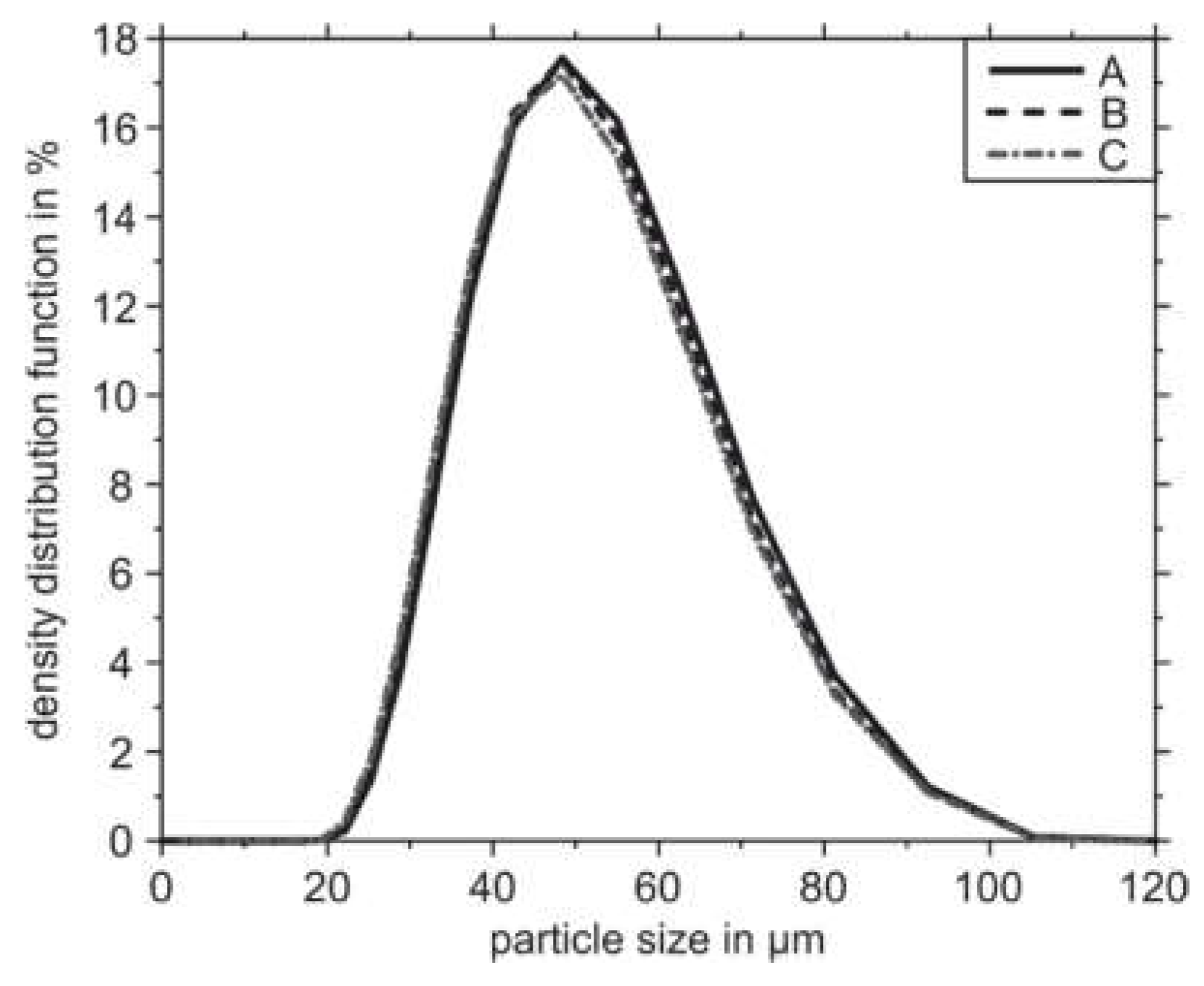
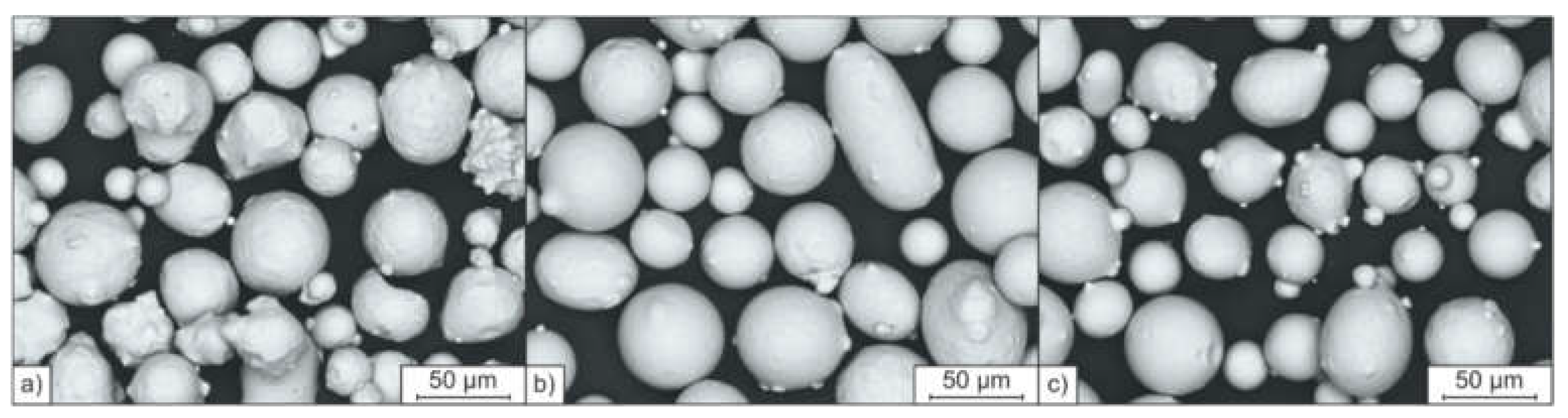
3.2. PBF-LB/M Densification and Microstructures
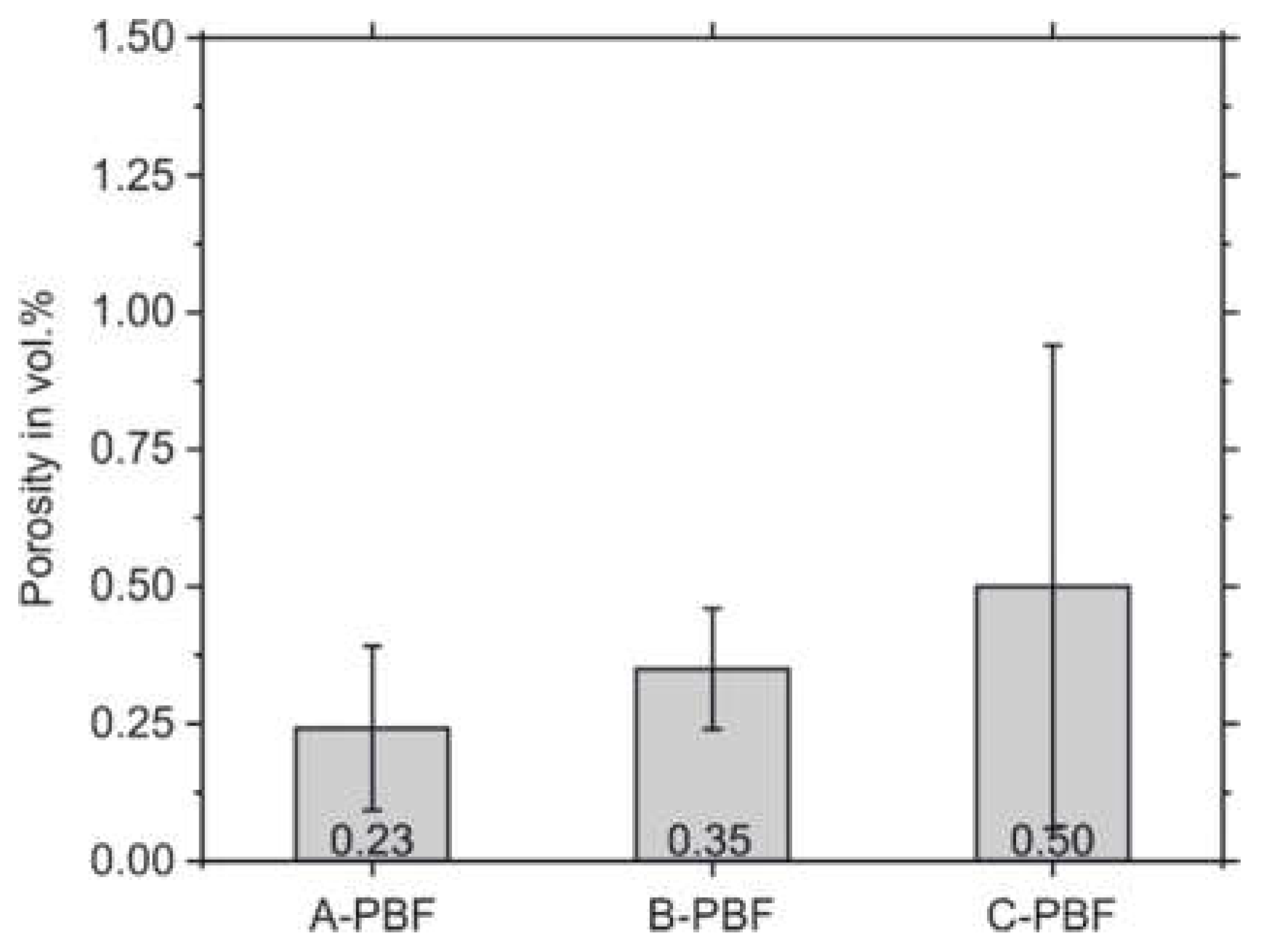
After PBF-LB/M-processing of the three starting powders, the remaining porosity of the cuboid specimens was determined by quantitative image analysis. The results are presented in
Figure 5. By applying the standard PBF-LB/M parameter set recommended by the system manufacturer (
Table 3), the samples, A-PBF, show the lowest porosity of 0.24% compared to the samples B-PBF and C-PBF. The samples B-PBF and C-PBF, which possess chemical compositions that are set at the boundaries of the allowed Cr and Ni equivalents (Cr
eq and Ni
eq), respectively, show higher porosities of 0.35% and 0.50%. The densities after PBF-LB/M cannot solely be related to the flow properties of the powders that are presented above. The evaluated powder properties seem to be less influencing the microstructure formation during the PBF-LB/M densification process. Thus, the different chemical compositions preferably determine the microstructure formation.
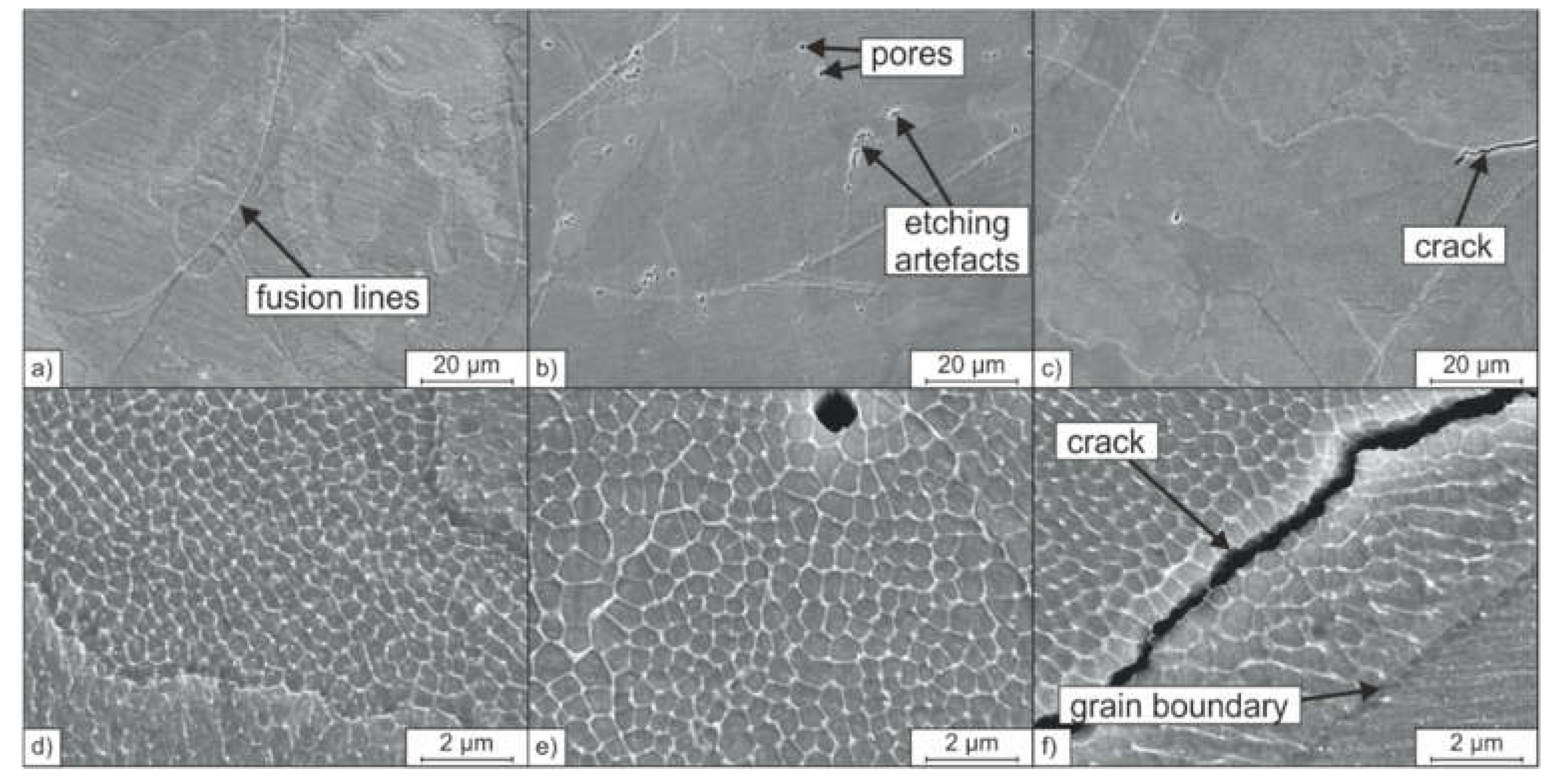
Figure 6 shows the associated microstructures of the PBF-LB/M-densified samples. All samples, A-PBF, B-PBF, and C-PBF, show a hierarchical microstructure as it was also reported for PBF-LB/-manufactured 316L steel in previous studies [
8,
26,
27]. Fusion lines resembling the laser-generated melt pools and large-angle grain boundaries can be observed on the mesoscale. The grains grow epitaxially in the direction of the heat gradient during the layer-wise built-up. Thereby, the crystallographic orientation of the mother grains is passed on to newly solidifying grains at the fusion lines [
27]. At higher magnifications, a fine cellular substructure inside these grains or colonies can be observed (see
Figure 6d–f). Krakhmalev et al. [
27,
28] showed that the cells inside the respective colonies share a continuous crystallographic plane. Therefore, the borders between adjacent cells should not be interpreted as additional large-angle grain boundaries. Still, the borders between the cells are characterized by a high dislocation density, as transmission electron microscopy investigations made by Voisin et al. and others showed [
