SBR Vulcanizates Filled with Modified Ground Tire Rubber
Abstract
:1. Introduction
2. Experimental
2.1. Methods of GTR Powder Modification
2.1.1. Oxidative Treatment
2.1.2. Silane Treatment
2.1.3. Hybrid Treatment
2.2. Preparation of Rubber Mixes
2.3. Characterization of GTR
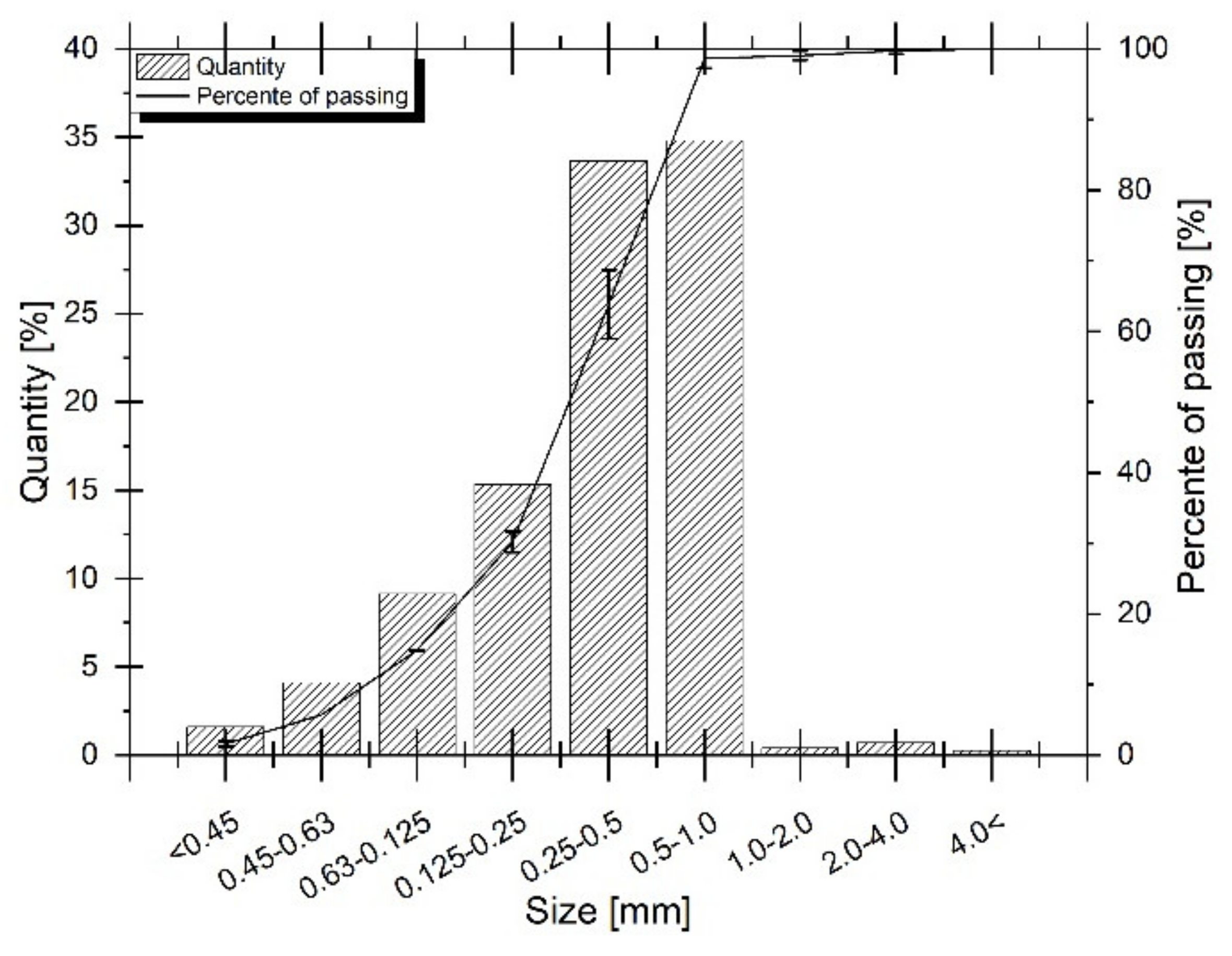
2.3.1. Particle Size Distribution
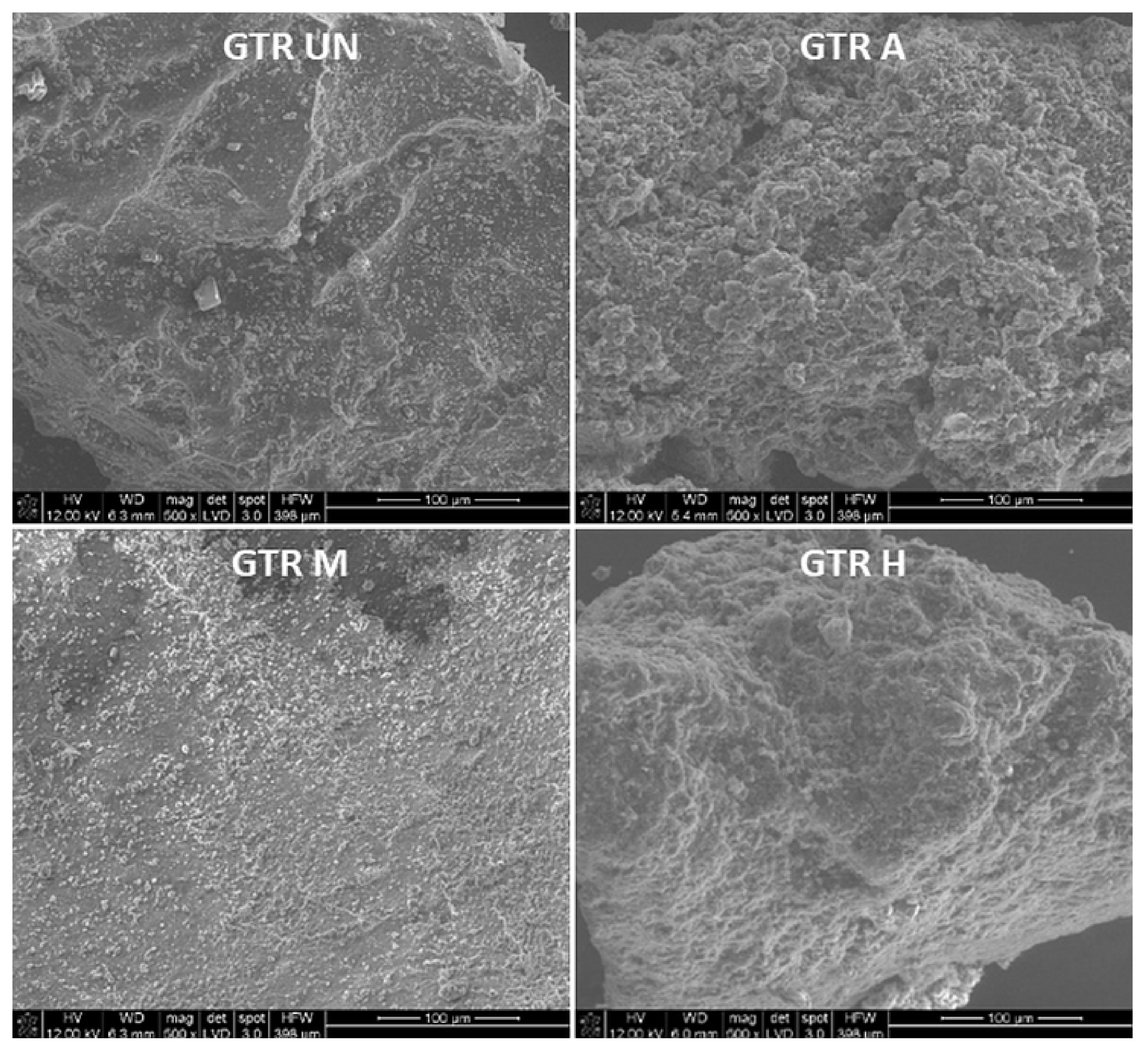
2.3.2. Particle Shape
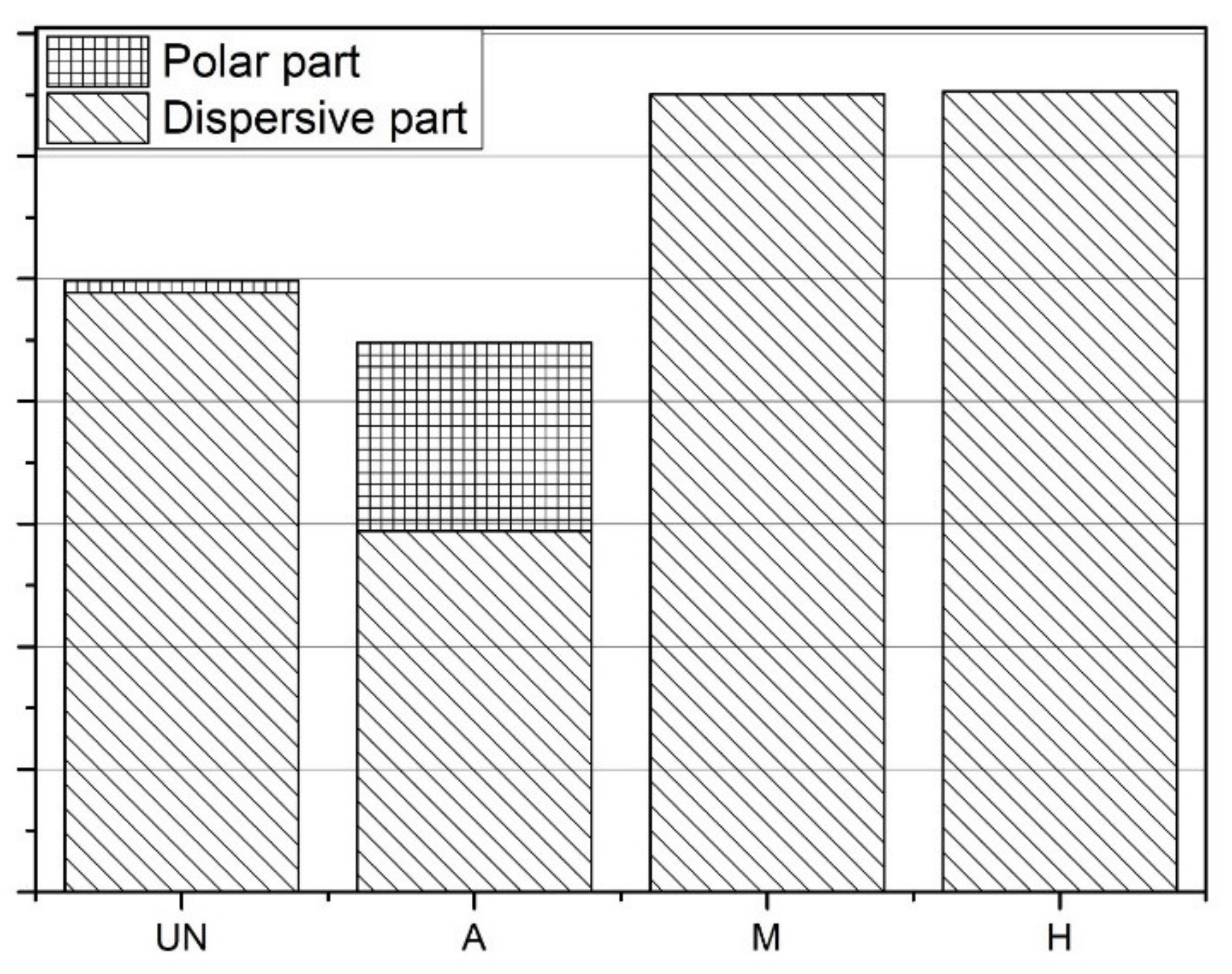
2.3.3. Surface Polarity
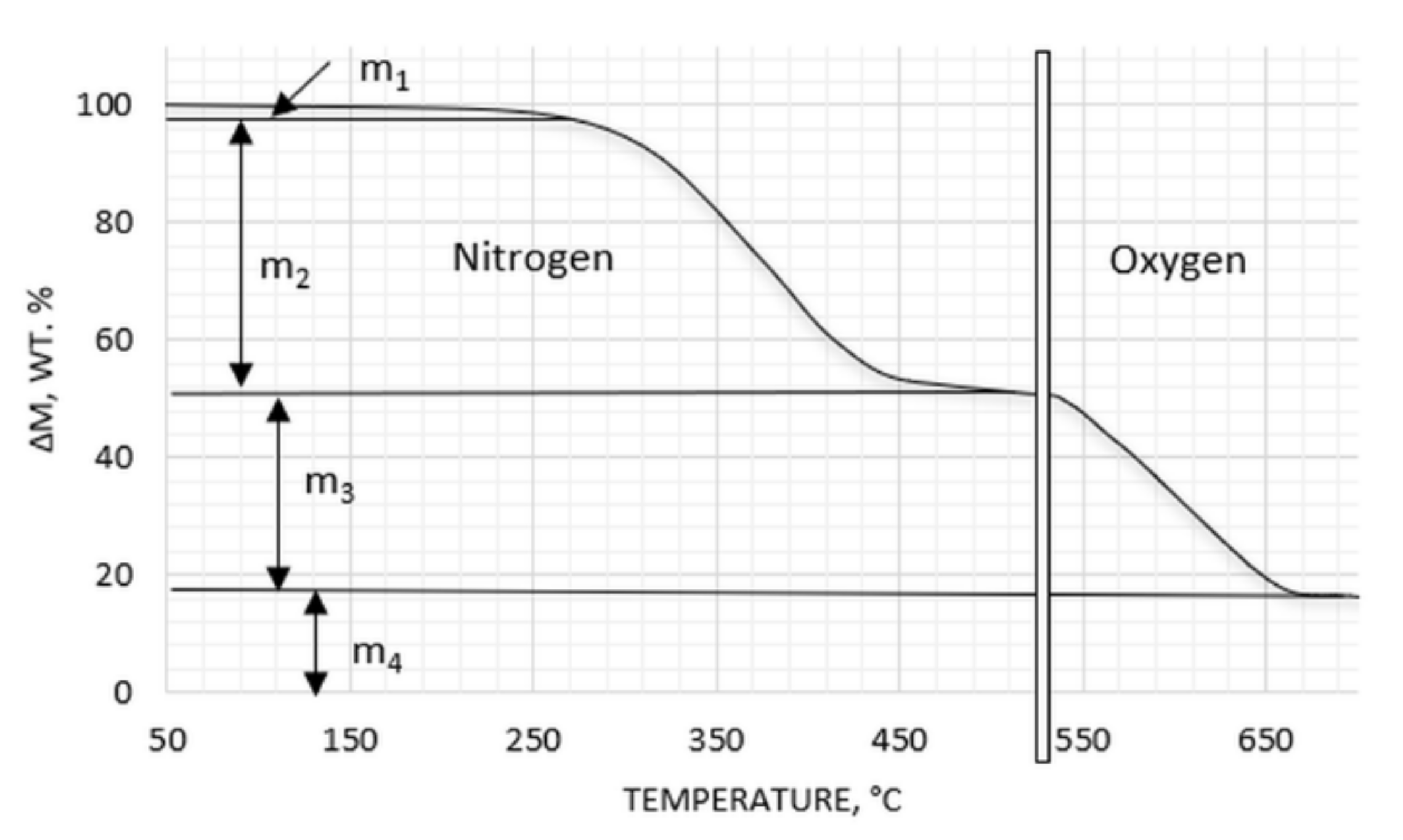
2.3.4. Chemical Activity
2.3.5. Specific Surface Area
2.3.6. Morphology and Composition of the Surface
2.3.7. Composition
2.4. Characterization of Rubber Vulcanizates
2.4.1. Kinetic of Vulcanization
2.4.2. Sample Preparation
2.4.3. Degree of Rubber Crosslinking
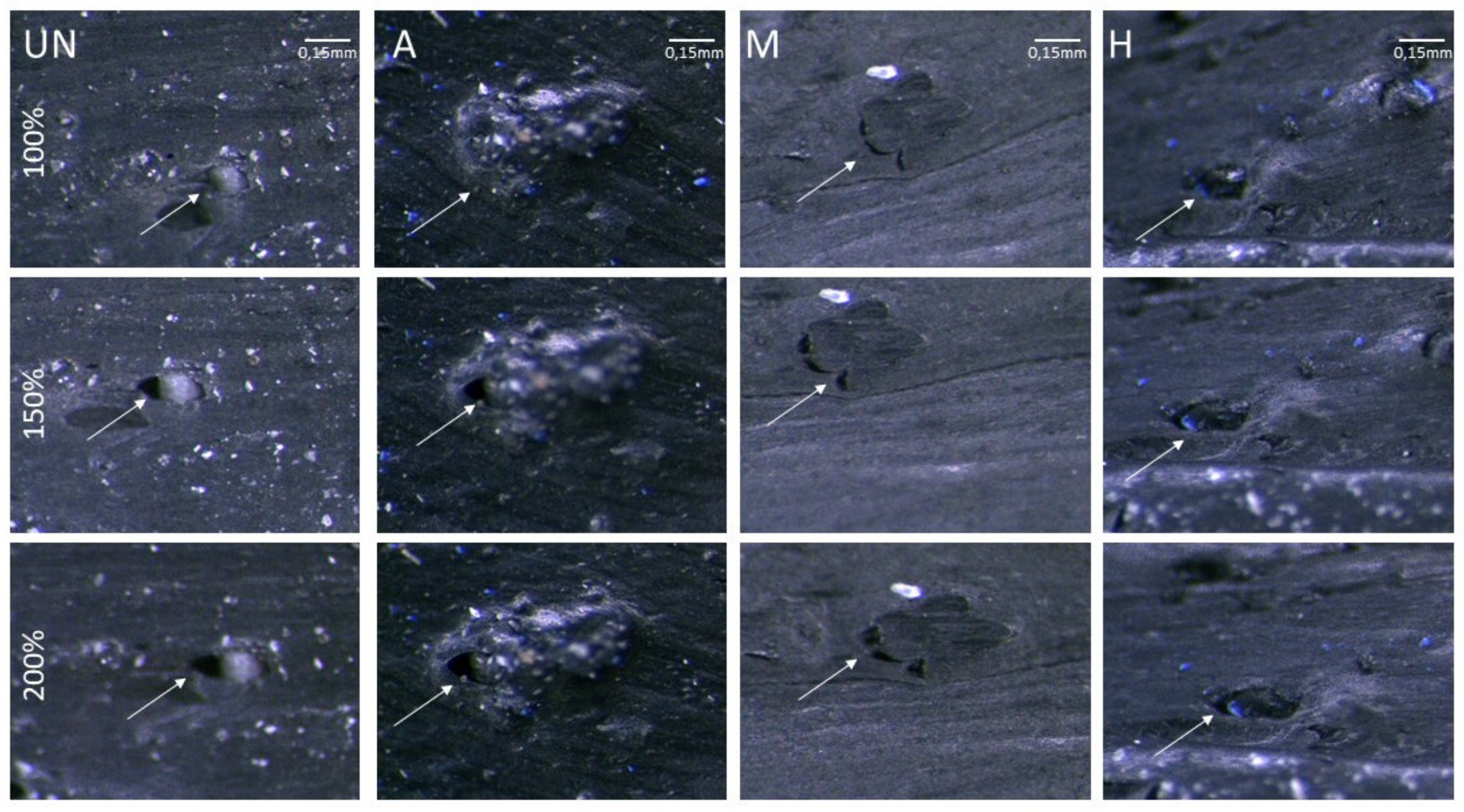
2.4.4. GTR–Polymer Matrix Compatibility
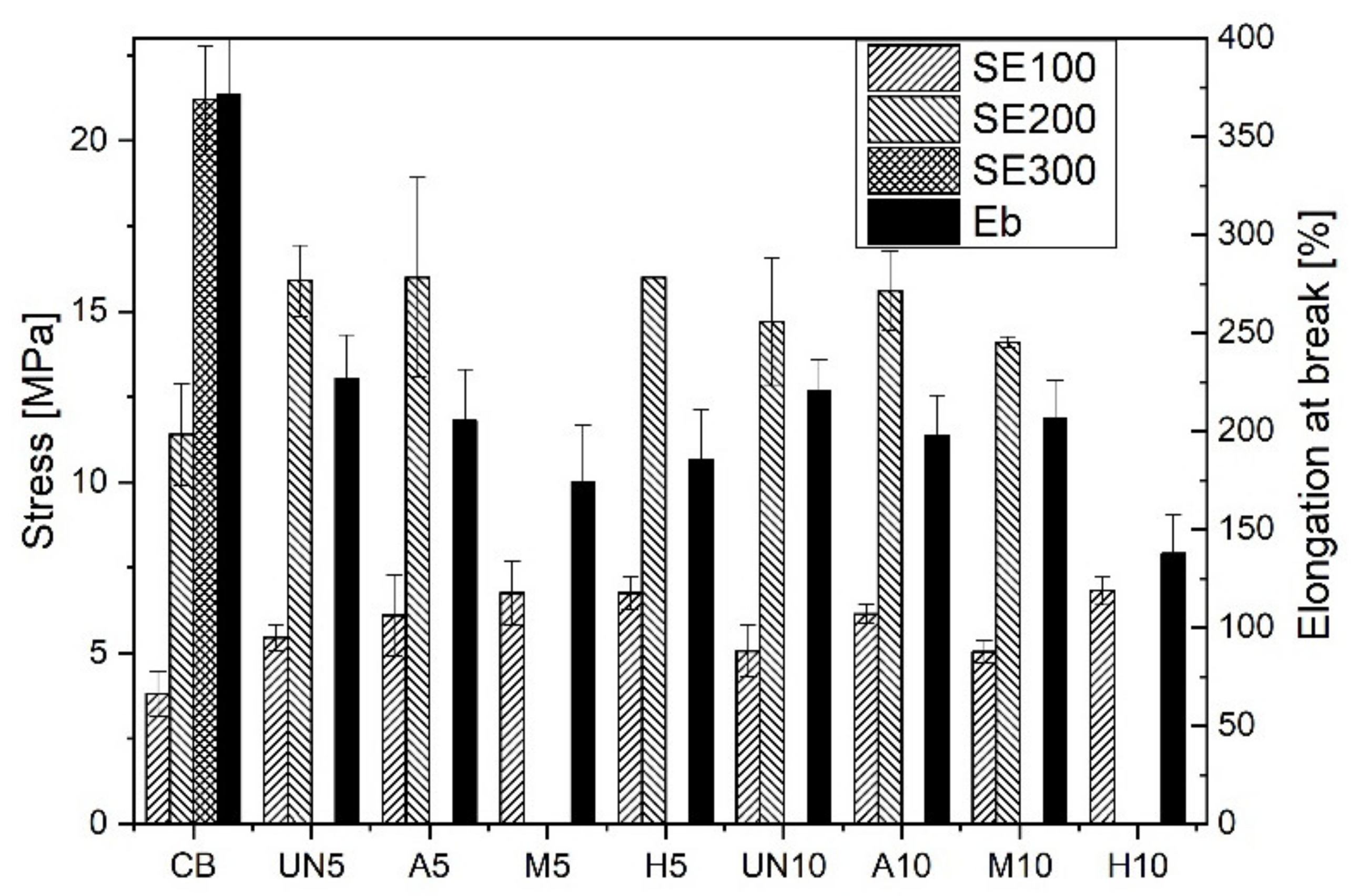
2.4.5. Mechanical Properties of Rubber Vulcanizates
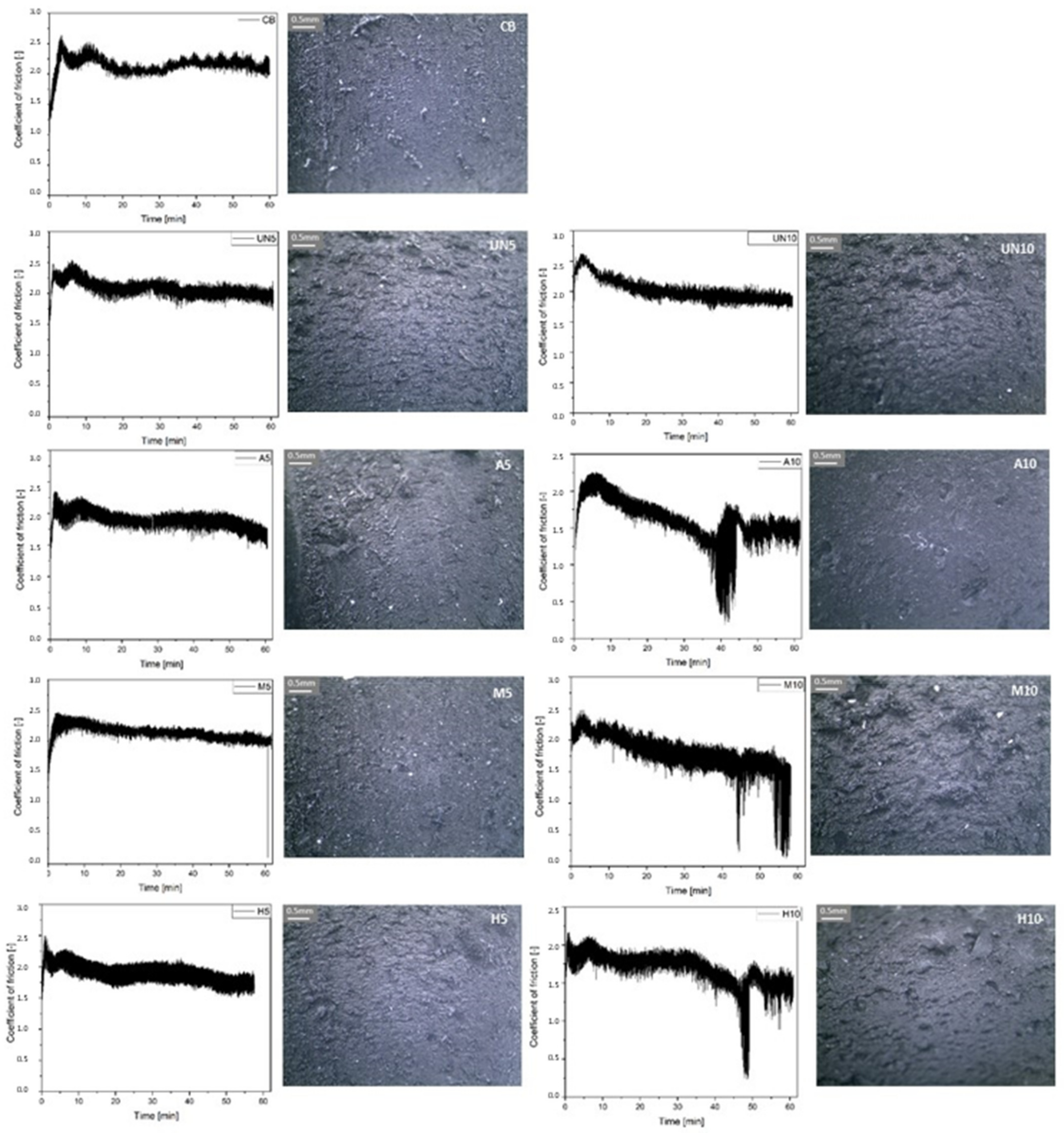
2.4.6. Tribological Properties of Rubber Vulcanizates
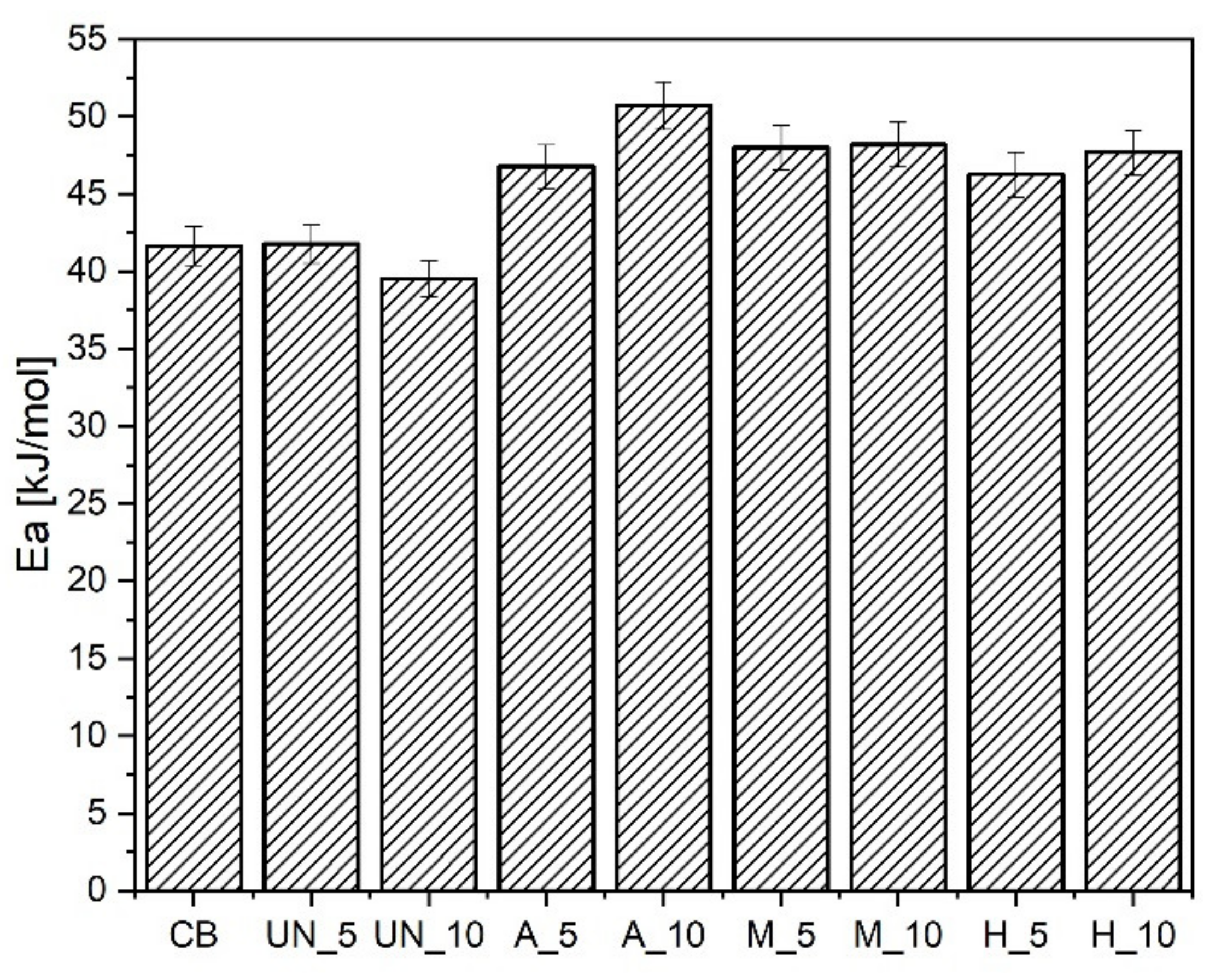
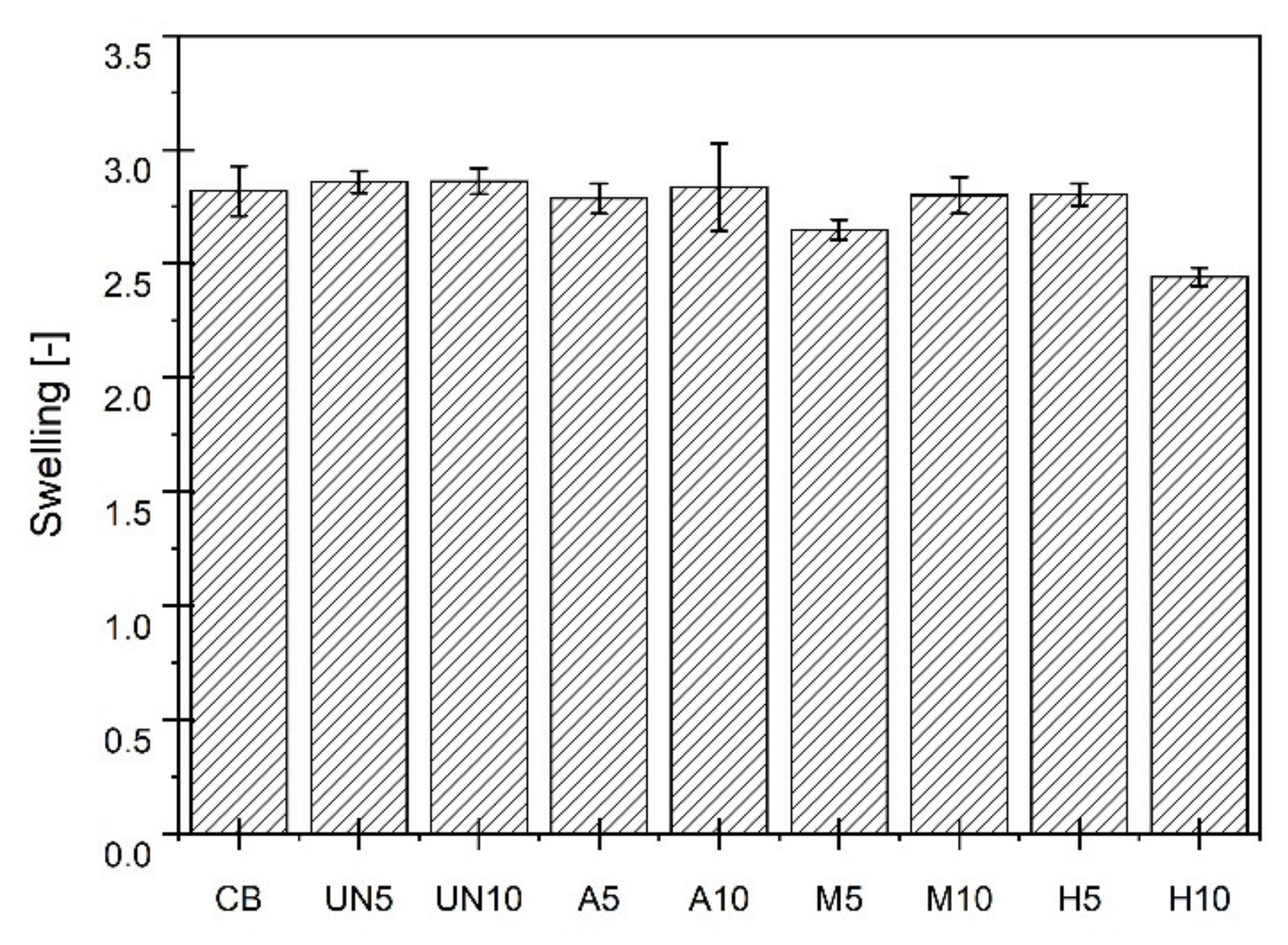
