Influence of Laser Treatment on the Corrosion Resistance of Cr3C2-25(Ni20Cr) Cermet Coating
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Vickers Hardness of Material
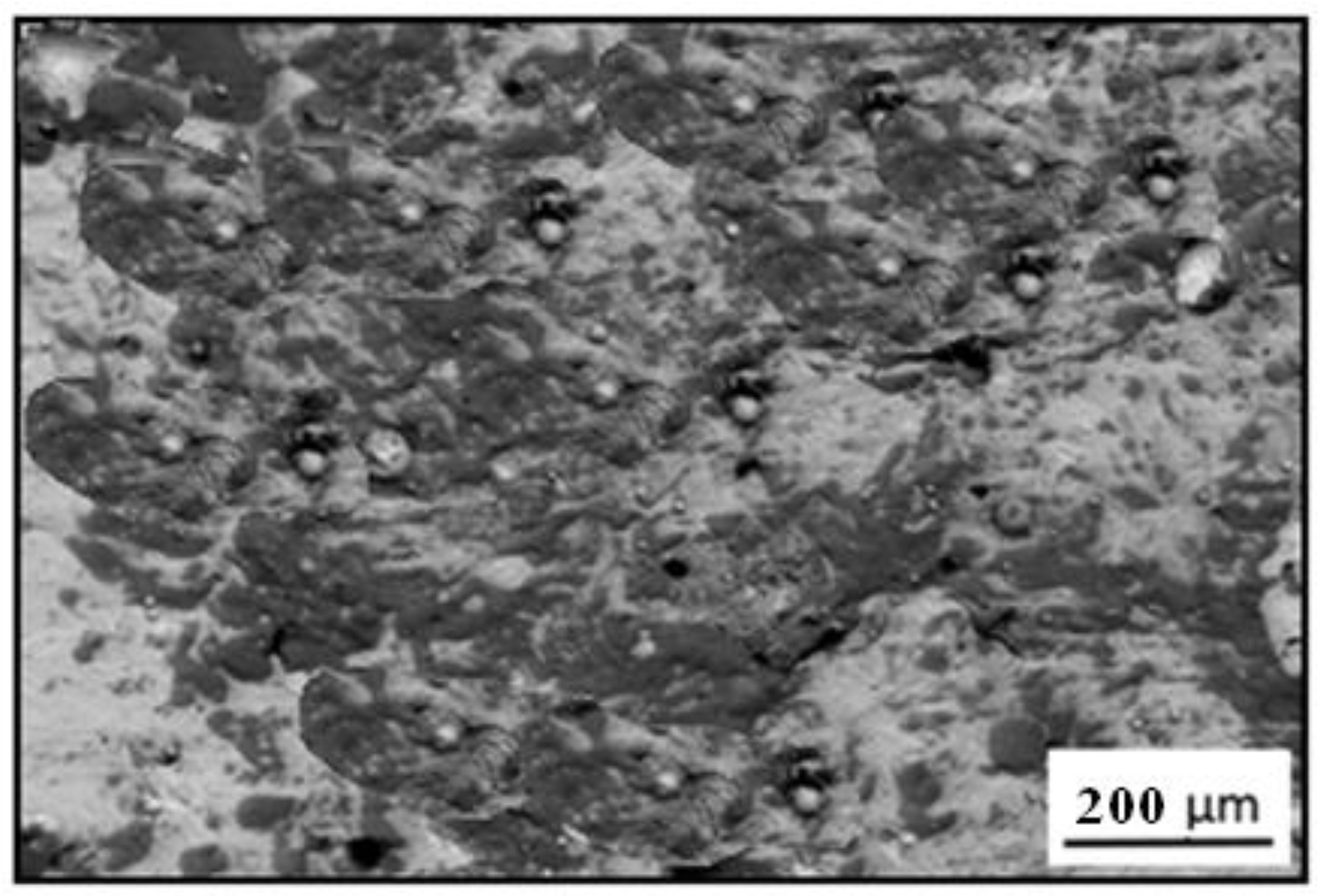
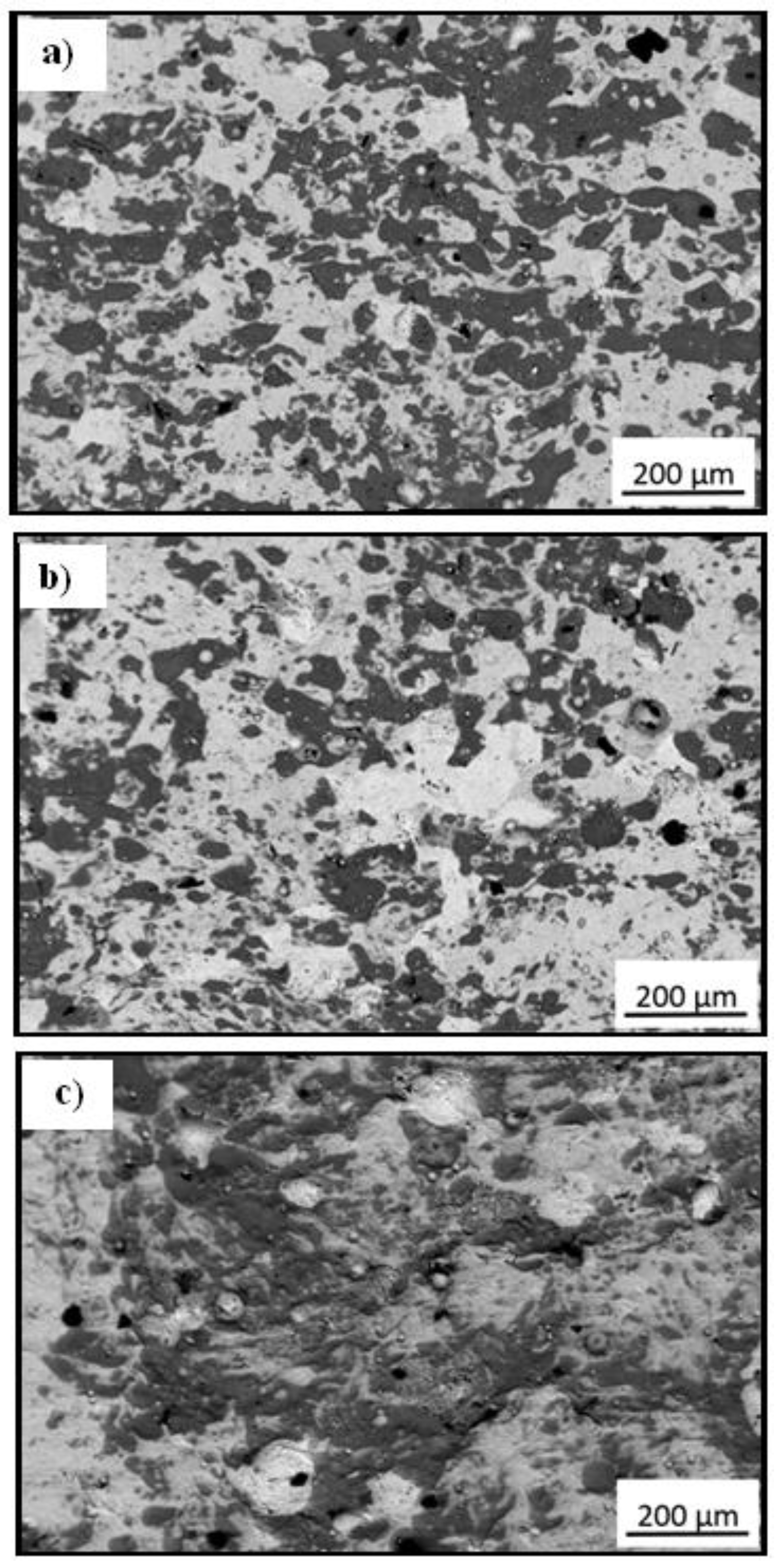
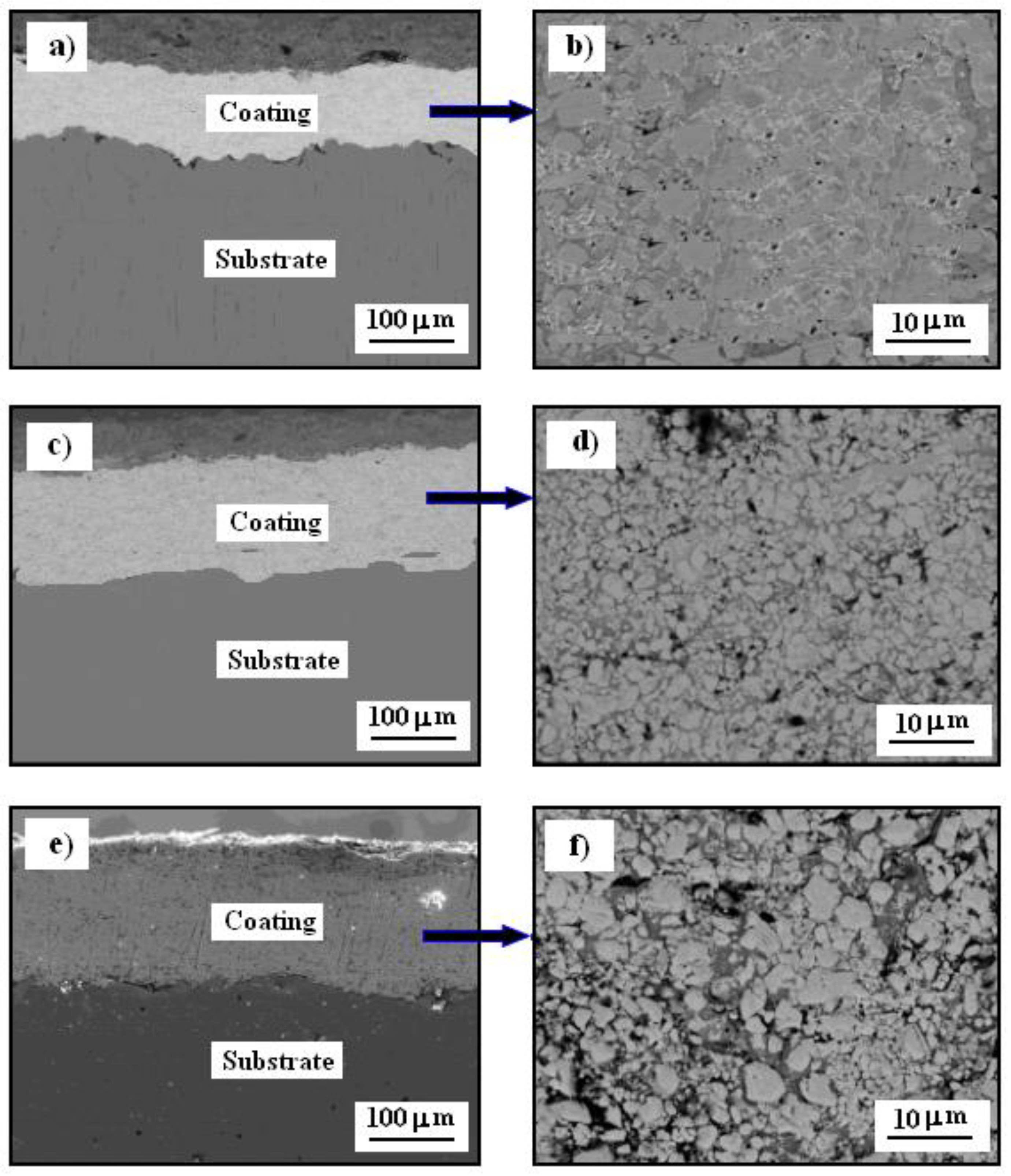
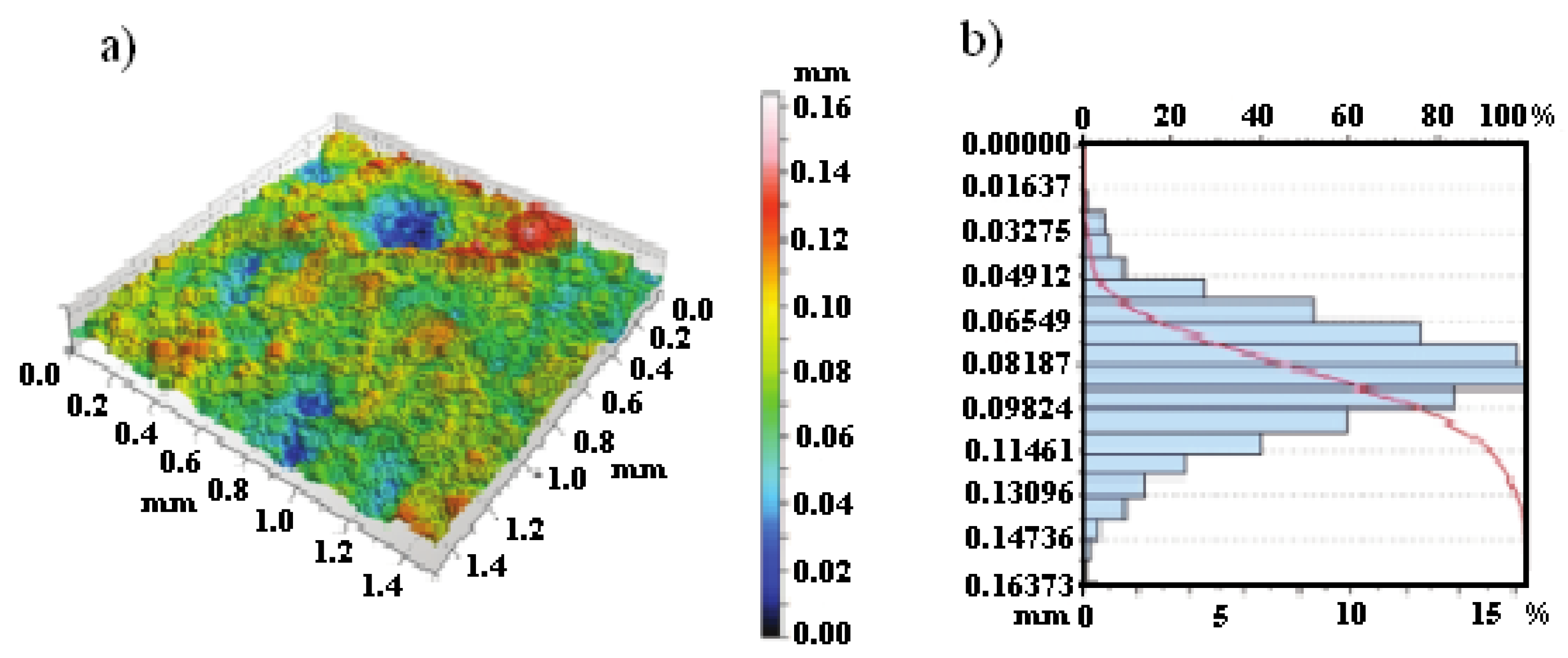
3.2. Scanning Electron Microscopy Images
3.3. Microstructure of Material
3.4. Corrosion Test
3.4.1. Open-Circuit Potential Measurements
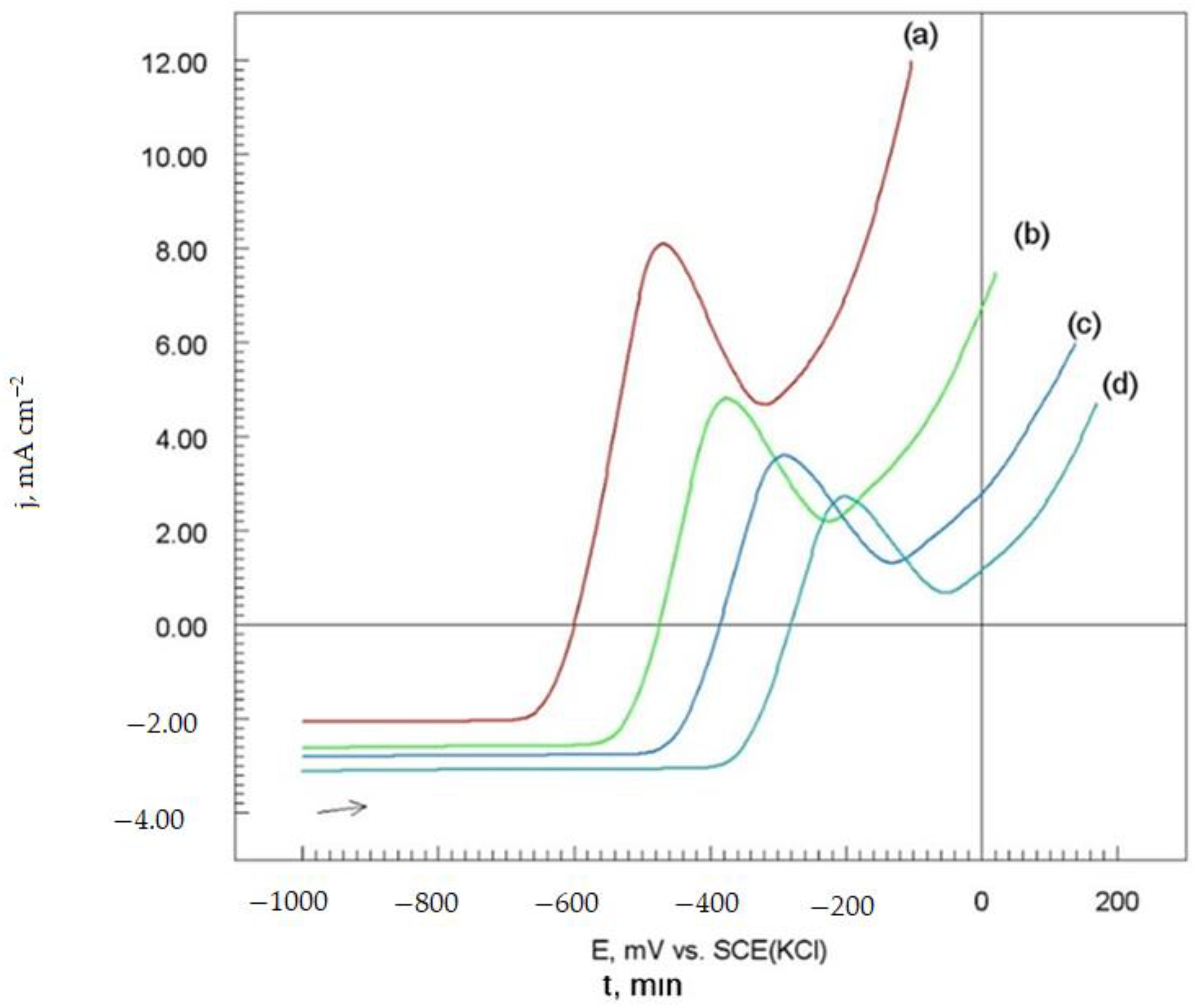
3.4.2. Potentiodynamic Polarization Measurements
3.4.3. Corrosion Electrochemical Parameters
3.4.4. Polarization Resistance and Corrosion Rate
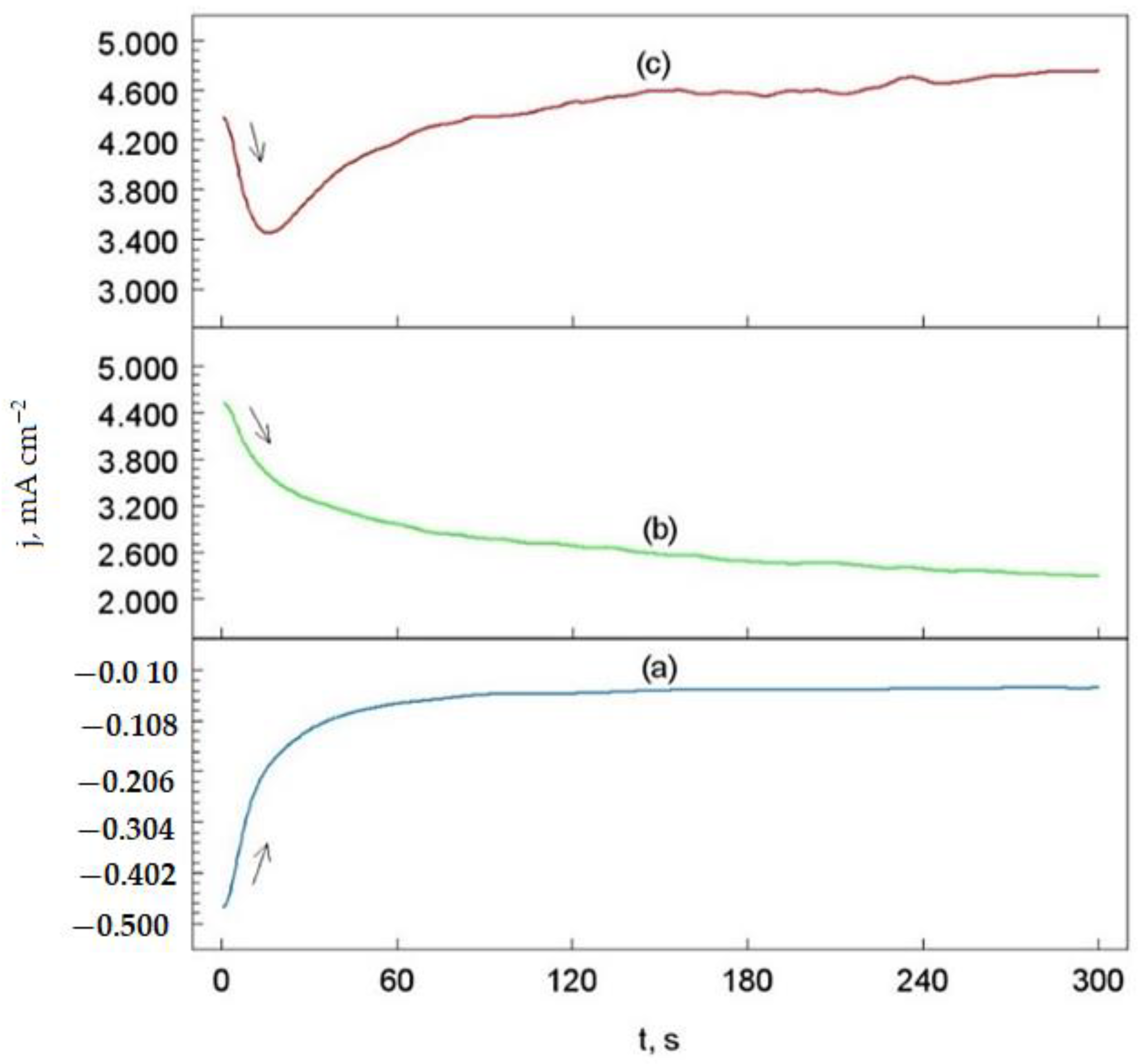
3.5. Chronoamperometric Measurements
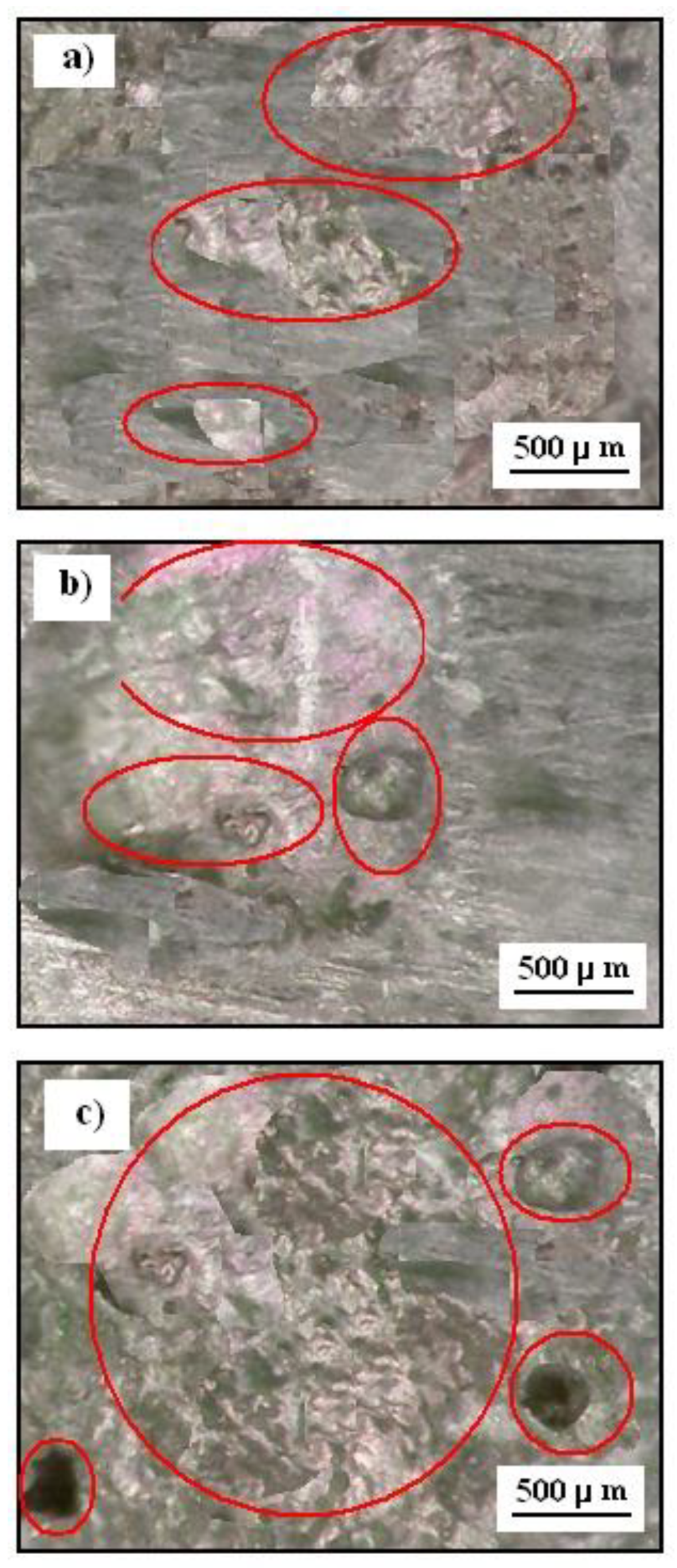
3.6. Photographic Images after Corrosion Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karthikeyan, J. Cold spray technology. Adv. Mater. Process. 2005, 163, 33–35. [Google Scholar]
- Scendo, M.; Zorawski, W.; Goral, A. Influence of nickel powders on corrosion resistance of cold sprayed coatings on Al7075 substrate. Metals 2019, 9, 890. [Google Scholar] [CrossRef] [Green Version]