8,
27,
29]. The formation of the cellular substructure is based on the rapid solidification rate resulting from the typical small melt pools generated during the PBF-LB/M process. This is also associated with high thermal gradients and thermal stresses [
8,
27]. In addition, the areas between the cells are prone to microsegregation of the alloying elements such as Cr and Mo [
8,
27].
Although the obtained microstructures of the samples A-PBF, B-PBF, and C-PBF appear to be similar, differences can be found in the formation of volumetric defects. The defects found in all the samples are primarily spherical pores and few irregularly shaped binding defects. Binding defects can be traced back to voids in the applicated powder bed and insufficient local wetting owing to insufficient energy input [
7,
30]. Spherical pores can be related to evaporation or outgassing of chemical elements due to excessive local energy inputs [
31]. The element N can recombine to gaseous N
2 and thus forms spherical gas pores during PBF-LB/M [
32]. This behavior can be one reason for the higher porosities of B-PBF and C-PBF compared to the material A-PBF. Powders B and C both possess higher N contents than material A (compare
Table 2).
Furthermore, unlike the samples A-PBF and B-PBF, the sample C-PBF shows cracks that reduce the relative density (
Figure 6). The cracks run intergranular through the cellular substructure, as can be seen in
Figure 6f. Cracks in austenitic steels are often associated with hot cracks [
33]. However, the cracks found cannot be interpreted as hot cracks, as these are formed on the large-angle grain boundaries owing to low melting phases (solidification cracks) [
34]. The intergranular crack morphology found in sample C-PBF is a result of solid-state cracking. Moreover, the contents of the hot crack promoting elements S and P, which form low melting phases, are low in starting powder C (0.01 mass% each, compare
Table 2).
Solid-state cracking in austenitic steels is reported in welding technology [
