Polymer-Based Carriers in Dental Local Healing—Review and Future Challenges
Abstract
1. Introduction
2. Topical Administration of Dental Carriers
2.1. Oral Mucosa
2.2. Periodontal Pockets
2.3. Other Needs for Topical Treatment of Oral Diseases
3. Carriers and Biodegradable Polymeric Materials in Dentistry
3.1. Natural Polysaccharides
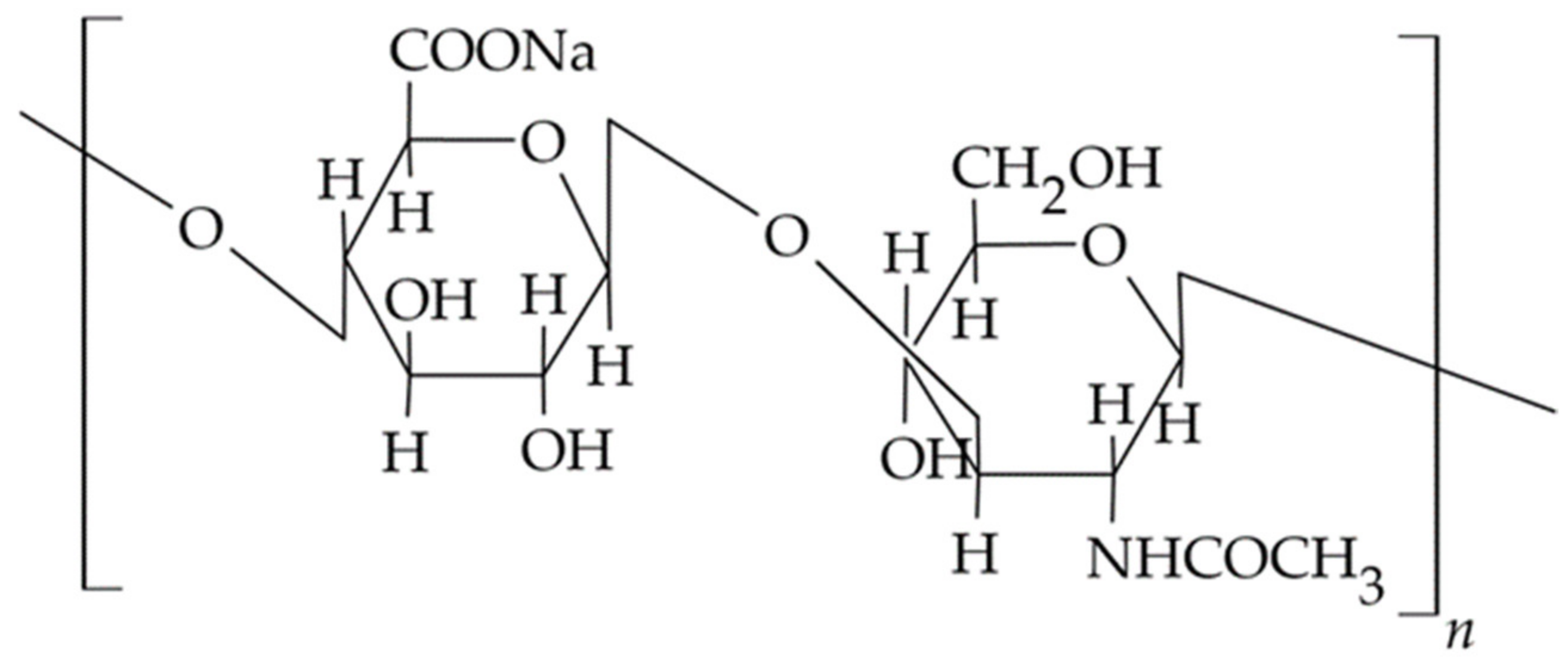
3.1.1. Chitosan and Its Derivatives
3.1.2. Hyaluronic acid (HA) and Hyaluronate
3.1.3. Gums and Pectins
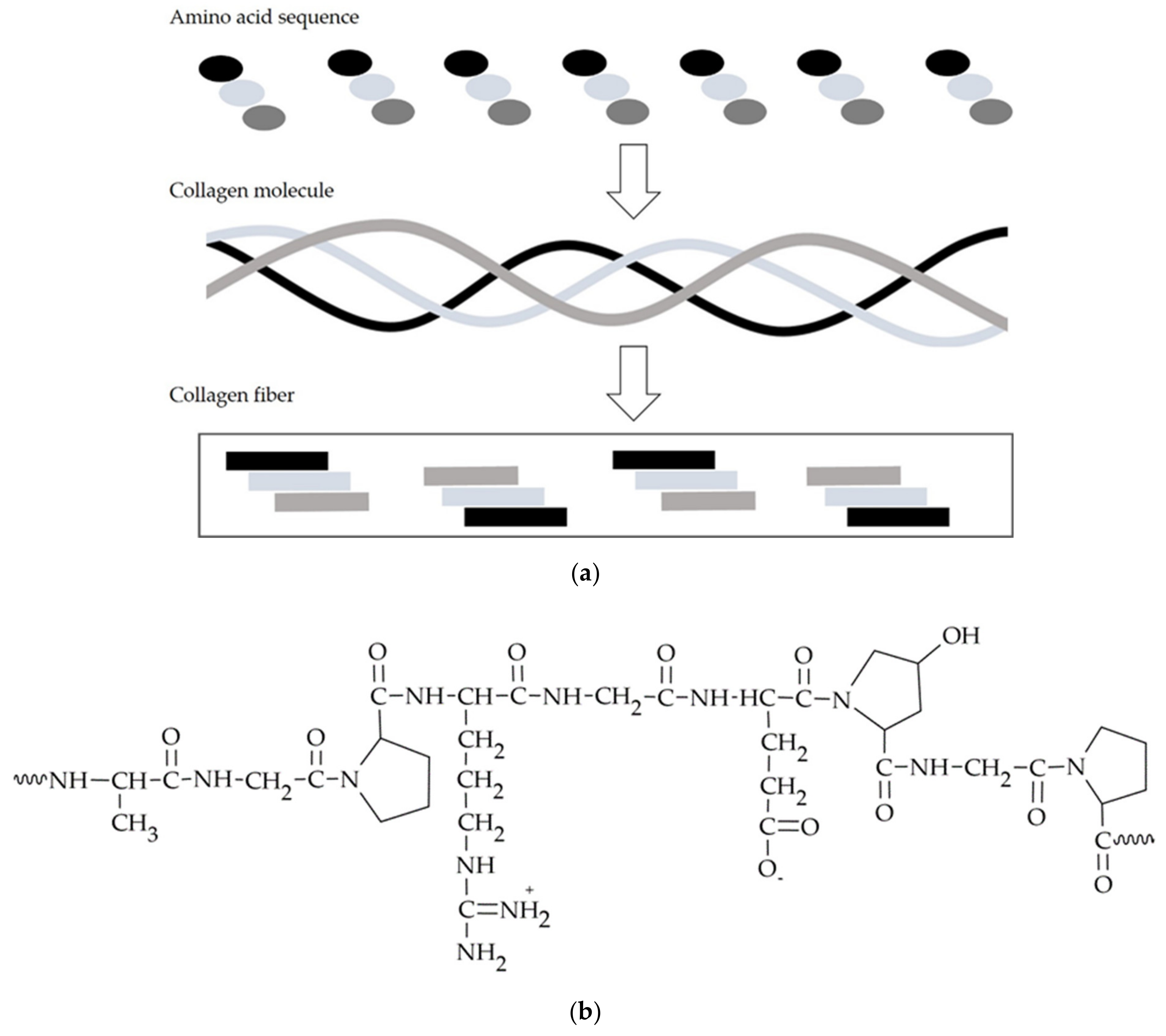
3.2. Peptides—Collagen, Gelatin
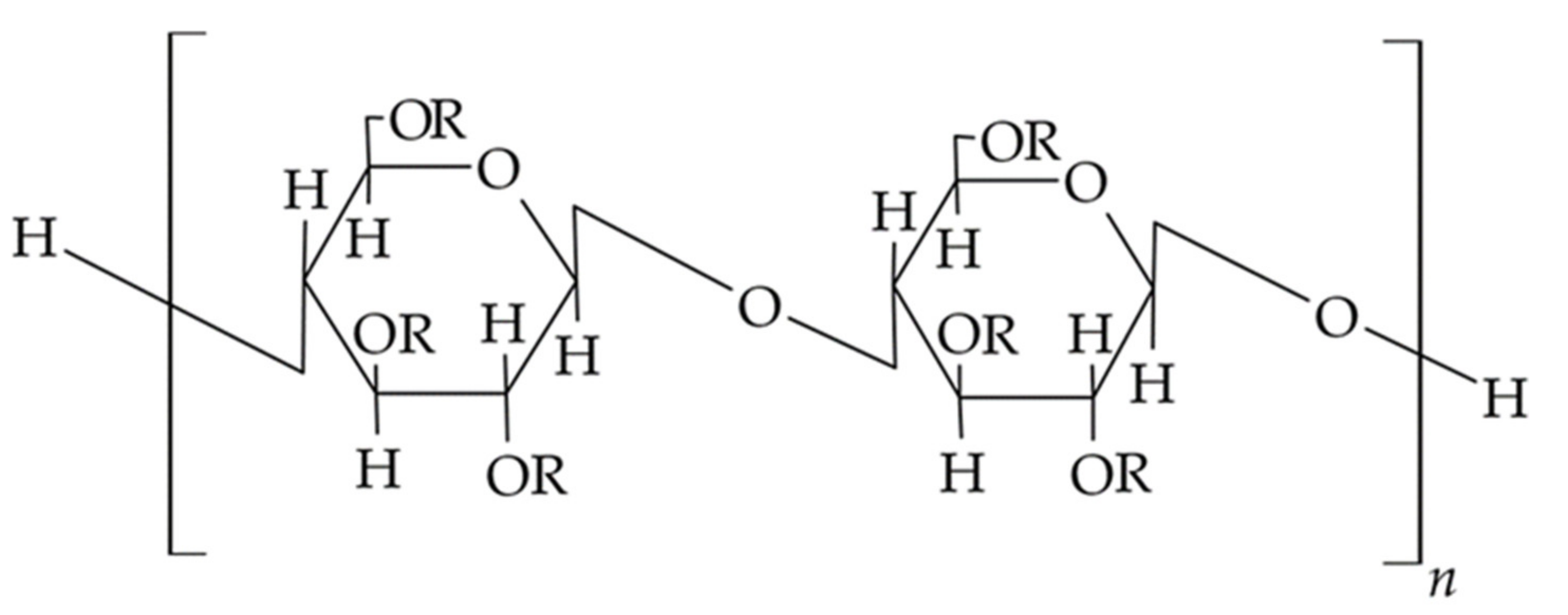
3.3. Semi-Synthetic Polysaccharides—Cellulose Derivatives
3.4. Biodegradable Synthetic Polymers
3.4.1. Polyvinyl Alcohol (PVA)
3.4.2. Polylactides (PLGA, PLA)
3.4.3. Polycaprolactone (PCL)
3.4.4. Poloxamers
| Polymer | Carrier | Drug |
|---|---|---|
| PVA | film | ornidazole [179] econazole [155] pvpi [180] lodocaine [159] |
| Polylactides | fibers | lidocaine and epinephrine [117] |
| films | metronidazole [181] | |
| implant | secnidazole/doxycycline [161] | |
| microspheres | metro, mino, ciprofloxacin [162] chlorhexidine digluconate [163] | |
| nanoparticles | methylene blue [164] minocycline/arestin [165] | |
| PCL | microfibers | metronidazole [173] |
| gels membrane | metronidazole [182] ZnO [170] tetracycline [183] | |
| Poloxamer | nanoemulsion hydrogel | quercetin [175] mebeverini hydrochloridum [177] |
| Ethylene-vinyl acetate (EVA) | fibers | tetracycline [184] |
4. Directions of Development of Polymer-Based Carriers and Outlook in Dental Administration
- The search for new biodegradable polymers for the development of carriers that release the substance in the oral cavity,
- The inclusion of mucoadhesive polymers that provide additional lubrication and physical protection of the ulcerated oral mucosa and alleviate the symptoms of ongoing inflammation,
- The use of multifunctional polymers, which, in addition to the basic function of an pharmaceutical excipient, may have additional properties such as pharmacological activity,
- The use of in situ sensitive polymers, i.e., temperature changes (poloxamers, PVA, cellulose derivatives, alginate, gellan), pH changes (Carbopol®, chitosan), resulting in stimuli-responsive drug carriers,
- Design of carriers tailored to the place of application in an individual dose obtained, for example, by means of 3D technology, electrospinning, realizing the idea of personalized therapy, and making it possible to obtain an effective therapeutic concentration even with a small dose of the drug,
- The administration of biologic drugs, which are difficult to deliver using conventional dosage forms and are currently almost exclusively limited to systemic administration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karolewicz, B. A review of polymers as multifunctional excipients in drug dosage form technology. Saudi Pharm. J. 2016, 24, 525–536. [Google Scholar] [CrossRef]
- Deepak, H.; Gaurav, S. Novel excipients as different polymers: A review. J. Drug Delivery. Ther. 2013, 3, 202–207. [Google Scholar]
- Aleeva, G.N.; Zhuravleva, M.V.; Khafiz’yanova, R.K. The role of excipients in determining the pharmaceutical and therapeutic properties of medicinal agents (Review). Pharm. Chem. J. 2009, 43, 230–234. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, A.M.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Ogaji, I.J.; Nep, E.I.; Audu-Peter, J.D. Advances in natural polymers as pharmaceutical excipients. Pharm. Anal. Acta 2011, 3, 146. [Google Scholar] [CrossRef]
- Sene, C.; Dupont, A.; Corning, D. Characterising polymeric excipients. Innovat. Pharmaceut. Tech. 2003, 87–91. Available online: http://www.iptonline.com/articles/public/IPTTWELVE87NoPrint.pdf (accessed on 12 July 2021).
- Paderini, C.; Compilato, D.; Giannola, L.I.; Campisi, G. Oral local drug delivery and new perspectives in oral drug formulation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 25–34. [Google Scholar] [CrossRef]
- Bruschi, M.L.; De Freitas, O. Oral bioadhesive drug delivery systems. Drug Dev. Ind. Pharm. 2005, 31, 293–310. [Google Scholar] [CrossRef]
- Russo, E.; Selmin, F.; Baldassari, S.; Gennari, C.G.M.; Caviglioli, G.; Cilurzo, F.; Minghetti, P.; Parodi, B. A focus on mucoadhesive polymers and their application in buccal dosage forms. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Ribeiro, L.N.M.; Volpato, M.C.; Cereda, C.M.S.; Groppo, F.C.; Tofoli, G.R.; Ribeiro de Araújo, D.; Santi, P.; Padula, C.; de Paula, E. Recent advances and perspectives in topical oral anesthesia. Expert Opin. Drug Deliv. 2017, 14, 673–684. [Google Scholar] [CrossRef]
- Sudhakar, Y.; Kuotsu, K.; Bandyopadhyay, A.K. Buccal bioadhesive drug delivery—A promising option for orally less efficient drugs. J. Control. Release 2006, 114, 15–40. [Google Scholar] [CrossRef]
- Akca, G.; Ozdemir, A.; Oner, Z.G.; Senel, S. Comparison of different types and sources of chitosan for the treatment of infections in the oral cavity. Res. Chem. Intermediat. 2018, 44, 4811–4825. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in oral transmucosal drug delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef]
- Collins, L.M.C.; Dawes, C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J. Dent. Res. 1987, 66, 1300–1302. [Google Scholar] [CrossRef]
- Naumova, E.A.; Dierkes, T.; Sprang, J.; Arnold, W.H. The oral mucosal surface and blood vessels. Head Face Med. 2013, 9, 8. [Google Scholar] [CrossRef]
- Lee, V.H.L. Mucosal drug delivery. J. Natl. Cancer Inst. Monogr. 2001, 29, 41–44. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, 68–77. [Google Scholar] [CrossRef]
- Goodson, J.M. Gingival crevice fluid flow. Periodontolology 2000, 31, 43–54. [Google Scholar] [CrossRef]
