Assessment of the Soft-Tissue Seal at the Interface between the Base of the Fixed Denture Pontic and the Oral Mucosa
Abstract
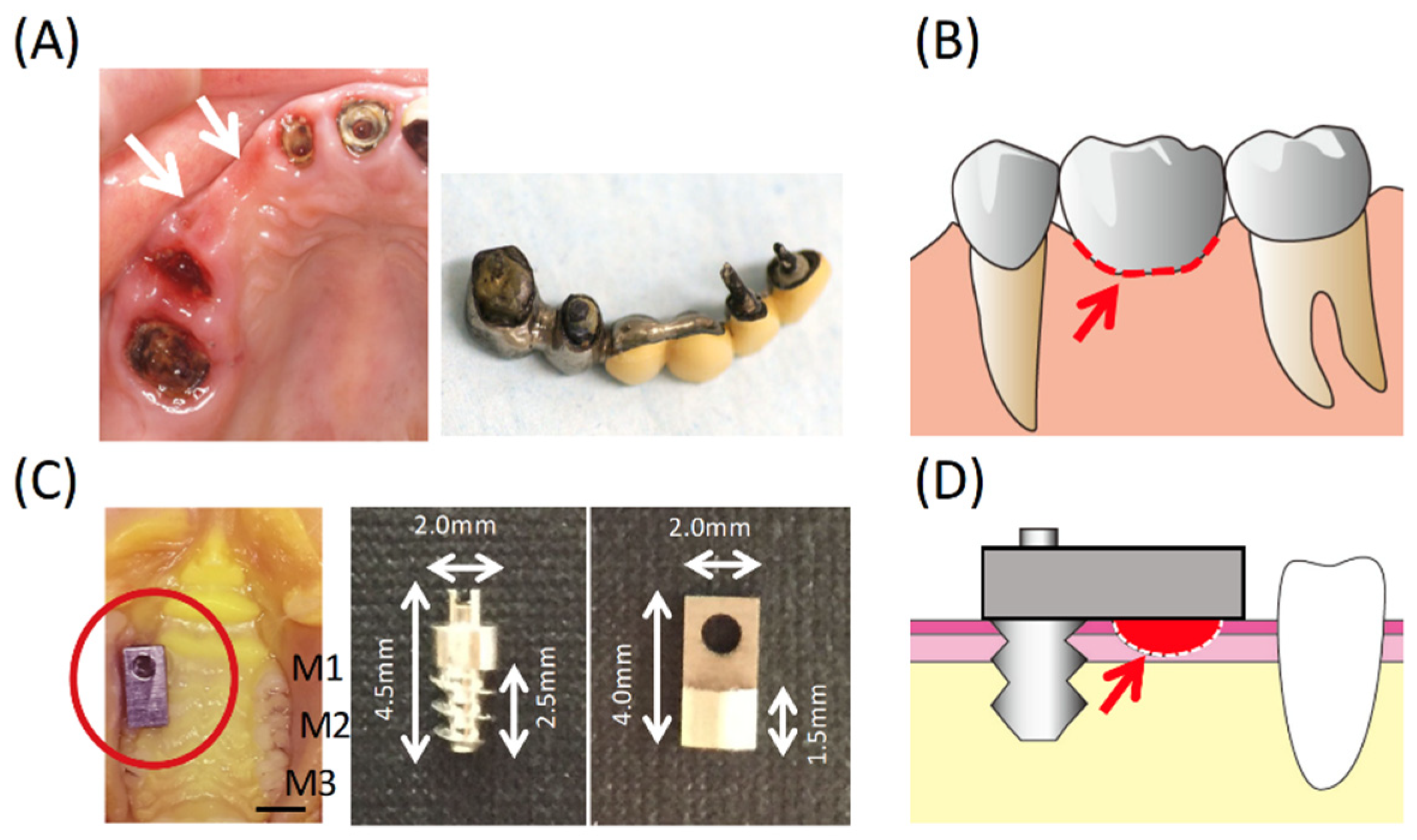
:1. Introduction
2. Materials and Methods
2.1. Experimental Bridge Model
2.2. Animals
2.3. Experimental Groups
2.4. Immunohistochemistry (Light Microscopy)
2.5. Immunohistochemistry (Electron Microscopy)
2.6. Horseradish Peroxidase (HRP) Test
2.7. Culture Experiments
2.8. Immune or Chemical-Fluorescence Staining for Adhesion Proteins
2.9. Western Blotting
2.10. Adhesion Assay
2.11. Statistical Analysis
3. Results
3.1. Condition of the Oral Mucosa at the Bridge Pontic Base
3.2. Epithelial Adhesion to the Base of the Bridge Pontic
3.3. Influence of Materials on Epithelial Cells
3.4. Influence of Material Used for the Bridge Pontic Base
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Bragger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implants Res. 2004, 15, 667–676. [Google Scholar] [CrossRef]
- Abrams, L. Augmentation of the deformed residual edentulous ridge for fixed prosthesis. Compend. Contin. Educ. Gen. Dent. 1980, 1, 205–213. [Google Scholar] [PubMed]
- Garber, D.A.; Rosenberg, E.S. The edentulous ridge in fixed prosthodontics. Compend. Contin. Educ. Dent. 1981, 2, 212–223. [Google Scholar] [PubMed]
- Ikeda, H.; Shiraiwa, M.; Yamaza, T.; Yoshinari, M.; Kido, M.A.; Ayukawa, Y.; Inoue, T.; Koyano, K.; Tanaka, T. Difference in penetration of horseradish peroxidase tracer as a foreign substance into the peri-implant or junctional epithelium of rat gingivae. Clin. Oral Implants Res. 2002, 13, 243–251. [Google Scholar] [CrossRef]
- Grusovin, M.G.; Coulthard, P.; Jourabchian, E.; Worthington, H.V.; Esposito, M.A. Interventions for replacing missing teeth: Maintaining and recovering soft tissue health around dental implants. Cochrane Database Syst. Rev. 2010, CD003069. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Ultrastructural localization of laminin-5 (gamma2 chain) in the rat peri-implant oral mucosa around a titanium-dental implant by immuno-electron microscopy. Biomaterials 2005, 26, 6280–6287. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Goto, T.; Kido, M.A.; Kagiya, T.; Mino, S.; Shimono, M.; Tanaka, T. Changes in the distribution of laminin-5 during peri-implant epithelium formation after immediate titanium implantation in rats. Biomaterials 2005, 26, 1751–1760. [Google Scholar] [CrossRef]
- Oshiro, W.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Yamazoe, J.; Kondo, R.; Sakaguchi, M.; Matsuura, Y.; Tsukiyama, Y.; Koyano, K. Effects of CaCl2 hydrothermal treatment of titanium implant surfaces on early epithelial sealing. Colloids Surf. B Biointerfaces 2015, 131, 141–147. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Narimatsu, I.; Kondo, R.; Oshiro, W.; Koyano, K. Epithelial sealing effectiveness against titanium or zirconia implants surface. J. Biomed. Mater. Res. A 2019, 107, 1379–1385. [Google Scholar] [CrossRef]
