Effect of Sm Doping on the Microstructure, Mechanical Properties and Shape Memory Effect of Cu-13.0Al-4.0Ni Alloy
Abstract
:1. Introduction
2. Materials and Methods
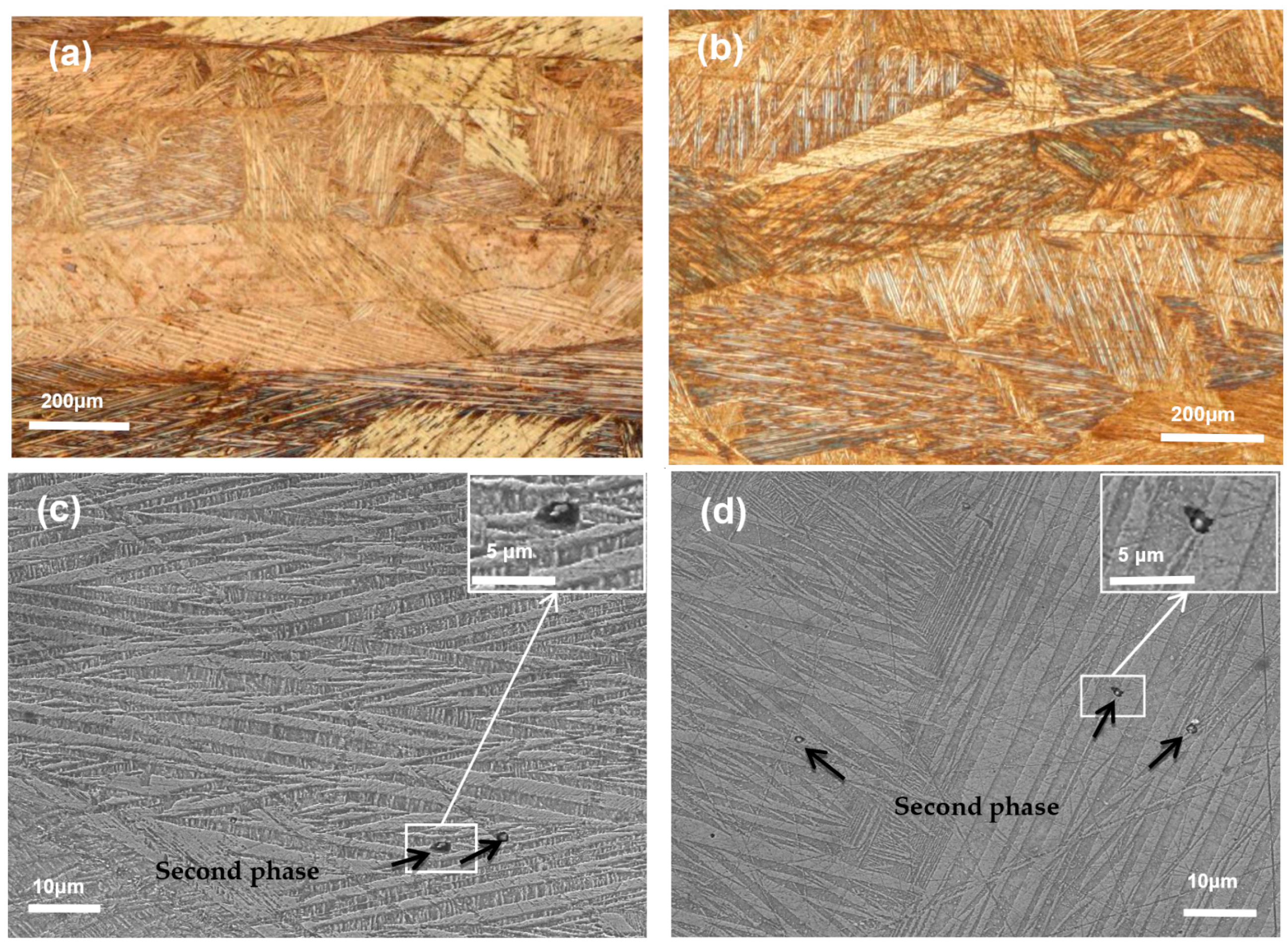
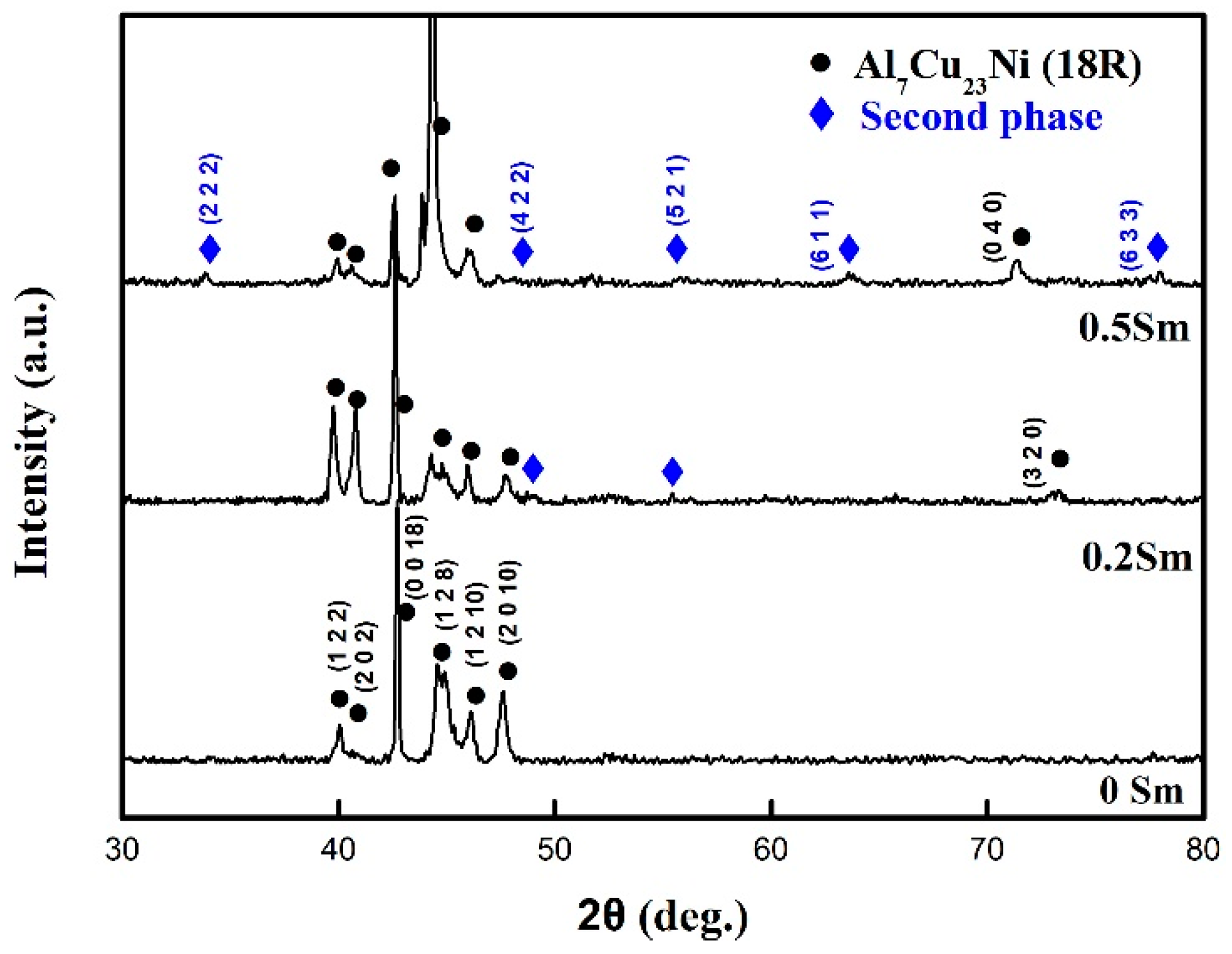
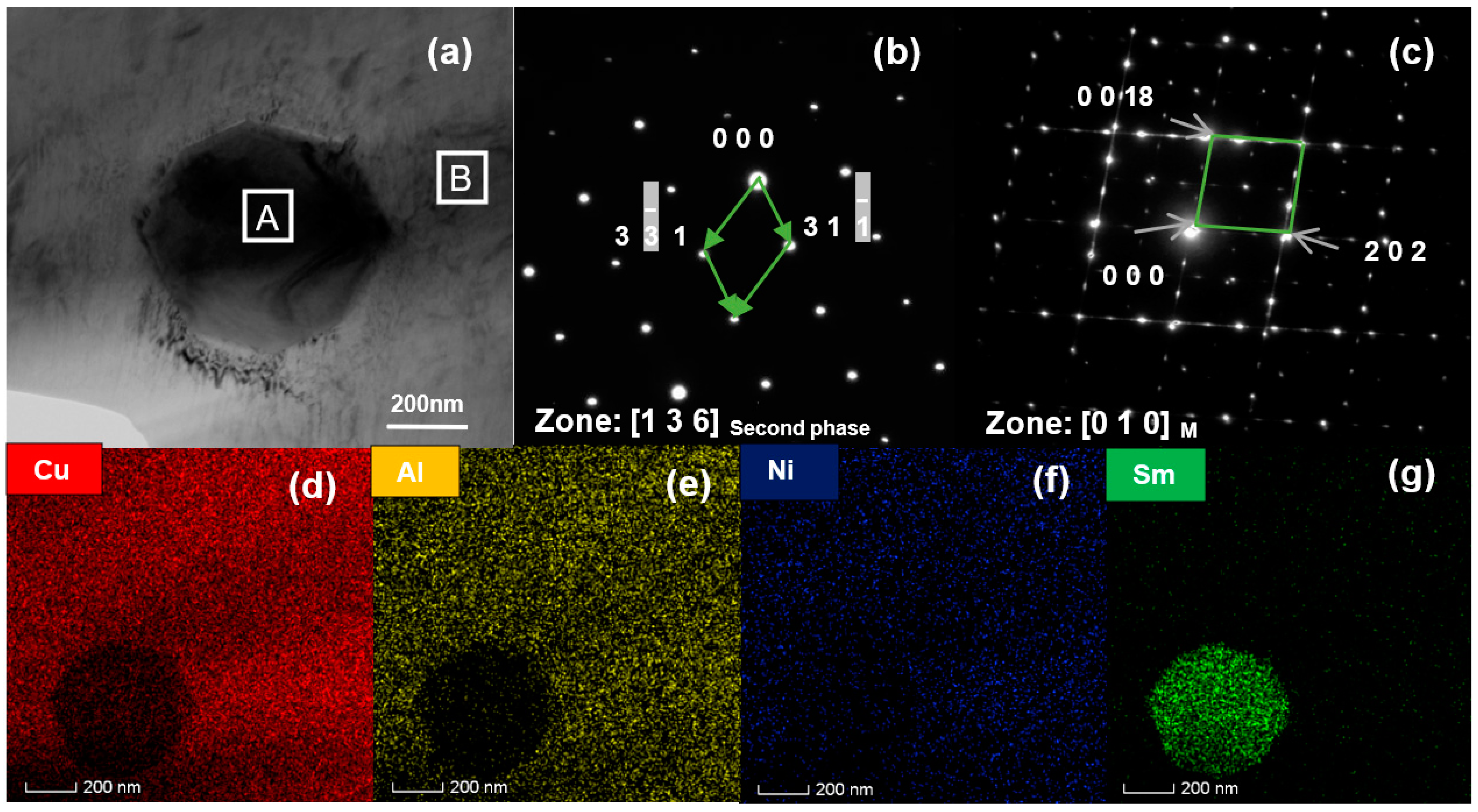
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otsuka, K.; Ren, X. Martensitic transformations in nonferrous shape memory alloys. Mater. Sci. Eng. A 1999, 273–275, 89–105. [Google Scholar] [CrossRef]
- Van Humbeeck, J. Shape Memory Alloys: A material and a Technology. Adv. Eng. Mater. 2001, 3, 837–850. [Google Scholar] [CrossRef]
- Wayman, C.; Duerigt, T.W. Engineering aspects of shape memory alloys. Eng. Asp. Shape Mem. Alloy. 1990, 11, 3–20. [Google Scholar] [CrossRef]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Frenzel, J.; George, E.P.; Dlouhy, A.; Somsen, C.; Wagner, M.F.X.; Eggeler, G. Influence of Ni on martensitic phase transformations in NiTi shape memory alloys. Acta Mater. 2010, 58, 3444–3458. [Google Scholar] [CrossRef]
