Surface Adsorption of the Cancer Biomarker Lysophosphatidic Acid in Serum Studied by Acoustic Wave Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
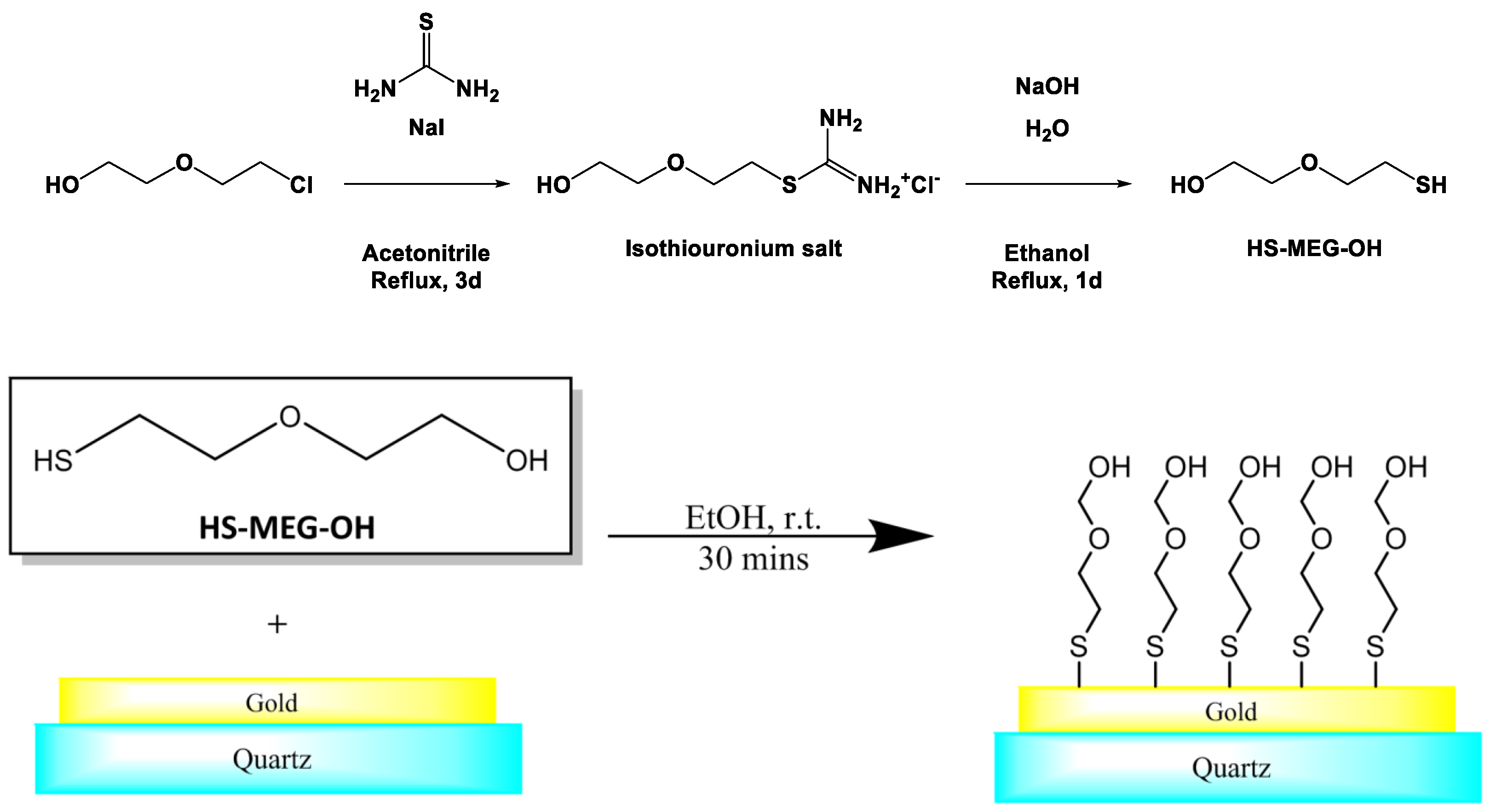
2.2. Surface Modification of TSM Gold Electrodes
2.3. TSM Operation and Measurements
3. Results
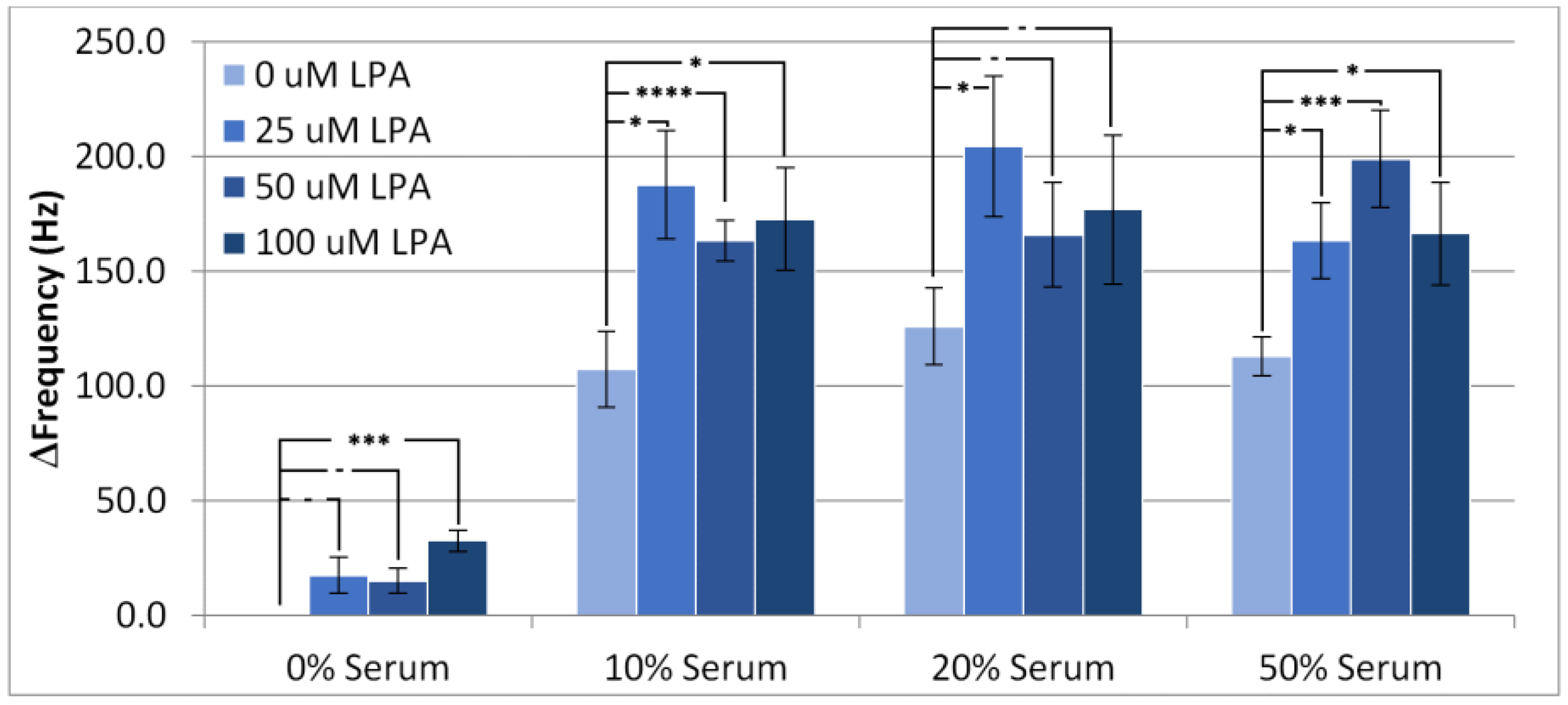
3.1. Resonant Frequency Responses
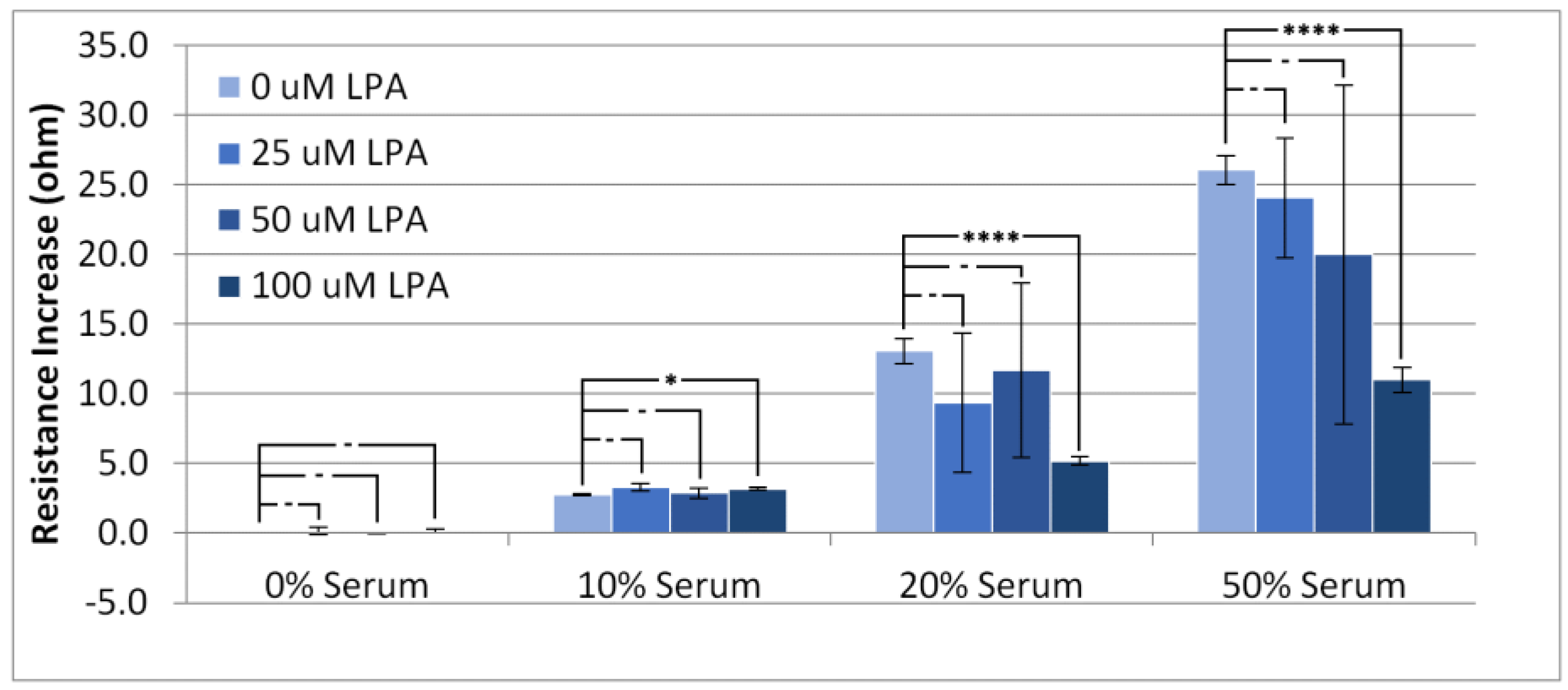
3.2. Responses in Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Sullivan, C.K.; Guilbault, G.G. Commercial quartz crystal microbalances—Theory and applications. Biosens. Bioelectron. 1999, 14, 663–670. [Google Scholar] [CrossRef]
- Johannsmann, D. The Quartz Crystal Microbalance in Soft Matter Research: Fundamentals and Modelling (Soft and Biological Matter); Springer: New York, NY, USA, 2014. [Google Scholar]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Surface chemistry to minimize fouling from blood-based fluids. Chem. Soc. Rev. 2012, 41, 5599–5612. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Tripathi, S.; Ethiraj, K.R.; Chandrasekaran, N.; Mukherjee, A. Human serum albumin corona on functionalized gold nanorods modulates doxorubicin loading and release. New J. Chem. 2018, 42, 16555–16563. [Google Scholar] [CrossRef]
- Uehara, N.; Sonoda, N.; Haneishi, C. Specific turn-on near infrared fluorescence from non-fluorescent gold nanoclusters bearing sulfhydryl oligopeptides. Colloid. Surf. A Physicochem. Eng. Aspects. 2018, 538, 14–22. [Google Scholar] [CrossRef]
- Thompson, M.; Blaszykowski, C.; Sheikh, S.; Rodriguez-Emmenegger, C.; de los Santos, A. Biological Fluid-Surface Interactions in Detection and Medical Devices; Royal Society of Chemistry: Cambridge, UK, 2017; Chapter 5; pp. 184–265. [Google Scholar]
