Functional Materials Based on Cyclometalated Platinum(II) β-Diketonate Complexes: A Review of Structure–Property Relationships and Applications
Abstract
:1. Introduction
2. Structure–Property Relationships
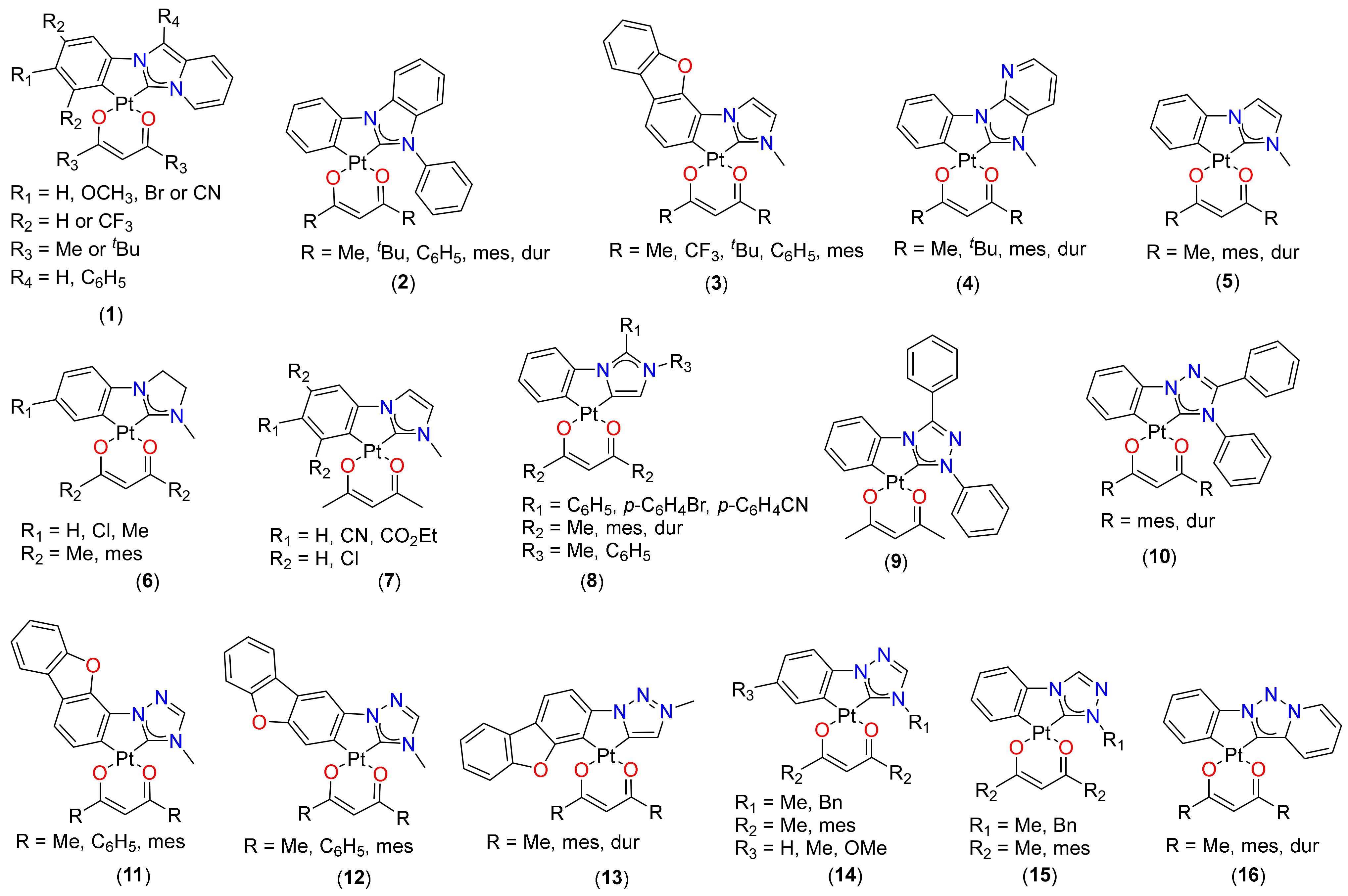
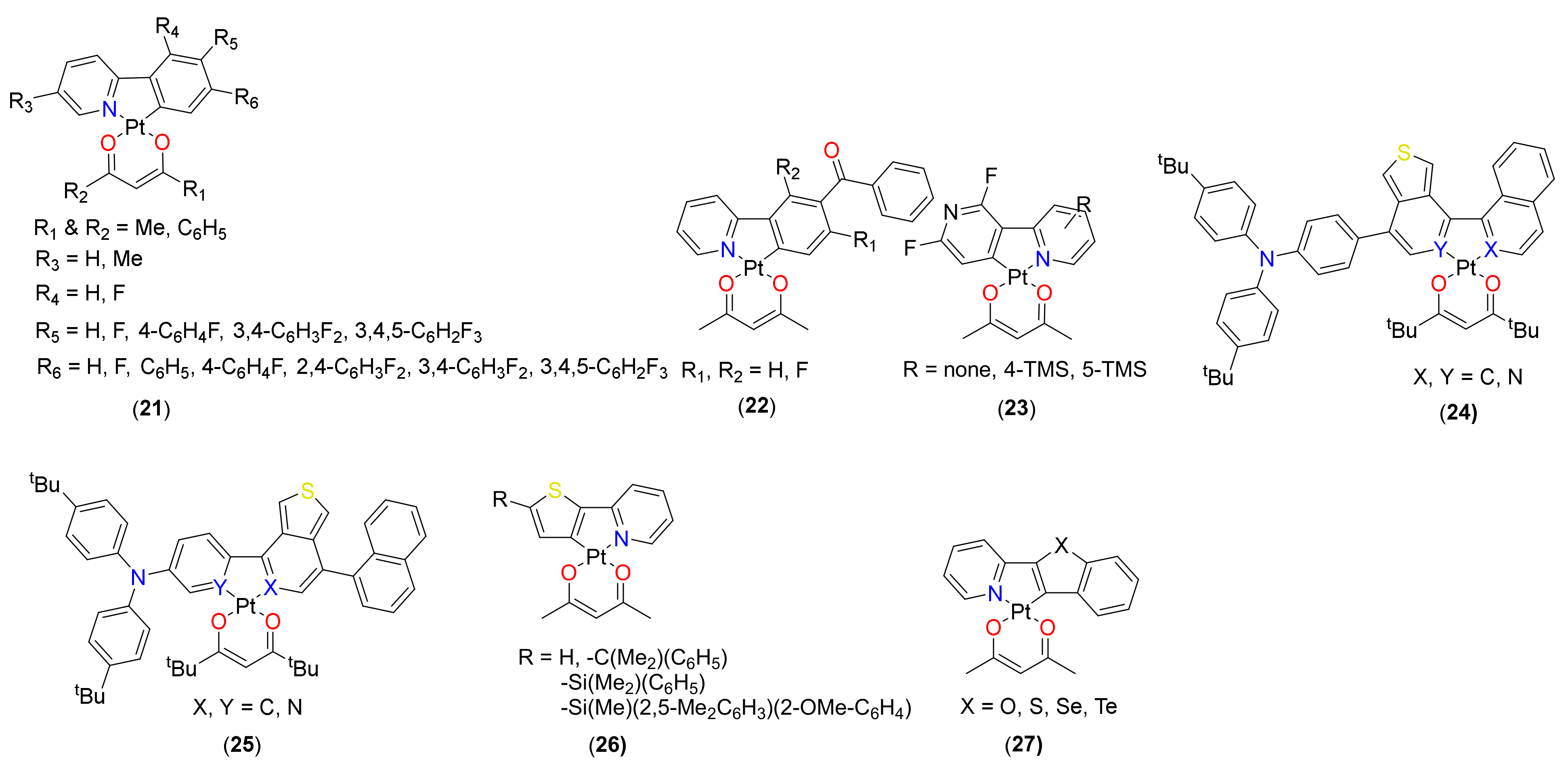
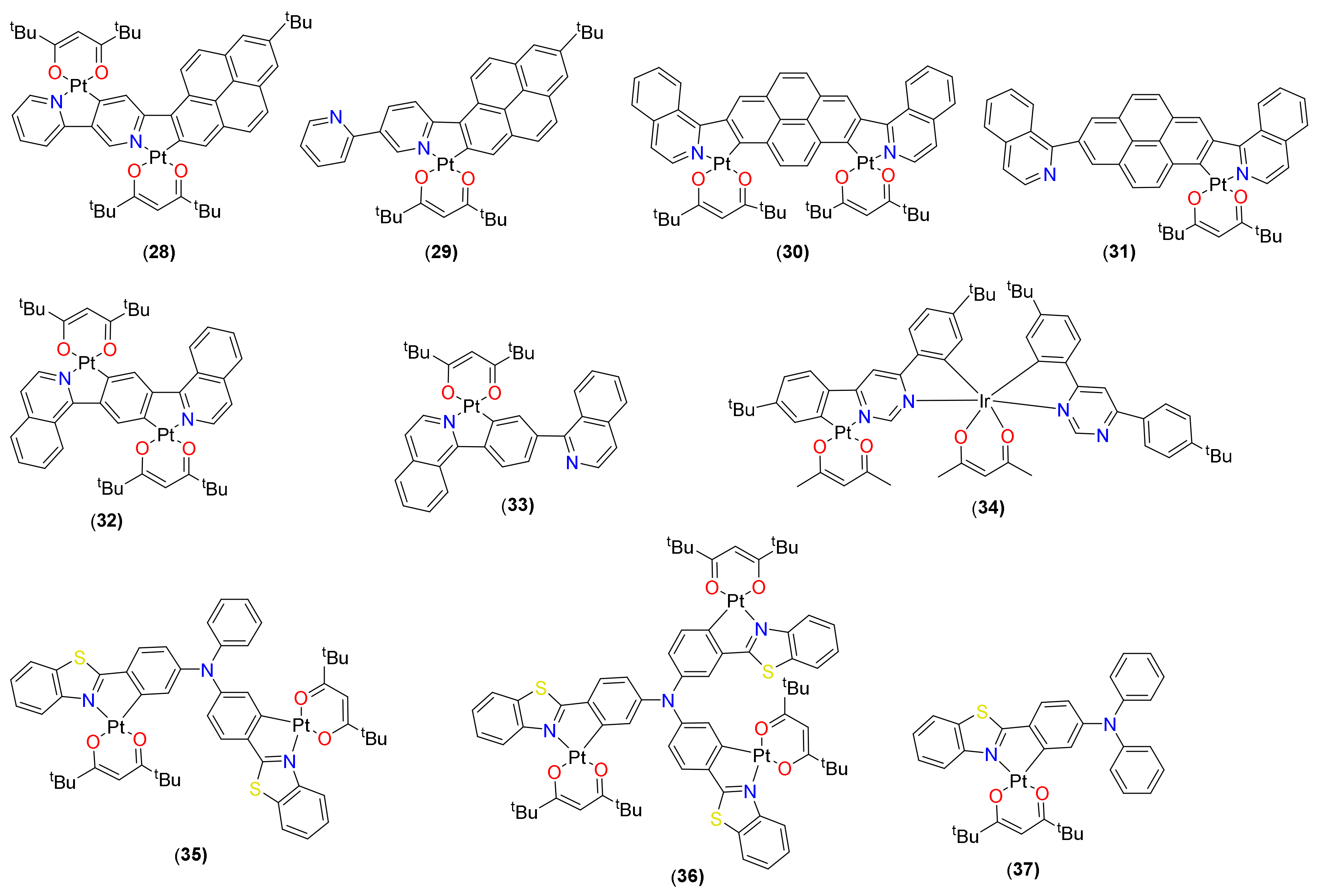
2.1. (C^C)Pt(O^O) Type Complexes
| Complex | λexc | λem c | Φ | τ0 (μs) | Ref. |
|---|---|---|---|---|---|
| 3 (R = tBu) a | 355 | 465 | 0.83 | 27.4 | [30] |
| 3 (R = C6H5) a | 370 | 530 | 0.51 | 4.7 | [30] |
| 3 (R = mes) a | 335 | 466 | 0.91 | 18.9 | [30] |
| 3 (R = Me) a | 355 | 463, 497 | 0.90 | 23 | [31] |
| 4 (R = Me) a | 340 | 458, 547 | 0.64 | 4.8 | [32] |
| 4 (R = tBu) a | 340 | 456 | 0.74 | 5.8 | [32] |
| 4 (R = mes) a | 340 | 472 | 0.91 | 2.9 | [32] |
| 4 (R = dur) a | 355 | 466 | 0.88 | 3.3 | [32] |
| 5 (R = Me) a | 320 | 441 | 0.07 | - | [31] |
| 5 (R = mes) a | 320 | 482 | 0.82 | 3.1 | [17] |
| 5 (R = dur) a | 320 | 479 | 0.72 | 3.3 | [17] |
| 6 (R1 = H, R2 = Me) a | 305 | 470 | 0.29 | 23 | [11] |
| 6 (R1 = H, R2 = mes) a | 305 | 486 | 0.71 | 5 | [11] |
| 6 (R1 = Cl, R2 = Me) a | 305 | 473 | 0.38 | 25 | [11] |
| 6 (R1 = Cl, R2 = mes) a | 305 | 476 | 0.7 | 7 | [11] |
| 6 (R1 = Me, R2 = Me) a | 305 | 481 | 0.24 | 23 | [11] |
| 6 (R1 = Me, R2 = mes) | 305 | 490 | 0.42 | 8 | [11] |
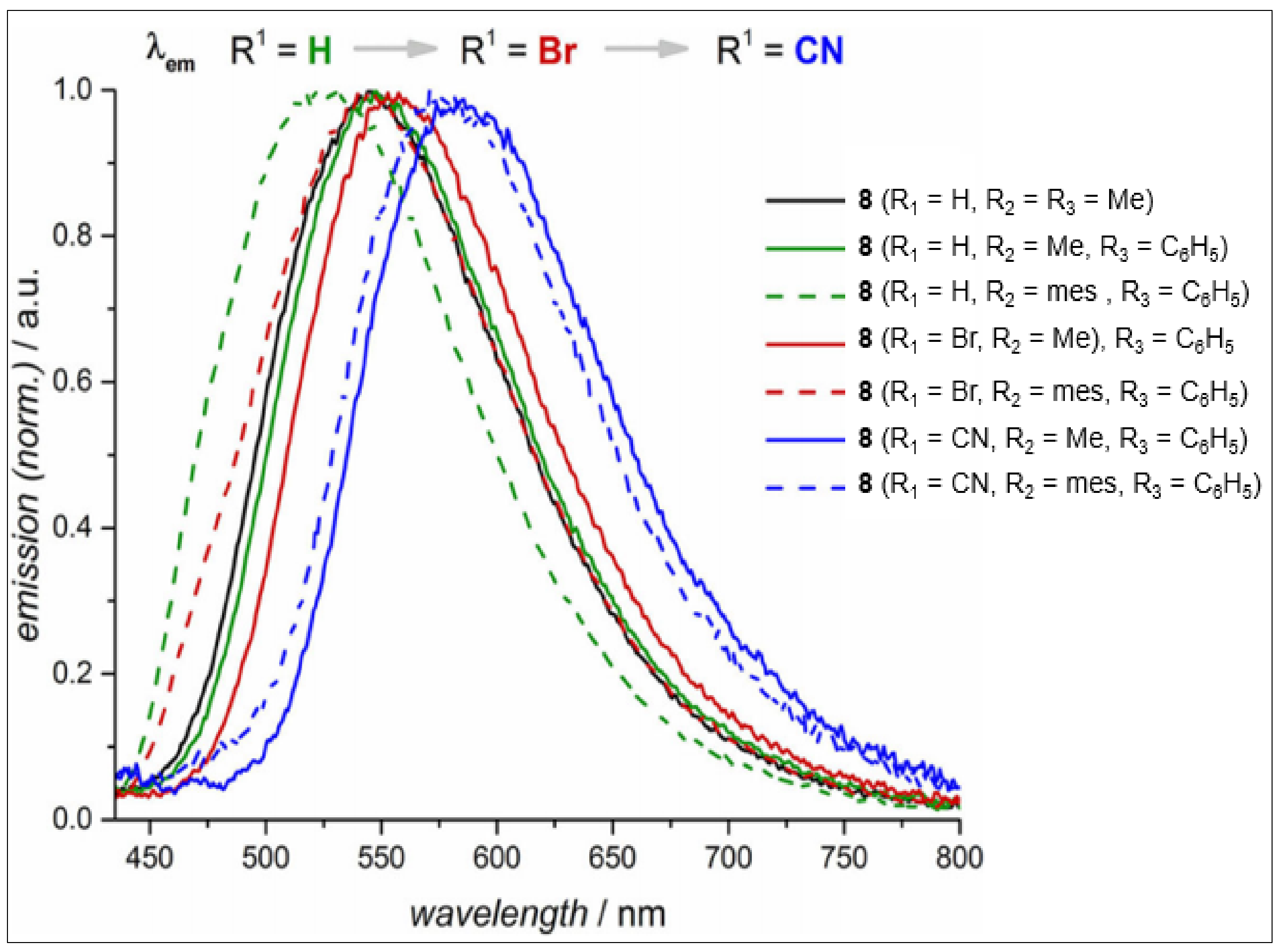
| 7 (R1 = CN, R2 = H) b | 330 | 470 | 0.98 | - | [33] |
| 7 (R1 = COOEt, R2 = H) b | 330 | 474 | 0.93 | - | [33] |
| 7 (R1 = H, R2 = Cl) b | 330 | 450 | 0.04 | - | [33] |
| 9a | 355 | 441, 465 | 0.42 | 35.6 | [37] |
| 10 (R = mes) a | 330 | 470 | 0.82 | 7.2 | [37] |
| 10 (R = dur) a | 330 | 467 | 0.87 | 8.5 | [37] |
| 11 (R = Me) a | 370 | 474, 506, 542 | 0.70 | 26 | [23] |
| 11 (R = C6H5) a | 360 | 526 | 0.41 | 6 | [23] |
| 11 (R = mes) a | 355 | 473, 505, 541 | 0.76 | 26 | [23] |
| 12 (R = Me) a | 330 | 446, 474 | 0.71 | 24 | [23] |
| 12 (R = C6H5) a | 370 | 524 | 0.45 | 7 | [23] |
| 12 (R = mes) a | 340 | 445, 474 | 0.87 | 10 | [23] |
| 13 (R = Me) a | 380 | 490 | 0.67 | 20.5 | [38] |
| 13 (R = mes) a | 380 | 488 | 0.69 | 17.9 | [38] |
| 13 (R = Dur) a | 380 | 488 | 0.78 | 17.7 | [38] |
| 14 (R1 = Bn, R2 = Me, R3 = H) a | 330 | 432 | 0.13 | 15 | [39] |
| 14 (R1 = Me, R2 = mes, R3 = H) a | 340 | 478 | 0.82 | 4 | [39] |
| 14 (R1 = Me, R2 = mes, R3 = OMe) a | 355 | 477 | 0.82 | 5 | [39] |
| 14 (R1 = Me, R2 = mes, R3 = Me) a | 355 | 475 | 0.82 | 3 | [39] |
| 14 (R1 = Bn, R2 = mes, R3 = H) a | 370 | 477 | 0.72 | 5 | [39] |
| 14 (R1 = Bn, R2 = mes, R3 = OMe) a | 370 | 478 | 0.70 | 7 | [39] |
| 14 (R1 = Bn, R2 = mes, R3 = Me) a | 340 | 471 | 0.81 | 5 | [39] |
| 15 (R1 = Bn, R2 = Me) a | 330 | 455 | 0.35 | 15 | [39] |
| 15 (R1 = Me, R2 = mes) a | 345 | 477 | 0.82 | 4 | [39] |
| 15 (R1 = Bn, R2 = mes) a | 355 | 477 | 0.83 | 4 | [39] |
| 16 (R = Me) a | 380 | 538 | 0.47 | 25.4 | [40] |
| 16 (R = mes) a | 380 | 537 | 0.53 | 22 | [40] |
| 16 (R = dur) a | 380 | 537 | 0.54 | 22.6 | [40] |
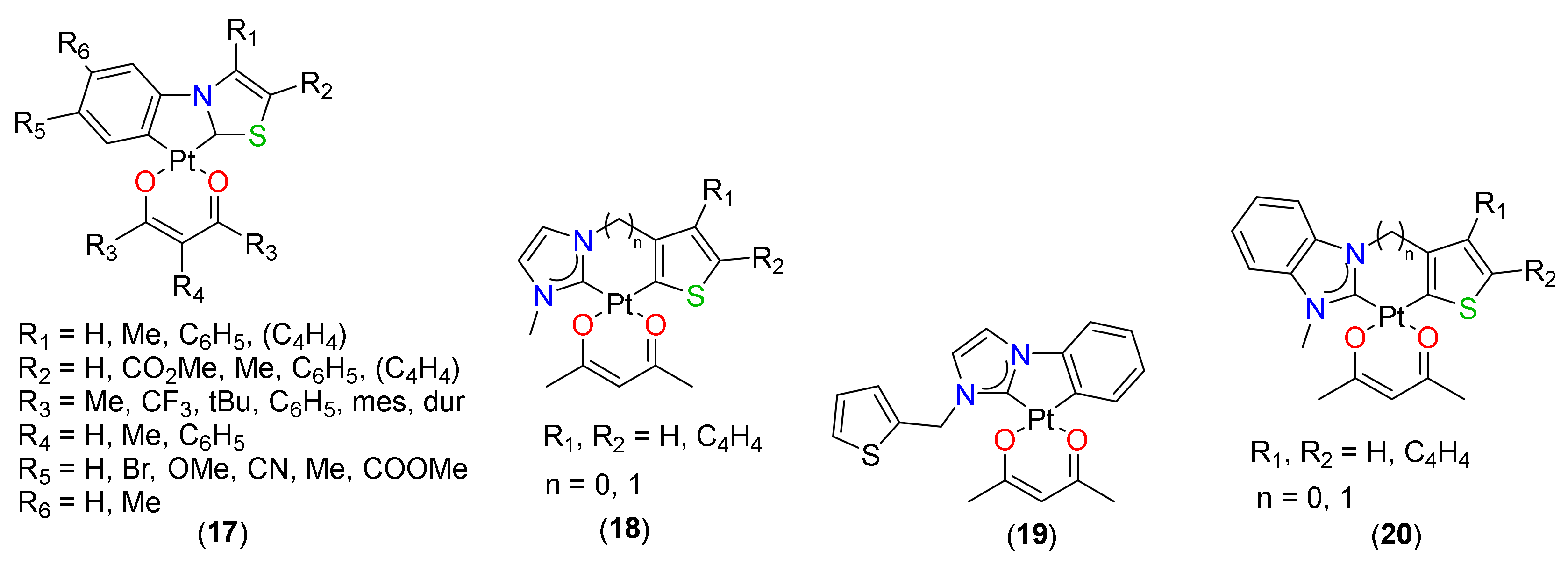
2.2. (C^N)Pt(O^O) Type Complexes
| S. No. | PL Parametres | Ref. | |||
|---|---|---|---|---|---|
| λabs a nm, (ε × 10−4 M−1 cm−1) | λPL a (nm) | Φ a (%) | τ0 (µs) | ||
| 30 | 601 (0.44), 435 (1.64), 383 (7.75), 318 (3.79), 275 (4.27) | 704 | 16.94 | 0.39 b | [61] |
| 31 | 504 (0.48), 364 (9.09), 329 (4.73), 285 (5.43) | 630 | 21.44 | 0.45 b | [61] |
| 32 | 570 (6.6), 535 (6.1), 450 (1.3),424 (1.2), 392 (2.8),375 (2.0), 310 (3.4), 286 (4.0) | 730 | 0.77 | 0.26 c | [62] |
| 33 | 413 (0.97), 369 (2.15), 352 (1.81), 325 (2.22), 270 (3.06), 280 (2.87) | 618 | 2.42 | 0.37 c | [62] |
3. Applications