- Grujicic, M.; Saylor, J.R.; Beasely, D.E.; De-Rosset, W.S.; Helfritch, D. Computational analysis of the interfacial bonding between feed-powder particles and the substrate in the cold gas dynamic-spray process. Appl. Surf. Sci. 2003, 219, 211–227. [Google Scholar] [CrossRef]
- Sevillano, F.; Poza, P.; Munez, C.J.; Vezzu, S.; Rech, S.; Trentin, A. Cold-sprayed Ni-Al2O3 coatings for applications in power generation industry. J. Therm. Spray Techn. 2013, 22, 772–782. [Google Scholar] [CrossRef]
- Luo, X.-T.; Li, Y.-J.; Li, C.-J. A comparison of cold spray deposition behavior between gas atomized and dendritic porous electrolytic Ni powders under the same spray conditions. Mater. Lett. 2016, 163, 58–60. [Google Scholar] [CrossRef]
- He, J.; Schoenung, J.M. Nanostructured coatings. Mater. Sci. Eng. 2002, A336, 274–319. [Google Scholar] [CrossRef]
- Goral, A.; Zorawski, W.; Makrenek, M. The Effect of the stand of distance on the microstructure and mechanical properties of cold sprayed Cr3C2-25(Ni20Cr) coating. Surf. Coat. Technol. 2019, 361, 9–18. [Google Scholar] [CrossRef]
