavalu, S.; Fritea, L.; Brocks, M.; Barbaro, K.; Murvai, G.; Costea, T.O.; Antoniac, I.; Verona, C.; Romani, M.; Latini, A.; et al. Novel hybrid composites based on PVA/SeTiO2 nanoparticles and natural hydroxyapatite for orthopedic applications: Correlations between structural, morphological and biocompatibility properties. Materials 2020, 13, 2077. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Thumbigere-Math, V.; Tay, F.R.; Fouad, A.F.; Oates, T.W.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Novel bioactive and therapeutic root canal sealers with antibacterial and remineralization properties. Materials 2020, 13, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, K.; Moeini, A.; Tay, F.R.; Niu, L.N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef]
- Yitzhak, R.; Noel, M.E. Carbon nanotubes in drug delivery: Focus on infectious diseases. Expert Opin. Drug Deliv. 2009, 6, 517–530. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Chen, Q.-Y.; Xiao, S.-L.; Shi, S.Q.; Cai, L.-P. A One-pot synthesis and characterization of antibacterial silver nanoparticle–cellulose film. Polymers 2020, 12, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalu, S.; Simon, V.; Goller, G.; Akin, I. Bioactivity and antimicrobial properties of PMMA/Ag2O acrylic bone cement collagen coated. Dig. J. Nanomater. Biostruct. 2011, 6, 779–790. [Google Scholar]
- Rema, T.; Lawrence, J.R.; Dynes, J.J.; Hitchcock, A.P.; Korber, D.R. Microscopic and spectroscopic analyses of chlorhexidine tolerance in Delftia acidovorans biofilms. Antimicrob. Agents Chemother. 2014, 58, 5673–5686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés, M.E.; Sinesterra, R.D.; Avila-Campos, M.J.; Tortamano, N.; Rocha, R.G. The chlorhexidine:β-cyclodextrin inclusion compound: Preparation, characterization and microbiological evaluation. J. Incl. Phenom. Macrocycl. Chem. 2001, 40, 297–302. [Google Scholar] [CrossRef]
- Maity, P.; Kasisomayajula, S.V.; Parameswaran, V.; Basu, S.; Gupta, N. Improvement in surface degradation properties of polymer composites due to pre-processed nanometric alumina fillers. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 63–72. [Google Scholar] [CrossRef]
- Choi, Y.J.; Luo, T.J.M. Electrochemical properties of silver nanoparticle doped aminosilica nanocomposite. Int. J. Electrochem. 2011, 2011, 404937. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Mujahid, M.; Gul, I.H.; Hussain, A. Electrochemical study of magnetic nanogel designed for controlled re-lease of chlorhexidine gluconate. Electrochim. Acta 2019, 295, 113–123. [Google Scholar] [CrossRef]
- de Lima, A.P.; Stefano, J.S.; Montes, R.H.O.; Cunha, R.R.; Silva, L.A.J.; Richter, E.M.; Muñoz, R.A.A. Electrochemical oxidation of chlorhexidine and its amperometric determination by flow-injection analysis. J. Braz. Chem. Soc. 2014, 25, 448–452. [Google Scholar]
- Sousa, C.P.; de Oliveira, R.C.; Freire, T.M.; Fechine, P.B.A.; Salvador, M.A.; Homem-de-Mello, P.; Morais, S.; Lima-Neto, P.; Correia, A.N. Chlorhexidine digluconate on chitosan-magnetic iron oxide nanoparticles modified electrode: Electroanalysis and mechanistic insights by computational simulations. Sens. Actuators B Chem. 2017, 240, 417–425. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Vieira Santos, R.; Araujo dos Santos, E.C.; Avila Campos Rocha, T.L.; Domingues Junior, N.S.; Mendes Moraes, C.A. Replacement of commercial silica by rice husk ash in epoxy composites: A comparative analysis. Mater. Res. 2018, 21, 0562. [Google Scholar] [CrossRef] [Green Version]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naeb, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694. [Google Scholar] [CrossRef] [Green Version]
- Jen, Y.-M.; Chang, H.-H.; Lu, C.-M.; Liang, S.-Y. Temperature-dependent synergistic effect of multi-walled carbon nanotubes and graphene nanoplatelets on the tensile quasi-static and fatigue properties of epoxy nanocomposites. Polymers 2021, 13, 84. [Google Scholar] [CrossRef]
- Cavalu, S.; Banica, F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Majeed Khan, M.A.; Kumar, S.; Ahamed, M.; Alrokayan, S.A.; AlSalhi, M.S. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res. Lett. 2011, 6, 434. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Jeelani, M.I.; Jeelani, S. Development of photo micro-graph method to characterize dispersion of CNT in epoxy. Mater. Sci. Eng. A. 2009, 506, 39–44. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Yousefi, A.A.; Naderi, G. Effect of type and aspect ratio of different carbon nanotubes on cure behavior of epoxy-based nanocomposites. Iran. Polym. J. 2015, 24, 1–12. [Google Scholar] [CrossRef]
- Atmeh, A.R.; AlSwaimi, E. The effect of heating time and temperature on epoxy resin and calcium silicate-based endodontic sealer. J. Endod. 2017, 43, 2112–2118. [Google Scholar] [CrossRef]
