Calcination of Clay Raw Materials in a Fluidized Bed
Abstract
:1. Introduction
2. Research Methodology
- Technical analysis of clay materials;
- Analysis of the particle size distribution of clay materials;
- Determination of the velocity of fluidization;
- Preliminary experimental tests at an ambient temperature;
- Preliminary tests of the calcination process of raw clay material at the temperature of 850 °C in a fluidized bed.
- The degree of calcination was determined;
- Particle distribution was compared before and after the calcination process.
2.1. Granulation Process of Enriched Clay Material
- Granules of 0.5–5 mm when rubbed through a 6.0 mm sieve;
- Granules of 0.5–3 mm when rubbed through a 6.0 mm sieve;
- Granules ≤1 mm when rubbing a 1.0 mm through a 3.0 mm sieve;
- Granules of 1.0–3.0 mm when rubbed through a 4.0 mm sieve;
- Granules ≤3 mm when rubbing a 3.0 mm sieve through a 4.0 mm sieve.
2.2. Characteristics of the Research Material
- A—kaolin industrially enriched at the division boundary of 100 μm. The useful ingredient has a high Al2O3 content (about 40%):
- Granules < 2 mm
- B—clay raw material comprehensively enriched with high-alumina kaolin accompanying deposits of vein quartz. In the enriched product, the mass ratio of substrates is 1:1. The enrichment aims to the Al2O3 content and reduce the Fe2O3 content:
- Granules < 5 mm;
- Granules < 3 mm;
- Granules < 1 mm;
- About 1–3 mm granules;
- C—material sample with a very high degree of carbonization, laboratory enriched at a division limit of 100 μm:
- Granules <2 mm.
- D—clay raw material aerated and carburized with coal suspension, stabilized with bone glue, electrokinetically dehydrated, sieve granulated:
- Granules 1–4 mm;
- Granules <1 mm.
2.3. Results of Technical Analysis of Clay Raw Materials
2.4. Results of Particle Analysis of Clay Materials and Determination of Fluidization Parameters
2.5. Determination of Fluidization Parameters
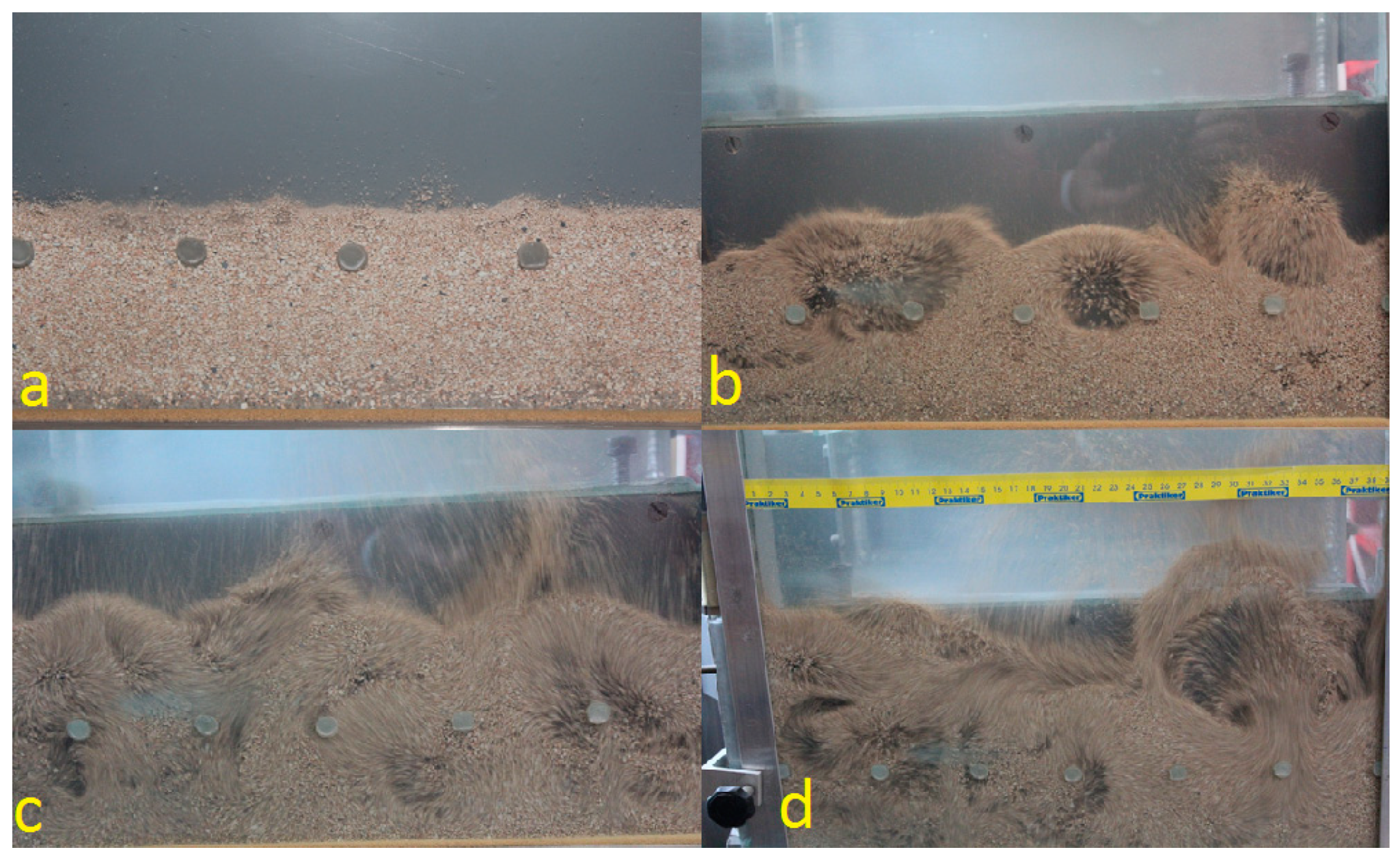
3. Preliminary Experimental Studies at Ambient Temperature
4. Preliminary Tests of the Calcination Process of Raw Clay Material at a Temperature of 850 °C in a Fluidized Bed