35]. A solid-state crack type known from austenitic and Ni-base welds is the so-called ductility dip crack (DDC). Theories for the DDC formation are provided by Lippold et al. [
36]. DDCs occur in a temperature range between 60% and 90% of the solidus temperature (T
SOL; approximately 800° to 1150 °C in austenitic steels). The crack mechanism is a namesake dip in the material’s ductility in this temperature range. The ductility is reduced due to grain growth and sliding below the recrystallization temperature, leading to the localization of tensile stresses at the grain boundaries [
37,
38,
39]. If these stresses exceed the material’s strength at the grain boundaries, crack formation at the grain boundaries takes place. Owing to multiple heating during subsequent weld passes in multilayer welds or additive manufacturing processes such as PBF-LB/M, the crystallographic grain boundaries move away from the initial solidification-related grain boundaries. For this reason, DDCs typically occur in a mostly straight line next to the original grain boundary at a migrated crystallographic grain boundary. This behavior can also be observed in
Figure 6f for the sample C-PBF. The crack runs parallel to the solidification-related grain boundary at a distance of approximately 3 µm.
It is assumed that the cracks formed in sample C-PBF are DDC. The other samples, A-PBF and B-PBF, do not show cracks while the starting powder properties are comparable, and the PBF-LB/M process parameters stayed untouched. Therefore, the reason for the DDC formation must be ground in the specific chemical composition of powder C as this is the only significant difference between the used starting powders. Powder C possesses a higher Ni
eq compared to powders A and B (
Table 2). Therefore, the fcc austenite is stabilized at the cost of the stability of the bcc ferrite phase [
40].
Alloys that consist of only one phase (e. g., only austenite) in the temperature range of the ductility dip and do not form precipitates at the grain boundaries are highly susceptible for DDC. Such alloys suffer from increased grain growth and sliding, promoting stress localization at the grain boundaries [
37,
38,
39].