- Marian, E.; Jurca, T.; Banica, F.; Morgovan, C.; Bratu, I. Thermal analysis and raman spectrometry of some complexes of theophylline with transitional. Rev. Chim. 2010, 61, 569–574. [Google Scholar]
- Hsieh, Y.C.; Chou, Y.C.; Lin, C.P.; Hsieh, T.F.; Shu, C.M. Thermal analysis of multi-walled carbon nanotubes by kissinger’s corrected kinetic equation. Aerosol Air Qual. Res. 2010, 10, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Priyadarshini, B.; Selvan, S.; Narayanan, K.; Fawzy, A. Characterization of chlorhexidine-loaded calcium-hydroxide microparticles as a potential dental pulp-capping material. Bioengineering 2017, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, R.; Guerreiro-Tanomaru, J.; Tanomaru-Filho, M.; Camilleri, J. Interface of dentine to root canal sealers. J. Dent. 2014, 42, 336–350. [Google Scholar] [CrossRef]
- Chow, J.W. Aminoglycoside resistance in enterococci. Clin. Infect. Dis. 2000, 31, 586–589. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high young modulus observed for individual nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Kapralos, V.; Rukke, H.V.; Koutroulis, D.O.A.; Camilleri, J.; Sunde, P.T. Antimicrobial and physicochemical characterization of endodontic sealers after exposure to chlorhexidine digluconate. Dent. Mater. 2021, 37, 249–263. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Gun’ko, Y.K. Mechanical reinforcement of polymers using CNTs. Adv. Mater. 2006, 18, 689–706. [Google Scholar] [CrossRef]
- Kalagi, S.; Feitosa, S.A.; Münchow, E.A.; Martins, V.M.; Karczewski, A.E.; Cook, N.B.; Diefenderfer, K.; Eckert, G.J.; Geraldeli, S.; Bottino, M.C. Chlorhexidine-modified nanotubes and their effects on the polymerization and bonding performance of a dental adhesive. Dent. Mater. 2020, 36, 687–697. [Google Scholar] [CrossRef]
- Stanislawczuk, R.; Reis, A.; Loguercio, A.D. A 2-year in vitro evaluation of chlorhexidine-containing acid on the durability of resin-dentin interfaces. J. Dent. 2011, 39, 40–47. [Google Scholar] [CrossRef]
- Rodrigues, C.T.; de Andrade, F.B.; de Vasconcelos, L.R.S.M.; Midena, R.Z.; Pereira, T.C.; Kuga, M.C.; Duarte, M.A.H.; Bernardineli, N. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int. Endod. J. 2018, 51, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. “Enterococcus faecalis”: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Kumar, J.; Sharma, R.; Sharma, M.; Prabhavathi, V.; Paul, J.; Chowdary, C.D. Presence of candida albicans in root canals of teeth with apical periodontitis and evaluation of their possible role in failure of endodontic treatment. J. Int. Oral Health 2015, 7, 42–45. [Google Scholar] [PubMed]
- AlShwaimi, E.; Bogari, D.; Ajaj, R.; Al-Shahrani, S.; Almas, K.; Majeed, A. In vitro antimicrobial effectiveness of root canal sealers against enterococcus faecalis: A systematic review. J. Endod. 2016, 42, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Simion, V.; Banica, F. In vitro study of collagen coating by electrodeposition on acrylic bone cement with antimicrobial. Dig. J. Nanomater. Biostruct. 2010, 6, 89–97. [Google Scholar]
- Ceci, M.; Viola, M.; Rattalino, D.; Beltrami, R.; Colombo, M.; Poggio, C. Discoloration of different esthetic restorative materials: A spectrophotometric evaluation. Eur. J. Dent. 2017, 11, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, M.R.; Bezerra da Silva, L.A.; Filho, M.T.; Santana da Silva, R. Release of formaldehyde by 4 endodontic sealers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 1999, 88, 221–225. [Google Scholar] [CrossRef]
- Sinjari, B.; Santilli, M.; D’Addazio, G.; Rexhepi, I.; Gigante, A.; Caputi, S.; Traini, T. Influence of dentine pre-treatment by sandblasting with aluminum oxide in adhesive restorations. an in vitro study. Materials 2020, 13, 3026. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marica, A.; Fritea, L.; Banica, F.; Sinescu, C.; Iovan, C.; Hulka, I.; Rusu, G.; Cavalu, S. Carbon Nanotubes for Improved Performances of Endodontic Sealer. Materials 2021, 14, 4248. https://doi.org/10.3390/ma14154248
Marica A, Fritea L, Banica F, Sinescu C, Iovan C, Hulka I, Rusu G, Cavalu S. Carbon Nanotubes for Improved Performances of Endodontic Sealer. Materials. 2021; 14(15):4248. https://doi.org/10.3390/ma14154248
Chicago/Turabian StyleMarica, Andreea, Luminita Fritea, Florin Banica, Cosmin Sinescu, Ciprian Iovan, Iosif Hulka, Gerlinde Rusu, and Simona Cavalu. 2021. "Carbon Nanotubes for Improved Performances of Endodontic Sealer" Materials 14, no. 15: 4248. https://doi.org/10.3390/ma14154248
APA StyleMarica, A., Fritea, L., Banica, F., Sinescu, C., Iovan, C., Hulka, I., Rusu, G., & Cavalu, S. (2021). Carbon Nanotubes for Improved Performances of Endodontic Sealer. Materials, 14(15), 4248. https://doi.org/10.3390/ma14154248