- Okawachi, H.; Ayukawa, Y.; Atsuta, I.; Furuhashi, A.; Sakaguchi, M.; Yamane, K.; Koyano, K. Effect of titanium surface calcium and magnesium on adhesive activity of epithelial-like cells and fibroblasts. Biointerphases 2012, 7, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, H.; Yamaza, T.; Yoshinari, M.; Ohsaki, Y.; Ayukawa, Y.; Kido, M.A.; Inoue, T.; Shimono, M.; Koyano, K.; Tanaka, T. Ultrastructural and immunoelectron microscopic studies of the peri-implant epithelium-implant (Ti-6Al-4V) interface of rat maxilla. J. Periodontol. 2000, 71, 961–973. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Furuhashi, A.; Koyano, K. The role of phosphoinositide 3-kinase in adhesion of oral epithelial cells to titanium. Arch. Oral Biol. 2013, 58, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Takamori, Y.; Atsuta, I.; Nakamura, H.; Sawase, T.; Koyano, K.; Hara, Y. Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin. Oral Implants Res. 2017, 28, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kondo, R.; Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Therapeutic interaction of systemically-administered mesenchymal stem cells with peri-implant mucosa. PLoS ONE 2014, 9, e90681. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Ogino, Y.; Moriyama, Y.; Tsukiyama, Y.; Koyano, K. In vivo and in vitro studies of epithelial cell behavior around titanium implants with machined and rough surfaces. Clin. Implant Dent. Relat. Res. 2014, 16, 772–781. [Google Scholar] [CrossRef]
- Ayukawa, Y.; Oshiro, W.; Atsuta, I.; Furuhashi, A.; Kondo, R.; Jinno, Y.; Koyano, K. Long Term Retention of Gingival Sealing around Titanium Implants with CaCl2 Hydrothermal Treatment: A Rodent Study. J. Clin. Med. 2019, 8, 1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiraiwa, M.; Goto, T.; Yoshinari, M.; Koyano, K.; Tanaka, T. A study of the initial attachment and subsequent behavior of rat oral epithelial cells cultured on titanium. J. Periodontol. 2002, 73, 852–860. [Google Scholar] [CrossRef]
- Hu, J.; Atsuta, I.; Ayukawa, Y.; Zhou, X.; Dwi Rakhmatia, Y.; Koyano, K. The impact of surface alteration on epithelial tissue attachment after the mechanical cleaning of titanium or zirconia surface. J. Oral Rehabil. 2020, 62, 331–334. [Google Scholar] [CrossRef]
- Zhou, X.; Atsuta, I.; Ayukawa, Y.; Narimatsu, I.; Zhou, T.; Hu, J.; Koyano, K. Effects of Different Divalent Cation Hydrothermal Treatments of Titanium Implant Surfaces for Epithelial Tissue Sealing. Materials 2020, 13, 2038. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol. 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Ogino, Y.; Moriyama, Y.; Jinno, Y.; Koyano, K. Evaluations of epithelial sealing and peri-implant epithelial down-growth around “step-type” implants. Clin. Oral Implants Res. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Rabinovitz, I.; Mercurio, A.M. The integrin alpha6beta4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell Biol. 1997, 139, 1873–1884. [Google Scholar] [CrossRef]
- Giancotti, F.G. Integrin signaling: Specificity and control of cell survival and cell cycle progression. Curr. Opin. Cell Biol. 1997, 9, 691–700. [Google Scholar] [CrossRef]
- Ghannad, F.; Nica, D.; Fulle, M.I.; Grenier, D.; Putnins, E.E.; Johnston, S.; Eslami, A.; Koivisto, L.; Jiang, G.; McKee, M.D.; et al. Absence of alphavbeta6 integrin is linked to initiation and progression of periodontal disease. Am. J. Pathol. 2008, 172, 1271–1286. [Google Scholar] [CrossRef] [Green Version]
- Rezniczek, G.A.; Janda, L.; Wiche, G. Plectin. Methods Cell Biol. 2004, 78, 721–755. [Google Scholar]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Yamaza, T.; Tsukiyama, Y.; Koyano, K. Promotive effect of insulin-like growth factor-1 for epithelial sealing to titanium implants. J. Biomed. Mater. Res. A 2013, 101, 2896–2904. [Google Scholar] [CrossRef] [PubMed]