- Wang, Y.; Cui, M.; Jiao, M.; Luo, X. Antifouling and ultrasensitive biosensing interface based on self-assembled peptide and aptamer on macroporous gold for electrochemical detection of immunoglobulin E in serum. Analyt. Bioanalyt. Chem. 2018, 410, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chuang, T.L.; Chen, P.Z.; Lin, C.W.; Gu, F.X. Low-fouling characteristics of ultrathin zwitterionic cysteine SAMs. Langmuir 2018, 35, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Lee, W.K.; Park, J.H.; Lee, J.S.; Seo, J.H. One-pot synthesis of a zwitterionic small molecule bearing disulfide moiety for antibiofouling macro- and nanoscale gold surfaces. Langmuir 2018, 35, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, P.; Zhang, Z.; Yao, D. Selective response of dopamine on 3-thienylphosphonic acid modified gold electrode with high antifouling capability and long-term stability. Mat. Sci. Eng. C 2019, 94, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Sheng, J.C.C.; Blaszykowski, C.; Thompson, M. New oligoethylene glycol linkers for the surface modification of an ultra-high frequency acoustic wave biosensor. Chem. Sci. 2010, 1, 271–275. [Google Scholar] [CrossRef]
- Fedorov, K.; Blaszykowski, C.; Sheikh, S.; Reheman, A.; Romaschin, A.; Ni, H.; Thompson, M. Prevention of thrombogenesis from whole human blood on plastic polymer by ultrathin monoethylene glycol silane adlayer. Langmuir 2014, 30, 3217. [Google Scholar] [CrossRef]
- Fedorov, K.; Jankowski, A.; Sheikh, S.; Blaszykowski, C.; Reheman, A.; Romaschin, A.; Ni, H.; Thompson, M. Prevention of surface-induced thrombogenesis on poly(vinylchloride). J. Mater. Chem. B 2015, 3, 8623. [Google Scholar] [CrossRef]
- Fedorov, K.; Sheikh, S.; Romaschin, A.; Thompson, M. Enhanced long-term antithrombogenicity instigated by a covalently-attached surface modifier on biomedical polymers. Res. Prog. Mater. Spec. Issue Appl. Dev. Biomater. Med. 2020, 2. [Google Scholar] [CrossRef]