- Khoo, Z.X.; An, J.; Chua, C.K.; Shen, Y.F.; Kuo, C.N.; Liu, Y. Effect of Heat Treatment on Repetitively Scanned SLM NiTi Shape Memory Alloy. Materials 2018, 12, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapozhnikov, K.; Torrens-Serra, J.; Cesari, E.; Van Humbeeck, J.; Kustov, S. On the Effect of Hydrogen on the Low-Temperature Elastic and Anelastic Properties of Ni-Ti-Based Alloys. Materials 2017, 10, 1174. [Google Scholar] [CrossRef] [Green Version]
- Firstov, G.S.; Van, H.J.; Koval, Y.N. High-temperature shape memory alloys. Mater. Sci. Eng. A 2004, 378, 2–10. [Google Scholar] [CrossRef]
- Ma, J.; Karaman, I.; Noebe, R.D. High temperature shape memory alloys. Int. Mater. Rev. 2013, 55, 257–315. [Google Scholar] [CrossRef]
- Pérez-Landazábal, J.I.; Recarte, V.; Sánchez-Alarcos, V.; Nó, M.L.; Juan, J.S. Study of the stability and decomposition process of the β phase in Cu-Al-Ni shape memory alloys. Mater. Sci. Eng. A 2006, 438–440, 734–737. [Google Scholar] [CrossRef]
- Van, H.J. Shape memory alloys with high transformation temperatures. Mater. Res. Bull. 2012, 47, 2966–2968. [Google Scholar] [CrossRef]
- Zak, G.; Kneissl, A.C.; Zatulskij, G. Shape memory effect in cryogenic Cu-Al-Mn alloys. Scr. Mater. 1996, 34, 363–367. [Google Scholar] [CrossRef]