- Dusane, J.; Mogal, V.; Borse, P.; Thakare, P.; Kshirsagar, S. Recent trends in treatment of periodontitis. Pharm. Biol. Eval. 2016, 3, 19–31. [Google Scholar]
- Freitas-Blanco, V.; Franz-Montan, M.; Groppo, F.C.; Carvalho, J.E.; Figueira, G.M.; Serpe, L.; Sousa, I.M.; Damasio, V.A.; Yamane, L.T.; de Paula, E.; et al. Development and evaluation of a novel mucoadhesive film containing Acmella oleracea extract for oral mucosa topical anesthesia. PLoS ONE 2016, 14, e0162850. [Google Scholar] [CrossRef]
- Lope-Lopez, J.; Jan-Pallí, E.; González-Navarro, B.; Jané-Salas, E.; Estrugo-Devesa, A.; Milani, M. Efficacy of chlorhexidine, dexpanthenol, allantoin and chitosan gel in comparison with bicarbonate oral rinse in controlling post-interventional inflammation, pain and cicatrization in subjects undergoing dental surgery. Curr. Med. Res. Opin. 2015, 31, 2179–2183. [Google Scholar] [CrossRef]
- Yadav, S.K.; Khan, G.; Bansal, M.; Thokala, S.; Bonde, G.V.; Upadhyay, M.; Mishra, B. Multiparticulate based thermosensitive intra-pocket forming implants for better treatment of bacterial infections in periodontitis. Int. J. Biol. Macromol. 2018, 116, 394–408. [Google Scholar] [CrossRef]
- Iorgulescu, G. Saliva between normal and pathological. Important factors in determining systemic and oral health. J. Med. Life 2009, 2, 303–307. [Google Scholar]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Ramseier, C.A.; Rasperini, G.; Batia, S.; Giannobile, W.V. Advanced reconstructive technologies for periodontal tissue repair. Periodontolology 2012, 59, 185–202. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liua, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Edmans, J.G.; Clitherow, K.H.; Murdoch, C.; Hatton, P.V.; Spain, S.G.; Colley, H.E. Mucoadhesive electrospun fibre-based technologies for oral medicine. Pharmaceutics 2020, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Gupta, S.K.; Newaskar, V.; Chandra, A. Advances in dental local anesthesia techniques and devices: An update. Natl. J. Maxillofac. Surg. 2013, 4, 19–24. [Google Scholar] [CrossRef]
- Lee, H.S. Recent advances in topical anesthesia. J. Dent. Anesth. Pain Med. 2016, 16, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xue, Y.; Jia, B.; Bai, Y.; Zuo, Y.; Wang, S.; Zhao, S.; Yang, W.; Tang, H. The preparation of hyaluronic acid grafted pullulan polymers and their use in the formation of novel biocompatible wound healing film. Carbohydr. Polym. 2018, 188, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, Y.; Yu, Y.; Zhou, X.; Du, W.; Wan, M.; Fan, M.; Zhou, X.; Xu, X.; Zheng, L. Evaluation of chitosan hydrogel for sustained delivery of Vegf for odontogenic differentiation of dental pulp stem cells. Stem Cells Int. 2019, 2019, 1515040. [Google Scholar] [CrossRef]
- Özdoğan, A.I.; Akca, G.; Şenel, S. Development and in vitro evaluation of chitosan based system for local delivery of atorvastatin for treatment of periodontitis. Eur. J. Pharm. Sci. 2018, 124, 208–216. [Google Scholar] [CrossRef]
- Echazú, M.I.A.; Olivetti, C.E.; Anesini, C.; Perez, C.J.; Alvarez, G.S.; Desimone, M.F. Development and evaluation of thymol-chitosan hydrogels with antimicrobial-antioxidant activity for oral local delivery. Mater. Sci. Eng. Mater. Biol. Appl. 2017, 81, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, Y.; Ganji, F. Thermosensitive hydrogel for periodontal application: In vitro drug release, antibacterial activity and toxicity evaluation. J. Biomat. Appl. 2016, 30, 919–929. [Google Scholar] [CrossRef]
- Pignatello, R.; Basile, L.; Puglisi, G. Chitosan glutamate hydrogels with local anesthetic activity for buccal application. Drug Deliv. 2009, 16, 176–181. [Google Scholar] [CrossRef]
- Khajuria, D.K.; Patil, O.N.; Karasik, D.; Razdan, R. Development and evaluation of novel biodegradable chitosan based metformin intrapocket dental film for the management of periodontitis and alveolar bone loss in a rat model. Arch. Oral Biol. 2018, 85, 120–129. [Google Scholar] [CrossRef]
- Labib, G.S.; Aldawsari, H.M.; Badr-Eldin, S.M. Metronidazole and Pentoxifylline films for the local treatment of chronic periodontal pockets: Preparation, in vitro evaluation and clinical assessment. Expert Opin. Drug Deliv. 2014, 11, 855–865. [Google Scholar] [CrossRef]
- Samprasit, W.; Kaomongkolgit, R.; Sukma, M.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Mucoadhesive electrospun chitosan-based nanofibre mats for dentalcaries prevention. Carbohydr. Polym. 2015, 117, 933–940. [Google Scholar] [CrossRef]
- Qasim, S.B.; Delaine-Smith, R.M.; Fey, T.; Rawlinson, A.; Rehman, U.I. Freeze gelated porous membranes for periodontal tissue regeneration. Acta Biomater. 2015, 23, 317–328. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Rehman, U.I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Pillay, V.; Chetty, D.J.; Essack, S.Y.; Dangor, C.M.; Govender, T. Optimisation and characterisation of bioadhesive controlled release tetracycline microspheres. Int. J. Pharm. 2005, 306, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Pichayakorn, W.; Boonme, P. Evaluation of cross-linked chitosan microparticles containing metronidazole for periodontitis treatment. Mater. Sci. Eng. Mater. Biol. Appl. 2013, 33, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Yeom, J.; Oh, E.J.; Reddy, M.; Kim, J.Y.; Cho, D.W.; Lim, H.P.; Kim, N.S.; Park, S.W.; Shin, H.I.; et al. Guided bone regeneration by poly(lactic-co-glycolic acid) grafted hyaluronic acid bi-layer films for periodontal barrier applications. Acta Biomater. 2009, 5, 3394–3403. [Google Scholar] [CrossRef]