3. Results and Discussions
Characterization of GTR
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wojciechowski, A.; Doliński, A.; Żmuda, W. Thermal decomposition of tires—carbonizate treatment. In Proceedings of the XVIIIth International Scientific Conference “Computer Aided Systems for Science, Industry and Transport” TransComp 2014. Logistyka 2014, 6, 11279–11288. (In Polish) [Google Scholar]
- Duda, A.; Sobala, D. Testing tyre bales from compressed used car tyres for use in construction. Builder 2017, 12, 74–77. (In Polish) [Google Scholar]
- CWA 14243-2002. Products from Recycling of Used Tires and Their Applications. Available online: https://shop.bsigroup.com/ProductDetail?pid=000000000030078060 (accessed on 4 May 2021).
- Gronowicz, J.; Kubiak, T. Recycling of used tyres. J. Mach. Constr. Maint. 2007, 2, 5–17. (In Polish) [Google Scholar]
- Kamińska, E.; Skarbek-Żabkin, A. Eco-balance analysis in estimating environmental burdens. Mot. Transp. 2015, 1, 49–66. (In Polish) [Google Scholar]
- Mroziński, A.; Flizikowski, J. Product utility of tire shredding in recycling. Chem. Eng. Equip. 2012, 2, 37–38. (In Polish) [Google Scholar]