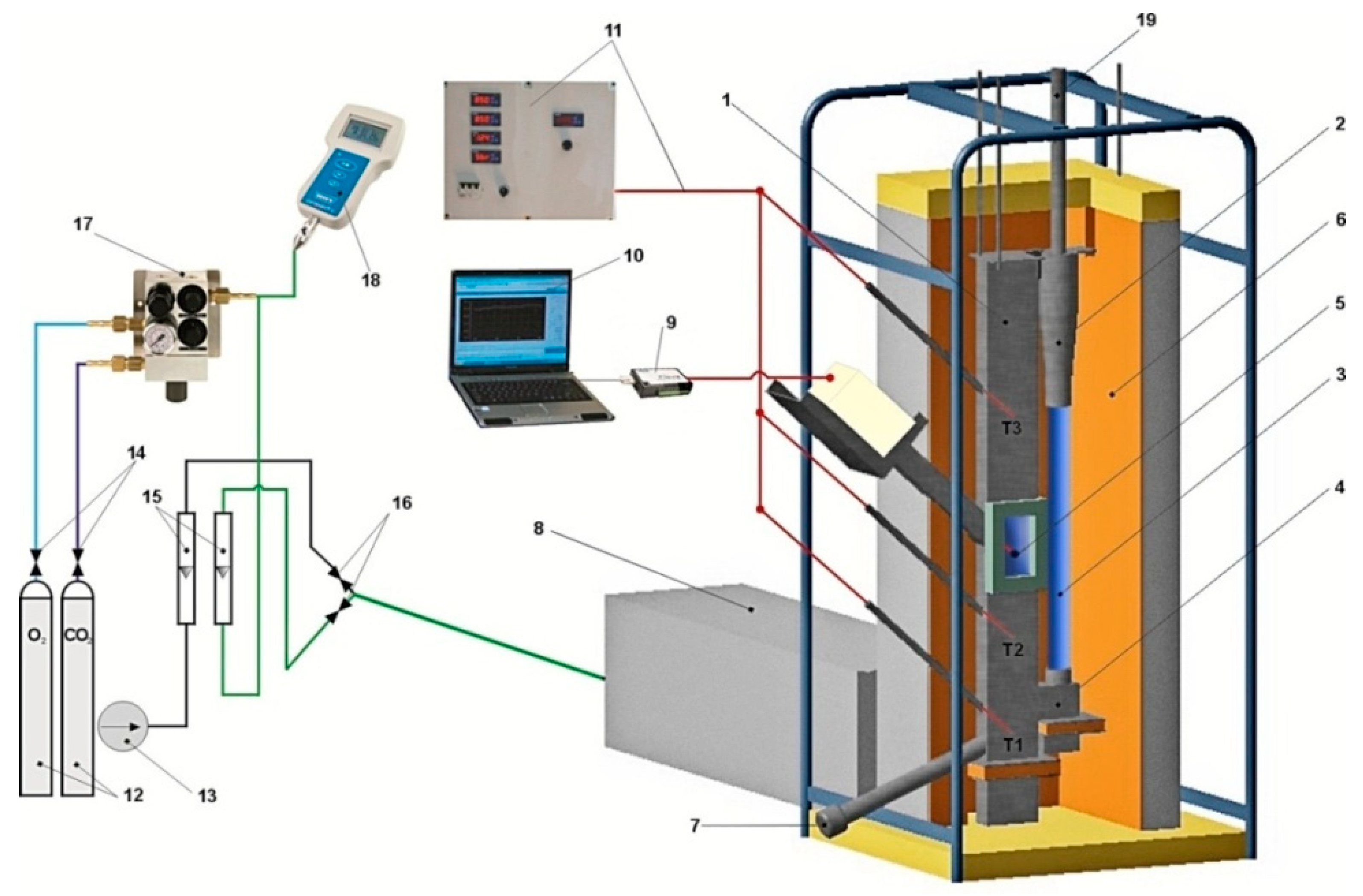
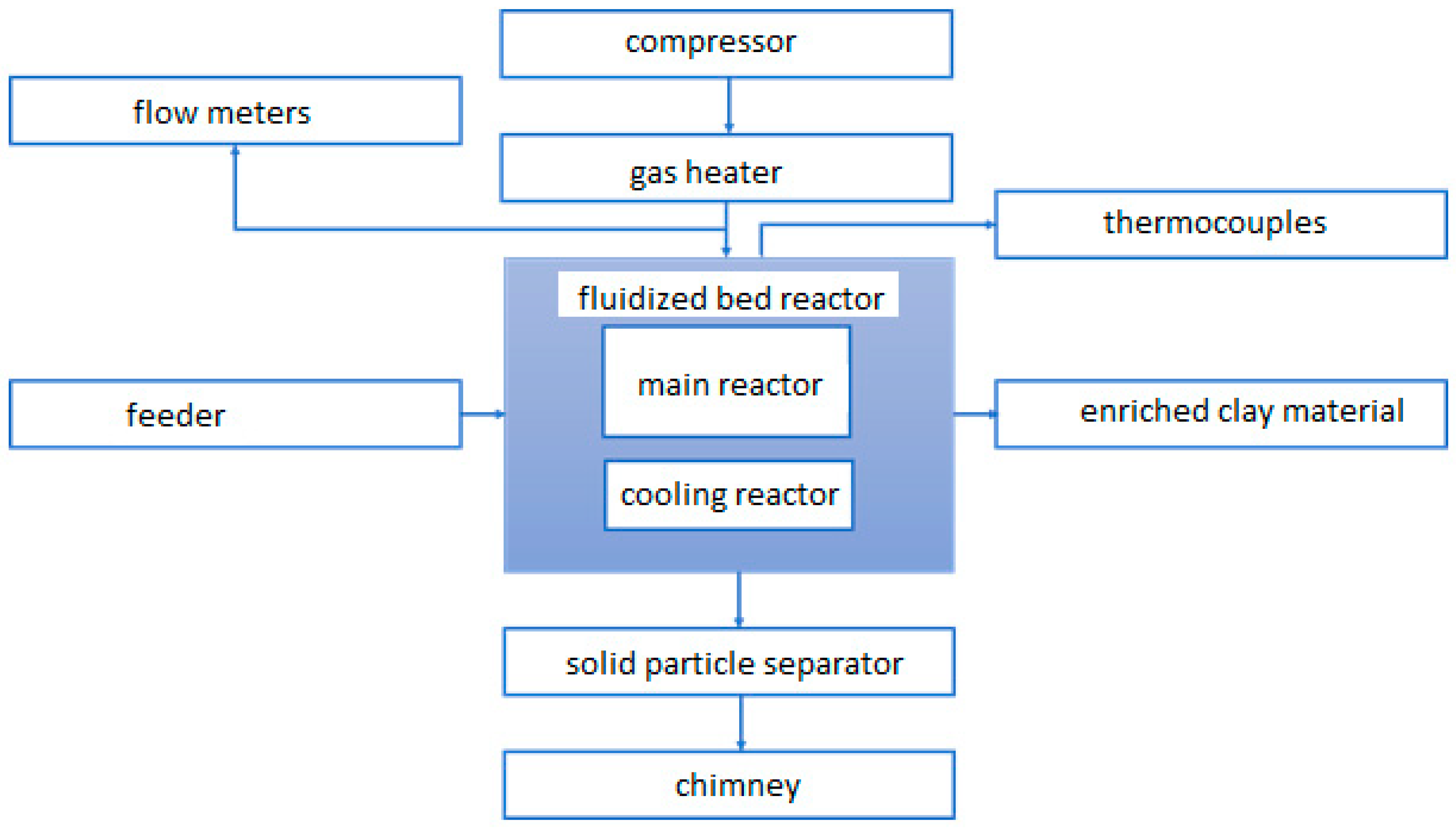
5. Experimental Studies of Fluidized Bed Calcination in a Fluidized Bed Reactor
- at a temperature of 850 °C: A, granule size < 2 mm; C, granule size < 2 mm; B, granule size < 5 mm; D, granule size 1–4 mm;
- at a temperature of 750 °C: B, granule size < 3 mm; TJW, granule size 1–3 mm;
- at 700 °C: B, granule size < 1 mm;
Experimental Stand
6. Results of BZ 1-103-3/2016/P Experimental Studies on Calcination in a Fluidized Bed Reactor
7. Conclusions
- The conducted analysis indicates that calcination of the clay granulate, which is highly aluminous in fluidized conditions, is possible while still preserving the appropriate granularity class with a range of between 0.5 and 3.0 mm. Fine-grained granulates above 0.5 mm are blown out with the exhaust fumes in the fluidization process, while granules over 3 mm do not create a constant fluid phase.
- In the case of performing calcination at a temperature of 850 °C, very good calcination results were obtained when the granulate was held in the fluidized bed reactor for a 10-min period of time spent (the degree of calcination was then 100%).
- It was also established that it is possible to calcine clay particles at lower temperatures, e.g., 750 °C, with a calcination degree of 96.5%, and at 700 °C, with a calcination degree of 87.6%.
- During the time period in which the clay material particles remain in the fluidized bed reactor, they are subject to fragmentation, as is visible by the increase in the number of particles with smaller particle sizes in the graphs that show the particle distribution of materials after calcination.
- Calcining in the fluidized bed does not change the chemical composition of the clay, as the oxides that make up the clay do not react to high temperatures, and the carbon contained in it is completely burnt out.
- The research conducted illustrates that the process of calcinations under the conditions described in this paper require a period of time that is several times shorter than when using a rotary furnace, thus shortening the process by tens of minutes.