- Singh, H.; Sidhu, T.S.; Karthikeyan, J.; Kalsi, S.B.S. Development and characterization of Cr3C2-NiCr coated super alloy by novel cold spray process. Mater. Manuf. Process. 2015, 31, 1476–1482. [Google Scholar] [CrossRef]
- Wolfe, D.E.; Eden, T.J.; Potter, J.K.; Jaroh, A.P. Investigation and characterization of Cr3C2-based wear-resistant coatings applied by the cold spray process. J. Therm. Spray Techn. 2006, 15, 400–412. [Google Scholar] [CrossRef]
- Stein, K.J.; Schorr, B.S.; Mader, A.R. Erosion of thermal spray MCr-CrC cermet coatings. Wear 1999, 224, 153–159. [Google Scholar] [CrossRef]
- Sidhu, T.S.; Prakash, S.; Agrawal, R.D. Characterizations of HVOF sprayed NiCrBSi coatings on Ni- and Fe-based superalloys and evaluation of cyclic oxidation behaviour of some Ni-based superalloys in molten salt environment. Thin Solid Film 2006, 515, 95–105. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Prakash, S. Studies on the behaviour of satellite-6 as plasma sprayed and laser remelted coatings in molten salt environment at 900 °C under cyclic conditions. J. Mater. Process. Technol. 2006, 172, 52–63. [Google Scholar] [CrossRef]