- Liu, H.S.; Mead, J.L.; Stacer, R.G. Environmental Effects of Recycled Rubber in Light-Fill Applications. Rubber Chem. Technol. 2000, 73, 551–564. [Google Scholar] [CrossRef]
- Herczeg, I.; Dietrich, O. Cold grinding—Recycling of used tyres and rubber scrap. Műanyag Gumi 2005, 42, 267–268. (In Hungarian) [Google Scholar]
- Burford, R.P.; Pittolo, M. Characterization and Performance of Powdered Rubber. Rubber Chem. Technol. 1982, 55, 1233–1249. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Meszaros, L.; Barany, T. Ground tyre rubber (GTR) in thermoplastics, thermosets, and rubbers. J. Mater. Sci. 2013, 48, 1–38. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Rodrigue, D. Highly filled thermoplastic elastomers from ground tire rubber, maleated polyethylene and high density polyethylene. Plast. Rubber Compos. 2013, 42, 115–122. [Google Scholar] [CrossRef]
- Carli, L.N.; Boniatti, R.; Teixeira, C.E.; Nunes, R.C.R.; Crespo, J.S. Development and characterization of composites with ground elastomeric vulcanized scraps as filler. Mater. Sci. Eng. C 2009, 29, 383–386. [Google Scholar] [CrossRef]