- Due to the possibility of reactor remaining in continuous operation, this technology can find application in industry (while maintaining the appropriate grain size distribution to be subjected to calcination).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ratajczak, T.; Hycnar, E. Accompanying minerals in lignite deposits. In Geological and Raw Materials Aspects of the Management of Accompanying Minerals; IGSMiE PAN: Krakow, Poland, 2017; Volume 1. [Google Scholar]
- Ratajczak, T.; Hycnar, E.; Bożęcki, P. Mineralogical criterion as a part of suitability assessment of some polish raw clay for hydro-insulating barriers construction. Górnictwo Odkryw. 2016, 57, 19–25. [Google Scholar]
- Zhou, L.; Slaný, M.; Bai, B.; Du, W.; Qu, C.; Zhang, J.; Tang, Y. Enhanced Removal of Sulfonated Lignite from Oil Wastewater with Multidimensional MgAl-LDH Nanoparticles. Nanomaterials 2021, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, P.Z.; Wang, X.H.; Geng, P.; Akhtar, F.; Zhang, H.F. Fabrication and properties of freeze-cast mullite foams derived from coal-series kaolin. Ceram. Int. 2016, 42, 12414–12421. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, L.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20, ISSN 0169-1317. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Recent progress of utilization of activated kaolinitic clay in cementitious construction materials. Compos. Part B Eng. 2021, 211, 108636. [Google Scholar] [CrossRef]
- Sanz, J.; Madani, A.; Serratosa, J.M.; Moya, J.S.; Aza, S. Aluminum-27 and Silicon-29 Magic-Angle Spinning Nuclear Magnetic Resonance Study of the Kaolinite-Mullite Transformation. J. Am. Ceram. Soc. 1988, 71, C418–C421. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; Pérez-Rodríguez, J.L.; Sobrados, I.; Sanz, J. Influence of Grinding in Pyrophyllite−Mullite Thermal Transformation Assessed by 29Si and 27Al MAS NMR Spectroscopies. Chem. Mater. 1997, 9, 677–684. [Google Scholar] [CrossRef]
- Liu, X.X.; Liu, X.W.; Hu, Y.H. Investigation of the thermal behaviour and decomposition kinetics of kaolinite. Clay Min. 2015, 50, 199–209. [Google Scholar] [CrossRef]
- Kurdowski, W. Chemistry of Cement and Concrete; Cement Producers Association, PWN: Krakow, Poland, 2010. [Google Scholar]
- Nadeem, A.; Memon, S.A.; Lo, T.Y. Mechanical performance, durability, qualitative and quantitative analysis of microstructure of fly ash and metakaolin mortar at elevated temperatures. Constr. Build. Mater. 2013, 38, 338–347. [Google Scholar] [CrossRef]
- Kuang, J.; Yuan, W.; Li, L.; Hu, J.; Xu, L. Effects of Er(NO3)3, Nd(NO3)3 and Y(NO3)3 on kinetics of dehydroxylation of kaolinite. Powder Technol. 2016, 301, 581–589. [Google Scholar] [CrossRef]
- Souri, A.; Golestani-Fard, F.; Naghizadeh, R.; Veiseh, S. An investigation on pozzolanic activity of Iranian kaolins obtained by thermal treatment. Appl. Clay Sci. 2015, 103, 34–39. [Google Scholar] [CrossRef]
- Rashad, A.M. Metakaolin as cementitious material: History, scours, production and composition—A comprehensive overview. Constr. Build. Mater. 2013, 41, 303–318. [Google Scholar] [CrossRef]
- Cao, Z.; Cao, Y.D.; Dong, H.J.; Zhang, J.S.; Sun, C.B. Effect of calcination condition on the microstructure and pozzolanic activity of calcined coal gangue. Int. J. Min. Process. 2016, 146, 23–28. [Google Scholar] [CrossRef]
- Khadilkar, A.; Rozelle, P.L.; Pisupati, S.V. Models of agglomerate growth in fluidized bed reactors: Critical review, status and applications. Powder Technol. 2014, 264, 216–228. [Google Scholar] [CrossRef]
- Scala, F.; Salatino, P. Dolomite attrition during fluidized-bed calcination and sulfation. Combust. Sci. Technol 2003, 175, 2201–2216. [Google Scholar] [CrossRef]
- Zhang, W. A Review of Techniques for the Process Intensification of Fluidized Bed Reactors. Chin. J. Chem. Eng. 2009, 17, 688–702. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, F.; Zhang, H.F.; Cui, L.J.; Yu, J.; Xu, G.W. Extraction of vanadium from stone coal by roasting in a fluidized bed reactor. Fuel 2015, 142, 180–188. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, T. Recovery of low grade haematite via fluidised bed magnetising roasting: Investigation of magnetic properties and liberation characteristics. Ironmak Steelmak 2012, 39, 112–120. [Google Scholar] [CrossRef]
- Shuai, Y.; Yuexin, H.; Yanjun, L.; Peng, G.; Jianwen, Y. Effect of calcination temperature on activation behaviors of coal-series kaolin by fluidized bed calcination, Physicochem. Probl. Miner. Process. 2018, 54, 590–600. [Google Scholar]
- Haneklaus, N.; Zheng, Y.; Allelein, H.J. Stop Smoking—Tube-In-Tube Helical System for Flameless Calcination of Minerals. Processes 2017, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Internal Report Cewap sp. z o.o.; istotne.pl: Boleslawiec, Poland, 2017.
