Evaluation of the Possibility of Using 1.4462 and 1.4501 Steel as a Construction Material for Apparatus Operating at an Increased Temperature and with Corrosive Factors
Abstract
:1. Introduction
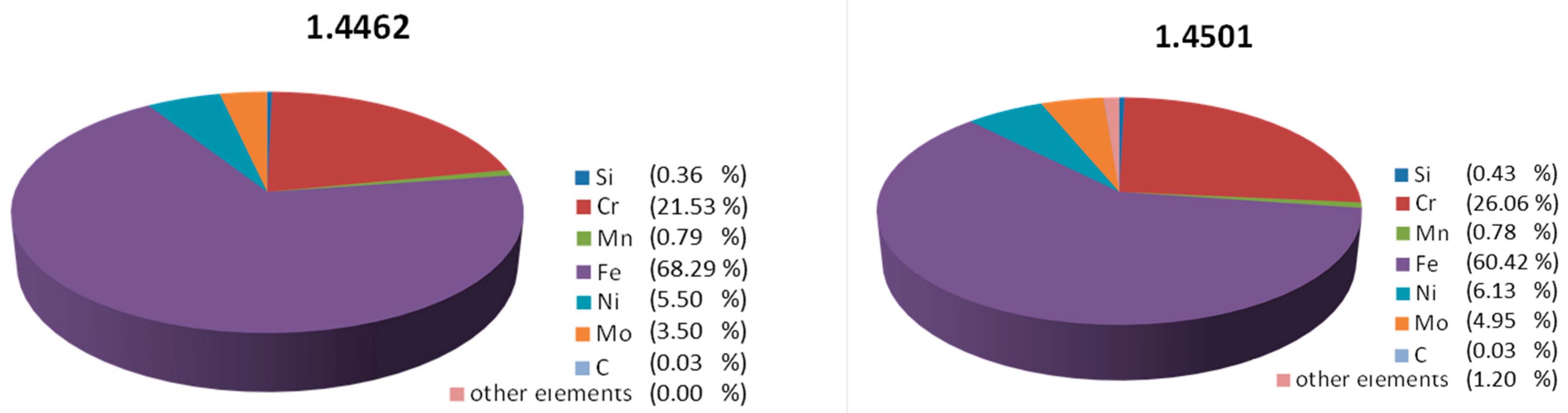
2. Materials and Methods
3. Results
4. Conclusions
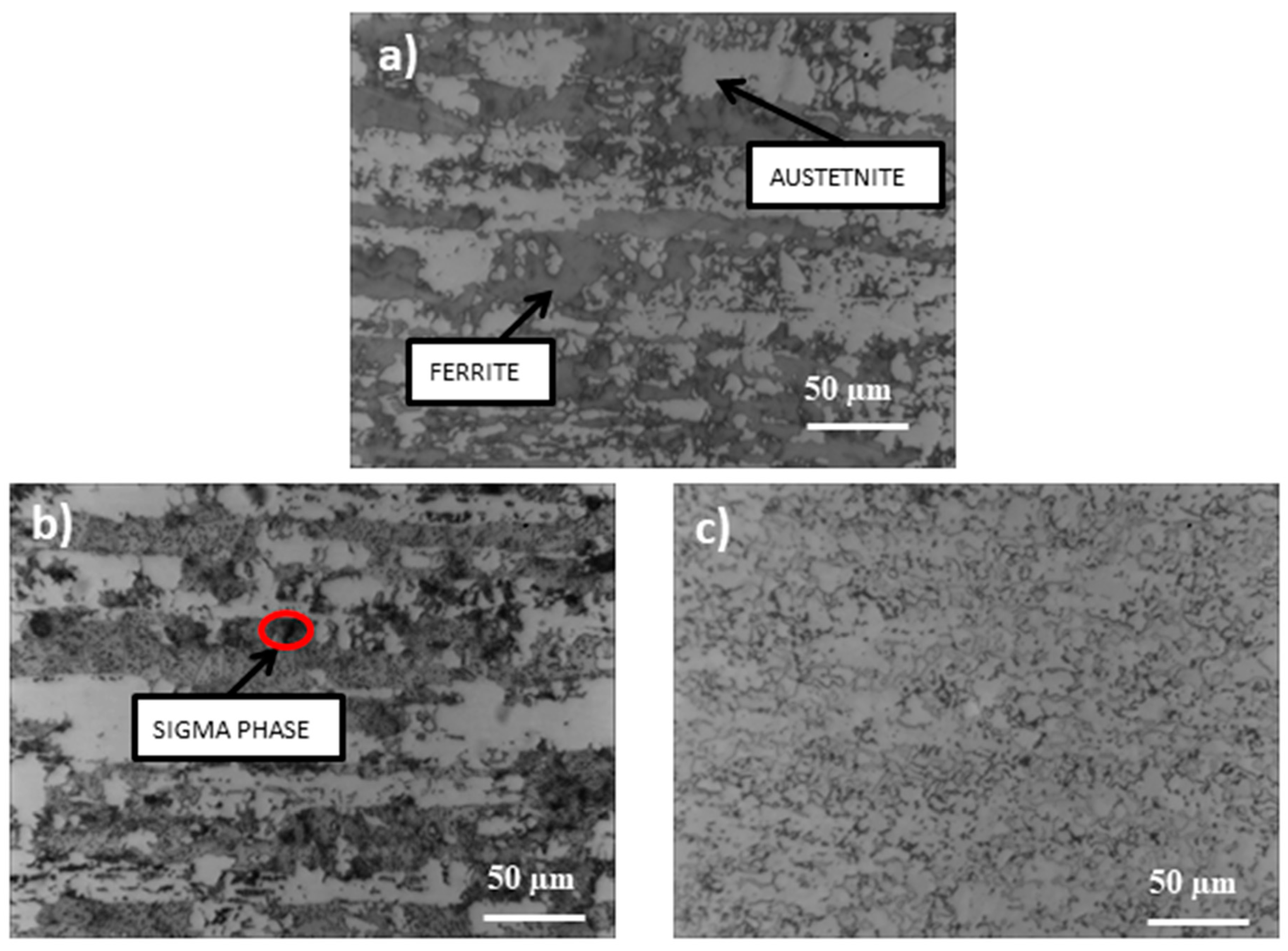
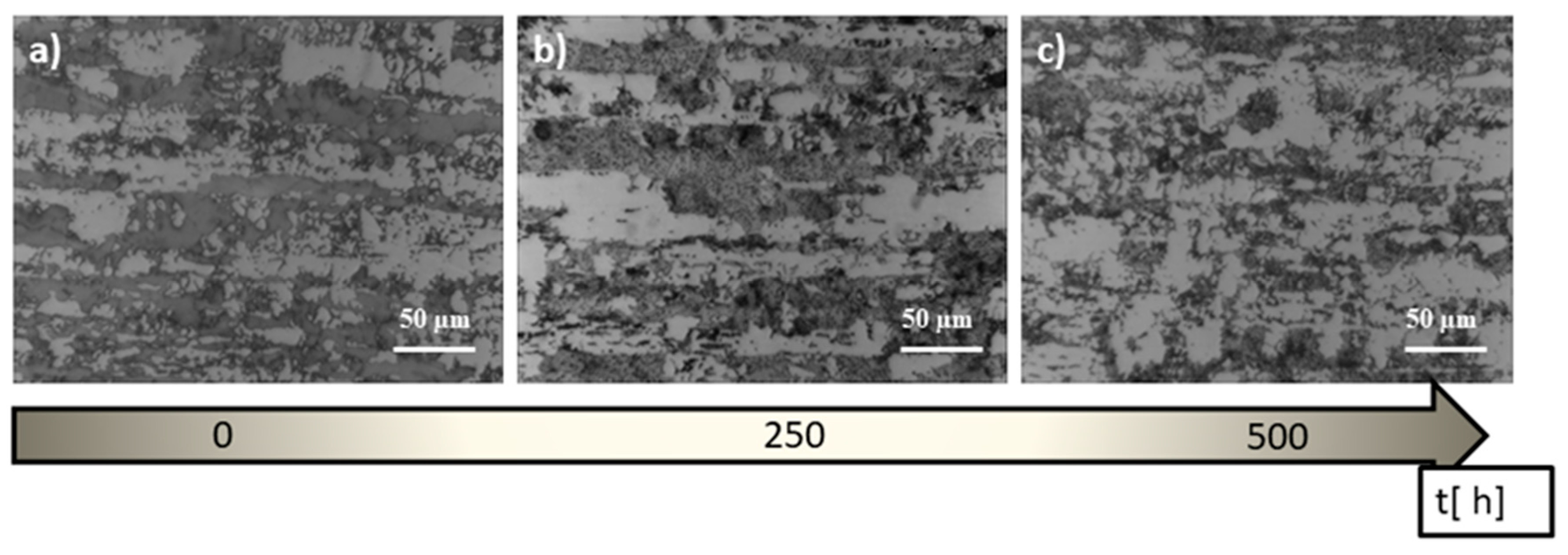
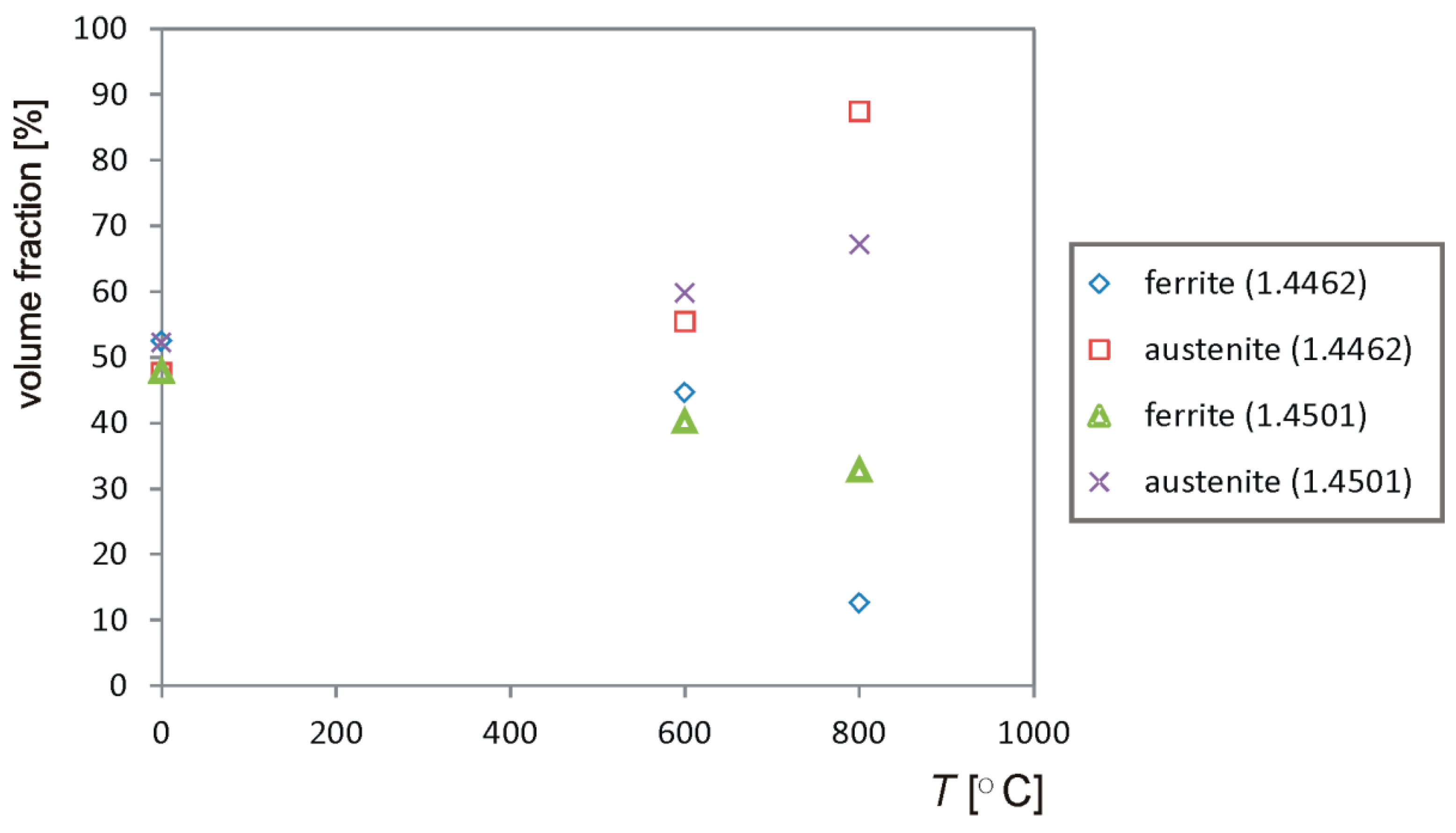
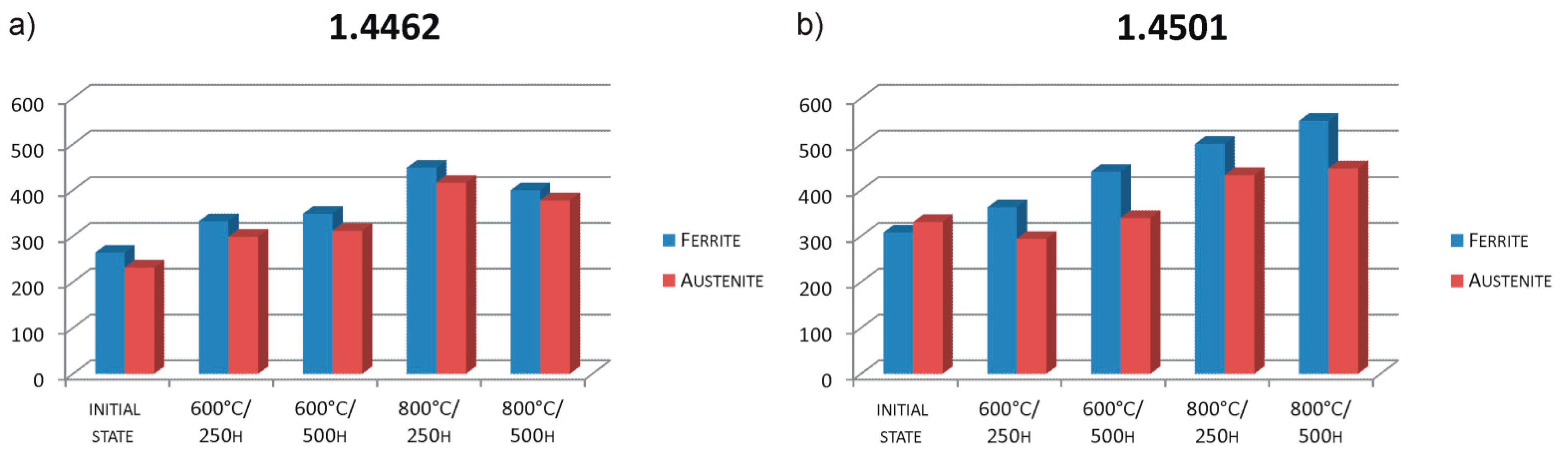
- The performed heat treatment, even under the least restrictive conditions (600 °C, 250 h), resulted in the precipitation inside the ferrite of the harmful intermetallic phase—the σ phase and the growth of austenite areas—and secondary austenite was formed. These structural changes resulted in an increase in the hardness of the samples.
- During higher temperature annealing, the σ phase is less compared to the annealing effect at 600 °C. This is in line with the analysis of the Fe–Cr equilibrium diagram.
- Long-term annealing (for 500 h) reduced the amount of the σ phase and in-creased the occurrence of dark ferrite areas. Most likely, the σ phase had partially dissolved and the recovering ferrite was ferrite δ. According to the Fe–Fe3C equilibrium diagram, ferrite δ is formed at 1394 °C. However, the high amount of alloying elements could reduce this temperature.