- Zhou, L.; Lan, J.; Liu, J.; Li, X.; Shi, B.; Zheng, S. Effect of Gradient Heat Treatment on Microstructure and Properties of Cu-Al-Mn Shape Memory Alloy. Materials 2019, 12, 2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, Q.S.; Zeng, X.; Sui, J.; Cai, W.; Wang, H.; Feng, Y. Microstructure, mechanical properties and shape memory effect of Ni-Mn-Ga-B high-temperature shape memory alloy. Intermetallics 2016, 68, 113–117. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, M.; Lai, J.K.L. Microstructure characterization and effect of thermal cycling and ageing on vanadium-doped Cu-Al-Ni-Mn high-temperature shape memory alloy. J. Mater. Sci. 1998, 33, 3579–3584. [Google Scholar] [CrossRef]
- Saud, S.N.; Hamzah, E.; Abubakar, T.; Bakhsheshi-Rad, H.R. Correlation of microstructural and corrosion characteristics of quaternary shape memory alloys Cu-Al-Ni-X (X=Mn or Ti). Trans. Nonferrous Met. Soc. China 2015, 25, 1158–1170. [Google Scholar] [CrossRef]
- Vajpai, S.K.; Dube, R.K.; Sangal, S. Application of rapid solidification powder metallurgy processing to prepare Cu-Al-Ni high temperature shape memory alloy strips with high strength and high ductility. Mater. Sci. Eng. A 2013, 570, 32–42. [Google Scholar] [CrossRef]