- Basu, P.; Fraser, S.A. Circulating Fluidized Bed Boilers, Design and Operations; Butterworth-Heinemann: Boston, MA, USA, 1991. [Google Scholar]
- Bis, Z. Fluidized Bed Boilers: Theory and Practice; Publisher of the Częstochowa University of Technology: Czestochowa, Poland, 2010. [Google Scholar]





| Component | A | B | C |
|---|---|---|---|
| SiO2 wt.% | 54.10 | 58.50 | 58.90 |
| Al2O3 wt.% | 41.70 | 32.70 | 32.80 |
| Fe2O3 wt.% | 1.07 | 2.51 | 2.01 |
| TiO2 wt.% | 0.58 | 2.01 | 1.52 |
| CaO wt.% | 0.16 | 0.33 | 0.22 |
| MgO wt.% | 0.24 | 0.61 | 0.45 |
| K2O wt.% | 0.37 | 2.99 | 2.16 |
| Na2O wt.% | 0.05 | 0.11 | 0.05 |
| other wt.% | 1.73 | 0.24 | 1.89 |
| total wt.% | 100 | 100 | 100 |
| Material | Granulation [mm] | Moisture [%] | Volatile Matter Content [%] | Clay Content [%] |
|---|---|---|---|---|
| A | <2 | 6.20 | 1.78 | 92.02 |
| B | <5 | 8.54 | 1.15 | 98.63 |
| <1 | 0.12 | 1.01 | 98.87 | |
| <3 | 0.08 | 1.06 | 98.45 | |
| 1–3 | 0.11 | 0.61 | 97.43 | |
| C | <2 | 7.00 | 2.05 | 90.95 |
| D | 1–4 | 2.44 | 19.42 | 78.14 |
| <1 | 0.93 | 18.36 | 80.71 |
| Material | Granulation [mm] | Mass [g] | Number of Particles [–] | Time [min] | dn 1 [μm] | ds 2 [μm] | dv 3 [μm] | da 4 [μm] | dgeo 5 [μm] | Dmed 6 [μm] | Dmod 7 [μm] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | <2 | 6.7 | 545,435 | 96 | 164.7 | 224.7 | 291 | 492 | 102.5 | 134.6 | 24.2 |
| B | <5 | 14.5 | 151,073 | 18.3 | 57.5 | 200 | 434.3 | 2059.3 | 33 | 26.6 | 24.2 |
| <1 | 12 | 1,300,381 | 157.7 | 177 | 226.7 | 265.7 | 366 | 113.7 | 174 | 24.2 | |
| <3 | 14.5 | 882,081 | 102.7 | 159.7 | 219.7 | 288.3 | 496.3 | 97.2 | 119.1 | 24.2 | |
| 1–3 | 13.3 | 443,342 | 162 | 242.7 | 179.7 | 288 | 1311 | 40.2 | 24.9 | 24.2 | |
| C | <2 | 10.6 | 385,442 | 32.7 | 80.2 | 193.7 | 343.7 | 1103 | 40.1 | 24.9 | 24.2 |
| D | 1–4 | 8.7 | 22,676 | 22 | 165.3 | 353.3 | 579.3 | 1363 | 74.1 | 43.4 | 51.6 |
| <1 | 10.1 | 146,958 | 28.7 | 155.7 | 199.3 | 242.7 | 360.3 | 115.3 | 113.7 | 51.6 |
| Material | Granulation (mm) | Ar (–) | Re (–) | Umf (m/s) | Ut (m/s) | Utr (m/s) | Umb (m/s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | 27 | 850 | 27 | 850 | 27 | 850 | 27 | 850 | 27 | 850 | 27 | 850 | |||