3.1. Organic Photovoltaics (OPVs)
3.2. Organic Light-Emitting Diodes (OLEDs)
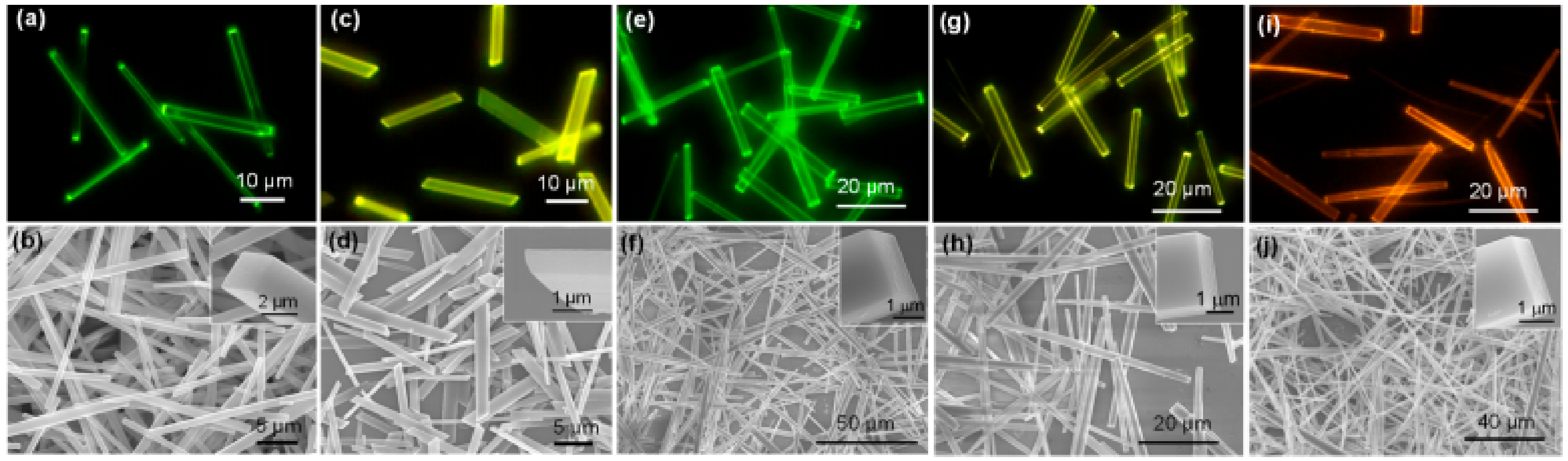
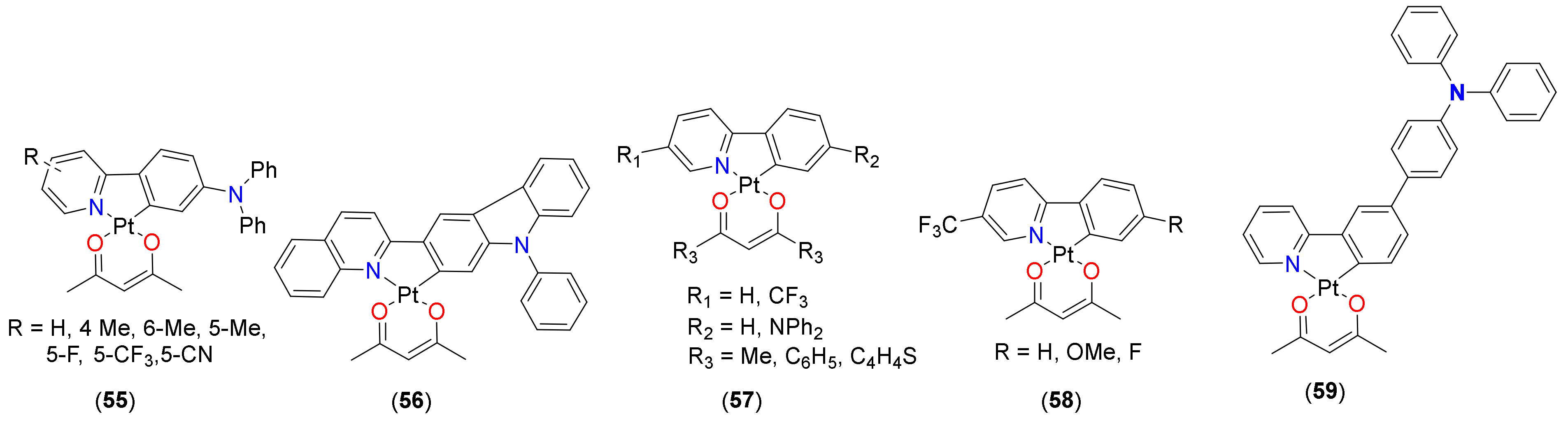
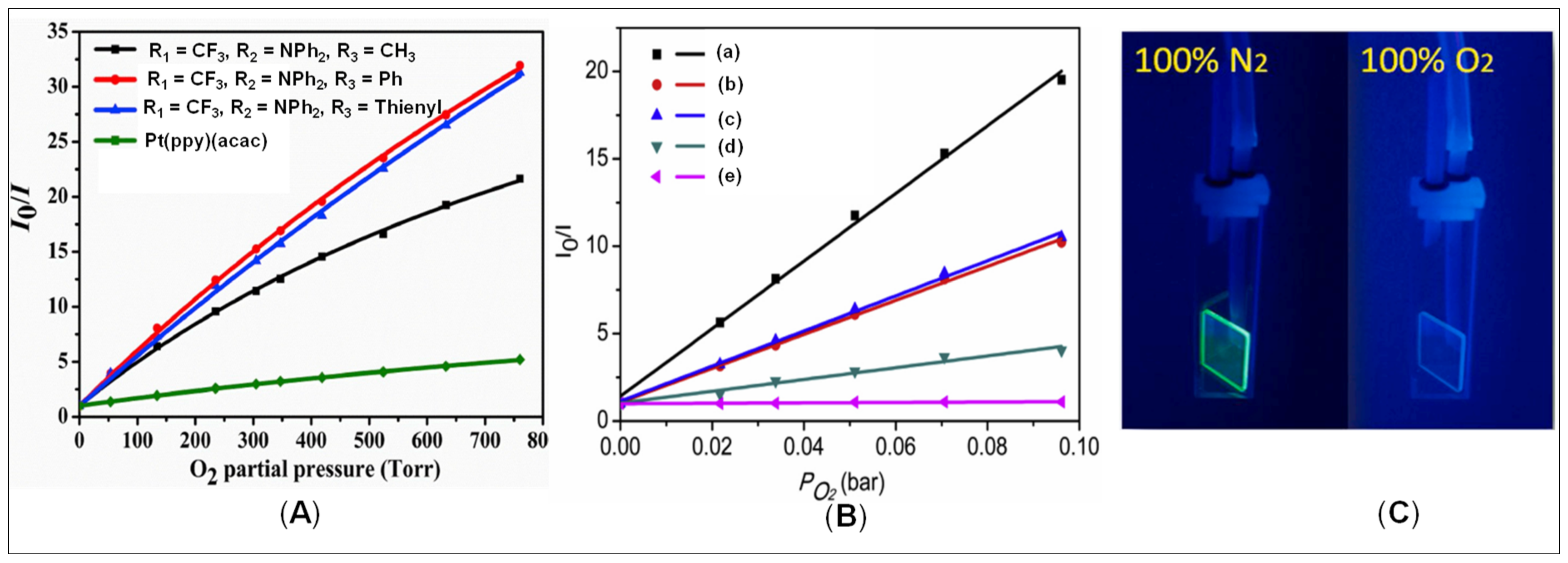
3.3. Oxygen Sensing
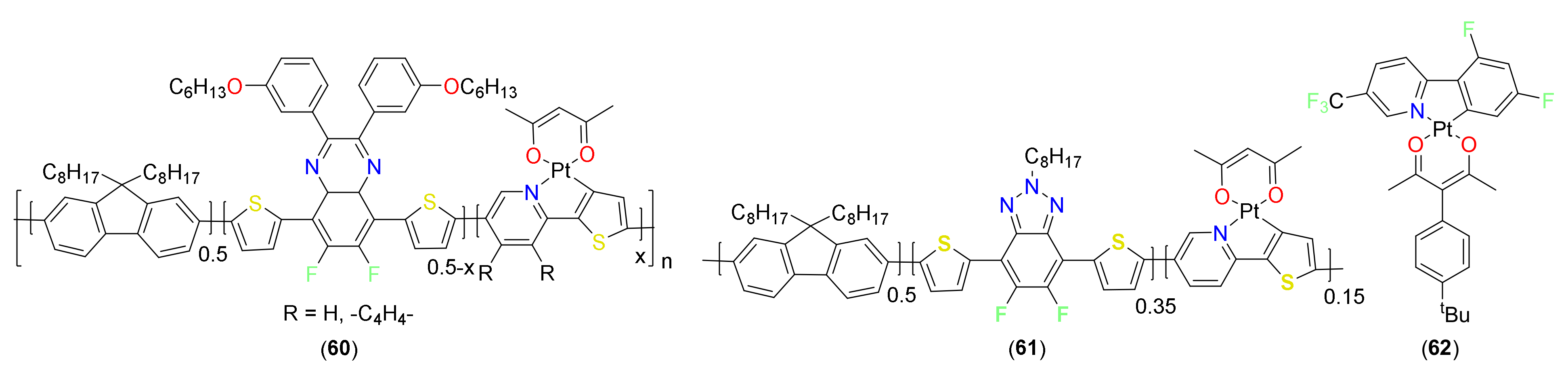
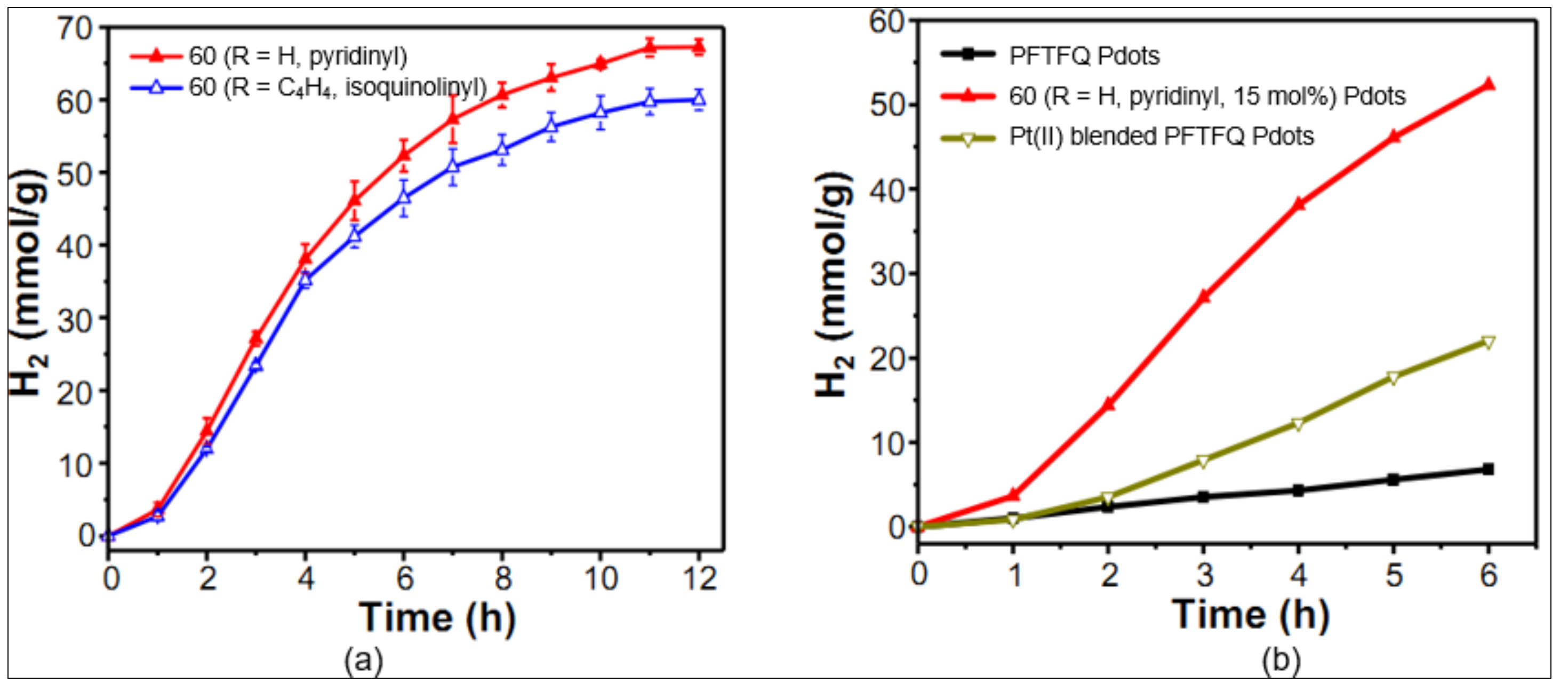
3.4. Miscellaneous
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bischoff, L.; Baudequin, C.; Hoarau, C.; Urriolabeitia, E.P. Organometallic fluorophores of d8 metals (Pd, Pt, Au). Adv. Organomet. Chem. 2018, 69, 73–134. [Google Scholar]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal–organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, Y.; Chou, P.-T. Transition-metal phosphors with cyclometalating ligands: Fundamentals and applications. Chem. Soc. Rev. 2010, 39, 638–655. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.-C.; Chan, A.K.-W.; Chan, M.-Y.; Yam, V.W.-W. Platinum and gold complexes for OLEDs. Photolumin. Mater. Electrolumin. Devices 2017, 374, 67–109. [Google Scholar]
- Cebrián, C.; Mauro, M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar] [CrossRef]
- Haque, A.; Xu, L.; Al-Balushi, R.A.; Al-Suti, M.K.; Ilmi, R.; Guo, Z.; Khan, M.S.; Wong, W.-Y.; Raithby, P.R. Cyclometalated tridentate platinum (II) arylacetylide complexes: Old wine in new bottles. Chem. Soc. Rev. 2019, 48, 5547–5563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, A.; Al-Balushi, R.A.; Al-Busaidi, I.J.; Khan, M.S.; Raithby, P.R. Rise of conjugated poly-ynes and poly(metalla-ynes): From design through synthesis to structure–property relationships and applications. Chem. Rev. 2018, 118, 8474–8597. [Google Scholar] [CrossRef]
- Yersin, H.; Rausch, A.F.; Czerwieniec, R.; Hofbeck, T.; Fischer, T. The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs. Coord. Chem. Rev. 2011, 255, 2622–2652. [Google Scholar] [CrossRef]
- Tseng, P.-J.; Chang, C.-L.; Chan, Y.-H.; Ting, L.-Y.; Chen, P.-Y.; Liao, C.-H.; Tsai, M.-L.; Chou, H.-H. Design and synthesis of cycloplatinated polymer dots as photocatalysts for visible-light-driven hydrogen evolution. ACS Catal. 2018, 8, 7766–7772. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, W.; Zhu, C. Theoretical study of the substituent effect controlling the radiative and non-radiative decay processes of platinum (II) complexes. Phys. Chem. Chem. Phys. 2017, 19, 23532–23540. [Google Scholar] [CrossRef]
- Stipurin, S.; Strassner, T. Phosphorescent cyclometalated platinum (II) imidazolinylidene complexes. Eur. J. Inorg. Chem. 2021, 9, 804–813. [Google Scholar] [CrossRef]
- Hang, X.C.; Fleetham, T.; Turner, E.; Brooks, J.; Li, J. Highly efficient blue-emitting cyclometalated platinum (II) complexes by judicious molecular design. Angew. Chem. 2013, 125, 6885–6888. [Google Scholar] [CrossRef]
- Koshevoy, I.O.; Krause, M.; Klein, A. Non-covalent intramolecular interactions through ligand-design promoting efficient photoluminescence from transition metal complexes. Coord. Chem. Rev. 2020, 405, 213094. [Google Scholar] [CrossRef]
- Strassner, T. Phosphorescent platinum (II) complexes with C^C* cyclometalated NHC ligands. Acc. Chem. Res. 2016, 49, 2680–2689. [Google Scholar] [CrossRef]
- Goswami, S.; Winkel, R.W.; Alarousu, E.; Ghiviriga, I.; Mohammed, O.F.; Schanze, K.S. Photophysics of organometallic platinum (II) derivatives of the diketopyrrolopyrrole chromophore. J. Phys. Chem. A 2014, 118, 11735–11743. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zhang, K.; Chen, K.; Wang, P.; Lu, Z.; Zhu, W.; Liu, Y. More efficient spin–orbit coupling: Adjusting the ligand field strength to the second metal ion in asymmetric binuclear platinum (II) configurations. Dalton Trans. 2020, 49, 8722–8733. [Google Scholar] [CrossRef]