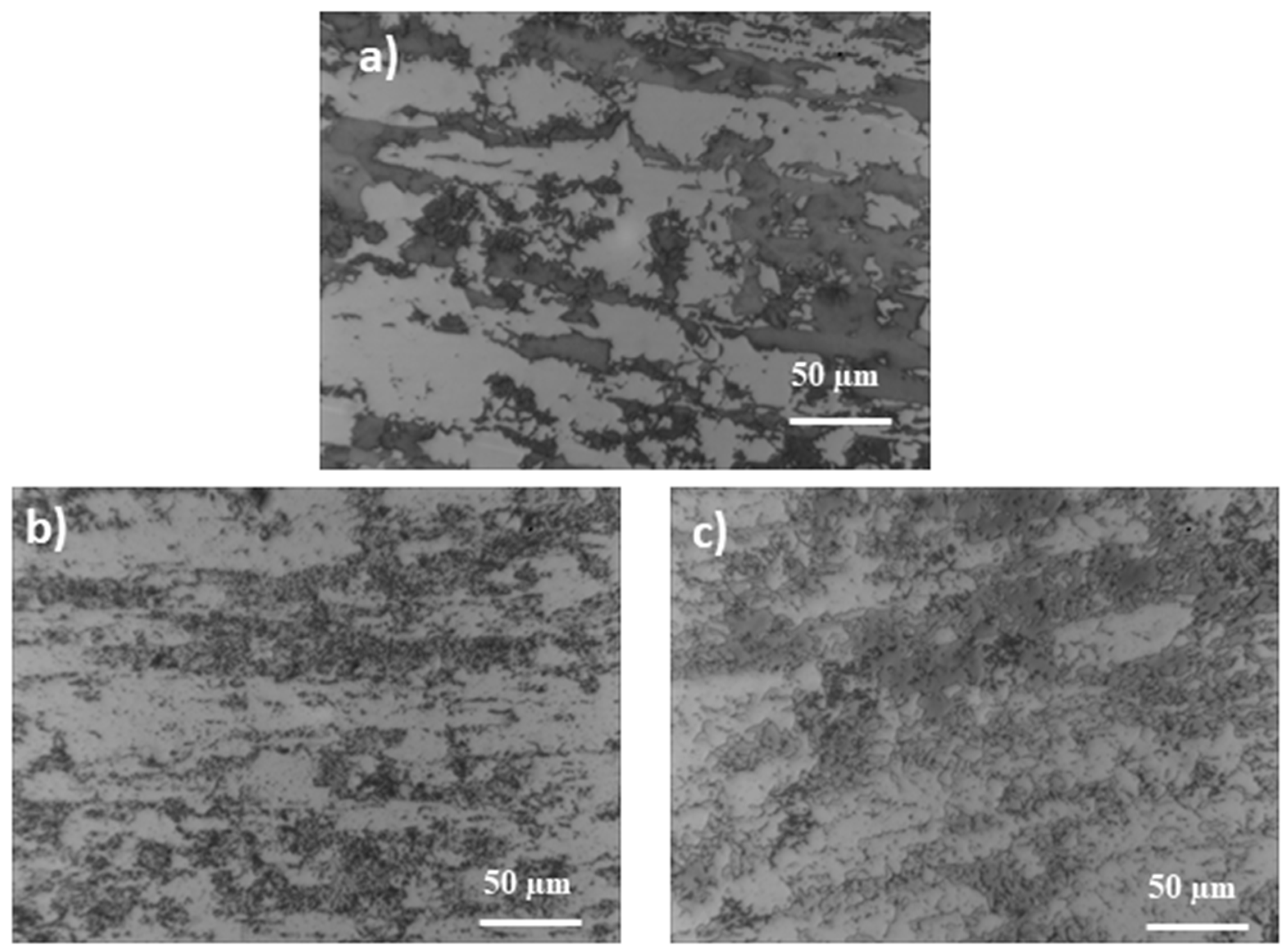
- During the annealing of steels, a temperature of 800 °C was observed, leading to the disappearance of the banded structure (uniform structure). It can be due to this that the grain size became coarse, and banded austenite gradually evolved into the structure, which is similar to “island” and “bamboo”.
- The loss of magnetic properties of both samples after long-term annealing, both at 600 and 800 °C, may result from the formation of a large amount of paramagnetic secondary austenite and probably also δ ferrite devoid of magnetic properties.
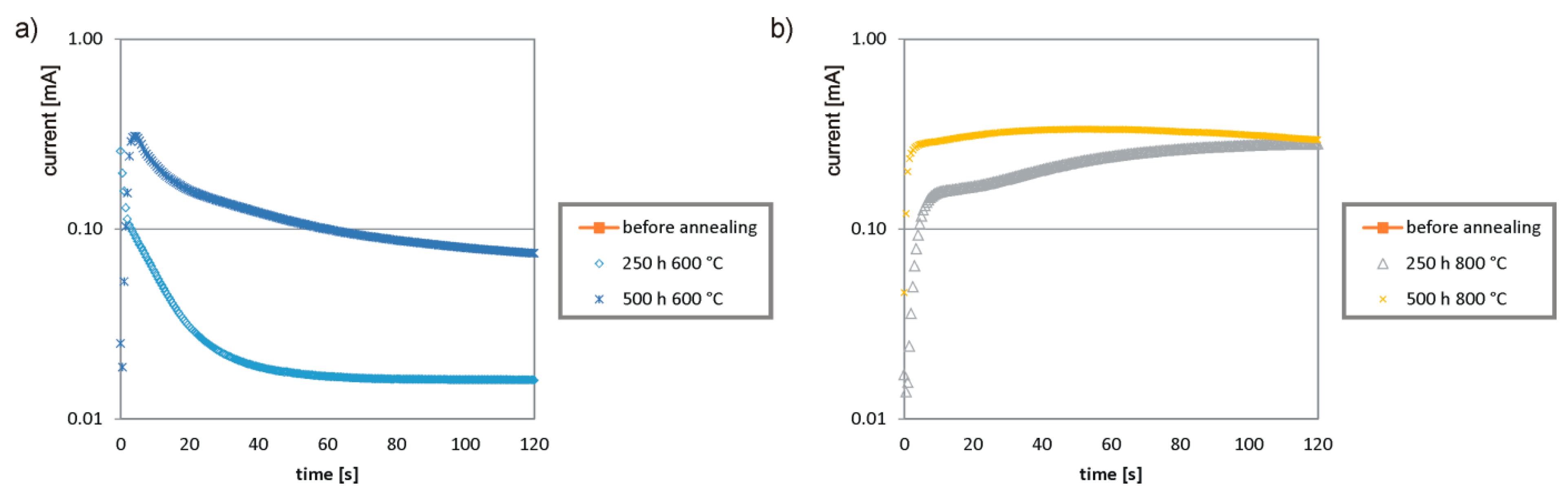
- The decrease in corrosion resistance compared to the initial state was visible for all samples, and the material annealed at 600 °C was passivated. The samples annealed at a higher temperature (800 °C) showed worse corrosion resistance than the samples annealed at 600 °C. This is due to the transformation of ferrite into secondary austenite and precipitates of excess chromium in the form of the σ phase.