- De La Franier, B.; Asker, D.; van den Berg, D.; Hatton, B.; Thompson, M. Reduction of microbial adhesion on polyurethane by a sub-nanometer covalently-attached surface modifier. Colloids Surf. B Biointerfaces 2021, 200, 111579. [Google Scholar] [CrossRef]
- Sheikh, S.; Yang, D.Y.; Blaszykowski, C.; Thompson, M. Single ether group in a glycol-based ultra-thin layer prevents surface fouling from undiluted serum. Chem. Commun. 2012, 48, 1305–1307. [Google Scholar] [CrossRef]
- Yang, T.; De La Franier, B.; Thompson, M. Anti-Thrombogenicity Study of a Covalently-Attached Monolayer on Stent-Grade Stainless Steel. Materials 2021, 14, 2342. [Google Scholar] [CrossRef]
- Pawlowska, N.M.; Fritzsche, H.; Blaszykowski, C.; Sheikh, S.; Vezvaie, M.; Thompson, M. Probing the hydration of ultrathin antifouling organosilane adlayers using neutron reflectometry. Langmuir 2014, 30, 1199–1203. [Google Scholar] [CrossRef]
- Sheikh, S.; Blaszykowski, C.; Nolan, R.; Thompson, D.; Thompson, M. On the hydration of subnanometric antifouling organosilane adlayers: A molecular dynamics simulation. J. Colloid Interface Sci. 2015, 437, 197–204. [Google Scholar] [CrossRef]
- Avci, C.; Sheikh, S.; Blaszykowski, C.; Thompson, M. Critical role of surface hydration on the dynamics of serum adsorption studied with monoethylene glycol adlayers on gold. Chem. Commun. 2013, 49, 466–468. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, Z.; Wiper, D.W.; Wu, M.; Morton, R.E.; Elson, P.; Kennedy, A.W.; Belinson, J.; Markman, M.; Casey, G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA 1998, 280, 719–723. [Google Scholar] [CrossRef] [Green Version]
