Abstract
Ca-P coatings on Ti implants have demonstrated good osseointegration capability due to their similarity to bone mineral matter. Three databases (PubMed, Embase, and Web of Science) were searched electronically in February 2021 for preclinical studies in unmodified experimental animals, with at least four weeks of follow-up, measuring bone-to-implant contact (BIC). Although 107 studies were found in the initial search, only eight experimental preclinical studies were included. Adverse events were selected by two independent investigators. The risk of bias assessment of the selected studies was evaluated using the Cochrane Collaboration Tool. Finally, a meta-analysis of the results found no statistical significance between implants coated with Ca-P and implants with etched conventional surfaces (difference of means, random effects: 5.40; 99% CI: −5.85, 16.65). With the limitations of the present review, Ca-P-coated Ti surfaces have similar osseointegration performance to conventional etched surfaces. Future well-designed studies with large samples are required to confirm our findings.
1. Introduction
Titanium (Ti) is one of the most widely used materials for the manufacture of dental and orthopaedic implants due to its mechanical properties, chemical stability, and excellent biocompatibility [1]. The quality of implants depends on the properties of their surfaces; therefore, the modification of these surfaces, with the aim of achieving optimal osseointegration and shortening waiting times for functional loading, has become an area of great interest for researchers and is under constant evolution.
The osseointegration of implants has been defined as a direct and functional connection between the bone and the implant, where the macroscopic and microscopic characteristics of the implant surface are of great importance. Lack of osseointegration is often due to poor bone formation around the implant surface, leading to insufficient fixation of the implant [2].
The deposition of calcium-phosphate (Ca-P) coatings on the implant surface has received significant attention due to the chemical similarity to natural bone mineral. Ca-P-based coatings show the ability to adhere directly to bone tissue and to increase the biochemical anchorage between bone and the coating material [3]. Ca-P coatings on titanium implants have been shown to improve their biofunctionality by facilitating osseointegration and longevity, hence the existing philosophy regarding this type of coating is that biological integration is improved when the structure mimics bone [4,5,6].
Ca-P, in the form of apatite, is the main mineral content (~69%) of natural bone [7]. However, it is not osteoinductive [8], and its activity is limited to osteoconduction, although it has been shown that, in combination with growth factors and bioactive proteins, it can be osteoinductive [9].
Ultrastructural observations have shown that Ca-P coatings partially dissolve, saturating body fluids in the peri-implant area and leading to a double precipitation of biological apatite, which could serve as a substrate for bone-forming cells, the only difficulty being matching the dissolution of the coating with the rate of healing to achieve ideal bone apposition on the titanium surface [10].
Although previous reviews on this topic have been published, none of them compared in vivo, Ca-P-coated Ti surfaces with conventional etched surfaces (sandblasted large grit acid etched, SLA, surfaces). Therefore, the aim of the present systematic review and meta-analysis was to bring together preclinical studies in experimental animals to determine whether Ca-P-coated Ti implant surfaces possess increased osseointegration capability.
2. Materials and Methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [11].
2.1. Protocol and Registration
A search was carried out for any registered protocols on a similar topic in the International Prospective Register of Systematic Reviews (PROSPERO). No systematic review protocols were found in this database. Therefore, this review was pre-registered in the PROSPERO platform under the identification number CRD-REGISTER-2-ID255185.
The Population, Intervention, Comparison, Outcome and Study Design framework (PICOS) was used as a basis to formulate the research question, which was: “Do Ca-P-coated Ti surfaces have a higher osseointegration capacity than etched Ti surfaces?”. (P) Population: animals receiving endosseous Ti implants. (I) Intervention: Ti implants with Ca-P incorporation. (C) Comparison: Ti implants with conventional surface. (O) Outcome: bone formation around the implant surface. (S) Study design: preclinical studies in unmodified experimental animals (Table 1).

Table 1.
PICOS items.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria for the study selection were:
- -
- Preclinical studies in unmodified animals (osteoporotic, diabetic…), using endosseous implants with Ca-P incorporation;
- -
- Studies with at least six animals and 4 weeks of follow-up;
- -
- Studies published in English.
The exclusion criteria for the study selection were:
- -
- In vitro studies;
- -
- Narrative and systematic reviews;
- -
- Clinical cases;
- -
- Studies that did not meet the established inclusion criteria.