Additively manufactured 316L stainless steel is known for being fully austenitic in the PBF-LB/M processed condition [
41]. However, the solidification path and phase formation in the solid-state of 316L steel can also include the bcc phase ferrite [
42].
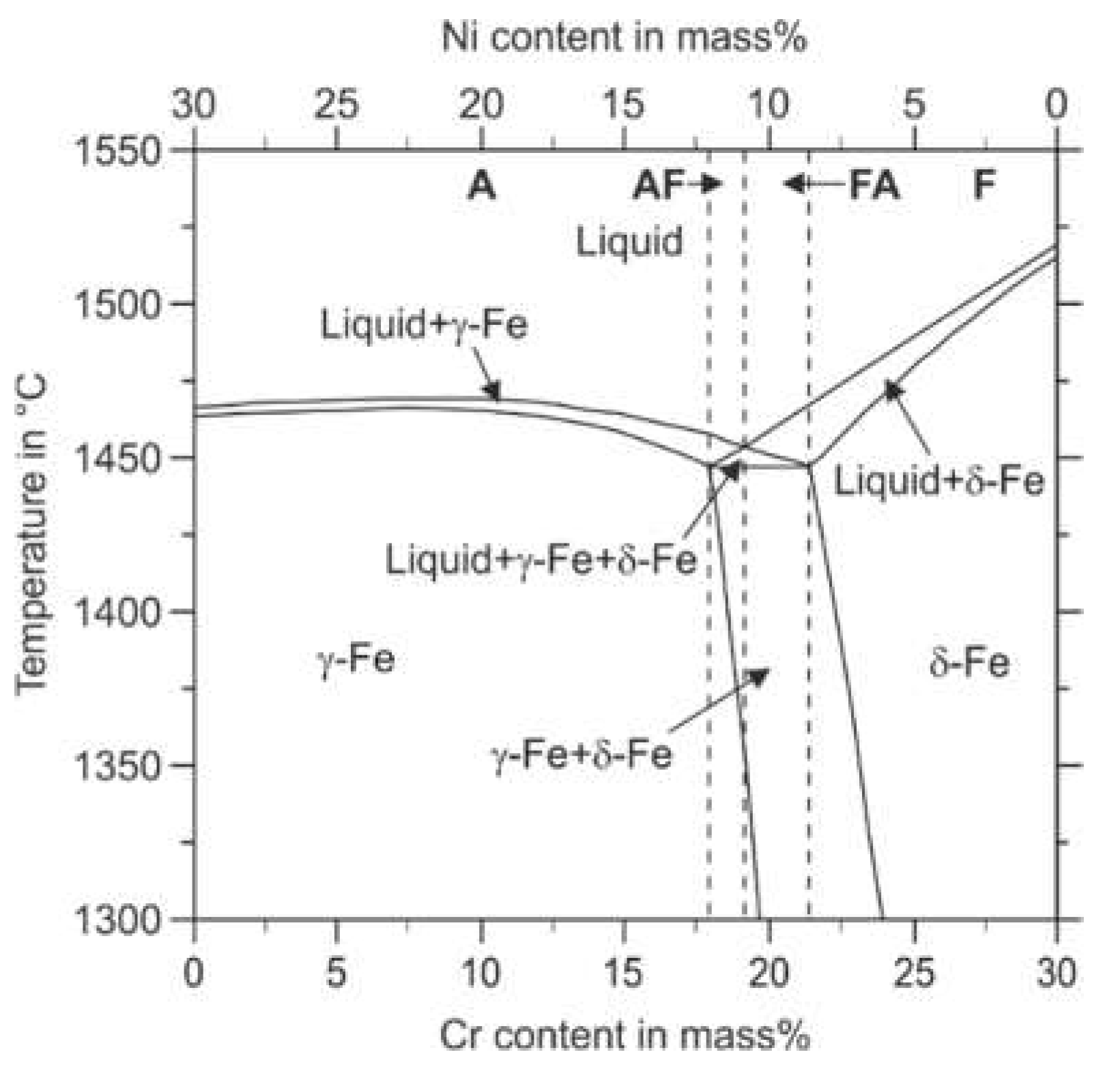
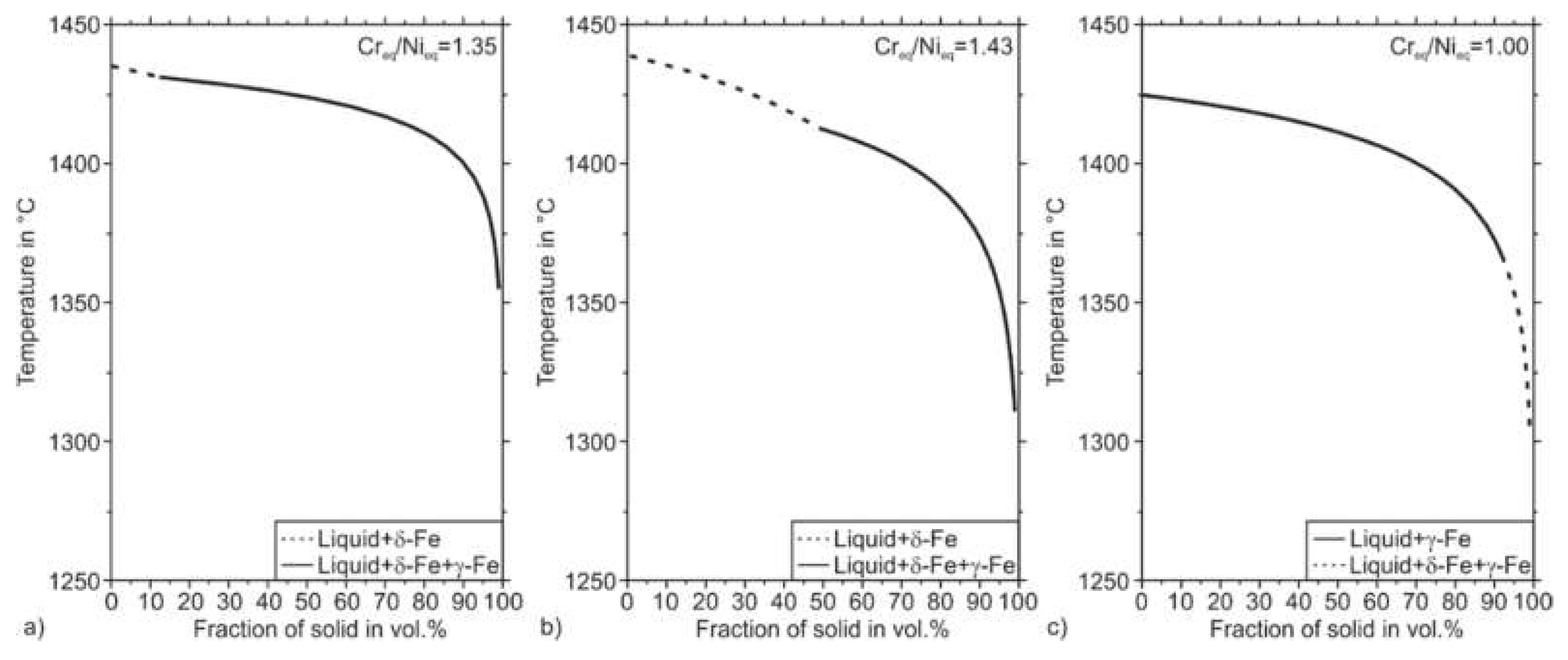
Figure 7 illustrates the thermodynamically stable phases in the Fe–Cr–Ni system concerning the Cr and the Ni content, respectively. Fe–Cr–Ni alloys can solidify in four different modes called A, AF, FA, and F. Mode A means primary austenitic solidification with no ferrite involved. According to the thermodynamic calculation, this mode occurs for lower Cr to Ni ratios. According to Faulkner et al., in welding processes, the fully austenitic solidification mode A is enabled for steels that possess a Cr
eq/Ni
eq ratio smaller than 1.3 [
43]. Higher Cr
eq/Ni
eq ratios result in primary austenitic solidification with crystallization of ferrite in the latter solidification path (AF-Mode). However, the ferrite stability decreases with decreasing temperature leading to a ferrite to austenite transformation in the solid-state during the cooling period. The intrinsic heat treatment during the deposition of subsequent material layers in the PBF-LB/M process can promote such phase transformations during the build-up of a component [