- Sedláková, I.; Vávrová, J.; Tošner, J.; Hanousek, L. Lysophosphatidic acid (LPA)—A prospective marker in ovarian cancer. Tumor Biol. 2011, 32, 311–316. [Google Scholar] [CrossRef]
- Sutphen, R.; Xu, Y.; Wilbanks, G.D.; Fiorica, J.; Grendys, E.C.; LaPolla, J.P.; Arango, H.; Hoffman, M.S.; Martino, M.; Wakeley, K.; et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1185–1191. [Google Scholar]
- Meleh, M.; Pozlep, B.; Mlakar, A.; Meden-Vrtovec, H.; Zupancic-Kralj, L. Determination of Serum lysophosphatidic acid as a potential biomarker for ovarian cancer. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 858, 287–291. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure-property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Gong, P.; Grainger, D.W. Non-fouling surfaces: A review of principles and applications for microarray capture assay designs. Methods Mol. Biol. 2007, 381, 59–92. [Google Scholar] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Franier, B.; Thompson, M. Surface Adsorption of the Cancer Biomarker Lysophosphatidic Acid in Serum Studied by Acoustic Wave Biosensor. Materials 2021, 14, 4158. https://doi.org/10.3390/ma14154158
De La Franier B, Thompson M. Surface Adsorption of the Cancer Biomarker Lysophosphatidic Acid in Serum Studied by Acoustic Wave Biosensor. Materials. 2021; 14(15):4158. https://doi.org/10.3390/ma14154158
Chicago/Turabian StyleDe La Franier, Brian, and Michael Thompson. 2021. "Surface Adsorption of the Cancer Biomarker Lysophosphatidic Acid in Serum Studied by Acoustic Wave Biosensor" Materials 14, no. 15: 4158. https://doi.org/10.3390/ma14154158
APA StyleDe La Franier, B., & Thompson, M. (2021). Surface Adsorption of the Cancer Biomarker Lysophosphatidic Acid in Serum Studied by Acoustic Wave Biosensor. Materials, 14(15), 4158. https://doi.org/10.3390/ma14154158