- Afat, I.M.; Akdogan, E.T.; Gonul, O. Effects of leukocyte- and platelet-rich fibrin alone and combined with hyaluronic acid on early soft tissue healing after surgical extraction of impacted mandibular third molars: A prospective clinical study. J. Craniomaxillofac. Surg. 2019, 47, 280–286. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Kobayashi, E.; Hernandez, M.; Zhang, Y.; Miron, R. Hyaluronic acid gel-based scaffolds as potential carrier for growth factors: An in vitro biossay on its osteogenic potential. J. Clinic. Med. 2016, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.P.; Fortes, A.C.; Gonsalves da Cruz Fonseca, S.; Breitkreutz, J.; Ferraz, H.G. Manufacture and characterization of mucoadhesive buccal films based on pectin and gellan gum containing triamcinolone acetonide. Int. J. Polym. Sci. 2018, 2018, 2403802. [Google Scholar] [CrossRef]
- Chang, S.J.; Huan, Y.T.; Yang, S.C.; Kuo, S.M.; Lee, M.W. In vitro properties of gellan gum sponge as the dental filling to maintain alveolar space. Carbohydr. Polym. 2012, 88, 684–689. [Google Scholar] [CrossRef]
- Souza Filho, M.D.; Medeiros, J.V.R.; Vasconcelos, D.F.P.; Silva, D.A.; Leodido, A.C.M.; Fernandes, H.F.; Silva, F.R.P.; Franca, L.F.C.; Lenardo, D.; Pinto, G.R.; et al. Orabase formulation with cashew gum polysaccharide decreases inflammatory and bone loss hallmarks in experimental periodontitis. Int. J. Biol. Macromol. 2018, 107, 1093–1101. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Babii, O.; Wang, Z.; Liu, G.; Martinez, E.C.; Littel van den Hurk, S.; Chen, L. Low molecular weight chitosan nanoparticles for CpG oligodeoxynucleotides delivery: Impact of molecular weight, degree of deacetylation, and mannosylation on intracellular uptake and cytokine induction. Int. J. Biol. Macromol. 2020, 159, 46–56. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Madrazo-Jiménez, M.; Rodríguez-Caballero, A.; Serrera-Figallo, M.; Garrido-Serrano, R.; Gutiérrez-Corrales, A.; Gutiérrez-Pérez, J.; Torres-Lagares, D. The effects of a topical gel containing chitosan, 0.2% chlorhexidine, allantoin and despanthenol on the wound healing process subsequent to impacted lower third molar extraction. Medicina Oral Patologia Oral y Cirugia Bucal 2016, 21, 696–702. [Google Scholar]
- Dai, T.; Tanaka, T.; Huang, M.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Davydova, V.N.; Kalitnik, A.A.; Markov, P.A.; Volodko, A.V.; Popov, S.V.; Ermak, I.M. Cytokine-inducing and anti-inflammatory activity of chitosan and its low-molecular derivative. Appl. Biochem. Microbiol. 2016, 52, 476–482. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mengíbar, M.; Heras, A. Effect of chito-oligosaccharides over human faecal microbiota during fermentation in batch cultures. Carbohydr. Polym. 2016, 137, 617–624. [Google Scholar]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Baldrick, P. Pharmaceutical excipient development: The need for preclinical guidance. Regul. Toxicol. Pharmacol. 2000, 32, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, S.; Kesharwani, P.; Jain, K.; Tekade, R.K.; Jain, N.K. Mucoadhesion: A promising approach in drug delivery system. React. Funct. Polym. 2016, 100, 151–172. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Roldo, M.; Hornof, M.; Caliceti, P.; Bernkop-Schnürch, A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. Eur. J. Pharm. Biopharm. 2004, 57, 115–121. [Google Scholar] [CrossRef]
- Neufeld, L.; Bianco-Peled, H. Pectin-chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Borchard, G.; Lueβen, H.L.; de Boer, A.G.; Verhoef, J.C.; Lehr, C.M.; Junginger, H.E. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions In vitro. J. Control. Release 1996, 39, 131–138. [Google Scholar] [CrossRef]
- Atai, Z.; Atai, M.; Amini, J.; Salehi, N. Original In vivo study of antifungal effects of low-molecular-weight chitosan against Candida albicans. J. Oral Sci. 2017, 59, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Pragłowska, J.; Piątkowski, M.; Deineka, V.; Janus, Ł.; Korniienko, V.; Husak, E.; Holubnycha, V.; Liubchak, I.; Zhurba, V.; Sierakowska, A.; et al. Chitosan-based bioactive hemostatic agents with antibacterial properties—Synthesis and characterization. Molecules 2019, 24, 2629. [Google Scholar] [CrossRef]
- Boynueǧri, D.; Özcan, G.; Şenel, S.; Uc, D.; Uraz, A.; Ögus, E.; Çakilci, B.; Karaduman, B. Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: A pilot study. J. Biomed. Mater. Res. Appl. Biomater. 2009, 90, 461–466. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Uysal, T.; Akkurt, M.D.; Amasyali, M.; Ozcan, S.; Yagci, A.; Basak, F.; Sagdic, D. Does a chitosan-containing dentifrice prevent demineralization around orthodontic brackets. Angle Orthod. 2011, 81, 319–325. [Google Scholar] [CrossRef]
- Schlueter, N.; Klimek, J.; Ganss, C. Effect of a chitosan additive to a Sn2+-containing toothpaste on its anti-erosive/anti-abrasive efficacy—a controlled randomised in situ trial. Clin. Oral Invest. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Shuo, X.; Zhou, O.; Jiang, Z.; Wang, Y.; Yang, K.; Qiu, X.; Ji, O. The effect of doxycycline-containing chitosan/carboxymethyl chitosan nanoparticles on NLRP3 inflammasome in periodontal disease. Carbohydr. Polym. 2020, 237, 116163. [Google Scholar]
- Wohlfahrt, J.; Aass, A.M.; Koldsland, O.C. Treatment of peri-implant mucositis with a chitosan brush—A pilot randomized clinical trial. Int. J. Dent. Hyg. 2019, 17, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Investig. Dermatol. 2007, 127, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Hascall, V.; Esko, J.D. Chapter 15. Hyaluronan. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor Laboratory Press: San Diego, CA, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1908/ (accessed on 4 May 2021).