- The conducted experiments have shown that the working temperature of duplex and super duplex steels must be clearly lower than 600 °C. The literature data define the operating temperature range for these steels from −50 to +300 or +500 °C. The operating temperature of most of the analyzed groups of apparatuses is below +500 °C. This confirms the possibility of using duplex steel as a construction material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Environment. Regulation of the Minister of Climate of September 24, 2020 on Emission Standards for Certain Types of Installations, Fuel Combustion Sources and Waste Incineration or Co-Incineration Devices; Ministry of Environment: Warsaw, Poland, 2020.
- European Commission. Euro 6D ISC-FCM, Commission Regulation (EU) 2018/1832 of 5 November 2018; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Integrated Technologies, Inc. Part 1: Material Selection for Chemical Process Equipment—Metals. 2019. Available online: https://www.processengineer.com/material-selection-for-chemical-process-equipment-metals/ (accessed on 15 June 2018).
- Proszak-Miąsik, D.; Rybak-Wilusz, E.; Rabczak, S. Ecological and Financial Effects of Coal-Fired Boiler Replacement with Alternative Fuels. J. Ecol. Eng. 2020, 21, 1–7. [Google Scholar] [CrossRef]
- U.S. Department of Energy. A Best Practices Process Heating Technical Brief. Materials Selection Considerations for Thermal Process Equipment. Industrial Technologies Program Energy Efficiency and Renewable Energy. 2004. Available online: https://www.energy.gov/sites/prod/files/2014/05/f15/proc_heat_tech_brief.pdf (accessed on 20 January 2019).
- Olszak, A.; Migus, M.; Kesy, Z.; Kesy, A. New materials and technologies used in chemical equipment design. Mechanik 2015, 3, 190/53–190/58. [Google Scholar] [CrossRef] [Green Version]
- Billingham, M.; Lee, C.-H.; Smith, L.; Haines, M.; James, S.; Goh, B.; Dvorak, K.; Robinson, L.; Davis, C.; Peralta-Solorio, D. Corrosion and materials selection issues in carbon capture plants. Energy Procedia 2011, 4, 2020–2027. [Google Scholar] [CrossRef] [Green Version]
- Łabanowski, J. Duplex stainless steels—New material for chemical industry. Inż. Ap. Chem 1997, 36, 3–10. (in Polish). [Google Scholar]
- Bonollo, F.; Tiziani, A.; Ferro, P. Duplex Stainless Steels; Alvarez_Armas, I., Degallaix-Moreuil, S., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 141–155. [Google Scholar]
- Charles, J. Composition and properties of duplex stainless steels. In Welding in the World/Le Soudage Dans Le Monde; Springer: Berlin/Heidelberg, Germany, 1995; Volume 36, pp. 43–54. [Google Scholar]