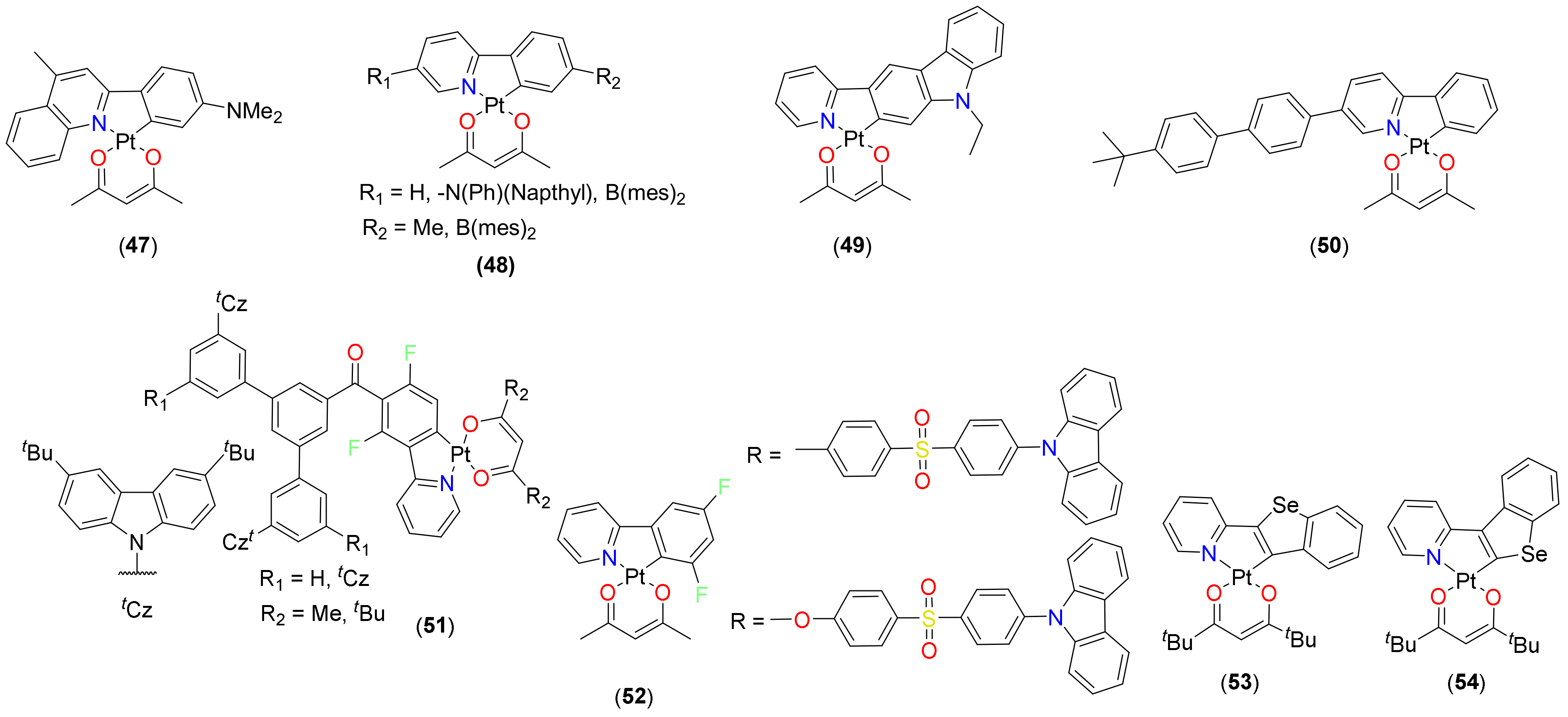
- Pittkowski, R.; Strassner, T. Enhanced quantum yields by sterically demanding aryl-substituted β-diketonate ancillary ligands. Beilstein J. Org. Chem. 2018, 14, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leopold, H.; Heinemeyer, U.; Wagenblast, G.; Münster, I.; Strassner, T. Changing the emission properties of phosphorescent C^C*-cyclometalated thiazol-2-ylidene platinum (II) complexes by variation of the β-diketonate ligands. Chem. Eur. J. 2017, 23, 1118–1128. [Google Scholar] [CrossRef]
- Yan, Y.; Yu, Z.; Liu, C.; Jin, X. Effects of phenyl/thienyl substituents at acetylacetone auxiliary ligands on the properties of cyclometalated platinum (II) complexes. Dye. Pigm. 2020, 173, 107949. [Google Scholar] [CrossRef]
- Qian, G.; Yang, X.; Wang, X.; Herod, J.D.; Bruce, D.W.; Wang, S.; Zhu, W.; Duan, P.; Wang, Y. Chiral platinum-based metallomesogens with highly efficient circularly polarized electroluminescence in solution-processed organic light-emitting diodes. Adv. Opt. Mat. 2020, 8, 2000775. [Google Scholar] [CrossRef]
- Heil, A.; Marian, C.M. Structure–emission property relationships in cyclometalated Pt (II) β-diketonate complexes. Inorg. Chem. 2019, 58, 6123–6136. [Google Scholar] [CrossRef]
- Huo, S.; Carroll, J.; Vezzu, D.A. Design, synthesis, and applications of highly phosphorescent cyclometalated platinum complexes. Asian J. Org. Chem. 2015, 4, 1210–1245. [Google Scholar] [CrossRef]
- Tenne, M.; Metz, S.; Wagenblast, G.; Münster, I.; Strassner, T. C^C* Cyclometalated platinum (II) complexes with dibenzofuranyl-1, 2, 4-triazol-5-ylidene ligands: Synthesis, characterization, and photoluminescent properties. Organometallics 2015, 34, 4433–4440. [Google Scholar] [CrossRef]
- Pinter, P.; Strassner, T. Prediction of emission wavelengths of phosphorescent NHC based emitters for OLEDs. Tetrahedron 2019, 75, 130431. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, G.-H.; Gu, M.-Q.; Wang, Q.; Wu, D. Theoretical study and design of phosphorescent cyclometalated (C^C*) PtII (acac) Complexes: The substituent effect controls the radiative and nonradiative decay processes. J. Phys. Chem. A 2017, 121, 6231–6242. [Google Scholar] [CrossRef]
- Lanoë, P.-H.; Moreno-Betancourt, A.; Wilson, L.; Philouze, C.; Monnereau, C.; Jamet, H.; Jouvenot, D.; Loiseau, F. Neutral heteroleptic cyclometallated platinum (II) complexes featuring 2-phenylbenzimidazole ligand as bright emitters in solid state and in solution. Dye. Pigment. 2019, 162, 967–977. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Tronnier, A.; Schleicher, D.; Strassner, T. (C^C*)-cyclometalated platinum (II) imidazo [1, 5-a] pyridine NHC complexes—Synthesis and characterization. J. Organomet. Chem. 2015, 775, 155–163. [Google Scholar] [CrossRef]
- Tronnier, A.; Heinemeyer, U.; Metz, S.; Wagenblast, G.; Muenster, I.; Strassner, T. Heteroleptic platinum (II) NHC complexes with a C^C* cyclometalated ligand—Synthesis, structure and photophysics. J. Mater. Chem. C 2015, 3, 1680–1693. [Google Scholar] [CrossRef] [Green Version]
- Tronnier, A.; Nischan, N.; Metz, S.; Wagenblast, G.; Münster, I.; Strassner, T. Phosphorescent C^C* Cyclometalated PtII dibenzofuranyl-NHC complexes—An auxiliary ligand study. Eur. J. Inorg. Chem. 2014, 2014, 256–264. [Google Scholar] [CrossRef]
- Unger, Y.; Meyer, D.; Molt, O.; Schildknecht, C.; Münster, I.; Wagenblast, G.; Strassner, T. Green–blue emitters: NHC-based cyclometalated Pt (C^C*)(acac) complexes. Angew. Chem. Int. Ed. 2010, 49, 10214–10216. [Google Scholar] [CrossRef]