44]. The further increased Cr
eq/Ni
eq ratios enable the FA solidification mode. In the FA-mode, ferrite crystallizes primarily from the melt, followed by the austenite phase. However, due to decreasing stability of the phase ferrite with decreasing temperature, the FA-Mode can also result in a fully austenitic microstructure at room temperature (compare
Figure 7). A further increased Cr
eq/Ni
eq ratio leads to a fully ferritic solidification when the mode F is entered.
To estimate the obtained austenite and ferrite contents of the solidified material concerning the Cr
eq and Ni
eq after welding processes (fast non-equilibrium solidification), the diagrams of Schaeffler and DeLong were introduced [
40,
45].
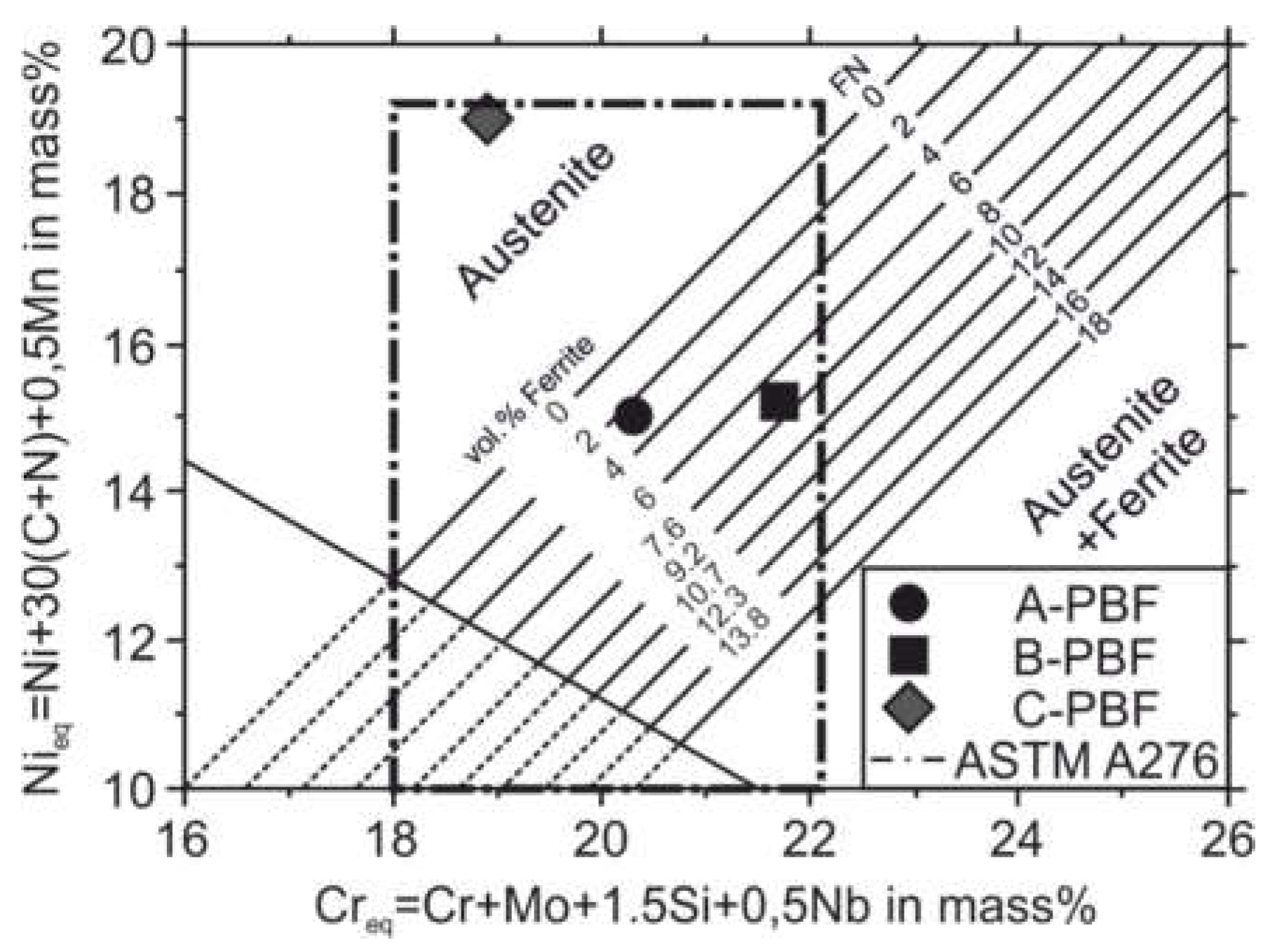
Figure 8 shows the positions in the DeLong diagram of the three investigated sample alloys A, B, and C within the compositional range of the AISI 316L steel. According to the DeLong diagram, the wide compositional range allowed for steel 316L by the ASTM A276 standard can promote fully austenitic and austenitic-ferritic microstructures. A fully austenitic microstructure is predicted for alloy C, while for the alloys A and B ferrite contents of approximately 2.5 vol.% and 6.5 vol.%, respectively, are predicted.