2.3. Search Strategy
The following search strategy was used: Two independent researchers conducted electronic searches in the PubMed, Embase, and Web of Science (WoS) databases up to February 2021 with the Medical Subject Headings (MeSH) terms: “Titanium implants”, “biocompatible coated materials”, “osseointegration”, “calcium phosphate”, “animal model”. Boolean operators “AND” and “OR” were used to refine the search (Table A1).
2.4. Selection of Studies
Two independent reviewers (N.L.-V., A.L.-V.) carried out the study selection by obtaining full text data from the selected articles, including general information, animal parameters (total number, species), methods of Ca-P incorporation, timing of assessment, methods of analysis, conclusions, and implant parameters (total number, length, diameter, shape, location, and surface characteristics of implant and control). After eliminating duplicates, studies were selected according to the inclusion criteria. Cohen’s kappa statistic was calculated to measure the level of agreement between the two reviewers. Disagreement on the eligibility of studies was resolved by discussion between the two reviewers.
2.5. Risk of Bias
The Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) risk of bias tool, an adapted version of the Cochrane RoB tool with specific biases in animal studies), was used to assess the methodology of the scientific evidence in all selected studies [12].
2.6. Quality of the Reports of the Selected Articles
Animal Research: Reporting of In Vivo Experiments (ARRIVE) [13] guidelines were used, with a total of 23 items. Each item was scored by reviewers N.L.-V. and A.L.-V. with scores of 0 (not reported) or 1 (reported), with an overall inventory of all included studies (Table 1).
2.7. Statistical Analysis
Odds ratios (ORs) with 95% confidence intervals (CI) were used for adverse event outcomes. The mean difference (MD) and standard deviation (SD) for BIC were used to estimate effect size. The meta-analysis was performed using RevMan software (Review Manager version 5.3; The Cochrane Collaboration, Copenhagen, Denmark). The random-effects model was selected because of the expected methodological heterogeneity in the included studies; furthermore, significant heterogeneity was interpreted when the I2 value was > 50% [14]. The threshold for statistical significance was defined as p < 0.05. A funnel plot was used to assess publication bias.
3. Results
3.1. Selection and Description of Studies
The initial electronic search yielded 107 references. After eliminating duplicates and irrelevant articles based on their title and abstracts (in vitro studies, systematic reviews, modified animals, non-Ti implants, and articles in other languages), 18 full texts were selected [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The concordance between reviewers (N.L.-V., A.L.-V.) was 100% with a Cohen’s kappa index of 1 (overall concordance). The reasons for rejecting 10 studies out of the 18 selected were the following: use of unconventional implants [23,26,29,31], comparing different apatite veneers [25,27,30], assessing parameters after occlusal loading [28], assessing the antimicrobial activity of the Ca-P veneer [24], and not providing data for meta-analysis [32]. Finally, eight studies were selected for the meta-analysis [15,16,17,18,19,20,21,22] (Figure 1).
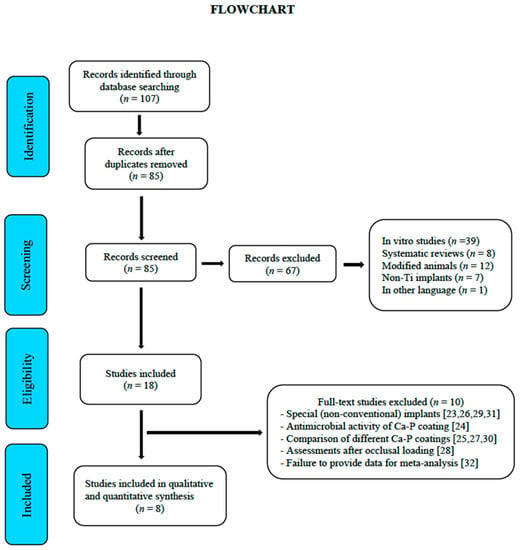
Figure 1.
Flowchart.
Table 2 provides the assessment of the ARRIVE criteria in animal studies, with a mean score of 17.25 ± 0.46. All studies provided adequate information in terms of title, abstract, introduction, ethical statement, species, surgical procedure, outcome assessment, and statistical analysis. Items 5 (rationale for animal models), 19 (3Rs, replace, reduce and refine), 20 (adverse events), 21 (limitations of the study) and 22 (generalizability/applicability) were not reported in any of the included studies.