- Sarı, U.; Kırındı, T. Effects of deformation on microstructure and mechanical properties of a Cu-Al-Ni shape memory alloy. Mater. Charact. 2008, 59, 920–929. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kawai, T.; Otsuka, K. On the origin of intergraunlar fracture in β phase shapememory alloys. Scr. Met. 1982, 16, 431–436. [Google Scholar] [CrossRef]
- Agrawal, A.; Dube, R.K. Methods of fabricating Cu-Al-Ni shape memory alloys. J. Alloys Compd. 2018, 750, 235–247. [Google Scholar] [CrossRef]
- Lara-Rodriguez, G.A.; Gonzalez, G.; Flores-Zúñiga, H.; Cortés-Pérez, J. The effect of rapid solidification and grain size on the transformation temperatures of Cu-Al-Be melt spun alloys. Mater. Charact. 2006, 57, 154–159. [Google Scholar] [CrossRef]
- Morris, M.A. Influence of boron additions on ductility and microstructure of shape memory Cu-Al-Ni alloys. Scr. Metall. Et Mater. 1991, 25, 2541–2546. [Google Scholar] [CrossRef]
- Morris, M.A. High temperature properties of ductile Cu-Al-Ni shape memory alloys with boron additions. Acta Metall. Et Mater. 1992, 40, 1573–1586. [Google Scholar] [CrossRef]