- Pinter, P.; Mangold, H.; Stengel, I.; Münster, I.; Strassner, T. Enhanced photoluminescence quantum yields through excimer formation of cyclometalated platinum (II) N-heterocyclic carbene complexes. Organometallics 2016, 35, 673–680. [Google Scholar] [CrossRef]
- Fuertes, S.; Chueca, A.J.; Martín, A.; Sicilia, V. Pt2Tl building blocks for two-dimensional extended solids: Synthesis, crystal structures, and luminescence. Cryst. Growth Des. 2017, 17, 4336–4346. [Google Scholar] [CrossRef]
- Soellner, J.; Strassner, T. Phosphorescent cyclometalated platinum (II) aNHC complexes. Chem. Eur. J. 2018, 24, 15603–15612. [Google Scholar] [CrossRef]
- Fantasia, S.; Petersen, J.L.; Jacobsen, H.; Cavallo, L.; Nolan, S.P. Electronic properties of N-heterocyclic carbene (NHC) ligands: Synthetic, structural, and spectroscopic studies of (NHC) platinum (II) complexes. Organometallics 2007, 26, 5880–5889. [Google Scholar] [CrossRef]
- Strassner, T.; Unger, Y.; Meyer, D.; Molt, O.; Münster, I.; Wagenblast, G. The “enders triazole”: A well known molecule, but still a new ligand. Inorg. Chem. Commun. 2013, 30, 39–41. [Google Scholar] [CrossRef]
- Soellner, J.; Strassner, T. The “enders triazole” revisited: Highly efficient, blue platinum (II) emitters. Organometallics 2018, 37, 1821–1824. [Google Scholar] [CrossRef]
- Soellner, J.; Strassner, T. Cyclometalated platinum (II) complexes with mesoionic dibenzofuranyl-1, 2, 3-triazol-4-ylidene ligands: Synthesis, characterization and photophysical properties. Chem. Photo. Chem. 2019, 3, 554–558. [Google Scholar] [CrossRef]
- Tenne, M.; Metz, S.; Wagenblast, G.; Münster, I.; Strassner, T. C^C* cyclometalated platinum (II) N-heterocyclic carbene complexes with a sterically demanding β-diketonato ligand—Synthesis, characterization and photophysical properties. Dalton Trans. 2015, 44, 8444–8455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soellner, J.; Strassner, T. Mesoionic 1, 2, 3-Triazolo [1, 5-a] pyridine-3-ylidenes in phosphorescent platinum (II) complexes. Chem. Photo. Chem. 2019, 3, 1000–1003. [Google Scholar] [CrossRef]
- Leopold, H.; Strassner, T. 4, 5-Substituted C^C* cyclometalated thiazol-2-ylidene platinum (II) complexes–Synthesis and photophysical properties. Dalton Trans. 2017, 46, 7800–7812. [Google Scholar] [CrossRef]
- Leopold, H.; Tronnier, A.; Wagenblast, G.; Münster, I.; Strassner, T. Photoluminescence of a new material: Cyclometalated C^C* thiazole-2-ylidene platinum (II) complexes. Organometallics 2016, 35, 959–971. [Google Scholar] [CrossRef]
- Mastrocinque, F.; Anderson, C.M.; Elkafas, A.M.; Ballard, I.V.; Tanski, J.M. Synthesis, characterization, and photophysical properties of cyclometalated N-Heterocyclic carbene platinum (II) complexes. J. Organomet. Chem. 2019, 880, 98–107. [Google Scholar] [CrossRef]
- Shigehiro, T.; Chen, Q.; Yagi, S.; Maeda, T.; Nakazumi, H.; Sakurai, Y. Substituent effect on photo-and electroluminescence properties of heteroleptic cyclometalated platinum (II) complexes based on a 2-(dibenzo [b, d] furan-4-yl) pyridine ligand. Dye. Pigment. 2016, 124, 165–173. [Google Scholar] [CrossRef]
- Jackel, A.; Linseis, M.; Häge, C.; Winter, R.F. Turning-on of coumarin phosphorescence in acetylacetonato platinum complexes of cyclometalated pyridyl-substituted coumarins. Inorganics 2015, 3, 55–81. [Google Scholar] [CrossRef] [Green Version]