Table 2.
Checklist of ARRIVE criteria reported by the included studies. Each item was judged as “0” (not reported) or “1” (reported). The total score of each of included studies was also recorded.
3.2. Risk of Bias Assessment
Although item 2 was mentioned in several studies, the lack of information resulted in a high and unclear risk of bias for most of the included studies (Figure 2).
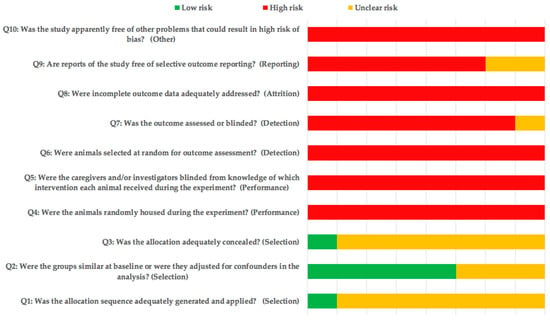
Figure 2.
SYRCLE’s risk of bias tool.
3.3. Qualitative Synthesis
The most commonly used animal model was rabbit [15,16,17,20], and all included studies evaluated BIC (Table 3); two of the included studies [17,21] evaluated bone density (BD) and two bone area (BA) [15,18]. All implants used were commercial threaded implants and only one of the studies used hydroxyapatite (HA) in combination with calcium oxide (CaO) as a coating [15]. The methods of Ca-P incorporation to the surface of the experimental implants were different in all selected studies (Table 4).

Table 3.
Characteristics of the studies included.

Table 4.
Characteristics of implants.
3.4. Quantitative Synthesis (Meta-Analysis)
The same studies included in the qualitative synthesis were used to perform a meta-analysis comparing Ca-P-coated Ti implants with etched Ti implants, with a total of 455 implants being evaluated. A meta-analysis of adverse outcomes could not be performed due to lack of data. All included studies [15,16,17,18,19,20,21,22] assessed BIC 4 weeks after placement. Heterogeneity was very high (I2 = 99%) (Table 5)). Figure 3 shows the forest plot for the meta-analysis.

Table 5.
Meta-analysis of BIC according to random-effects model.
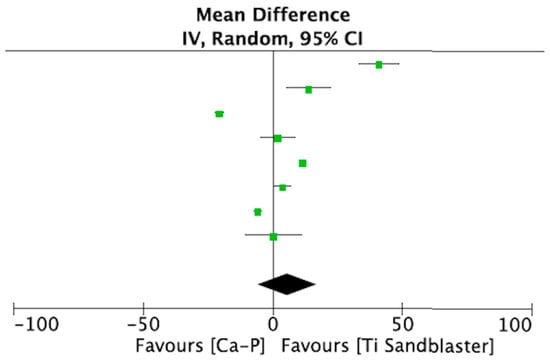
Figure 3.
Forest plot for meta-analysis of studies evaluating BIC at 4 weeks after placement, assuming a random-effects model.
3.5. Publication Bias and Heterogeneity
The experimental studies show graphical signs of publication bias, as can be observed in the funnel plot (Figure 4).
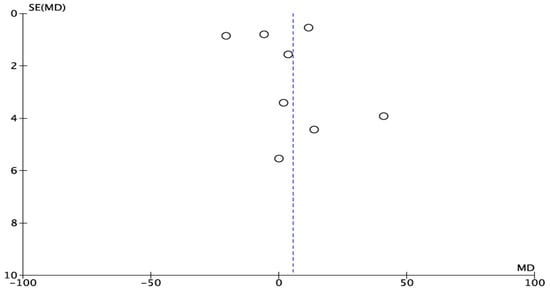
Figure 4.
Funnel plot for BIC. The asymmetry proves publication bias.
4. Discussion
The purpose of the present study was to answer the following clinical question: “Do Ca-P-coated Ti surfaces have a higher osseointegration capacity than etched Ti surfaces?”. To quantify the potential effect of Ca-P-containing surfaces on peri-implant bone apposition, a meta-analysis of BIC was performed. Our meta-analysis found no statistical significance between implants coated with Ca-P and implants with conventional etched surfaces (SLA type).