- Souza, R.C.; Voorwald, H.J.C.; Cioffi, M.O.H. Fatigue strength of HVOF sprayed Cr3C2-25CrNi and WC-10Ni on AISI 4340 steel. Surf. Coat. Technol. 2008, 203, 191–198. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Ferna’ndez, J.; Delgado, J.; Benedetti, A.V.; Climent, F. Effects of thickness coating on the electrochemical behaviour of thermal spray Cr3C2-NiCr coatings. Surf. Coat. Technol. 2002, 153, 107–113. [Google Scholar] [CrossRef]
- Matthews, S.; Hyland, M.; James, B. Microhardness variation in relation to carbide development in heat treated Cr3C2-NiCr thermal spray coatings. Acta Mater. 2003, 51, 4267–4277. [Google Scholar] [CrossRef]
- Bolelli, G.; Cannillo, V.; Lusvarghi, L.; Montorsi, M.; Mantini, F.P.; Barletta, M. Microstructural and tribological comparison of HVOF-sprayed and post-treated M-Mo-Cr-Si (M = Co, Ni) alloy coatings. Wear 2007, 263, 1397–1416. [Google Scholar] [CrossRef] [Green Version]
- Verdon, C.; Karimi, A.; Martin, J.-L. A study of high velocity oxy-fuel thermally sprayed tungsten carbide based coatings. Microstructures. Mater. Sci. Eng. A 1998, 246, 11–24. [Google Scholar] [CrossRef]
- Toma, D.; Brandl, W.; Marginean, G. Wear and corrosion behaviour of thermally sprayed cermet coatings. Surf. Coat. Technol. 2001, 138, 149–158. [Google Scholar] [CrossRef]
- Sundararajan, G.; Sudharshan, P.P.; Jyothirmayi, A.; Gundarkaram, R.C. The influence of heat treatment on the micro structural, mechanical and corrosion behaviour of cold sprayed SS 316L coatings. J. Mater. Sci. 2009, 44, 2320–2326. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Cho, T.-Y.; Yoon, J.-H.; Fang, W.; Song, K.-O.; Li, M.-X.; Joo, Y.-K.; Lee, C.G. Characterization of microstructure and surface properties of hybrid coatings of WC-CoCr prepared by laser heat treatment and high velocity oxygen fuel spraying. Mater. Charact. 2008, 59, 1412–1418. [Google Scholar] [CrossRef]
- Scendo, M.; Trela, J.; Radek, N. Influence of laser power on the corrosive resistance of WC-Cu coating. Surf. Coat. Technol. 2014, 259, 401–407. [Google Scholar] [CrossRef]