- Carli, L.N.; Bianchi, O.; Mauler, R.S.; Crespo, J.S. Accelerated aging of elastomeric composites with vulcanized ground scraps. J. Appl. Polym. Sci. 2012, 123, 280–285. [Google Scholar] [CrossRef]
- Yehia, A.A.; Mull, M.A.; Ismail, M.N.; Hefeny, Y.A.; Abdel-Bary, E.M. Effect of chemically modified waste rubber powder as a filler in natural rubber vulcanizates. J. Appl. Polym. Sci. 2004, 93, 30–36. [Google Scholar] [CrossRef]
- Kolinski, A.; Barnes, T.; Paszkowski, G.; Haber, A. Modified tyre-rubber crumb as a base compound for rubber parts manufacturing. Conf. Preprints of the 156th ACS Rubber Division Meeting—Fall 1999, Orlando (FL) 21-23.09.1999, Paper 106. In Tyre Compounding for Improved Performance; Evans, M.S., Ed.; iSmithers Rapra Publishing: Shropshire, UK, 2002; vol. 12. [Google Scholar]
- Formela, K.; Hejna, A.; Zedler, Ł.; Colom, X.; Cañavate, J. Microwave treatment in waste rubber recycling—recent advances and limitations. Express Polym. Lett. 2019, 13, 565–588. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Liang, M.; Lu, C. Improvement of the properties of ground tire rubber (GTR)—filled nitrile rubber vulcanizates through plasma surface modification of GTR powder. J. Appl. Polym. Sci. 2009, 114, 1118–1125. [Google Scholar] [CrossRef]
- Kosmalska, A.; Zaborski, M.; Ślusarski, L. Adsorption of curatives and activity of silica toward elastomers. Macromol. Symp. 2003, 194, 269–276. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Carrillo, F.; Velasco, J.I.; Pagès, P.; Mujal, R.; Noguésa, F. Structural and mechanical studies on modified reused tyres composites. Eur. Polym. J. 2006, 42, 2369–2378. [Google Scholar] [CrossRef]
- Colom, X.; Martin-Genesca, M.; Formela, K.; Cañavate, J. Surface Treatment of Rubber Waste. Ch. 2 in Green Chem. Ser. No. 59: Rubber Recycling: Challenges and Developments; Kim, J.K., Saha, P., Haponiuk, J.T., Aswathi, M.K., Thomas, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 39–43. [Google Scholar]
- Borggreve, R.J.M.; Gaymans, R.J. Impact behaviour of nylon-rubber blends: 4. Effect of the coupling agent, maleic anhydride. Polymer 1989, 30, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Stark, F.J.; Wagner, D.P.; Fesus, E.; Jakush, E. Surface