- Shafikov, M.Z.; Kozhevnikov, D.N.; Bodensteiner, M.; Brandl, F.; Czerwieniec, R. Modulation of intersystem crossing rate by minor ligand modifications in cyclometalated platinum (II) complexes. Inorg. Chem. 2016, 55, 7457–7466. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Xiao, M.; Zhong, P.; Liu, Y.; Tan, H.; Zhu, M.; Zhu, W. Luminescent metallomesogens based on platinum complex containing triphenylene unit. Tetrahedron 2015, 71, 463–469. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, C.; Song, X.; Li, J. Photostable trifluoromethyl-substituted platinum (II) emitters for continuous monitoring of molecular oxygen. J. Mater. Chem. C 2015, 3, 2166–2174. [Google Scholar] [CrossRef]
- Zeng, W.; Sun, M.-J.; Gong, Z.-L.; Shao, J.-Y.; Zhong, Y.-W.; Yao, J. Effect of the fluoro-substituent position on the crystal structure and photoluminescence of microcrystals of platinum β-diketonate complexes. Inorg. Chem. 2020, 59, 11316–11328. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-J.; Liu, Y.; Zeng, W.; Zhao, Y.S.; Zhong, Y.-W.; Yao, J. Photoluminescent anisotropy amplification in polymorphic organic nanocrystals by light-harvesting energy transfer. J. Am. Chem. Soc. 2019, 141, 6157–6161. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, C.; Xiu, J.-H.; Li, J.-Y. Photostable fluorophenyl-substituted cyclometalated platinum (II) emitters for monitoring of molecular oxygen in real time. Inorg. Chem. 2015, 54, 7783–7790. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wang, L.; Liu, C.; Jin, X. Effects of fluorine and phenyl substituents on oxygen sensitivity and photostability of cyclometalated platinum (II) complexes. Sens. Actuator B Chem. 2020, 304, 127378. [Google Scholar] [CrossRef]
- Okamura, N.; Maeda, T.; Fujiwara, H.; Soman, A.; Unni, K.N.; Ajayaghosh, A.; Yagi, S. Photokinetic study on remarkable excimer phosphorescence from heteroleptic cyclometalated platinum (II) complexes bearing a benzoylated 2-phenylpyridinate ligand. Phys. Chem. Chem. Phys. 2018, 20, 542–552. [Google Scholar] [CrossRef]
- Kang, J.; Zaen, R.; Park, K.-M.; Lee, K.H.; Lee, J.Y.; Kang, Y. Cyclometalated Platinum (ii) β-diketonate complexes as single dopants for high-efficiency white OLEDs: The relationship between intermolecular interactions in the solid state and electroluminescent efficiency. Cryst. Growth Des. 2020, 20, 6129–6138. [Google Scholar] [CrossRef]
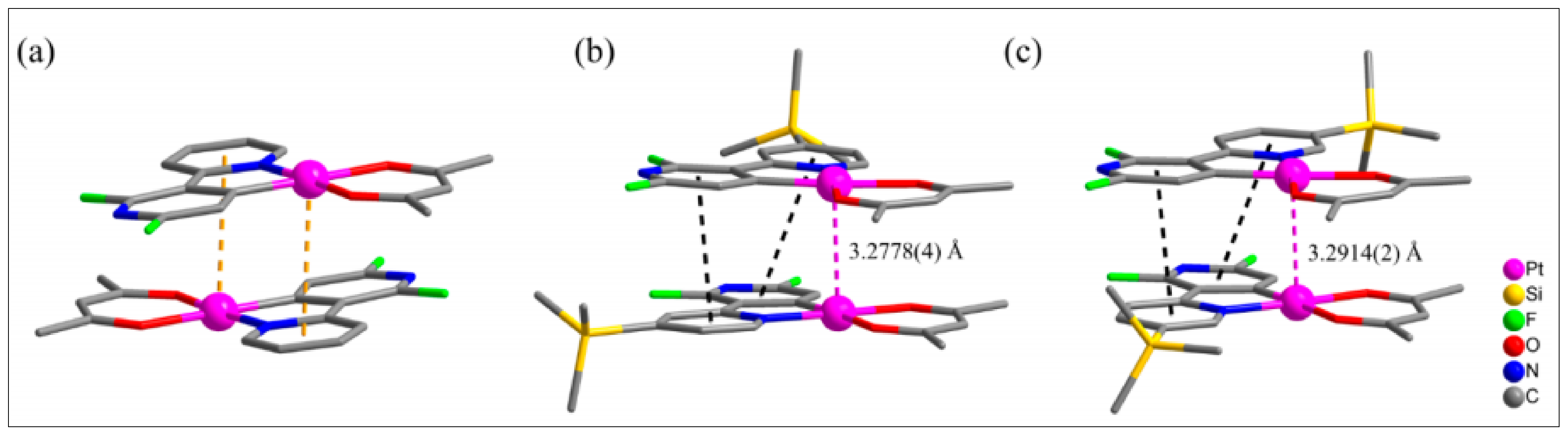
- Rozhkov, A.V.; Ananyev, I.V.; Gomila, R.M.; Frontera, A.; Kukushkin, V.Y. π-Hole··· dz2 [PtII] interactions with electron-deficient arenes enhance the phosphorescence of PtII-based luminophores. Inorg. Chem. 2020, 59, 9308–9314. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Wang, X.; He, J.; Wu, J.; Liu, H.; Song, J.; Qu, J.; Chan, W.T.-K.; Wong, W.-Y. Achieving NIR emission for donor–acceptor type platinum (II) complexes by adjusting coordination position with isomeric ligands. Inorg. Chem. 2018, 57, 14208–14217. [Google Scholar] [CrossRef] [PubMed]
- Usuki, T.; Uchida, H.; Omoto, K.; Yamanoi, Y.; Yamada, A.; Iwamura, M.; Nozaki, K.; Nishihara, H. Enhancement of the photofunction of phosphorescent Pt (II) cyclometalated complexes driven by substituents: Solid-state luminescence and circularly polarized luminescence. J. Org. Chem. 2019, 84, 10749–10756. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Yang, H.; Zhong, D.; Liu, B.; Yang, X.; Yue, L.; Zhou, G.; Ma, M.; Wu, Z. The synthesis of cyclometalated platinum (II) complexes with benzoaryl-pyridines as C^N ligands for investigating their photophysical, electrochemical and electroluminescent properties. Dalton Trans. 2020, 49, 15633–15645. [Google Scholar] [CrossRef]
- Berry, J.F.; Thomas, C.M. Multimetallic complexes: Synthesis and applications. Dalton Trans. 2017, 46, 5472–5473. [Google Scholar] [CrossRef]