- Morris, M.A.; Lipe, T. Microstructural influence of Mn additions on thermoelastic and pseudoelastic properties of Cu-Al-Ni alloys, Acta Metall. Mater. Acta Metall. Et Mater. 1994, 42, 1583–1594. [Google Scholar] [CrossRef]
- Saud, S.N.; Hamzah, E.; Abubakar, T.; Bakhsheshi-Rad, H.R.; Farahany, S.; Abdolahi, A.; Taheri, M.M. Influence of Silver nanoparticles addition on the phase transformation, mechanical properties and corrosion behaviour of Cu-Al-Ni shape memory alloys. J. Alloys Compd. 2014, 612, 471–478. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Cui, T.; Li, J.; Liu, Q.S.; Wang, H. The enhancement of the mechanical properties and the shape memory effect for the Cu-13.0Al-4.0Ni alloy by boron addition. J. Alloys Compd. 2019, 776, 326–333. [Google Scholar] [CrossRef]
- Zhu, M.; Ye, X.; Li, C.; Song, G.; Zhai, Q. Preparation of single crystal CuAlNiBe SMA and its performances. J. Alloys Compd. 2009, 478, 404–410. [Google Scholar] [CrossRef]
- Sarı, U. Influences of 2.5wt% Mn addition on the microstructure and mechanical properties of Cu-Al-Ni shape memory alloys. Int. J. Miner. Metall. Mater. 2010, 17, 192–198. [Google Scholar] [CrossRef]