treated recycled crumb rubber. Conf. Preprints of the 156th ACS Rubber Division Meeting—Fall 1999, Orlando (FL) 21-23.09.1999, Paper 84. In Tyre Compounding for Improved Performance; Evans, M.S., Ed.; iSmithers Rapra Publishing: Shropshire, UK, 2002; vol. 12. [Google Scholar]
- Sahakaro, K.; Beraheng, S. Reinforcement of maleated natural rubber by precipitated silica. J. Appl. Polym. Sci. 2008, 109, 3839–3848. [Google Scholar] [CrossRef]
- Adhikari, J.; Das, A.; Sinha, T.; Saha, P.; Kim, J.K. Rubber Recycling: Challenges and Developments: Grinding of Waste Rubber; Royal Society of Chemistry: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Ajay, K.; Manna, A.K.; Bhattacharyya, A.K.; De, P.P.; Tripathy, D.K.; De, S.K.; Peiffer, D.G. Effect of surface oxidation of filler and silane coupling agent on the chemorheological behavior of epoxidized natural rubber filled with ISAF carbon black. J. Appl. Polym. Sci. 1999, 71, 557–563. [Google Scholar] [CrossRef]
- Hassa-Załoba, A.; Tomaszewska, M.; Mężyński, J.; Bieliński, D.M.; Gozdek, T.; Sicinski, M. Rubber mixes and rubber with devulcanized rubber powder. Part 2. Modification of rubber powder and granulate. Elastomery 2015, 19, 8–17. Available online: http://elastomery.pl/wp-content/uploads/streszczenia-pdf/E2015_4_Hassa-Zaloba.pdf (accessed on 2 February 2021). (In Polish).
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Esmizadeh, E.; Naderi, G.; Barmar, M. Effect of Organo-clay on Properties and Mechanical Behavior of Fluorosilicone Rubber. Fiber Polym. 2014, 15, 2376–2385. [Google Scholar] [CrossRef]
- Vahidifar, A.; Esmizadeh, E.; Rodrigue, D. Effect of the simultaneous curing and foaming kinetics on the morphology development of polyisoprene closed cell foams. Elastomery 2018, 22, 3–18. Available online: http://elastomery.pl/wp-content/uploads/streszczenia-pdf/E2018_1_Rodrigue.pdf (accessed on 2 February 2021).
- Kamal, M.R.; Sourour, S. Kinetics and thermal characterization of thermoset cure. Polym. Eng. Sci. 1973, 13, 59–64. [Google Scholar] [CrossRef]
- T-05 Block-On Ring Wear Tester for Evaluation of Lubricants and Engineering Materials. Available online: http://www.katalog.itee.radom.pl/images/stories/karty/58.t-05_ang.pdf (accessed on 4 May 2021).