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Chen, G.; Sato, T.; Ohgushi, H.; Ushida, T.; Tateishi, T.; Tanaka, J. Culturing of skin fibroblasts in a thin PLGA—Collagen hybrid mesh. Biomaterials 2005, 26, 2559–2566. [Google Scholar] [CrossRef]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: Hyaluronic acid as a companion biomateriał for diverse dental aplications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef]
- Bertl, K.; Bruckmann, C.; Isberg, P.E.; Klinge, B.; Gotfresden, K.; Stavropoulos, A. Hyaluronan in non-surgical and surgical periodontal therapy: A systematic review. J. Clin. Periodontol. 2015, 42, 236–246. [Google Scholar] [CrossRef]
- Ferroni, L.; Gardin, C.; Sivolella, S.; Brunello, G.; Berengo, M.; Piattelli, A.; Bressan, E.; Zavan, B.A. Hyaluronan-Based scaffold for the in vitro construction of dental pulp like tissue. Int. J. Mol. Sci. 2015, 16, 4666–4681. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Pomowski, R.; Kundt, G.; Gocke, R. Treatment of gingivitis with hyauronan. J. Clin. Periodontol. 2003, 30, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Pistorius, A.; Martin, M.; Willershausen, B.; Rockmann, P. The clinical application of hyaluronic acid in gingivitis therapy. Quintessence Int. 2005, 36, 531–538. [Google Scholar] [PubMed]
- Shah, S.A. To compare the effect of the local delivery of hyaluronan as an adjunct to scaling and root planning in the treatment of chronic periodontitis. J. Indian Soc. Periodontol. 2016, 20, 549–556. [Google Scholar] [PubMed]
- Sapna, N.; Vandana, K.L. Evaluation of hyaluronian gel (Gengigel®) as topical applicant in the treatment of gingivitis. J. Investig. Clin. Dent. 2011, 2, 162–170. [Google Scholar] [CrossRef]
- Abdelraouf, S.A.; Dahab, O.A.; Elbarbary, A.; El-Din, A.M.; Mostafa, B. Assesment of hyaluronic acid gel injection in the reconstroction of interdental papilla: A randomized clinical trial. Open Access Maced. J. Med. Sci. 2019, 7, 1834–1840. [Google Scholar] [CrossRef]
- Pilloni, A.; Schmidlin, P.; Sahrmann, P.; Sculean, A.; Rojas, M. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Invest. 2018, 23, 1133–1141. [Google Scholar] [CrossRef]
- Lee, H.; Jung, J.Y.; Bang, D. The efficacy of topical 0.2% hyaluronic acid gel on recument oral ulcers: Comparision between reccurent aphthous ulcers and the oral ulcers of Behcet’s disease. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 590–595. [Google Scholar]
- Shetty, R.R.; Burde, K.N.; Guttal, K.S. The efficacy of topical hyaluronic acid 0.2% in the menagement of symptomatic oral lichen planus. J. Clin. Diagn. Res. 2016, 10, 46–50. [Google Scholar]
- Soriano-Lerma, A.; Magan-Fernandez, A.; Gijon, J.; Sanchez-Fernandez, E.; Soriano, M.; Garcia-Salcedo, J.A.; Mesa, F. Short-term effect of hyaluronic acid on the subgingival micribiome in peri-implantitis: A randomized controlled clinical trial. J. Periodontol. 2019, 91, 734–745. [Google Scholar] [CrossRef]
- Hosny, K.M.; Aldawsari, H.M.; Bahmdan, R.H.; Sindi, A.M.; Kurukula, M.; Alrobaian, M.M.; Aldryhim, A.Y.; Alchalidi, H.M.; Bahmdan, H.H.; Khallaf, R.A.; et al. Preparation, optimization and evaluation of hyaluronic acid-based hydrogel Loaded with miconazole self-nanoemulsion for the treatment of oral thrush. AAPS PharmSciTech 2019, 20, 297. [Google Scholar] [CrossRef]
- Sandhu, G.K.; Khinda, P.K.; Gill, A.S.; Kaira, H.S. Surgical re-entry evaluation of regenerative efficacy of bioactive Gengigel® and platet-rich fibrin in the treatment of grade II furcation: A novel approach. Contemp. Clin. Dent. 2015, 6, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Bertolami, C.N.; Bronson, R.E.; Damiani, P.J. Hyaluronidase activity of rabbit skin wound granulation tissue fibroblasts. J. Dent. Res. 1987, 66, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C.; Yoo, M.A.; Lee, H.J.; Son, D.H.; Jung, J.; Noh, I.; Kim, C.W. Modulation of biomechanical properities of hyaluronic acid hydrogels by crosslinking agents. J. Biomed. Mat. Res. Part A 2015, 103, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Dursun, E.; Tosun, E.; Bilgic, E.; Akman, A.C.; Orhan, K.; Celik, H.H.; Korkusuz, P.; Caglayan, F. Evaluation of hyaluronic matrix efficacy in sinus augmentation: A randomized-controlled histomorphometric and micro-computed tomography analysis. Int. J. Oral. Maxillofac. Surg. 2017, 7, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Cirligeriu, L.; Cimpean, A.; Calniceanu, H.; Viadau, M.; Sarb, S.; Raica, M.; Nica, L. Hyaluronic acid/bone substitute complex implanted on chick embryo chorioallantoic membrane induces osteoblastic differentiation and angiogenesis, but not inflammation. Int. J. Mol. Sci. 2018, 19, 4119. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Golchin, A.; Vargas, J.; Tayebi, L. Apllication of stem cell encapsulated hydrogel in dentistry. Appl. Biomed. Eng. Dent. 2019, 289–300. [Google Scholar] [CrossRef]
- Ahmadian, E.; Efekhari, A.; Dizaj, S.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.; Khalilov, R.; Samiei, M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behawior. Int. J. Biol. Macromol. 2019, 140, 245–254. [Google Scholar] [CrossRef]
- Donegan, G.C.; Hunt, J.A.; Rhodes, N. Investigating the importance of flow when utilizing hyaluronan scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 2010, 4, 83–95. [Google Scholar] [CrossRef]
- Catanzano, O.; D’Esposito, V.; Formisano, P.; Boateng, J.S.; Quaglia, F. Composite Alginate-Hyaluronan Sponges for the Delivery of Tranexamic Acid in Postextractive Alveolar Wounds. J. Pharm. Sci. 2018, 107, 654–661. [Google Scholar] [CrossRef]
- Milivojevic, M.; Pajic-Lijakovic, I.; Bugarski, B. Chapter 6—Gellan gum in drug delivery applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press Elsevier: London, UK, 2019; pp. 145–186. [Google Scholar]
- Noven Pharmaceuticals. DentiPatch (Lidocaine Hydrochloride) FDA Prescribing Information. May 1999. Available online: https://www.drugs.com/pro/dentipatch.html (accessed on 4 May 2020).