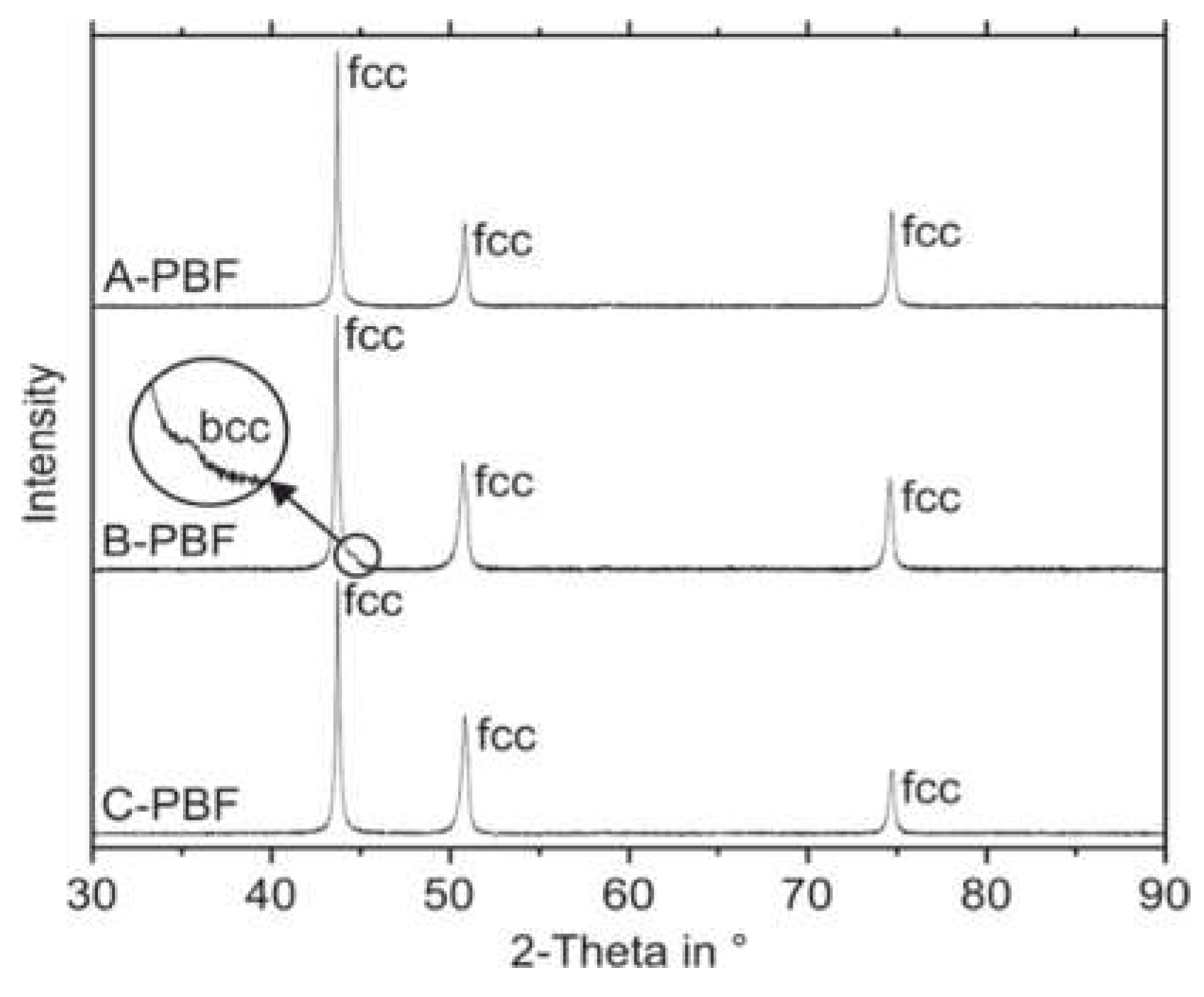
In contrast to the predictions according to the DeLong diagram, XRD measurements reveal a fully austenitic microstructure not only for the sample C-PBF but also for the sample A-PBF (
Figure 9). The XRD pattern of the sample B-PBF shows a small reflection of the bcc phase.
The patterns shown in
Figure 9 were measured at the ground top surface of the specimens. XRD measurements were also performed at the ground bottom surfaces of the samples to investigate a possible effect of the intrinsic heat treatment during the PBF-LB/M process on the ferrite contents. As these patterns appear similar to those obtained from the top surfaces, they are neglected in
Figure 9 for reasons of clarity. The actual ferrite contents of the samples were calculated from the XRD measurements by the Rietveld method for both the top and bottom surfaces. The results are given in
Table 5.
Additionally, magneto-inductive measurements of the ferrite contents were conducted using the Feritscope. The magneto-inductive measurements confirm the fully austenitic microstructure for the samples A-PBF and C-PBF. Furthermore, the Feritscope detects ferrite in the sample B-PBF, which is also in accordance with the results of the Rietveld analysis. The Rietveld analysis and the magneto-inductive measurements show increased ferrite contents at the top surface (0.5, respectively, 0.72 vol.%) compared to the bottom surface (0.18, respectively, 0.38 vol.%). These local differences in the ferrite content indicate that the intrinsic heat treatment during the repeated deposition of material layers promotes a transformation of primarily solidified ferrite to austenite in the underlying layers of the sample B-PBF.
The assumed transformation of the primarily solidified ferrite into austenite in the sample B-PBF that possesses a high Cr
eq/Ni
eq ratio during the further heat input also stands in accordance with an observation made in previous works regarding the rapid solidification of austenitic steels, such as in the PBF-LB/M process [
19,
20]. David et al. [
19] reported a shift from the mixed solidification modes (AF and FA) to the fully austenitic, respectively, fully ferritic solidification mode for higher solidification rates. High solidification rates counteract the compositional balancing by diffusion processes during solidification because these diffusion processes are dependent not only on temperature but also on time (kinetic). In general, the phase that crystallizes first is stabilized by rapid solidification. With the deposition of subsequent layers during the component build-up in PBF-LB/M, the rapid and almost diffusionless solidified material is heated. This heat can then enable diffusion processes and promote phase transformations so that more stable phases at lower temperatures are formed [
44,
46,
47]. This effect increases for earlier deposited layers as these are affected by more depositions of subsequent layers.