- Knyazeva, M.; Pohl, M. Duplex Steels: Part I: Genesis, Formation, Structure. Met. Microstruct. Anal. 2013, 2, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J.-O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Kim, D.-C.; Ogura, T.; Hamada, R.; Yamashita, S.; Saida, K. Establishment of a theoretical model based on the phase-field method for predicting the γ phase precipitation in Fe–Cr–Ni ternary alloys. Mater. Today Commun. 2021, 26, 101932. [Google Scholar] [CrossRef]
- Dille, J.; Areiza, M.; Tavares, S.; Pereira, G.; de Almeida, L.; Rebello, J. Microstructural evolution during aging at 800 °C and its effect on the magnetic behavior of UNS S32304 lean duplex stainless steel. J. Magn. Magn. Mater. 2017, 426, 102–107. [Google Scholar] [CrossRef]
- Cervo, R.; Ferro, P.; Tiziani, A.; Zucchi, F. Annealing temperature effects on superduplex stainless steel UNS S32750 welded joints. II: Pitting corrosion resistance evaluation. J. Mater. Sci. 2010, 45, 4378–4389. [Google Scholar] [CrossRef]
- Maamache, B.; Cheniti, B.; Belkessa, B.; Tahar-Chaouch, K.; Kouba, R.; Maamache, B.; Cheniti, B.; Belkessa, B.; Tahar-Chaouch, K.; Kouba, R. Effect of Aging Temperature on the Microstructure, Local Mechanical Properties, and Wear Behavior of a UNS S32750 Super Duplex Stainless Steel. J. Mater. Eng. Perform. 2021, 30, 546–555. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Y.; Chen, T.; Wang, X.; Liu, P.; Lu, Y. Microstructural Evolution and Mechanical Behavior of Thermally Aged Cast Duplex Stainless Steel. Materials 2020, 13, 5636. [Google Scholar] [CrossRef] [PubMed]
- Ferro, P.; Bonollo, F. A Semiempirical Model for Sigma-Phase Precipitation in Duplex and Superduplex Stainless Steels. Met. Mater. Trans. A 2011, 43, 1109–1116. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Singh, P.M. Effect of Heat Treatment on Corrosion and Stress Corrosion Cracking of S32205 Duplex Stainless Steel in Caustic Solution. Met. Mater. Trans. A 2009, 40, 1388–1399. [Google Scholar] [CrossRef]
- Otárola, T.; Hollner, S.; Bonnefois, B.; Anglada, M.; Coudreuse, L.; Mateo, A. Embrittlement of a superduplex stainless steel in the range of 550–700 °C. Eng. Fail. Anal. 2005, 12, 930–941. [Google Scholar] [CrossRef]
- Łabanowski, J. Mechanical properties and corrosion resistance of dissimilar stainless steel welds. Arch. Mater. Sci. Eng. 2007, 28, 27–33. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prałat, K.; Krupińska, A.; Ochowiak, M.; Włodarczak, S.; Matuszak, M.; Ciemnicka, J.; Koper, A.; Wójcicka, K. Evaluation of the Possibility of Using 1.4462 and 1.4501 Steel as a Construction Material for Apparatus Operating at an Increased Temperature and with Corrosive Factors. Materials 2021, 14, 4014. https://doi.org/10.3390/ma14144014