Certain thin Ca-P coatings have been shown to be amorphous and readily soluble in simulated body fluids [33], and several studies have found no difference in early osseointegration between CA-P-coated implants and implants with etched or Ti powder-blasted surfaces [34,35,36]. Koh and colleagues [15] in a study in rabbits concurred with these findings, finding no difference in bone apposition around Ca-P-coated surfaces compared to etched surfaces. Various forms of Ca-P differ in solubility and stability, which are characteristics that alter their biocompatibility. HA is a very poorly soluble but very stable Ca orthophosphate. Schliephake and colleagues [21] in a study in dogs compared the BIC of Ca-P and HA-coated implants and uncoated implants, finding no significant differences between the groups.
However, many studies have shown that Ca-P coatings improve the biocompatibility and fixation of implants; for example, Vercaigne and colleagues reported that Ca-P coatings are much more effective in stimulating the bone reaction than microroughness of surfaces [37], emphasizing that, in addition to the implant surface conditions, the bone reaction to an oral implant is determined by the local conditions at the implantation site, i.e., the presence of cortical or trabecular bone [38]. However, not all types of coatings achieve the same results. The coating technique is another important factor that can alter the solubility and stability of the coating [39]. Micro-coatings appear to improve fixation in the first few weeks by increasing the bone-to-implant contact surface [40], although these types of coatings tend to crack easily, detaching the coating and leading to implant failure [41,42]. Despite this, each technique has different advantages and disadvantages in terms of processing and outcome. However, coarse-grained coatings are the most prone to fracture of the bone-coating-metal substrate interface long after implantation, which has led to this type of implant falling into disuse in clinical practice. A study by Coelho and colleagues [43] compared the biological response of Ti alloy (Ti-6Al-4V) cylinders with Ca-P deposition cylinders in a dog model, determining the BIC using a computer program, and found no significant differences between the two surfaces compared in the first weeks of implantation.
Research seeks to improve the biomechanics of bone tissue by designing implants with improved biocompatibility, osteoconductivity and osteoinductivity, leading to faster and improved bone healing and turnover [44,45].
After implantation, the implant surfaces come into contact with biological fluids and tissues, and there are two types of host response: either forming an intermediate fibrous layer that does not guarantee adequate biomechanical fixation or direct bone-to-implant contact, ensuring osseointegration [4]. However, the actual process of osseointegration remains an unknown and little-studied mechanism, with genetics being identified as one of the inherent variables in the patient [46].
Numerous studies have shown that early fixation and long-term mechanical stability of fixtures are improved with rough profiles compared to smooth surfaces [47,48]; however, rough surfaces are more prone to generating pathologies around the implant tissues (peri-implantitis), and this would work against Ca-P-coated surfaces [49].
After implantation of Ca-P-coated fixtures, a layer of biological apatite is released on the implant surface that could serve as a matrix for adhesion and growth of osteogenic cells [50].
The studies included in our meta-analysis used different coating processes: oxidation [15,16], micro-coating (particle deposition) [18,19,20,21], and blasting (plasma spray) [20]. The study by Poulos and colleagues [17] used a proprietary coating method not described in the study; however, it has been described that the plasma spray technique is not very effective for coating dental implants with complex topographies [51], making it very difficult at this time to present a detailed discussion on the commercialization of Ca-P coatings, films, and layers on commercial Ti implants [52]. However, mechanical conditions are not the only requirement for promoting bone response. Implants with a thin Ca-P coating resulted in the highest amount of bone contact, but it is difficult to give a definitive explanation for the coating effect of Ca-P ceramics [36].
Finally, it should be added that we encountered some serious limitations related to this meta-analysis: firstly, the small number of studies included and therefore the limited number of implants studied; second, the high risk of bias of all studies; third, the substantial heterogeneity of the selected studies. This did not allow any solid conclusions to be drawn.
5. Conclusions
Within the aforementioned limitations, it can be concluded that Ca-P-coated Ti surfaces have a similar osseointegration power to conventional etched surfaces (SLA or similar). However, in order to confirm our results, well-structured, well-conducted studies with larger samples and longer follow-ups are necessary.