As shown so far, the rapid solidification in PBF-LB/M processing cannot be depicted by the DeLong diagram. However, a non-equilibrium and diffusionless solidification similar to the conditions during PBF-LB/M can be simulated by thermodynamic calculations after the approach by Scheil and Gulliver. The diffusionless solidification paths of the samples A-PBF, B-PBF, and C-PBF, calculated after the Scheil-Gulliver approach, are depicted in
Figure 10. The solidification simulations predict a primary solidification of bcc ferrite for the samples A-PBF and B-PBF, which possess Cr
eq/Ni
eq ratios of 1.35 and 1.43. For sample C-PBF containing a Cr
eq/Ni
eq ratio of 1.00, primary solidification of fcc austenite is predicted. The solidification simulations are in accordance with the results of Faulkner et al., who stated fully austenitic solidification of steels for Cr
eq/Ni
eq ratios smaller 1.3 [
43]. However, the microstructure of the PBF-LB/M solidified sample A-PBF is fully austenitic and does not include the bcc ferrite phase (compare
Table 5). The aforementioned intrinsic heat treatment can promote the absence of the phase ferrite in the sample A-PBF during the PBF-LB/M process, which allows the solidified ferrite to transform into austenite. However, as the top layers that were exposed to less or no intrinsic heat treatment are also free from the phase ferrite, it could be assumed that contrary to the Scheil–Gulliver simulation, the sample A-PBF already solidified fully austenitic. Kelly et al. also found a change from the FA solidification mode to the fully austenitic mode A instead of the mode F for rapid solidification and high undercooling of the melt [
48]. They related this behavior to a preferred crystallization of the fcc austenite owing to its formation kinetics.
It can be concluded that the microstructure formation of austenitic steels during PBF-LB/M is complex and, therefore, can hardly be predicted by the empirical approach of DeLong or by Scheil–Gulliver’s simulations. The alloys A and C with Cr
eq/Ni
eq ratios of 1.35, respectively, 1.00, showed a fully austenitic microstructure after PBF-LB/M densification. While the alloy B with a higher Cr
eq/Ni
eq ratio of 1.43 showed small amounts of ferrite (0.18–0.72 vol.%). In contrast to the fully austenitic sample A-PBF, the fully austenitic sample C-PBF with the lowest Cr
eq/Ni
eq ratio of 1.00 showed DDC in the as-built state. It is evident that Cr
eq/Ni
eq ratios as low as 1.00 should be avoided even if the susceptibility for solidification cracking (hot cracking) is eliminated by low P and S contents to prevent the crack formation at all (compare
Table 2).
3.3. Corrosion Resistance of the PBF-LB/M Densified Powders
In
Section 3.2, a connection between the microstructure formation and the microstructural defects depending on the different chemical compositions of the steel AISI 316L starting powders were pointed out. Now, these findings are to be linked with the corrosion behavior of the different variants represented in
Figure 11 and
Table 6, respectively. These show the current density/potential curves and the associated characteristic values of the materials under investigation. It can be stated that the results do not differ significantly concerning the detected characteristic potentials. Only slight differences can be seen in the passivation and passive current density. These values correlate with the anodic metal dissolution during the corrosion attack and therefore represent a direct indicator of the corrosion resistance of the respective material. The lowest metal dissolution and the highest corrosion resistance can be seen in the sample A-PBF, followed by B-PBF and C-PBF. The difference in the corrosion behavior of the three 316L variants could be attributed to the microstructural defects described in
Section 3.2. The higher the density of microstructural defects such as cracks and pores, the greater the specific surface area exposed to the corrosive attack. Consequently, higher passivation and passive current densities can be found for materials with lower relative density, such as the samples B-PBF and C-PBF in this study [
49,
50]. In addition, Otereo et al. [
51] showed that pitting and crevice corrosion phenomena can occur within pores, which can also have a negative impact on resistance to uniform surface corrosion. This is particularly favored by a deficient electrolyte exchange within the pore, which leads to the depletion of the oxygen necessary to maintain the passive layer. This effect could be even more pronounced in cracks such as those found in sample C-PBF because of the higher aspect ratio of a crack than a spherical pore, making electrolyte exchange even more difficult.
In addition to the defect density, the chemical composition is also a significant factor influencing the corrosion resistance of stainless steel. For instance, Yu et al. [
52] found that an increased Cr content leads to lower passivation and passive current densities due to the formation of a more stable passive layer.
Table 2 shows that the material C-PBF has the lowest Cr content of the steels examined. Accordingly, in addition to the higher defect density, the inferior corrosion resistance of C-PBF could be attributed to the lower Cr content.
It can be assumed that the effects of the higher defect density and the lower Cr content of the sample C-PBF and the associated worse corrosion behavior are superimposed. Therefore, it is not possible to specify which influence is responsible for the lower corrosion resistance of C-PBF exactly. However, it can be stated that the chemical composition of the steel processed by PBF-LB/M is directly related to the chemical properties. This is based on the one hand on chemical composition-dependent formation and stability of the passive layer, and on the other hand on the susceptibility, dictated by the chemical composition, to form microstructural defects such as solid-state or solidification cracks.
3.4. Mechanical Properties of the PBF-LB/M Densified Powders
The chemical composition and the microstructure are related to the composition and the processing route of a material and affect, not only the corrosion resistance but also the mechanical properties of components. To evaluate the mechanical properties of the three investigated 316L variants A, B, and C in the PBF-LB/M-processed condition, tensile and hardness testing were performed. Because the starting powder properties were comparable and the PBF-LB/M process parameters were kept constant for all samples produced, the differences in the mechanical behavior can be entirely related to the changes in the chemical composition and the associated changes in the microstructure formation.