- Hao, Z.; Meng, F.; Wang, P.; Wang, Y.; Tan, H.; Pei, Y.; Su, S.; Liu, Y. Dual phosphorescence emission of dinuclear platinum (II) complex incorporating cyclometallating pyrenyl-dipyridine-based ligand and its application in near-infrared solution-processed polymer light-emitting diodes. Dalton Trans. 2017, 46, 16257–16268. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, T.; Wu, T.; Ding, Z.; Zhang, Q.; Zhu, W.; Liu, Y. An effective strategy to obtain near-infrared emission from shoulder to shoulder-type binuclear platinum (ii) complexes based on fused pyrene core bridged isoquinoline ligands. J. Mater. Chem. C 2021, 9, 2282–2290. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Hao, Z.; Lei, G.; Cui, S.; Zhu, W.; Liu, Y. A feasible approach to obtain near-infrared (NIR) emission from binuclear platinum (II) complexes containing centrosymmetric isoquinoline ligand in PLEDs. Org. Electron. 2020, 87, 105902. [Google Scholar] [CrossRef]
- Shafikov, M.Z.; Tang, S.; Larsen, C.; Bodensteiner, M.; Kozhevnikov, V.N.; Edman, L. An efficient heterodinuclear Ir(III)/Pt (II) complex: Synthesis, photophysics and application in light-emitting electrochemical cells. J. Mater. Chem. C 2019, 7, 10672–10682. [Google Scholar] [CrossRef]
- Yang, X.; Jiao, B.; Dang, J.-S.; Sun, Y.; Wu, Y.; Zhou, G.; Wong, W.-Y. Achieving high-performance solution-processed orange OLEDs with the phosphorescent cyclometalated trinuclear Pt (II) complex. ACS Appl. Mater. Interfaces 2018, 10, 10227–10235. [Google Scholar] [CrossRef]
- Rappaport, P. The photovoltaic effect and its utilization. Sol. Energy 1959, 3, 8–18. [Google Scholar] [CrossRef]
- Wright, M.; Uddin, A. Organic—Inorganic hybrid solar cells: A comparative review. Sol. Energy Mater. Sol. Cells 2012, 107, 87–111. [Google Scholar] [CrossRef]
- Thompson, B.C.; Fréchet, J.M. Polymer–fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef] [PubMed]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Wang, T.; Kupgan, G.; Brédas, J.L. Organic photovoltaics: Relating chemical structure, local morphology, and electronic properties. Trends Chem. 2020, 2, 535–554. [Google Scholar] [CrossRef]
- Al-Busaidi, I.J.; Haque, A.; Al Rasbi, N.K.; Khan, M.S. Phenothiazine-based derivatives for optoelectronic applications: A review. Synth. Met. 2019, 257, 116189. [Google Scholar] [CrossRef]
- Guo, F.; Kim, Y.-G.; Reynolds, J.R.; Schanze, K.S. Platinum–acetylide polymer based solar cells: Involvement of the triplet state for energy conversion. Chem. Commun. 2006, 17, 1887–1889. [Google Scholar] [CrossRef]
- Yang, C.-M.; Wu, C.-H.; Liao, H.-H.; Lai, K.-Y.; Cheng, H.-P.; Horng, S.-F.; Meng, H.-F.; Shy, J.-T. Enhanced photovoltaic response of organic solar cell by singlet-to-triplet exciton conversion. Appl. Phys. Lett. 2007, 90, 133509. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Piliego, C.; Kim, B.; Poulsen, D.A.; Ma, B.; Unruh, D.A.; Fréchet, J.M. Solution-processable crystalline platinum-acetylide oligomers with broadband absorption for photovoltaic cells. Chem. Mater. 2010, 22, 2325–2332. [Google Scholar] [CrossRef]
- Felter, K.M.; Caselli, V.M.; Günbaş, D.D.; Savenije, T.J.; Grozema, F.C. Interplay between charge carrier mobility, exciton diffusion, crystal packing, and charge separation in perylene diimide-based heterojunctions. ACS Appl. Energy Mater. 2019, 2, 8010–8021. [Google Scholar] [CrossRef]
- Clem, T.A.; Kavulak, D.F.; Westling, E.J.; Fréchet, J.M. Cyclometalated platinum polymers: Synthesis, photophysical properties, and photovoltaic performance. Chem. Mater. 2010, 22, 1977–1987. [Google Scholar] [CrossRef]
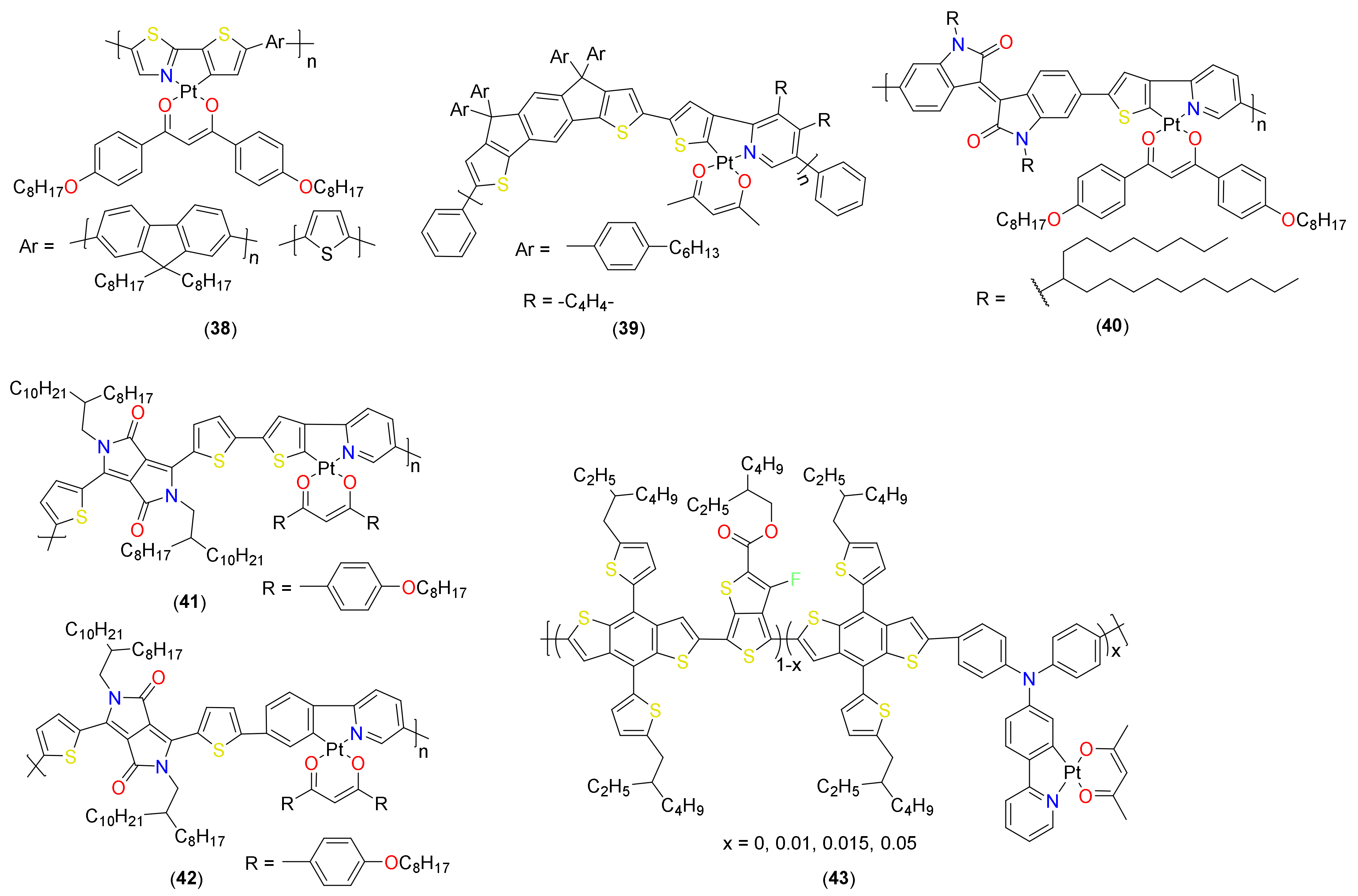