Author Contributions
Study concept and design, N.L.-V and A.L.-V.; data collection (literature search and study selection), N.L.-V., J.M.A. and J.M.R.; data analysis and interpretation (literature), B.M.d.S. and J.M.R.; drafting of the manuscript, N.L.-V.; A.L.-V. and B.M.d.S.; critical revision of the manuscript for important intellectual content, A.L.-V., M.J.R. and J.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| BA | Bone area |
| BCa-P | Bicalcium phosphate |
| BD | Bone density |
| BIC | Bone-to-implant contact |
| Ca-P | Calcium phosphate |
| CAO | Calcium oxide |
| HA | Hydroxyapatite |
| MeSH | Medical Subject Headings |
| SLA | Sandblasted large grit acid etched |
Appendix A

Table A1.
PRISMA Checklist.
Table A1.
PRISMA Checklist.
| Section/Topic | # | Checklist Item | Reported on Page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 2 |
| METHODS | |||
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 2 |
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 2 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 2 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 2 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 2 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 8 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 2 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 6 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 3 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 7,8 |
References
- Kaur, M.; Singh, K. Review on titanium and titanium-based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Bennardo, F.; Antonelli, A.; Barone, S.; Wagner, F.; Fortunato, L.; Traxler, H. Influence of clinician’s skill on primary implant stability with conventional and piezoelectric preparation techniques: An ex-vivo study. J. Biol. Regul. Homeost. Agents 2020, 34, 739–745. [Google Scholar]
- Orsini, G.; Piattelli, M.; Scarano, A.; Petrone, G.; Kenealy, J.; Piattelli, A.; Caputi, S. Randomized, controlledhistologic and histomorphometric evaluation of implants with nanometer-scale calcium phosphate added to the dual acid-etched surface in the human posterior maxilla. J. Periodontol. 2007, 78, 209–218. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Winge, M.I.; Reikerås, O.; Røkkum, M. Calcium phosphate bone cement: A possible alternative to autologous bone graft. A radiological and biomechanical comparison in rat tibial bone. Arch. Orthop. Trauma Surg. 2011, 131, 1035–1041. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Tache, A.; Valiquette, N.; Deporter, D.; Pilliar, R. Calcium phosphate sol–gel- derived thin films on porous-surfaced implants for enhanced osteoconductivity Part II: Short-term in vivo studies. Biomaterials 2004, 25, 5313–5321. [Google Scholar] [CrossRef]
- Rey, C. Calcium phosphate biomaterials and bone mineral. Differences in composition, structures and properties. Biomaterials 1990, 11, 13–15. [Google Scholar] [PubMed]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Wang, D.; Tabassum, A.; Wu, G.; Deng, L.; Wismeijer, D.; Liu, Y. Bone regeneration in critical-sized bone defect enhanced by introducing osteoinductivity to biphasic calcium phosphate granules. Clin. Oral Implant. Res. 2017, 28, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ambard, A.J.; Mueninghoff, L. Calcium phosphate cement: Review of mechanical and biological properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Stadlinger, B.; Pourmand, P.; Locher, M.C.; Schulz, M.C. Systematic review of animal models for the study of implant integration, assessing the influence of material, surface and design. J. Clin. Periodontol. 2012, 39, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Koh, J.W.; Kim, Y.S.; Yang, J.H.; Yeo, I.S. Effects of a calcium phosphate-coated and anodized titanium surface on early bone response. Int. J. Oral Maxillofac Implant. 2013, 28, 790–797. [Google Scholar] [CrossRef][Green Version]