| A | <2 | 2500 | 0.000492 | 10,003 | 458 | 6.68 | 0.34 | 0.22 | 0.10 | 5.23 | 0.45 | 5.43 | 0.81 | 0.05 | 0.03 |
| B | <5 | 650 | 0.002059 | 190,453 | 8724 | 65.05 | 5.90 | 0.50 | 0.41 | 8.89 | 0.76 | 5.40 | 0.81 | 0.20 | 0.14 |
| <1 | 2500 | 0.000366 | 4118 | 188 | 2.93 | 0.14 | 0.13 | 0.05 | 3.89 | 0.33 | 4.75 | 0.71 | 0.04 | 0.02 | |
| <3 | 2500 | 0.000496 | 10,267 | 470 | 6.84 | 0.35 | 0.22 | 0.10 | 5.28 | 0.45 | 5.45 | 0.81 | 0.05 | 0.03 | |
| 1–3 | 650 | 0.001311 | 49,140 | 2251 | 25.19 | 1.64 | 0.30 | 0.18 | 5.67 | 0.48 | 4.40 | 0.66 | 0.13 | 0.09 | |
| C | <2 | 650 | 0.001103 | 29,265 | 1341 | 16.78 | 0.99 | 0.24 | 0.13 | 4.77 | 0.41 | 4.07 | 0.61 | 0.11 | 0.07 |
| D | 1–4 | 650 | 0.001363 | 55,223 | 2530 | 27.51 | 1.84 | 0.32 | 0.19 | 5.89 | 0.50 | 4.48 | 0.67 | 0.13 | 0.09 |
| <1 | 2500 | 0.0003603 | 3928.46 | 179.78 | 2.80 | 0.13 | 0.12 | 0.05 | 3.83 | 0.33 | 4.72 | 0.70 | 0.04 | 0.02 | |
| Material | Granulation (mm) | Raw State | State after Fluidized Calcination | Degree of Calcination—State after Fluid Calcination | Fluidization Temperature | Fluidization Velocity |
|---|---|---|---|---|---|---|
| A | <2 | 12.85% | 0.00% | 100.00% | 850 °C | 0.12 m/s |
| B | <5 | 10.10% * | 0.00% * | 100.00% * | 850 °C | 0.45 m/s |
| <1 | 10.10% | 1.25% | 87.62% | 700 °C | 0.06 m/s | |
| <3 | 10.10% | 0.25% | 97.52% | 750 °C | 0.11 m/s | |
| 1–3 | 10.10% | 0.35% | 96.53% | 750 °C | 0.19 m/s | |
| C | <2 | 14.15% | 0.00% | 100.00% | 850 °C | 0.15 m/s |
| D | 1–4 | 26.00% | 0.03% | 99.88% | 850 °C | 0.21 m/s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczyńska, K.; Kaczyński, K.; Pełka, P. Calcination of Clay Raw Materials in a Fluidized Bed. Materials 2021, 14, 3989. https://doi.org/10.3390/ma14143989
Kaczyńska K, Kaczyński K, Pełka P. Calcination of Clay Raw Materials in a Fluidized Bed. Materials. 2021; 14(14):3989. https://doi.org/10.3390/ma14143989
Chicago/Turabian StyleKaczyńska, Katarzyna, Konrad Kaczyński, and Piotr Pełka. 2021. "Calcination of Clay Raw Materials in a Fluidized Bed" Materials 14, no. 14: 3989. https://doi.org/10.3390/ma14143989
APA StyleKaczyńska, K., Kaczyński, K., & Pełka, P. (2021). Calcination of Clay Raw Materials in a Fluidized Bed. Materials, 14(14), 3989. https://doi.org/10.3390/ma14143989