- Diener, A.; Nebe, B.; Luthen, F.; Becker, P.; Beck, U.; Neumann, H.G.; Rychly, J. Control of focal adhesion dynamics by material surface characteristics. Biomaterials 2005, 26, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.S.; Yin, J.; Xu, K.; Huang, J. Growth factors and corneal epithelial wound healing. Brain Res. Bull. 2010, 81, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Rogel, M.R.; Soni, P.N.; Troken, J.R.; Sitikov, A.; Trejo, H.E.; Ridge, K.M. Vimentin is sufficient and required for wound repair and remodeling in alveolar epithelial cells. FASEB J. 2011, 25, 3873–3883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuhashi, A.; Ayukawa, Y.; Atsuta, I.; Okawachi, H.; Koyano, K. The difference of fibroblast behavior on titanium substrata with different surface characteristics. Odontology 2012, 100, 199–205. [Google Scholar] [CrossRef]
- Kohal, R.J.; Finke, H.C.; Klaus, G. Stability of prototype two-piece zirconia and titanium implants after artificial aging: An in vitro pilot study. Clin. Implant Dent. Relat. Res. 2009, 11, 323–329. [Google Scholar] [CrossRef]
- Zhao, B.; van der Mei, H.C.; Subbiahdoss, G.; de Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Motro, P.F.; Kursoglu, P.; Kazazoglu, E. Effects of different surface treatments on stainability of ceramics. J. Prosthet. Dent. 2012, 108, 231–237. [Google Scholar] [CrossRef]
- Mores, R.T.; Borba, M.; Corazza, P.H.; Della Bona, A.; Benetti, P. Influence of surface finishing on fracture load and failure mode of glass ceramic crowns. J. Prosthet. Dent. 2017, 118, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Spahl, W.; Budzikiewicz, H.; Geurtsen, W. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J. Dent. 1998, 26, 137–145. [Google Scholar] [CrossRef]
- Durner, J.; Spahl, W.; Zaspel, J.; Schweikl, H.; Hickel, R.; Reichl, F.X. Eluted substances from unpolymerized and polymerized dental restorative materials and their Nernst partition coefficient. Dent. Mater. 2010, 26, 91–99. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atsuta, I.; Narimatsu, I.; Morimoto, T.; Cheng, C.-H.; Koyano, K.; Ayukawa, Y. Assessment of the Soft-Tissue Seal at the Interface between the Base of the Fixed Denture Pontic and the Oral Mucosa. Materials 2021, 14, 3997. https://doi.org/10.3390/ma14143997
Atsuta I, Narimatsu I, Morimoto T, Cheng C-H, Koyano K, Ayukawa Y. Assessment of the Soft-Tissue Seal at the Interface between the Base of the Fixed Denture Pontic and the Oral Mucosa. Materials. 2021; 14(14):3997. https://doi.org/10.3390/ma14143997
Chicago/Turabian StyleAtsuta, Ikiru, Ikue Narimatsu, Taichiro Morimoto, Chi-Hsiang Cheng, Kiyoshi Koyano, and Yasunori Ayukawa. 2021. "Assessment of the Soft-Tissue Seal at the Interface between the Base of the Fixed Denture Pontic and the Oral Mucosa" Materials 14, no. 14: 3997. https://doi.org/10.3390/ma14143997
APA StyleAtsuta, I., Narimatsu, I., Morimoto, T., Cheng, C.-H., Koyano, K., & Ayukawa, Y. (2021). Assessment of the Soft-Tissue Seal at the Interface between the Base of the Fixed Denture Pontic and the Oral Mucosa. Materials, 14(14), 3997. https://doi.org/10.3390/ma14143997