- Abbassa, M.K.; Radhia, M.M.; AhmedAdnan, R.S. The effect of Germanium addition on mechanical properties & microstructure of Cu-Al-Ni shape memory alloy. ScienceDirect 2017, 4, 224–233. [Google Scholar] [CrossRef]
- Abbass, M.K.; Adnan, R.S.A.; Alkubaisy, M. Computer Approach of Effect of 0.3 % of Ge, Te and Ce Additions on Thermodynamic Properties and Shape Memory Effect of Cu-14%Al-4.5%Ni Shape Memory Alloys. Mater. Today Proc. 2018, 5, 17602–17610. [Google Scholar] [CrossRef]
- Saud, S.N.; Abu Bakar, T.A.; Hamzah, E.; Ibrahim, M.K.; Bahador, A. Effect of Quarterly Element Addition of Cobalt on Phase Transformation Characteristics of Cu-Al-Ni Shape Memory Alloys. Metall. Mater. Trans. A 2015, 46, 3528–3542. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q. Cu-Al-Ni-V high-temperature shape memory alloys. Intermetallics 2018, 92, 108–112. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, J.; Liu, Q.S.; Cai, W. Effects of Gd addition on the microstructure, mechanical properties and shape memory effect of polycrystalline Cu-Al-Ni shape memory alloy. Mater. Lett. 2016, 180, 223–227. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, T.; Zhang, X.; Liu, Q.S.; Dong, Z.Z.; Cheng, M.H. Effect of Nd addition on the microstructure, mechanical properties, shape memory effect and corrosion behaviour of Cu-Al-Ni high-temperature shape memory alloys. J. Alloys Compd. 2021, 858, 157685–157713. [Google Scholar] [CrossRef]
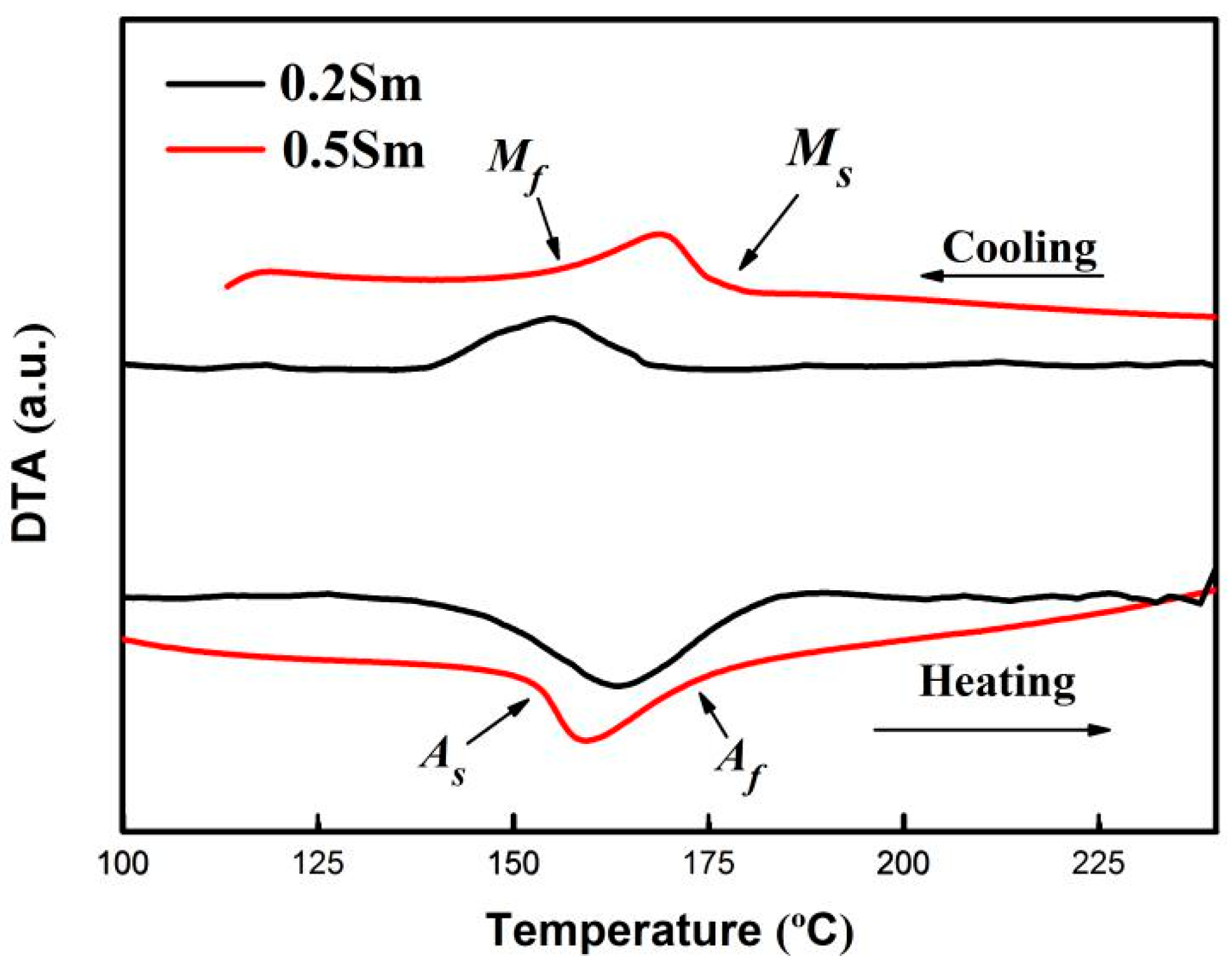
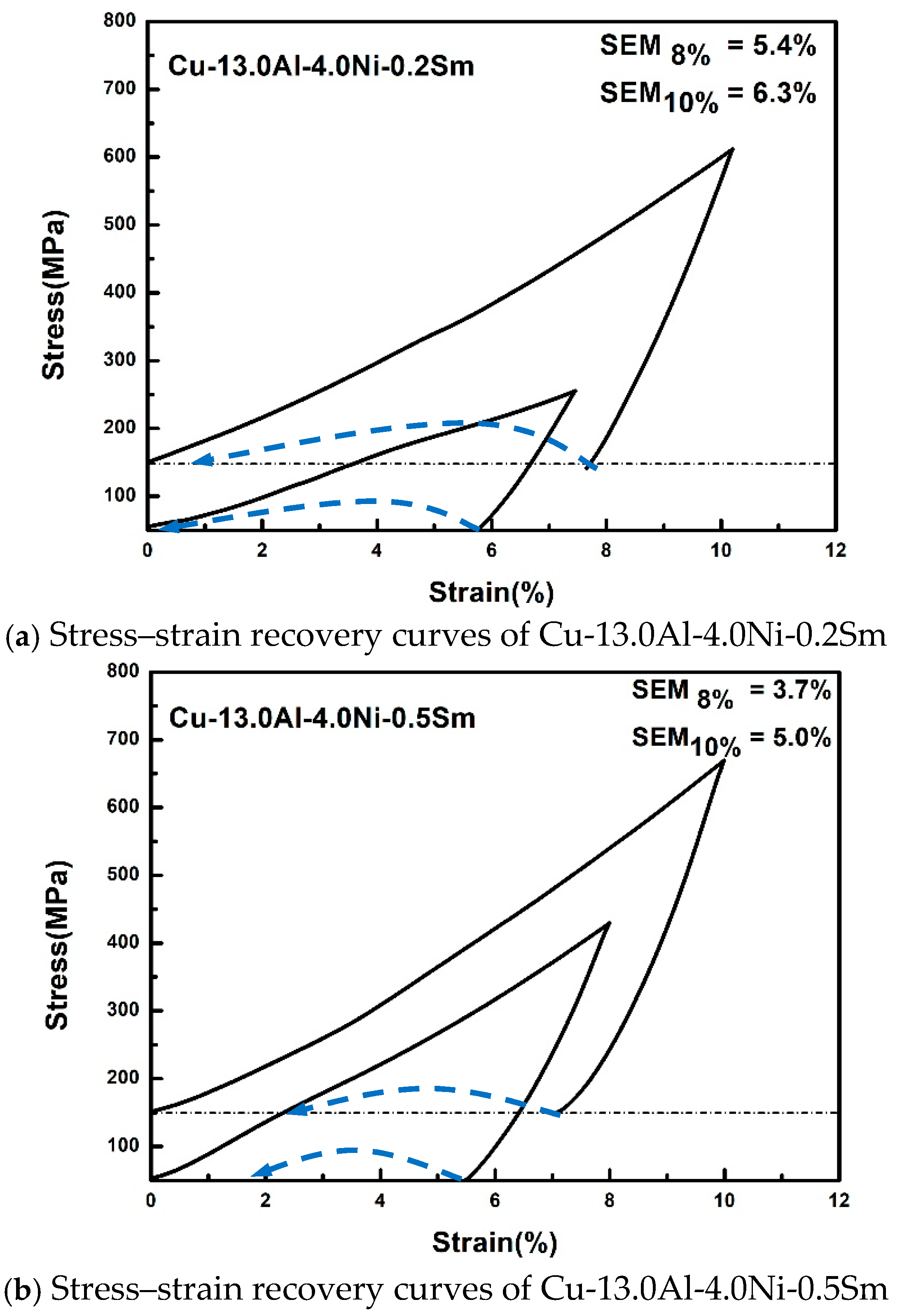
| Compositions | As | Af | Ms | Mf |
|---|---|---|---|---|
| Cu-13Al-4Ni [33] | 325 | 377 | 229 | 210 |
| Cu-13Al-4Ni-0.2Sm | 142 | 181 | 168 | 139 |
| Cu-13Al-4Ni-0.5Sm | 151 | 176 | 174 | 150 |
| Alloy | Ultimate Compression Strength (Fracture Stress)/MPa (σf) | Strength Improvement (%) | Maximum Strain (Fracture Strain)/% (εf) | Strain Improvement (%) |
|---|---|---|---|---|
| Cu-13Al-4.0Ni-2.0B [26] | 1180 | 103.4 | 15 | 42.9 |
| Cu-11.6Al-3.9Ni-2.5Mn [28] | 952 | 8.1 | 15 | 87.5 |
| Cu-14Al-4.5Ni-0.3Ge [30] | 1045 | 65.9 | 15.2 | 26.7 |
| Cu-14Al-4.5Ni-0.3Ce [30] | 1245 | 97.6 | 14.2 | 18.3 |
| Cu-14Al-4.5Ni-0.3Te [30] | 659 | 4.6 | 16.2 | 35.0 |
| Cu-13.0Al-4.0Ni-1.0V [32] | 1170 | 101.7 | 16.7 | 59.0 |
| Cu-13.0Al-4.0Ni-0.9Gd [33] | 950 | 63.8 | 16.5 | 57.1 |
| Cu-13.0Al-4.0Ni-0.5Nd [34] | 940 | 62.1 | 18.3 | 74.3 |