- Nguyen, S.; Hiorth, M.; Rykke, M.; Smistad, G. Polymer coated liposomes for dental drug delivery—Interactions with parotid saliva and dental enamel. Eur. J. Pharm. Sci. 2013, 50, 78–85. [Google Scholar] [CrossRef]
- Gracia, L.H.; Brown, A.; Rees, G.D.; Fowler, C.E. Studies on a novel combination polymer system: In vitro erosion prevention and promotion of fluoride uptake in human enamel. J. Dent. 2010, 38, 4–11. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Nokhodchi, A.; Ghanbarzadeh, S.; Kouhsoltani, M. Triamcinolone acetonide oromucoadhesive paste for treatment of aphthous stomatitis. Adv. Pharm. Bull. 2015, 5, 277–282. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Ann. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Mandlik, V.B.; Jha, A.K. Periochip. Med. J. Armed Forces India 2007, 63, 368–369. [Google Scholar] [CrossRef][Green Version]
- Fernandes, G.J.; Hatton, M.N. Prevention of alveolar osteitis. A Case report and review of literature. NY State Dent. J. 2016, 82, 21–25. [Google Scholar]
- Maani, S.; Saleh, M.; Melek, L.; Sadaka, M. Evaluation of colloidal silver Gelatin Sponge (Gelatamp) in patients receiving anticoagulant after tooth extraction (clinical study). Alexandria Dent. J. 2015, 40, 101–106. [Google Scholar] [CrossRef]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Ranganathan, M.; Balaji, M.; Krishnaraj, R.; Narayanan, V.; Thangavelu, A. Assessment of regeneration of bone in the extracted third molar sockets augmented using xenograft (CollaPlugTN Zimmer) in comparison with the normal healing on the contralateral side. J. Pharm. Bioall. Sci. 2017, 9, S180–S186. [Google Scholar] [CrossRef]
- Kondreddy, K.; Ambalavanan, N.; Ramakrishna, T.; Kumar, R.S. Effectiveness of a controlled release chlorhexidine chip (PerioCol™-CG) as an adjunctive to scaling and root planning when compared to scaling and root planing alone in the treatment of chronic periodontitis: A comparative study. J. Indian Soc. Periodontol. 2012, 16, 553–557. [Google Scholar] [CrossRef]
- Lee, F.Y.; Lee, D.; Lee, T.C.; Chen, J.K.; Wu, R.C.; Liu, K.C.; Liu, S.J. Fabrication of multi-layered lidocaine and epinephrine-eluting PLGA/collagen nanofibers: In vitro and in vivo study. Polymers 2017, 9, 416. [Google Scholar] [CrossRef]
- Libonati, F.; Nair, A.K.; Vergani, L.; Buehler, M.J. Mechanics of collagen–hydroxyapatite model nanocomposites. Mech. Res. Comm. 2014, 58, 17–23. [Google Scholar] [CrossRef]
- Elangovan, S. Dental implants placed in alveolar ridge augmented using guided bone regeneration procedure performed using resorbable collagen membranes and particulate bone grafts using simultaneous or staged approach exhibit a high survival rate. J. Evid. Based Dent. Pract. 2018, 18, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Gopalakrishna, V.; Shetty, A.; Nagraj, V.; Imran, M.; Kumar, P. Efficacy of PRF vs. PRF + Biodegradable Collagen Plug in Post-extraction Preservation of Socket. J. Contemp. Dent. Pract. 2019, 20, 1323–1328. [Google Scholar]
- Ikada, Y.; Tabata, Y. Protein release from gelatin matrices. Adv. Drug Deliv. Rev. 1998, 31, 287–301. [Google Scholar]
- Kabiri, M.; Emami, H.S.; Rafinia, M.; Tahriri, M. Preparation and characterization of absorbable hemostat crosslinked gelatin sponges for surgical application. Curr. Appl. Phys. 2011, 11, 457–461. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Basak, A.; Sheet, D. Characterization and scanning electron microscopic investigation of crosslinked freeze dried gelatin matrices for study of drug diffusivity and release kinetics. Micron 2012, 43, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Wang, X. Biomimetic synthesis of gelatin polypeptide-assisted noble-metal nanoparticles and their interaction study. Nanoscale Ress. Lett. 2011, 6, 22. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Rel. 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Saikhun, J.; Pholpramoo, C.; Misra, R.D.; Kitiyanant, Y. Chitosan gelatin scaffolds for tissue engineering: Physicochemical properties and biological response of buffalo embryonic stem cells and transfecant of GFP-buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hong, S.R.; Lee, Y.O.; Song, K.W.; Park, M.H.; Nam, S.Y. Study of gelatin containing artificial skin: I: Preparation and characteristics of novel gelatin—Alginate sponge. Biomaterials 1999, 20, 4019–4417. [Google Scholar] [CrossRef]
- Julius, L.L.; Hungerford, R.W.; Nelson, J.W.; McKercher, T.; Zellhoefer, R.W. Prevention of dry socket with local application of Terra-Cortril in gelfoam. Oral Maxillofac. Surg. 1982, 40, 285–286. [Google Scholar] [CrossRef]
- Rohanizadeh, R.; Swain, M.V.; Mason, R.S. Gelatin sponges (Gelfoam®) as a scaffold for osteoblasts. J. Mater. Sci. Mater. Med. 2008, 19, 1173–1182. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, W.; Lei, Y.; Wu, T.; Zhang, S.; Guo, Y.; Liu, Y.; Chen, D.; Yuan, Q.; Wang, Y. Effect of gelatin sponge with colloid silver on bone healing in infected cranial defects. Mater. Sci. Eng. C Mater Biol. Appl. 2017, 70, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, O.; Li, Y.; Guo, J.; Jiang, L.; Jia, M.; Deng, Y. Use of “gelatamp“ colloidal silver gelatin sponge to prevent dry socket after extracting mandibular impacted teeth. Shanghai Kou Qiang Yi Xue 2013, 22, 108–110. [Google Scholar] [PubMed]
- Komal, P.; Dodwad, V.; Kishore, B. Effect of controlled-release Periochip™ on clinical and microbiological parameters in patients of chronic periodontitis. Indian Soc. Periodontol. 2013, 17, 605–611. [Google Scholar]
- Medaiah, S.; Srinivas, M.; Melath, A.; Girish, S.; Polepalle, T.; Dasari, A.B. Chlorhexidine chip in the treatment of chronic periodontitis—A clinical study. J. Clin. Diagn. Res. 2014, 8, ZC22–ZC25. [Google Scholar]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, R.A.M.; Al, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.; Simöes, S. Oral films: Current status and future perspectives: I-Galenical development and quality attributes. J. Control. Rel. 2015, 206, 1–19. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Laffleur, F.; Krouská, J.; Tkacz, J.; Pekař, M.; Aghai, F. Netsomboon, K. Buccal adhesive films with moisturizer-the next level for dry mouth syndrome? Int. J. Pharm. 2018, 550, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kida, D.; Karolewicz, B.; Junka, A.; Sender-Janeczek, A.; Duś, I.; Marciniak, D.; Szulc, M. Metronidazole-Loaded Porous Matrices for Local Periodontitis Treatment: In vitro Evaluation and in vivo pilot study. Appl. Sci. 2019, 9, 4545. [Google Scholar] [CrossRef]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Hydroxypropylmethyl cellulose-based sponges loaded-microemulsifying curcumin: Preparation, characterization, and in vivo oral absorption studies. J. Appl. Polym. Sci. 2016, 133, 42966. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.M.; Mahmoud, A.A.; Shahin, H.I. Design and in vitro/in vivo evaluation of ultra-thin mucoadhesive buccal film containing fluticasone propionate. AAPS PharmSciTech. 2017, 18, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Anoop, K. Local antimicrobial delivery of satranidazole loaded cross linked periodontal chips using bio degradable polymers. Int. J. Pharm. 2013, 5, 839–847. [Google Scholar]
- Zhang, C.; Liu, Y.; Li, W.; Gao, P.; Xiang, D.; Ren, X.; Liu, D. Mucoadhesive buccal film containing ornidazole and dexamethasone for oral ulcers: In vitro and in vivo studies. Pharm. Dev. Technol. 2019, 24, 118–126. [Google Scholar] [CrossRef]
- Ceschel, G.C.; Maffei, P.; Lombardi Borgia, S.; Ronchi, C. Design and evaluation of buccal adhesive hydrocortisone acetate (HCA) tablets. Drug Deliv. 2001, 8, 161–167. [Google Scholar]
- Mohammed, F.A.; Khedr, H. Preparation and In vitro/In vivo evaluation of the buccal bioadhesive properties of slow-release tablets containing miconazole nitrate. Drug Dev. Ind. Pharm. 2003, 29, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Ramineni, S.K.; Dziubla, T.D.; Cunningham, L.L., Jr.; Puleo, D.A. Local delivery of imiquimod in hamsters using mucoadhesive films and their residence time in human patients. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Rawat, M.K.; Jain, A.; Rajput, A.; Chaturvedi, T.P.; Singh, S. Development of Satranidazole Mucoadhesive Gel for the Treatment of Periodontitis. AAPS PharmSciTech. 2009, 10, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Yotsuyanagi, T.; Okamoto, T.; Nabeshima, T. Pain relief of oral ulcer by dibucaine-film. Pain 1999, 83, 625–626. [Google Scholar] [CrossRef]
- Oguchi, M.; Shikama, N.; Sasaki, S.; Gomi, K.; Katsuyama, Y.; Ohta, S.; Hori, M.; Takei, K.; Arakawa, K.; Sone, S. Mucosa-adhesive water-soluble polymer film for treatment of acute radiation-induced oral mucositis. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 1033–1037. [Google Scholar] [CrossRef]
- Yildir, E.; Sjöholm, E.; Preis, M.; Trivedi, P.; Trygg, J.; Fardim, P.; Sandler, N. Investigation of dissolved cellulose in development of buccal discs for oromucosal drug delivery. Pharm. Dev. Technol. 2018, 23, 520–529. [Google Scholar] [CrossRef]