- Scendo, M.; Spadlo, S.; Staszewska-Samson, K.; Mlynarczyk, P. Influence of heat treatment on the corrosion resistance of aluminum-copper coating. Metals 2020, 10, 966. [Google Scholar] [CrossRef]
- Scendo, M.; Staszewska-Samson, K. Effect of surface modification on corrosion resistance of uncoated and DLC coated stainless steel surface. J. Mater. Eng. Perform. 2017, 26, 3946–3953. [Google Scholar] [CrossRef]
- Otmianowski, T.; Antoszewski, B.; Żórawski, W. Local laser treatment of tribological plasma sprayed coatings. In Proceedings of the15th International Thermal Spray Conference, Nice, France, 25–29 May 1998; pp. 1333–1336. [Google Scholar]
- Matthews, S.; James, B.; Hyland, M. Erosion of oxide scales formed on Cr3C2-NiCr thermal spray coatings. Corros. Sci. 2008, 50, 3087–3094. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, L.; Gan, F. High temperature naphthenic acid corrosion of steel in high TAN refining media. Anti-Corros. Methods Mater. 2008, 55, 257–263. [Google Scholar]
- Quej-Ake, L.M.; Contreras, A.; Aburto, J. The effect of non-ionic surfactant on the internal corrosion for X52 steel in extra-heavy crude oil-in-water emulsions. Anti-Corros. Methods Mater. 2018, 65, 234–248. [Google Scholar] [CrossRef]
- Yang, G.; Song, W.; Wang, F.; Ma, Y.; Hao, Y. Corrosion behavior of 20# steel in aqueous CO2 solution under stratified gas-liquid two-phase flow condition. Anti-Corros. Methods Mater. 2019, 66, 11–18. [Google Scholar]





| Parameter | Value |
|---|---|
| Power, kW | 6 |
| Protective gas | Argon |
| Laser spot speed, mm/min | 600, 800, 1000 |
| Spot size, mm | 10 × 1 |
| Laser Spot Speed mm/min | HV10 |
|---|---|
| Without laser remelting | 326 ± 4 |
| 1000 | 349 ± 2 |
| 800 | 361 ± 3 |
| 600 | 384 ± 3 |
| Laser Spot Speed mm/min | Ecorr mV vs. SCE(KCl) | −bc | ba | jcorr mA/cm2 |
|---|---|---|---|---|
| mV/dec | ||||
| Without laser remelting | −598 | 170 | 130 | 1.80 |
| 1000 | −472 | 130 | 90 | 0.90 |
| 800 | −385 | 90 | 80 | 0.60 |
| 600 | −279 | 70 | 60 | 0.40 |
| Laser Spot Speed mm/min | Rp mΩ cm2 |
|---|---|
| Without laser remelting | 17.8 |
| 1000 | 25.6 |
| 800 | 30.6 |
| 600 | 35.1 |