Prałat K, Krupińska A, Ochowiak M, Włodarczak S, Matuszak M, Ciemnicka J, Koper A, Wójcicka K. Evaluation of the Possibility of Using 1.4462 and 1.4501 Steel as a Construction Material for Apparatus Operating at an Increased Temperature and with Corrosive Factors. Materials. 2021; 14(14):4014. https://doi.org/10.3390/ma14144014
Chicago/Turabian StylePrałat, Karol, Andżelika Krupińska, Marek Ochowiak, Sylwia Włodarczak, Magdalena Matuszak, Justyna Ciemnicka, Artur Koper, and Karolina Wójcicka. 2021. "Evaluation of the Possibility of Using 1.4462 and 1.4501 Steel as a Construction Material for Apparatus Operating at an Increased Temperature and with Corrosive Factors" Materials 14, no. 14: 4014. https://doi.org/10.3390/ma14144014
APA StylePrałat, K., Krupińska, A., Ochowiak, M., Włodarczak, S., Matuszak, M., Ciemnicka, J., Koper, A., & Wójcicka, K. (2021). Evaluation of the Possibility of Using 1.4462 and 1.4501 Steel as a Construction Material for Apparatus Operating at an Increased Temperature and with Corrosive Factors. Materials, 14(14), 4014. https://doi.org/10.3390/ma14144014