- Repka, M.A.; Gutta, K.; Prodduturi, S.; Munjal, M.; Stodghill, S.P. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur. J. Pharm. Biopharm. 2005, 59, 189–196. [Google Scholar] [CrossRef]
- Kohda, Y.; Kobayashi, H.; Baba, Y.; Yuasa, H.; Ozeki, T.; Kanaya, Y.; Sagara, E. Controlled release of lidocaine hydrochloride from buccal mucosa-adhesive films with solid dispersion. Int. J. Pharm. 1997, 158, 147–155. [Google Scholar] [CrossRef]
- Ceschel, G.C.; Bergamante, V.; Calabrese, V.; Biserni, S.; Ronchi, C.; Fini, A. Design and evaluation in vitro of controlled release mucoadhesive tablets containing chlorhexidine. Drug Dev. Ind. Pharm. 2006, 32, 53–61. [Google Scholar] [CrossRef]
- Fini, A.; Bergamante, V.; Ceschel, G.C. Mucoadhesive gels designed for the controlled release of chlorhexidine in the oral cavity. Pharmaceutics 2011, 3, 665–679. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Halima, N.B. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Gajra, B.; Pandya, S.S.; Singh, S.; Rabari, H.A. Mucoadhesive hydrogel films of econazole nitrate: Formulation and optimization using factorial design. J. Drug Deliv. 2014, 2014, 305863. [Google Scholar]
- Oliveira, A.S.; Seidi, O.; Ribeiro, N.; Colaço, R.; Serro, A.P. Tribomechanical comparison between PVA hydrogels obtained using different processing conditions and human cartilage. Materials 2019, 12, 3413. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Chaker, A.; Jridi, M.; Maalej, H.; Jellouli, K.; Boufi, S.; Nasri, M. Structural analysis, and antioxidant and antibacterial properties of chitosan-poly (vinyl alcohol) biodegradable films. Environ. Sci. Pollut. Res. 2016, 23, 15310–15320. [Google Scholar] [CrossRef] [PubMed]
- Nafee, N.A.; Ismail, F.A.; Boraie, N.A.; Mortada, L.M. Mucoadhesive buccal patches of miconazole nitrate: In vitro/In vivo performance and effect of ageing. Int. J. Pharm. 2003, 264, 1–14. [Google Scholar] [CrossRef]
- Padula, C.; Pozzetti, L.; Traversone, V.; Nicoli, S.; Santi, P. In vitro evaluation of mucoadhesive films for gingival administration of lidocaine. AAPS PharmSciTech 2013, 14, 1279–1283. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Gad, H.A.; El-Nabarawi, M.A.; El-Hady, S.S.A. Formulation and Evaluation of PLA and PLGA In Situ Implants Containing Secnidazole and/or Doxycycline for Treatment of Periodontitis. AAPS PharmSciTech 2008, 9, 878–884. [Google Scholar] [CrossRef]
- Torshabi, M.; Nojehdehian, H.; Tabatabaei, F.S. In vitro behavior of poly-lactic-co-glycolic acid microspheres containing minocycline, metronidazole, and ciprofloxacin. J. Invest. Clin. Dent. 2016, 8, e12201. [Google Scholar] [CrossRef] [PubMed]
- Yue, I.C.; Poff, J.; Cortes, M.E.; Simisterra, R.D.; Faris, C.B.; Hildgen, P.; Langer, R.; Shastri, V.P. A novel polymeric chlorhexidine device for treatment of periodontal disease. Biomaterials 2004, 25, 3743–3750. [Google Scholar] [CrossRef]
- Klepac-Ceraj, V.; Patel, N.; Song, X.; Holewa, C.; Patel, C.; Kent, R.; Amiji, M.M.; Soukos, N.S. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Lasers Surg. Med. 2011, 43, 600–606. [Google Scholar] [CrossRef]
- Williams, R.C.; Paquette, D.W.; Offenbacher, S.; Adams, D.F.; Armitage, G.C.; Bray, K.; Caton, J.; Cochran, D.L.; Drisko, C.H.; Fiorellini, J.P.; et al. Treatment of periodontitis by local administration of minocycline microspheres: A controlled trial. J. Periodontol. 2001, 72, 1535–1544. [Google Scholar] [CrossRef]
- Cortelli, J.R.; Querido, S.M.; Aquino, D.R.; Ricardo, L.H.; Pallos, D. Longitudinal clinical evaluation of adjunct Minocycline in the treatment of chronic periodontitis. J. Periodontol. 2006, 77, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Deo, V.; Ansari, S.; Mandia, S.; Bhongade, M. Therapeutic efficacy of subgingivally delivered doxycycline hyclate as an adjunct to non-surgical treatment of chronic periodontitis. J. Oral Maxillofac. Res. 2011, 2, e3. [Google Scholar] [CrossRef][Green Version]
- Sandhya, Y.P.; Prabhuji, M.L.; Chandra, R.V. Comparative evaluation of the efficacy of 10% doxycycline hyclate in the periodontal treatment of smokers: A clinical and microbiological study. Oral Health Prev. Dent. 2011, 9, 59–65. [Google Scholar] [PubMed]
- Saarani, N.N.; Jamuna-Thevi, K.; Shahab, N.; Hermawan, H.; Saidin, S. Antibacterial efficacy of triple-layered poly (lactic-co-glycolic acid)/nanoapatite/lauric acid guided bone regeneration membrane on periodontal bacteria. Dent. Mater. J. 2017, 36, 260–265. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Nasajpour, A.; Ansari, S.; Rinoldi, C.; Rad, A.S.; Aghaloo, T.; Shin, S.R.; Mishra, Y.K.; Adelung, R.; Swieszkowski, W.; Annabi, N.; et al. A Multifunctional polymeric periodontal membrane with osteogenic and antibacterial characteristics. Adv. Funct. Mater. 2018, 28, 1703437. [Google Scholar] [CrossRef]
- Shim, J.H.; Won, J.Y.; Park, J.H.; Bae, J.H.; Ahn, G.; Kim, C.H.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Bae, E.B.; et al. Effects of 3D-printed polycaprolactone/β-tricalcium phosphate membranes on guided bone regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef]
- Xue, J.; Niu, Y.; Gong, M.; Shi, R.; Chen, D.; Zhang, L.; Lvov, Y. Electrospun microfiber membranes embedded with drug-loaded Clay. ACS Nano 2015, 9, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhih, M.; Formela, K.; et al. A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Karolewicz, B.; Górniak, A.; Owczarek, A.; Żurawska-Płaksej, E.; Piwowar, A.; Pluta, J. Thermal, spectroscopic, and dissolution studies of ketoconazole–Pluronic F127 system. J. Therm. Anal. 2014, 115, 2487–2493. [Google Scholar] [CrossRef]
- Aithal, G.C.; Nayak, U.Y.; Mehta, C.; Narayan, R.; Gopalkrishna, P.; Pandiyan, S.; Garg, S. Localized in situ nanoemulgel drug delivery system of quercetin for periodontitis: Development and computational simulations. Molecules 2018, 23, 1363. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Kamel, A.O.; Ezzat, O.M.; El Dessouky, H.F.; Sammour, O.A. Doxycycline hydrochloride-metronidazole solid lipid microparticles gels for treatment of periodontitis: Development, in-vitro and in-vivo clinical evaluation. Expert Opin. Drug Deliv. 2017, 14, 1241–1251. [Google Scholar] [CrossRef]
- Abdel-Hamid, S.M.; Abdel-Hady, S.E.; El-Shamy, A.A.; El-Dessoukyb, H.F. Formulation of an antispasmodic drug as a topical local anesthetic. Int. J. Pharm. 2006, 326, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Chen, X.G.; Zhong, D.Y.; Xu, X.C. Study on poly(vinyl alcohol)/carboxymethyl-chitosan blend film as local drug delivery system. J. Mater. Sci. Mater. Med. 2007, 18, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Kida, D.; Gładysz, O.; Szulc, M.; Zborowski, J.; Junka, A.; Janeczek, M.; Lipińska, A.; Skalec, A.; Karolewicz, B. Development and evaluation of a polyvinylalcohol—Cellulose derivative-based film with povidone-iodine predicted for wound treatment. Polymers 2020, 12, 1271. [Google Scholar] [CrossRef]
- Shifrovitch, Y.; Binderman, I.; Bahar, H.; Berdicevsky, I.; Zilberman, M. Metronidazole-Loaded Bioabsorbable Films as Local Antibacterial Treatment of Infected Periodontal Pockets. J. Periodontol. 2009, 80, 330–337. [Google Scholar] [CrossRef]
- Dabhi, M.R.; Sheth, N.R. Formulation development of physiological environment responsive periodontal drug delivery system for local delivery of metronidazole benzoate. Drug Dev. Ind. Pharm. 2013, 39, 425–436. [Google Scholar] [CrossRef]
- Alhusein, N.; De Bank, P.A.; Blagbrough, I.S.; Bolhuis, A. Killing bacteria within biofilms by sustained release of tetracycline from triple-layered electrospun micro/nanofibre matrices of polycaprolactone and poly (ethylene-co-vinyl acetate). Drug Deliv. Transl. Res. 2013, 3, 531–541. [Google Scholar] [CrossRef]
- Steinberg, D.; Friedman, M. Development of sustained release devices for modulation of dental plaque biofilm and treatment of oral infectious diseases. Drug Develop. Res. 2000, 50, 555–565. [Google Scholar] [CrossRef]
- Rehman, I.U.; Melo, M.A. Molecular research on dental materials and biomaterials 2018. Int. J. Mol. Sci. 2020, 21, 9154. [Google Scholar] [CrossRef]
- Di Pasquale, S.A.; Byrne, M.E. Controlled architecture for improved macromolecular memory within polymer networks. Curr. Opin. Biotechnol. 2016, 40, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.R.; Peppas, N.A. Molecular recognition with soft biomaterials. Soft Matter 2020, 16, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Tammaro, L.; Di Salle, A.; Calarco, A.; De Luca, I.; Riccitiello, F.; Peluso, G.; Vittoria, V.; Sorrentino, A. Multifunctional bioactive resin for dental restorative materials. Polymers 2020, 12, 332. [Google Scholar] [CrossRef]
- Intra-Oral Treatment of OLP With Rivelin®-CLO Patches. Available online: https://clinicaltrials.gov/ct2/show/NCT03592342 (accessed on 27 April 2021).