- Colom, X.; Carrillo, F.; Canavate, J. Composites reinforced with reused tyres: Surface oxidant treatment to improve the interfacial compatibility. Compos. Part A Appl. Sci. 2007, 38, 44–50. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Verdugo-Manzanares, R.; González, S.; Verdejo, R.; Lopez-Manchado, M.A.; Santana, M.H. On the Use of Mechano-Chemically Modified Ground Tire Rubber (GTR) as Recycled and Sustainable Filler in Styrene-Butadiene Rubber (SBR) Composites. J. Compos. Sci. 2021, 5, 68. [Google Scholar] [CrossRef]
- Jong-Yeop, L.; Nam, P.; Seokhwan, L.; Byeongkyu, A.; Woong, K.; Hyunsung, M.; Hyun-jong, P.; Wonho, K. Influence of the silanes on the crosslink density and crosslink structure of silica-filled solution styrene butadiene rubber compounds. Compos. Interfaces 2017, 24, 711–727. [Google Scholar] [CrossRef]
- Sae-oui, P.; Thepsuwan, U.; Hatthapanit, K. Effect of curing system on reinforcing efficiency of silane coupling agent. Polym. Test. 2004, 23, 397–403. [Google Scholar] [CrossRef]
- Gamez, J.F.H.; Hernandez, E.H.; Cespedes, R.I.N.; Velázquez, M.G.N.; Rosales, S.G.S.; Corral, F.S.; Morones, P.G.; Tavizón, S.F.; de Leon, R.D.; Cepeda, L.F. Mechanical reinforcement of thermoplastic vulcanizates using ground tyre rubber modified with sulfuric acid. Polym. Compos. 2016, 39. [Google Scholar] [CrossRef]
- Hernández, E.H.; Gámez, J.F.H.; Cepeda, L.F.; Muñoz, E.J.C.; Corral, F.S.; Rosales, S.G.S.; Velázquez, G.N.; Morones, P.G.; Martínez, D.I.S. Sulfuric acid treatment of ground tire rubber and its effect on the mechanical and thermal properties of polypropylene composites. J. Appl. Polym. Sci. 2017, 134, 44864. [Google Scholar] [CrossRef]
- Cacopardo, L.; Mattei, G.; Ahluwalia, A. A new load-controlled testing method for viscoelastic characterisation through stress-rate measurements. Materialia 2020, 9, 100552. [Google Scholar] [CrossRef]
- Torbati-Fard, N.; Hosseini, S.M.; Razzaghi-Kashani, M. Effect of the silica-rubber interface on the mechanical, viscoelastic, and tribological behaviors of filled styrene-butadiene rubber vulcanizates. Polym. J. 2020, 52, 1223–1234. [Google Scholar] [CrossRef]
- Bielinski, D.M.; Glab, P.; Slusarski, L.; Boiteux, G.; Chapel, J.-P. Surface migration of carboxylic acid in styrene–butadiene rubber and its tribological consequences. J. Appl. Polym. Sci. 2002, 86, 3368–3376. [Google Scholar] [CrossRef]
- Bieliński, D.M.; Mężyński, J.; Tomaszewska, M.; Hassa-Żałoba, A. Rubber Powder and Its Application to Elastomers; Institute for Engineering of Polymer Materials and Dyes: Toruń, Poland, 2015. [Google Scholar]



| CB | 5 Parts of Rubber from GTR | 10 Parts of Rubber from GTR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| UN | A | M | H | UN | A | M | H | ||
| SBR Ker 1500 | 100.0 | 95.0 | 90.0 | ||||||
| Stearic acid | 1.0 | ||||||||
| Zinc oxide | 3.0 | ||||||||
| CBS | 1.0 | ||||||||
| Sulfur | 2.0 | ||||||||
| Carbon Black N330 | 50.0 | 44.0 | 44.2 | 44.0 | 43.3 | 38.0 | 38.4 | 38.1 | 36.6 |
| GTR | - | 11.5 | 12.4 | 11.6 | 12.9 | 23.0 | 24.8 | 23.2 | 25.7 |
| GTR Sample | SSA [m2/g] |
|---|---|
| UN | 0.0731 ± 0.0075 |
| A | 0.6202 ± 0.0310 |
| M | 0.0402 ± 0.0097 |
| H | 1.2104 ± 0.0179 |
| Phase | GTR | |||
|---|---|---|---|---|
| UN | A | M | H | |
| Low molecular weight organic substances/water, wt. % | 4.5 | 13.0 | 5.5 | 9.0 |
| Rubber, wt. % | 43.4 | 40.2 | 43.1 | 38.9 |
| Carbon black and mineral substances, wt. % | 52.1 | 46.8 | 51.4 | 52.1 |
| Composition [at. %] | GTR | |||
|---|---|---|---|---|
| UN | A | M | H | |
| Carbon | 90.24 | 93.57 | 88.21 | 91.07 |
| Oxygen | 4.87 | 3.95 | 7.04 | 5.18 |
| Silicon | 0.49 | 0.31 | 1.93 | 0.74 |
| Sulfur | 1.01 | 1.06 | 1.33 | 1.66 |
| Parameter Sample | ML [dNm] | MH [dNm] | ∆M | ts2 [min] | t90 [min] | CRI [%/min] |
|---|---|---|---|---|---|---|
| CB | 2.6 | 20.6 | 18.0 | 4.6 | 18.2 | 8.6 |
| UN_5 | 2.6 | 19.1 | 16.5 | 4.4 | 14.5 | 9.9 |
| UN_10 | 2.7 | 18.4 | 15.7 | 4.1 | 13.3 | 10.8 |
| A_5 | 2.7 | 20.7 | 18.0 | 4.3 | 15.8 | 8.7 |
| A_10 | 3.0 | 20.8 | 17.8 | 4.0 | 15.7 | 8.6 |
| M_5 | 2.7 | 19.2 | 16.5 | 4.3 | 14.9 | 9.4 |
| M_10 | 3.1 | 17.6 | 14.5 | 3.9 | 13.4 | 10.5 |
| H_5 | 2.6 | 20.7 | 18.1 | 4.2 | 16.2 | 7.2 |
| H_10 | 2.8 | 19.5 | 16.7 | 4.2 | 16.5 | 8.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klajn, K.; Gozdek, T.; Bieliński, D.M.; Siciński, M.; Zarzecka-Napierała, M.; Pędzich, Z. SBR Vulcanizates Filled with Modified Ground Tire Rubber. Materials 2021, 14, 3991. https://doi.org/10.3390/ma14143991
Klajn K, Gozdek T, Bieliński DM, Siciński M, Zarzecka-Napierała M, Pędzich Z. SBR Vulcanizates Filled with Modified Ground Tire Rubber. Materials. 2021; 14(14):3991. https://doi.org/10.3390/ma14143991
Chicago/Turabian StyleKlajn, Katarzyna, Tomasz Gozdek, Dariusz M. Bieliński, Mariusz Siciński, Magdalena Zarzecka-Napierała, and Zbigniew Pędzich. 2021. "SBR Vulcanizates Filled with Modified Ground Tire Rubber" Materials 14, no. 14: 3991. https://doi.org/10.3390/ma14143991
APA StyleKlajn, K., Gozdek, T., Bieliński, D. M., Siciński, M., Zarzecka-Napierała, M., & Pędzich, Z. (2021). SBR Vulcanizates Filled with Modified Ground Tire Rubber. Materials, 14(14), 3991. https://doi.org/10.3390/ma14143991