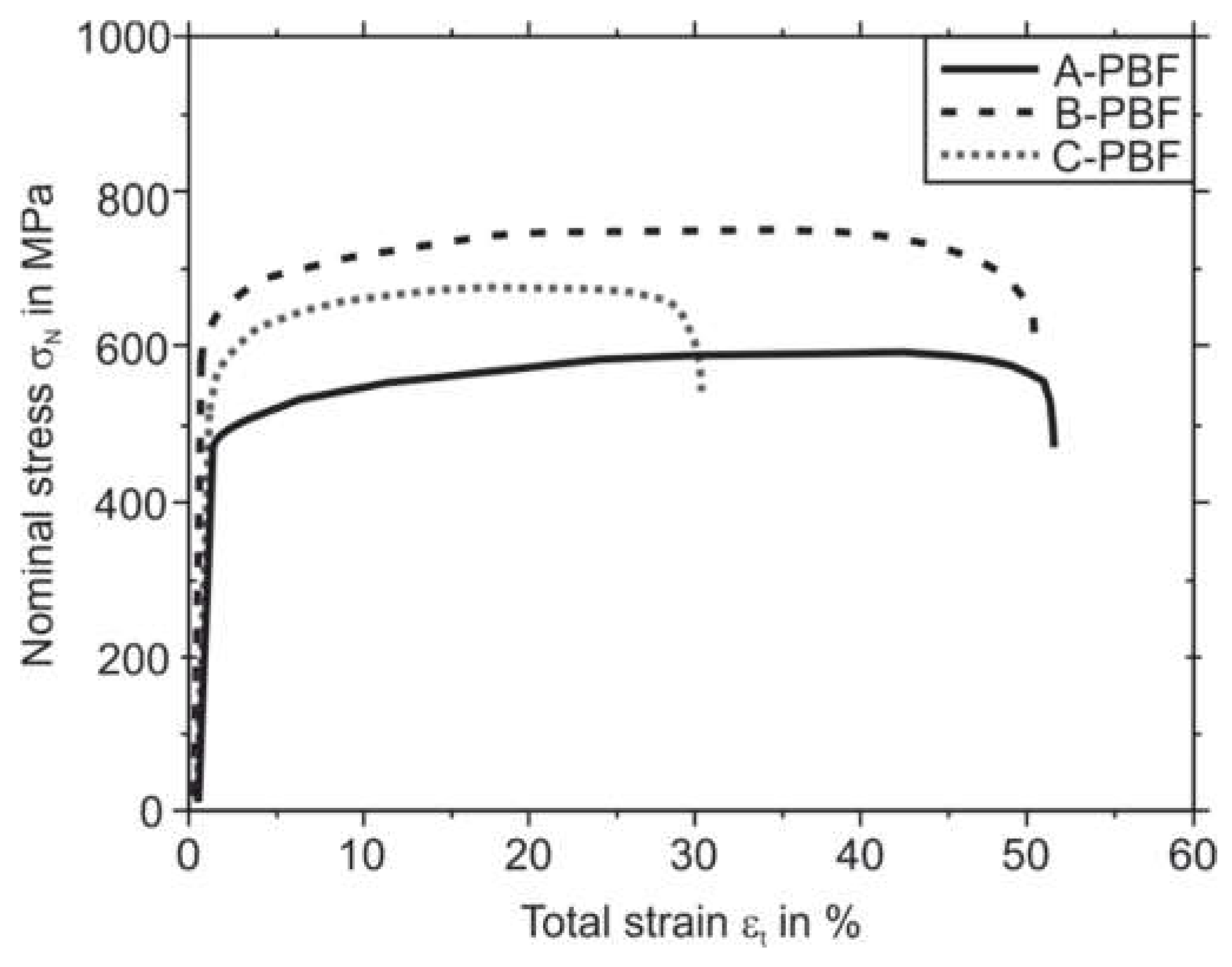
Figure 12 shows the results of the quasi-static tensile tests of the PBF-LB/M specimens. For every sample type, A-PBF, B-PBF, and C-PBF, the averaged curves of three tested samples are plotted. Sample B-PBF shows the highest strength with a yield strength R
p0.2 of 593 MPa and ultimate tensile strength R
m of 7522 MPa. The sample A-PBF possess the lowest yield strength R
p0.2 and tensile strength R
m of 465 MPa, respectively, 564. MPa. The samples A-PBF and B-PBF show almost identical elongations at fracture A
5 of 5.1% and 5.1%, respectively. Specimen A-PBF shows the lowest A
5 of 3.0%. The characteristic values obtained from the tensile test and the hardness tests are collected in
Table 7. The hardness of the PBF-LB/M specimens corresponds to the strength of the samples. Consequently, the sample B-PBF shows the relatively highest hardness of approximately 253 HV1, followed by the samples C-PBF (234 HV1) and A-PBF (184 HV1).
Considering the chemical compositions of the samples, which is given in
Table 2, the increased hardness and strength of the samples B-PBF and C-PBF compared to A-PBF can be ground in the increased contents of the elements C and N. These elements can be dissolved interstitially in the metal matrix and can therefore cause a strong solid-solution hardening effect [
53]. The strengthening effect of increased N contents in additively manufactured 316L stainless steel was also observed in a previous study by Boes et al. [
32]. The formation of nitrides was suppressed by the rapid solidification rate present in the PBF-LB/M process so that the increased N contents were dissolved only in the metal matrix [
32]. Boes et al. investigated nitrogen contents of 0.1 and 0.3 mass% and found a slight decrease in the elongation at fracture from 47.5% in a low nitrogen (0.03 mass%) 316L reference to 41.7% and 36.4%, respectively [
8,
32]. They attributed the loss in elongation at fracture in case of the high N content to an increase in porosity associated with the increased N contents. High N contents will promote the formation of gas pores due to the outgassing of recombined N
2 if the solubility in the laser-generated melt pool is exhausted. The N solubility decreases with increasing overheating of the melt pool [
54].
A decrease in the elongation at fracture can also be observed for C-PBF. The reason for this can also be given by an increased porosity (compare
Figure 5). However, this increase in porosity is not mainly linked to the outgassing of N
2 but also to the formation of solid-state cracks, namely the DDCs. However, it has to be mentioned that the increased Ni content of the starting material C can promote the outgassing of N
2 owing to a reduction in N solubility associated with increased Ni contents [
32,
54]. The main reason for the reduction in elongation at fracture for C-PBF is assumed to be the occurrence of the DDCs. These cracks provide a more pronounced notch effect than roundly shaped pores. Although increased contents of N and C increase the strength of the additively manufactured 316L steel by solid-solution hardening, these alloying elements also lead to an increase in the Ni
eq. According to the DeLong diagram, the elements N and C increase the Ni
eq 30 times stronger than Ni (see
Figure 8) [
40]. The strong increase in the Ni
eq then promotes the formation of the DDCs, which negatively affects the mechanical properties.