- Sankar, V.; Hearnden, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.; Lockhart, P.; Patton, L.; Porter, S.; Thornhill, M. Local drug delivery for oral mucosal diseases: Challenges and opportunities. Oral Dis. 2011, 17, 73–84. [Google Scholar] [CrossRef] [PubMed]


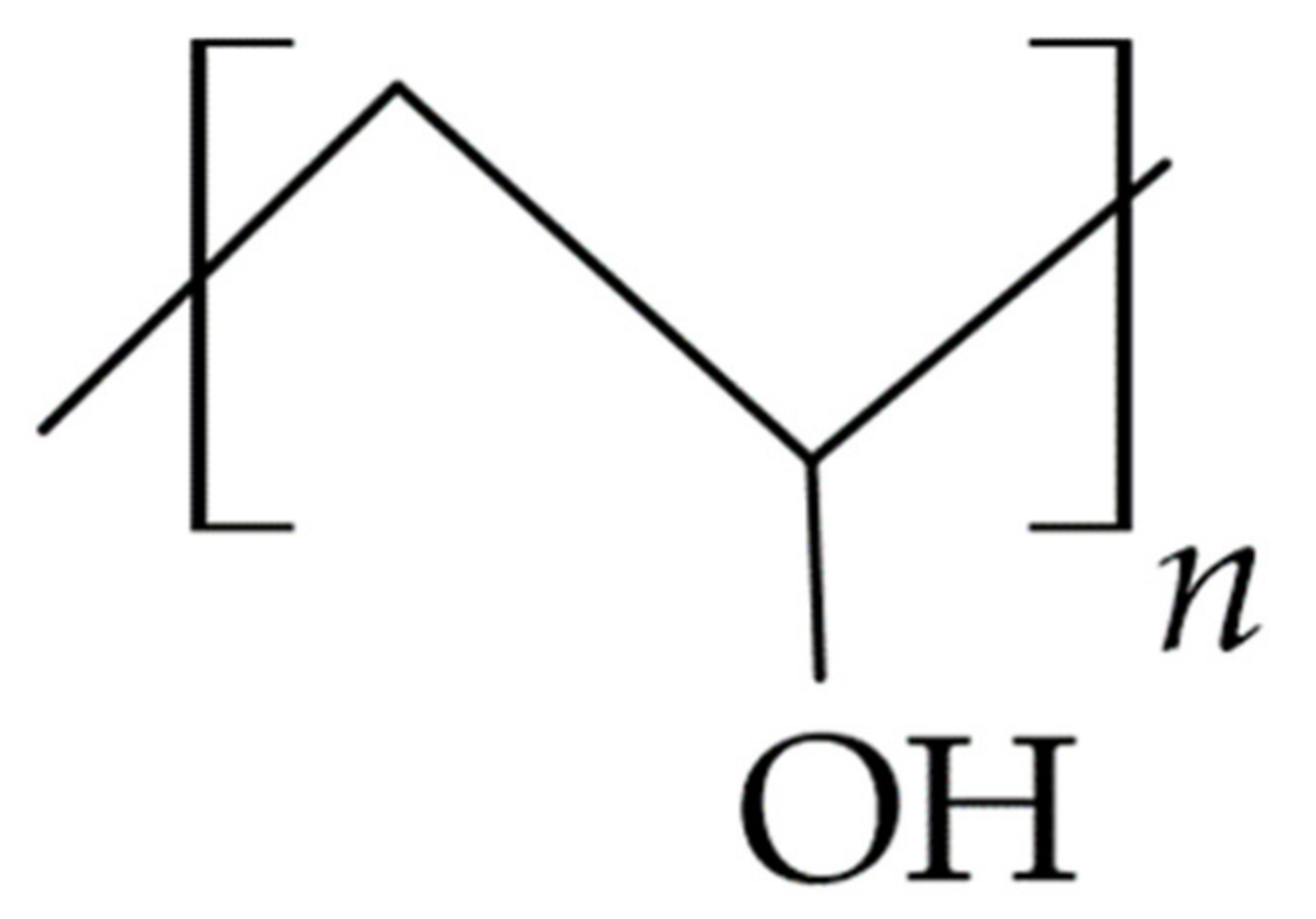
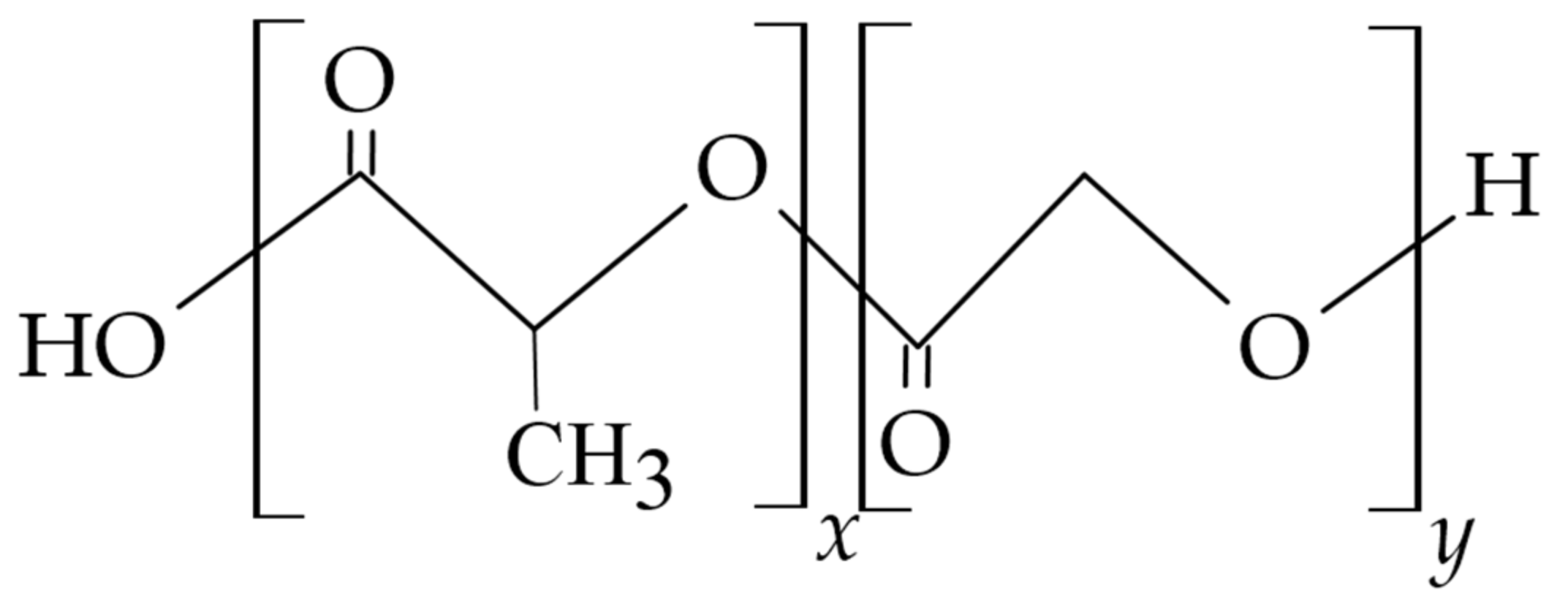
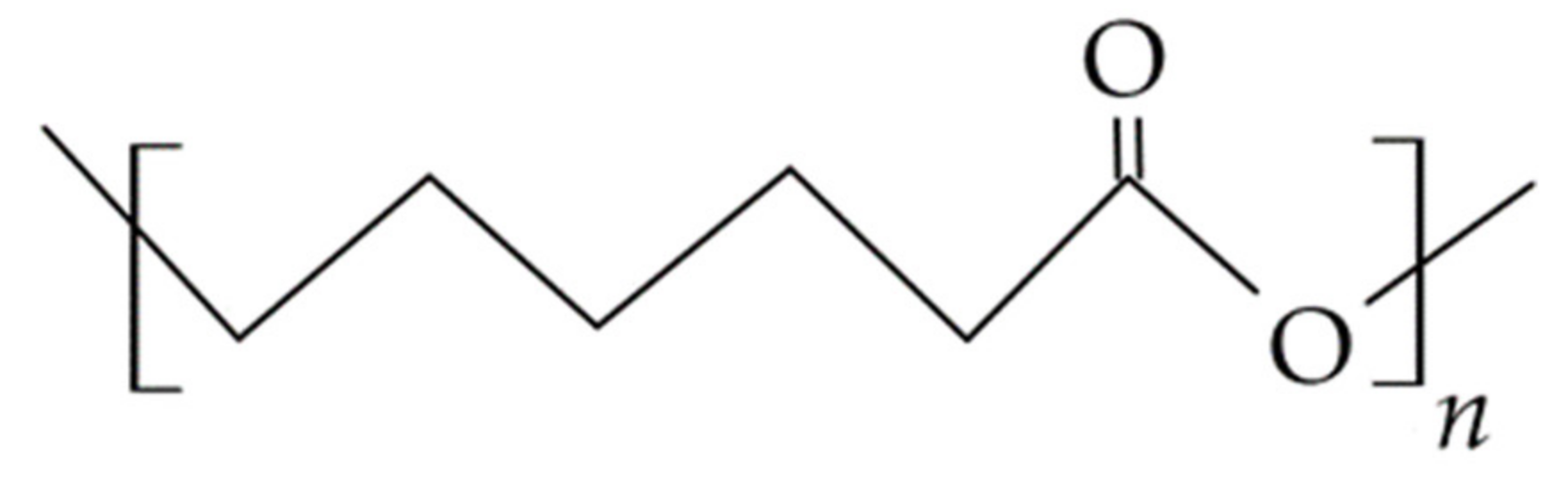
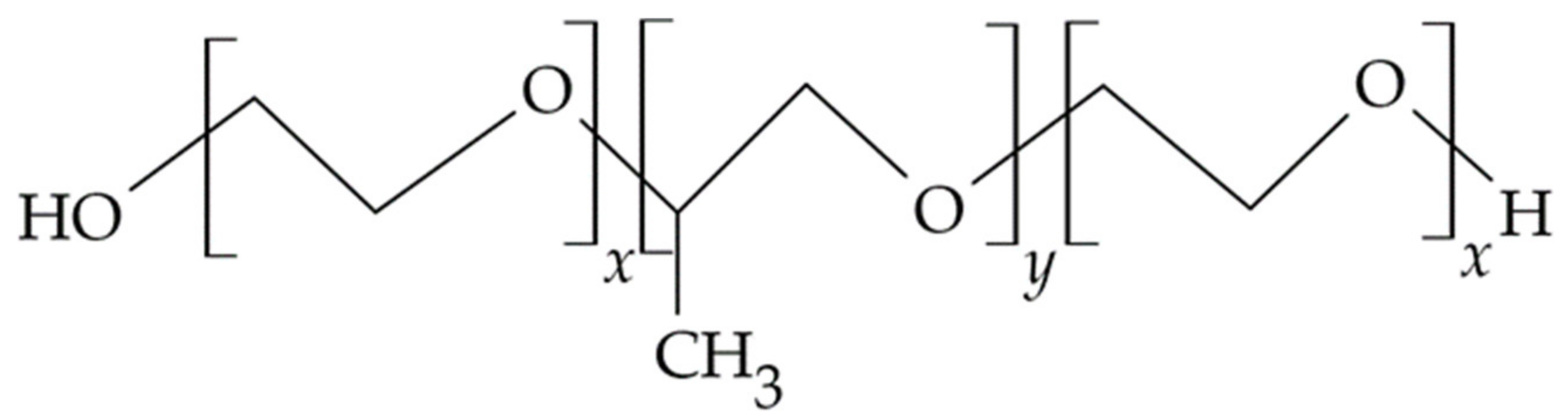
| Characteristics | Oral Cavity | Periodontal Pocket Depth |
|---|---|---|
| Administration area/size of the administration area | surface area of periodontal pockets 50–200 cm2 [14], 100 cm2 [13] | >4 mm during an inflammatory state [17] |
| Type of covering epithelium | keratinized [7,13] | non-keratinized [13] |
| Thickness of epithelium | 500–800 µm [11] | 100–600 µm [13] |
| Exchange/flow of saliva/fluid in the gingival groove | 0.5–2 L/day [8] | 3–137 µL/h [13,18] |
| Desired characteristics of the drug carrier | adhesion, moldability, and adaptability to the application site (plasticity), resistance to smearing, patient self-application, biodegradability, modified release [7] | fitting into the application site, penetration into tissue, formation of physical bonds with tissue, resistance to smearing, adequate retention time, ease of application, biodegradability, modified release [19] |
| Polymeric carriers of active substance applied | rinses, films [20], gels [21], bioadhesive tablets, matrices, scaffolds | fibers, microspheres [22], implants, gels, in situ gels, nanocarriers (nanoparticles, nanospheres) |
| Polymer | Carrier | Drug |
|---|---|---|
| Chitosan | gels | VEGF [32] atorvastatin [33] thymol [34] metronidazole/vancomycin [35] lidocaine [36] |
| films | Acmella oreacea [20] metformini hydrochloridum [37] pentoxifylline [38] | |
| membranes | extract from Garcinia mangostana [39] without substance [40] without substance [41] | |
| microspheres microparticles | ornidazole/doxycycline [22] tetracycline hydrochloride [42] metronidazole [43] | |
| Hyaluronic acid Hyaluronate | membranes matrices | without substance [44] L-PRF [45] rhBMP9 [46] |