- Fontana, F.; Rocchietta, I.; Addis, A.; Schupbach, P.; Zanotti, G.; Simion, M. Effects of a calcium phosphate coating on the osseointegration of endosseous implants in a rabbit model. Clin. Oral Implant. Res. 2011, 22, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Poulos, N.M.; Rodriguez, N.A.; Lee, J.; Rueggeberg, F.A.; Schüpbach, P.; Hall, J.; Susin, C.; Wikesjö, U.M. Evaluation of a novel calcium phosphate-coated titanium porous oxide implant surface: A study in rabbits. Int. J. Oral Maxillofac Implant. 2011, 26, 731–738. [Google Scholar]
- Quaranta, A.; Iezzi, G.; Scarano, A.; Coelho, P.G.; Vozza, I.; Marincola, M.; Piattelli, A. A histomorphometric study of nanothickness and plasma-sprayed calcium-phosphorous-coated implant surfaces in rabbit bone. J. Periodontol. 2010, 81, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Fügl, A.; Ulm, C.; Tangl, S.; Vasak, C.; Gruber, R.; Watzek, G. Long-term effects of magnetron-sputtered calcium phosphate coating on osseointegration of dental implants in non-human primates. Clin. Oral Implant. Res. 2009, 20, 183–188. [Google Scholar]
- Le Guehennec, L.; Goyenvalle, E.; Lopez-Heredia, M.A.; Weiss, P.; Amouriq, Y.; Layrolle, P. Histomorphometric analysis of the osseointegration of four different implant surfaces in the femoral epiphyses of rabbits. Clin. Oral Implant. Res. 2008, 19, 1103–1110. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D.; Roesseler, S.; Dard, M.; Sewing, A.; Aref, A. Biomimetic calcium phosphate composite coating of dental implants. Int. J. Oral Maxillofac Implant. 2006, 21, 738–746. [Google Scholar]
- Caulier, H.; van der Waerden, J.P.; Wolke, J.G.; Kalk, W.; Naert, I.; Jansen, J.A. A histological and histomorphometrical evaluation of the application of screw-designed calciumphosphate (Ca-P)-coated implants in the cancellous maxillary bone of the goat. J. Biomed. Mater. Res. 1997, 35, 19–30. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; van Oirschot, B.A.; Bosco, R.; van den Beucken, J.J.; Aldosari, A.A.; Anil, S.; Jansen, J.A. Biological response to titanium implants coated with nanocrystals calcium phosphate or type 1 collagen in a dog model. Clin. Oral Implant. Res. 2013, 24, 475–483. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Noordin, S.; Masri, B.A.; Garbuz, D.S.; Duncan, C.P.; Hancock, R.E.; Wang, R. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Tovar, N.; Witek, L.; Marin, C.; Silva, N.R.; Bonfante, E.A.; Coelho, P.G. Osseointegration assessment of chairside argon-based nonthermal plasma-treated Ca-P coated dental implants. J. Biomed. Mater. Res. A 2013, 101, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, C.; Wang, Y.; Wang, Y. Early bone apposition and 1-year performance of the electrodeposited calcium phosphate coatings: An experimental study in rabbit femora. Clin. Oral Implant. Res. 2010, 21, 951–960. [Google Scholar] [CrossRef]
- Junker, R.; Manders, P.J.; Wolke, J.; Borisov, Y.; Jansen, J.A. Bone reaction adjacent to microplasma-sprayed CaP-coated oral implants subjected to occlusal load, an experimental study in the dog. Part I: Short-term results. Clin. Oral Implant. Res. 2010, 21, 1251–1263. [Google Scholar] [CrossRef]
- Junker, R.; Manders, P.J.; Wolke, J.; Borisov, Y.; Braceras, I.; Jansen, J.A. Bone reaction adjacent to microplasma-sprayed calcium phosphate-coated oral implants subjected to an occlusal load, an experimental study in the dog. Clin. Oral Implant. Res. 2011, 22, 135–142. [Google Scholar] [CrossRef]
- Schwarz, M.L.; Kowarsch, M.; Rose, S.; Becker, K.; Lenz, T.; Jani, L. Effect of surface roughness, porosity, and a resorbable calcium phosphate coating on osseointegration of titanium in a minipig model. J. Biomed. Mater. Res. A 2009, 89, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Manders, P.J.; Wolke, J.G.; Jansen, J.A. Bone response adjacent to calcium phosphate electrostatic spray deposition coated implants: An experimental study in goats. Clin. Oral Implant. Res. 2006, 17, 548–553. [Google Scholar] [CrossRef]
- Schopper, C.; Moser, D.; Goriwoda, W.; Ziya-Ghazvini, F.; Spassova, E.; Lagogiannis, G.; Auterith, A.; Ewers, R. The effect of three different calcium phosphate implant coatings on bone deposition and coating resorption: A long-term histological study in sheep. Clin. Oral Implant. Res. 2005, 16, 357–368. [Google Scholar] [CrossRef]