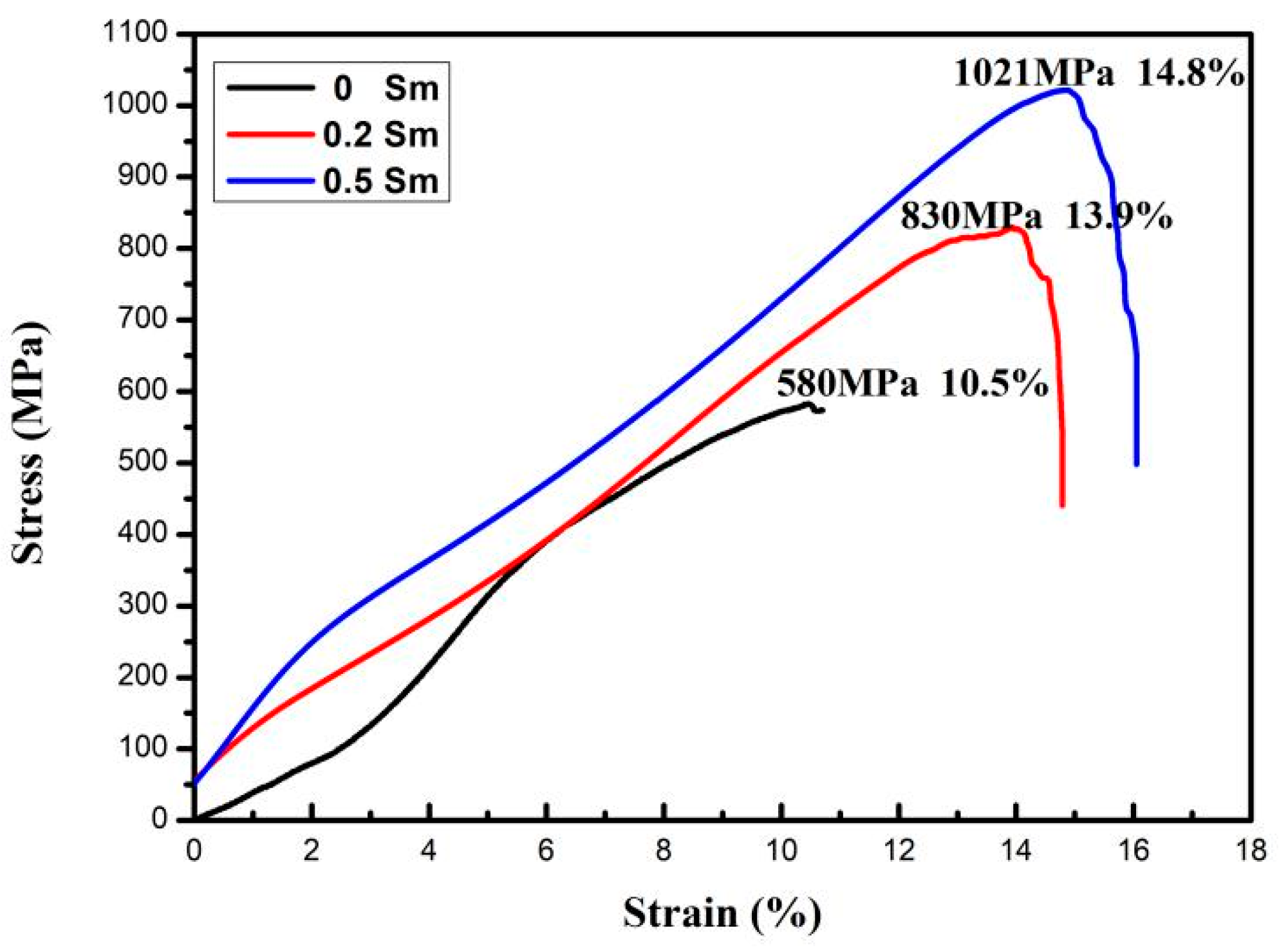
| Present alloy (Cu-13.0Al-4.0Ni-0.5Sm) | 1021 | 76.0 | 14.8 | 41.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Cui, B.; Sun, B.; Zhang, X.; Dong, Z.; Liu, Q.; Cui, T. Effect of Sm Doping on the Microstructure, Mechanical Properties and Shape Memory Effect of Cu-13.0Al-4.0Ni Alloy. Materials 2021, 14, 4007. https://doi.org/10.3390/ma14144007
Zhang Q, Cui B, Sun B, Zhang X, Dong Z, Liu Q, Cui T. Effect of Sm Doping on the Microstructure, Mechanical Properties and Shape Memory Effect of Cu-13.0Al-4.0Ni Alloy. Materials. 2021; 14(14):4007. https://doi.org/10.3390/ma14144007
Chicago/Turabian StyleZhang, Qimeng, Bo Cui, Bin Sun, Xin Zhang, Zhizhong Dong, Qingsuo Liu, and Tianyu Cui. 2021. "Effect of Sm Doping on the Microstructure, Mechanical Properties and Shape Memory Effect of Cu-13.0Al-4.0Ni Alloy" Materials 14, no. 14: 4007. https://doi.org/10.3390/ma14144007
APA StyleZhang, Q., Cui, B., Sun, B., Zhang, X., Dong, Z., Liu, Q., & Cui, T. (2021). Effect of Sm Doping on the Microstructure, Mechanical Properties and Shape Memory Effect of Cu-13.0Al-4.0Ni Alloy. Materials, 14(14), 4007. https://doi.org/10.3390/ma14144007