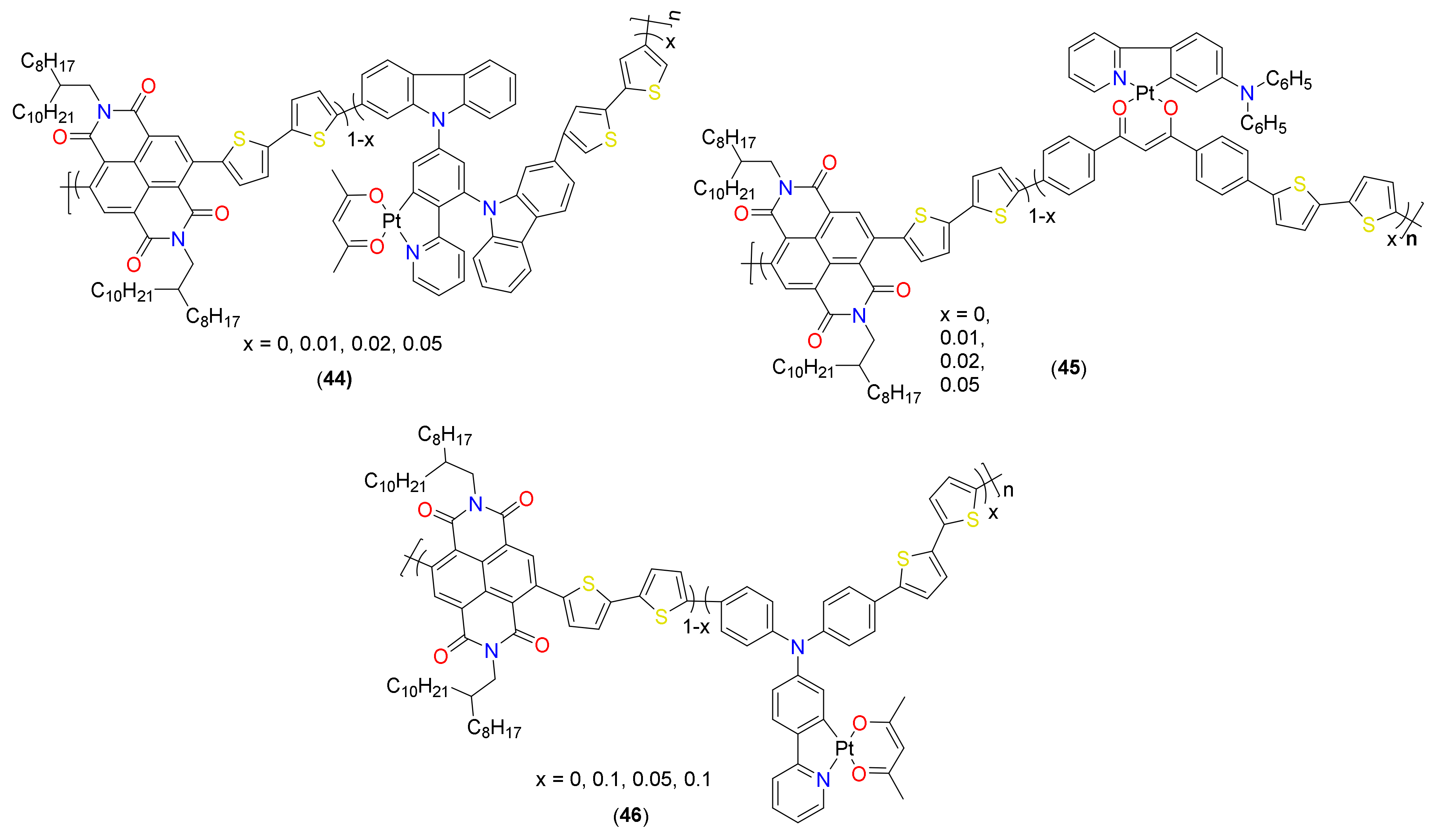
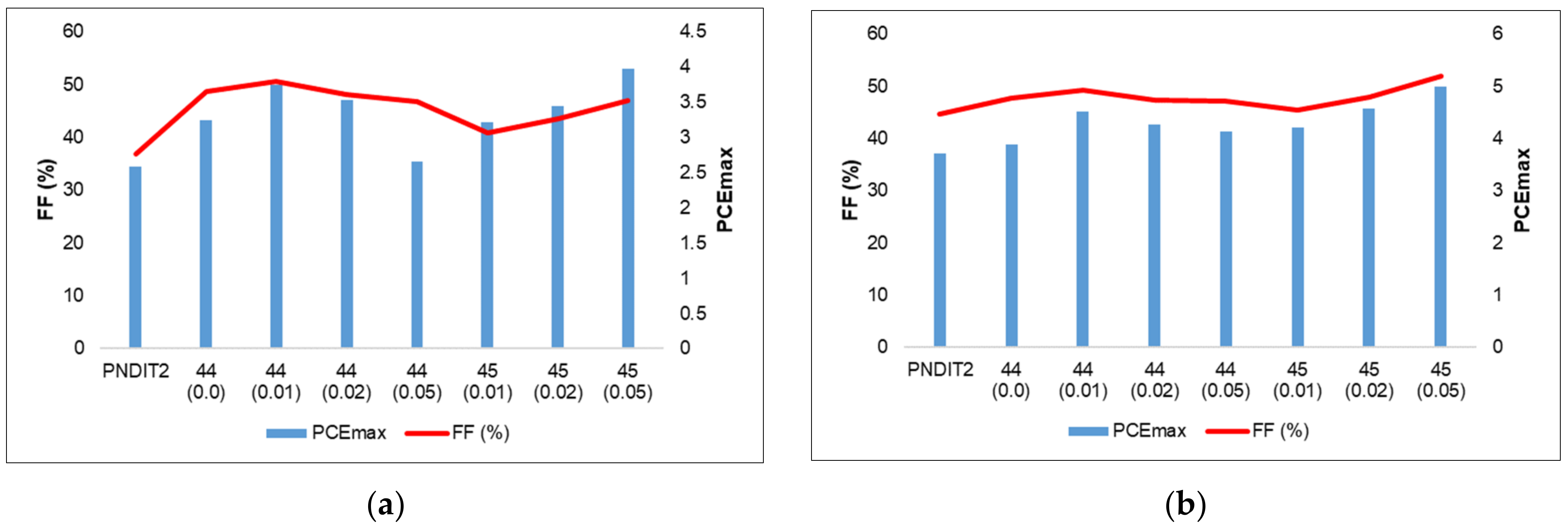
- Liao, C.-Y.; Chen, C.-P.; Chang, C.-C.; Hwang, G.-W.; Chou, H.-H.; Cheng, C.-H. Synthesis of conjugated polymers bearing indacenodithiophene and cyclometalated platinum (II) units and their application in organic photovoltaics. Sol. Energy Mater. Sol. Cells 2013, 109, 111–119. [Google Scholar] [CrossRef]
- Goswami, S.; Gish, M.K.; Wang, J.; Winkel, R.W.; Papanikolas, J.M.; Schanze, K.S. π-Conjugated organometallic isoindigo oligomer and polymer chromophores: Singlet and triplet excited state dynamics and application in polymer solar cells. ACS Appl. Mater. Interfaces 2015, 7, 26828–26838. [Google Scholar] [CrossRef]
- Goswami, S.; Hernandez, J.L.; Gish, M.K.; Wang, J.; Kim, B.; Laudari, A.P.; Guha, S.; Papanikolas, J.M.; Reynolds, J.R.; Schanze, K.S. Cyclometalated platinum-containing diketopyrrolopyrrole complexes and polymers: Photophysics and photovoltaic applications. Chem. Mater. 2017, 29, 8449–8461. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, J.; Liu, Y.; Wang, S.; Zhong, Y.; Li, C.; Zhang, Z.; Xing, G.; Huettner, S.; Tao, Y.; et al. Cyclometalated Pt complex-based random terpolymers for efficient polymer solar cells. Polym. Chem. 2017, 8, 4729–4737. [Google Scholar] [CrossRef]
- Gao, X.; Liang, Y.; Wang, H.; Yang, T.; Huettner, S.; Wang, J.; Zhu, F.; Tao, Y. Terpolymer acceptors based on an organic ligand or corresponding cyclometalated Pt complex for all polymer solar cells. Org. Electron. 2019, 70, 93–100. [Google Scholar] [CrossRef]
- Gao, X.; Wang, M.; Cao, X.; Yang, J.; Zhong, Y.; Zhang, Z.; Li, C.; Huettner, S.; Tao, Y.; Li, Y. Cyclometalated Pt complex based random terpolymers as electron acceptors for all polymer solar cells. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Shi, D.; Wang, M.; Xue, Z.; Hu, Y.; Tao, Y.; Huang, W. Pt complex-based terpolymer acceptors linked through ancillary ligand for all-polymer solar cells. J. Mater. Chem. C 2018, 6, 9903–9913. [Google Scholar] [CrossRef]
- Fan, C.; Yang, C. Yellow/orange emissive heavy-metal complexes as phosphors in monochromatic and white organic light-emitting devices. Chem. Soc. Rev. 2014, 43, 6439–6469. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Guo, Y.; Feng, Z.; Zhou, G.; Chen, Z.; Yang, X. Triphenylamine-based trinuclear Pt (II) complexes for solution-processed OLEDs displaying efficient pure yellow and red emissions. Org. Electron. 2021, 91, 106101. [Google Scholar] [CrossRef]
- Tronnier, A.; Strassner, T. (C^C*) Cyclometalated binuclear N-heterocyclic biscarbene platinum (II) complexes—Highly emissive phosphorescent emitters. Dalton Trans. 2013, 42, 9847–9851. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Egawa, K.; Maeda, T.; Yagi, S. Control of excimer phosphorescence by steric effects in cyclometalated platinum (ii) diketonate complexes bearing peripheral carbazole moieties towards application in non-doped white OLEDs. New J. Chem. 2018, 42, 11583–11592. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, K.; Kim, C.K.; Tan, S.; Wang, S.; Wang, L.; Li, J.; Wang, Y. Stimuli-responsive cyclometalated platinum complex bearing bent molecular geometry for highly efficient solution-processable OLEDs. Chin. Chem. Lett. 2021, 32, 493–496. [Google Scholar] [CrossRef]