| gels | 0.2% [4] | |
| Gums Pectins | film sponge matrix | triamcinolone acetonide [47] without substance [48,49] lidocaine [10] |
| Polymer | Carrier | Drug |
|---|---|---|
| Gelatin | implant (Periochip®) | chlorhexidine digluconate [110] |
| hemostatic sponge | Gelfoam® without substance [111] Gelatamp Ag ions [112] | |
| Collagen | membranes implant (PerioCol™-CG) | without substance [113] chlorhexidine digluconate [114] |
| Cellulose Ethers | R Groups |
|---|---|
| Methylcellulose | H, CH3 |
| Ethylcellulose | H, CH2CH3 |
| Hydroxyethyl methylcellulose | H, CH3, [CH2CH2O]nH |
| Hydroxypropyl cellulose | H, [CH2CH(CH3)O]H |
| Carboxymethyl cellulose | H, CH2COONa |
| Polymer | Carrier | Drug |
|---|---|---|
| HPMC | sponge | curcumin [138] |
| film | fluticasone propionate [139] satranidazole [140] ornidazole/dexamethasone [141] metronidazole [38] | |
| mucoadhesive tablet | chlorhexidine digluconate [142] miconazole [143] | |
| CMC | film | imiquimod [144] allantoin [136] |
| CMCNa | gel matrix | satranidazole [145] metronidazole [137] |
| HPC | film | dibucaine [146] tetracaine/ofloxacin [147] |
| EC | matrix | lidocaine/triamcinolone [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kida, D.; Zakrzewska, A.; Zborowski, J.; Szulc, M.; Karolewicz, B. Polymer-Based Carriers in Dental Local Healing—Review and Future Challenges. Materials 2021, 14, 3948. https://doi.org/10.3390/ma14143948
Kida D, Zakrzewska A, Zborowski J, Szulc M, Karolewicz B. Polymer-Based Carriers in Dental Local Healing—Review and Future Challenges. Materials. 2021; 14(14):3948. https://doi.org/10.3390/ma14143948
Chicago/Turabian StyleKida, Dorota, Aneta Zakrzewska, Jacek Zborowski, Małgorzata Szulc, and Bożena Karolewicz. 2021. "Polymer-Based Carriers in Dental Local Healing—Review and Future Challenges" Materials 14, no. 14: 3948. https://doi.org/10.3390/ma14143948
APA StyleKida, D., Zakrzewska, A., Zborowski, J., Szulc, M., & Karolewicz, B. (2021). Polymer-Based Carriers in Dental Local Healing—Review and Future Challenges. Materials, 14(14), 3948. https://doi.org/10.3390/ma14143948