- Stewart, M.; Welter, J.F.; Goldberg, V.M. Effect of hydroxyapatite/tricalcium-phosphate coating on osseointegration of plasma-sprayed titanium alloy implants. J. Biomed. Mater. Res. A. 2004, 69, 1–10. [Google Scholar] [CrossRef]
- Yoshinari, M.; Watanabe, Y.; Ohtsuka, Y.; Dérand, T. Solubility control of thin calcium-phosphate coating with rapid heating. J. Dent. Res. 1997, 76, 1485–1494. [Google Scholar] [CrossRef]
- Fuming, H.; Guoli, Y.; Xiaoxiang, W.; Shifang, Z. The removal torque of titanium implant inserted in rabbit femur coated with biomimetic deposited Ca-P coating. J. Oral Rehabil. 2008, 35, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Schouten, C.; Meijer, G.J.; van den Beucken, J.J.; Leeuwenburgh, S.C.; de Jonge, L.T.; Wolke, J.G.; Spauwen, P.H.; Jansen, J.A. In vivo bone response and mechanical evaluation of electrosprayed CaP nanoparticle coatings using the iliac crest of goats as an implantation model. Acta Biomater. 2010, 6, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Yoshinari, M.; Kiba, H.; Yamamoto, H.; Nemoto, K.; Jansen, J.A. Trabecular bone response to surface roughened and calcium phosphate (Ca-P) coated titanium implants. Biomaterials 2002, 23, 1025–1031. [Google Scholar] [CrossRef]
- Vercaigne, S.; Wolke, J.G.C.; Naert, I.; Jansen, J.A. Bone healing capacity of titanium plasma-sprayed and hydroxylapatite-coated oral implants. Clin. Oral Implant. Res. 1998, 9, 261–271. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yoshinari, M.; Nemoto, K.; Wolke, J.G.C.; Jansen, J.A. Effect of surface roughness and calcium phosphate coating on the implant/bone response. Clin. Oral Implant. Res. 2000, 11, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Sergey, V.D. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar]
- Stellino, G.; Landi, L. A 6-year unloaded hydroxyapatite-coated dental implant placed into an extraction socket in conjunction with nonresorbable hydroxyapatite grafting material: Histologic evaluation. Int. J. Periodontics Restor. Dent. 2002, 22, 575–581. [Google Scholar]
- Yang, Y.L.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef]
- Lacefield, W.R. Current status of ceramic coatings for dental implants. Implant. Dent. 1998, 7, 315–322. [Google Scholar] [CrossRef]
- Coelho, P.G.; Cardaropoli, G.; Suzuki, M.; Lemons, J.E. Early healing of nanothickness bioceramic coatings on dental implants. An experimental study in dogs. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 88, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Abrahamsson, I.; Albouy, J.P.; Lindhe, J. Bone healing at implants with a fluoride-modified surface: An ex- perimental study in dogs. Clin. Oral Implant. Res. 2007, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.C.; Moineddin, R.; Davies, J.E. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials 2007, 28, 4748–4755. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Zarb, G.A. On osseointegration: The healing adaptation principle in the context of osseosufficiency, osseoseparation, and dental implant failure. Int. J. Prosthodont. 2012, 25, 48–52. [Google Scholar] [PubMed]
- Buser, D.; Schenk, R.; Steinemann, S.; Fiorellini, J.; Fox, C.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Gotfredsen, K.; Wennerberg, A.; Johansson, C.; Skovgaard, L.T.; Hjorting-Hansen, E. Anchorage of TiO2 -blasted, HA-coated, and machined implants: An experimental study with rabbits. J. Biomed. Mater. Res. 1995, 29, 1223–1231. [Google Scholar] [CrossRef]
- De Bruyn, H.; Christiaens, V.; Doornewaard, R.; Jacobsson, M.; Cosyn, J.; Jacquet, W.; Vervaeke, S. Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontology 2000 2017, 73, 218–227. [Google Scholar] [CrossRef]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings, films and layers. Prog. Biomater. 2012, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Paital, S.R.; Dahotre, N.B. Wettability and kinetics of hydroxyapatite precipitation on a laser-textured Ca–P bioceramic coating. Acta Biomater. 2009, 5, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).