- Petrenko, A.; Leitonas, K.; Volyniuk, D.; Baryshnikov, G.V.; Belyakov, S.; Minaev, B.F.; Ågren, H.; Durgaryan, H.; Gražulevičius, J.V.; Arsenyan, P. Benzoselenophenylpyridine platinum complexes: Green versus red phosphorescence towards hybrid OLEDs. Dalton Trans. 2020, 49, 3393–3397. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhao, S.N.; Zang, S.Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, C.; Wu, W.; Guo, H.; Ji, S.; Song, P.; Han, K.; Zhao, J.; Zhang, X.; Wu, Y. Tuning the emission colour of triphenylamine-capped cyclometallated platinum (II) complexes and their application in luminescent oxygen sensing and organic light-emitting diodes. Eur. J. Inorg. Chem. 2010, 2010, 4683–4696. [Google Scholar] [CrossRef]
- Liu, C.; Song, X.; Wang, Z.; Qiu, J. 2-Phenylquinoline-Based cyclometalated platinum (II) complexes: Synthesis and structure–photoelectric properties relationship in oxygen sensing. Chem. Plus. Chem 2014, 79, 1472–1481. [Google Scholar] [CrossRef]
- Liu, C.; Song, X.; Rao, X.; Xing, Y.; Wang, Z.; Zhao, J.; Qiu, J. Novel triphenylamine-based cyclometalated platinum (II) complexes for efficient luminescent oxygen sensing. Dye. Pigment. 2014, 101, 85–92. [Google Scholar] [CrossRef]
- Di, L.; Xia, Z.; Wang, H.; Xing, Y.; Yang, Z. Switchable and adjustable AIE activity of Pt (II) complexes achieving swift-responding and highly sensitive oxygen sensing. Sens. Actuator B Chem. 2021, 326, 128987. [Google Scholar] [CrossRef]
- Chang, C.-L.; Lin, W.-C.; Jia, C.-Y.; Ting, L.-Y.; Jayakumar, J.; Elsayed, M.H.; Yang, Y.-Q.; Chan, Y.-H.; Wang, W.-S.; Lu, C.-Y. Low-toxic cycloplatinated polymer dots with rational design of acceptor co-monomers for enhanced photocatalytic efficiency and stability. Appl. Catal. B Environ. 2020, 268, 118436. [Google Scholar] [CrossRef]
- Gao, L.; Ni, J.; Su, M.; Kang, J.; Zhang, J. Luminescence switching property of cycloplatinated (II) complexes bearing 2-phenylpyridine derivatives and the application for data security storage. Dye. Pigment. 2019, 165, 231–238. [Google Scholar] [CrossRef]
- Liao, M.-Y.; Elsayed, M.H.; Chang, C.-L.; Chiang, Y.-C.; Lee, W.-Y.; Chen, W.-C.; Chou, H.-H.; Chueh, C.-C. Realizing nonvolatile photomemories with multilevel memory behaviors using water-processable polymer dots-based hybrid floating gates. ACS Appl. Electron. Mater. 2021, 3, 1708–1718. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, J.; Yan, C.; Xie, Z.; Zhang, G.; Shao, X.; Zhang, D.; Zhou, S. Diketopyrrolopyrrole based donor–acceptor π-conjugated copolymers with near-infrared absorption for 532 and 1064 nm nonlinear optical materials. J. Mater. Chem. C. 2020, 8, 12993–13000. [Google Scholar] [CrossRef]
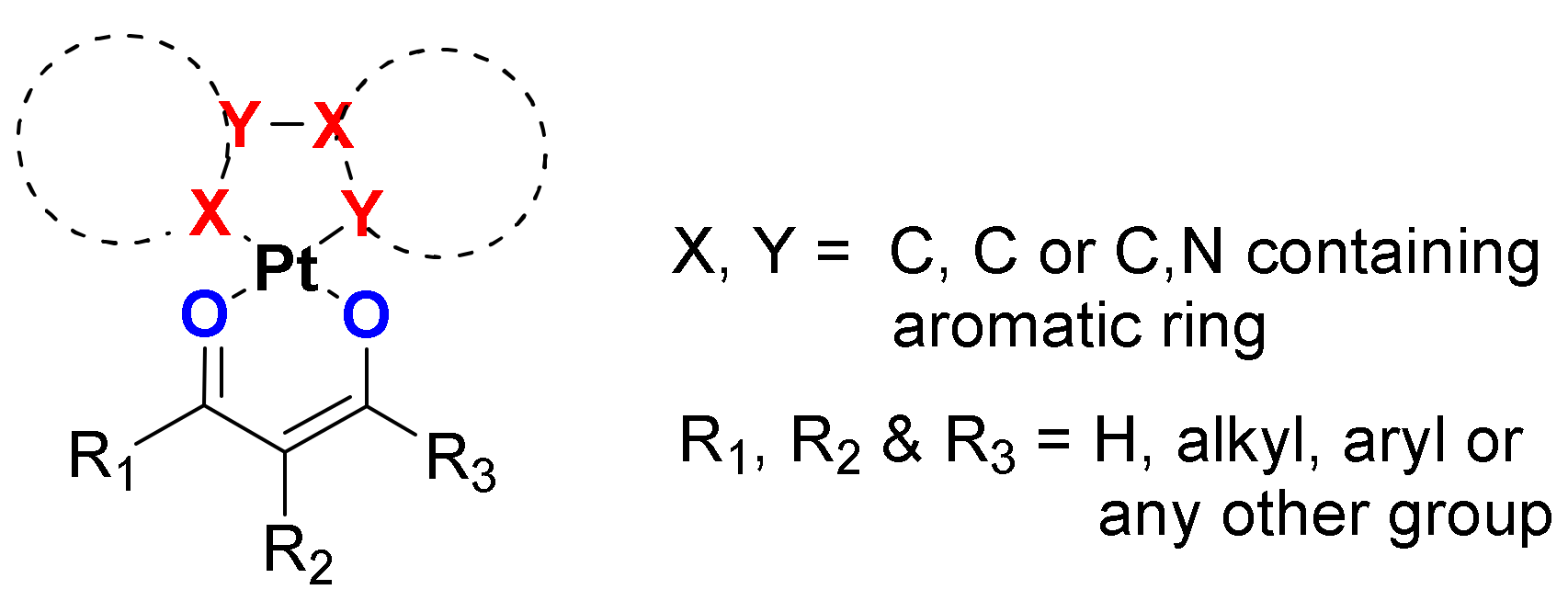

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, A.; El Moll, H.; Alenezi, K.M.; Khan, M.S.; Wong, W.-Y. Functional Materials Based on Cyclometalated Platinum(II) β-Diketonate Complexes: A Review of Structure–Property Relationships and Applications. Materials 2021, 14, 4236. https://doi.org/10.3390/ma14154236
Haque A, El Moll H, Alenezi KM, Khan MS, Wong W-Y. Functional Materials Based on Cyclometalated Platinum(II) β-Diketonate Complexes: A Review of Structure–Property Relationships and Applications. Materials. 2021; 14(15):4236. https://doi.org/10.3390/ma14154236
Chicago/Turabian StyleHaque, Ashanul, Hani El Moll, Khalaf M. Alenezi, Muhammad S. Khan, and Wai-Yeung Wong. 2021. "Functional Materials Based on Cyclometalated Platinum(II) β-Diketonate Complexes: A Review of Structure–Property Relationships and Applications" Materials 14, no. 15: 4236. https://doi.org/10.3390/ma14154236
APA StyleHaque, A., El Moll, H., Alenezi, K. M., Khan, M. S., & Wong, W.-Y. (2021). Functional Materials Based on Cyclometalated Platinum(II) β-Diketonate Complexes: A Review of Structure–Property Relationships and Applications. Materials, 14(15), 4236. https://doi.org/10.3390/ma14154236