| Laser Spot Speed mm/min | CR mm/year |
|---|---|
| Without laser remelting | 2.08 |
| 1000 | 1.04 |
| 800 | 0.70 |
| 600 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scendo, M.; Zorawski, W.; Staszewska-Samson, K.; Goral, A. Influence of Laser Treatment on the Corrosion Resistance of Cr3C2-25(Ni20Cr) Cermet Coating. Materials 2021, 14, 4078. https://doi.org/10.3390/ma14154078
Scendo M, Zorawski W, Staszewska-Samson K, Goral A. Influence of Laser Treatment on the Corrosion Resistance of Cr3C2-25(Ni20Cr) Cermet Coating. Materials. 2021; 14(15):4078. https://doi.org/10.3390/ma14154078
Chicago/Turabian StyleScendo, Mieczyslaw, Wojciech Zorawski, Katarzyna Staszewska-Samson, and Anna Goral. 2021. "Influence of Laser Treatment on the Corrosion Resistance of Cr3C2-25(Ni20Cr) Cermet Coating" Materials 14, no. 15: 4078. https://doi.org/10.3390/ma14154078
APA StyleScendo, M., Zorawski, W., Staszewska-Samson, K., & Goral, A. (2021). Influence of Laser Treatment on the Corrosion Resistance of Cr3C2-25(Ni20Cr) Cermet Coating. Materials, 14(15), 4078. https://doi.org/10.3390/ma14154078







