Low-Diffusion Fricke Gel Dosimeters with Core-Shell Structure Based on Spatial Confinement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of FPX Precursor Solution
2.3. Preparation and Fabrication of Hydrogel Pellets
2.3.1. Preparation of FPX Hydrogel Pellets
2.3.2. Fabrication of Core-Shell FPX@PDMS Pellets
2.4. Preparation of 3D FPX@PDMS in PVA Substrate Dosimeter
2.5. Characterization of FPX Pellets, FPX@PDMS Pellets and PDMS Coatings
2.6. Rheological Test of PVA Hydrogels Support
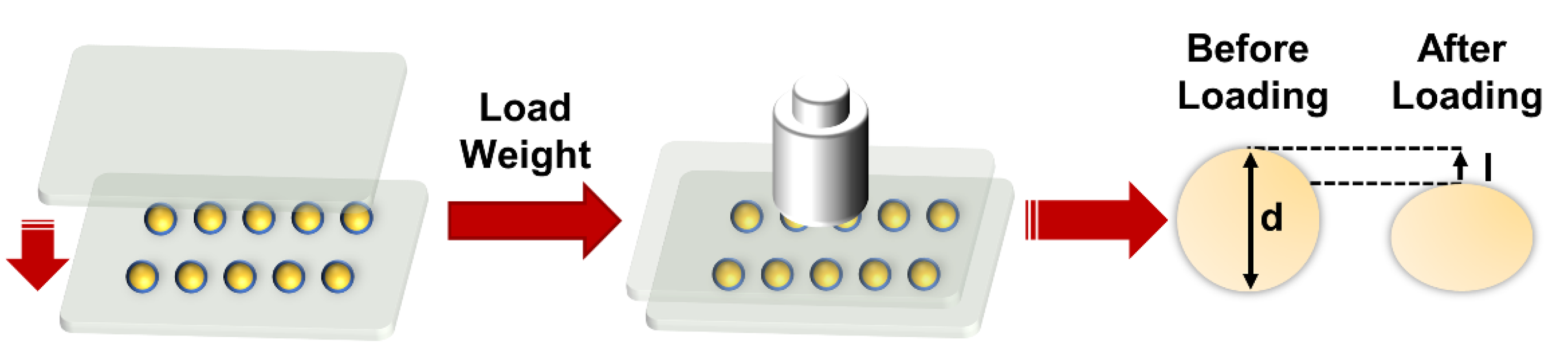
2.7. Mechanical Strain Analysis at the Micro-Level
2.8. Diffusion Measurements
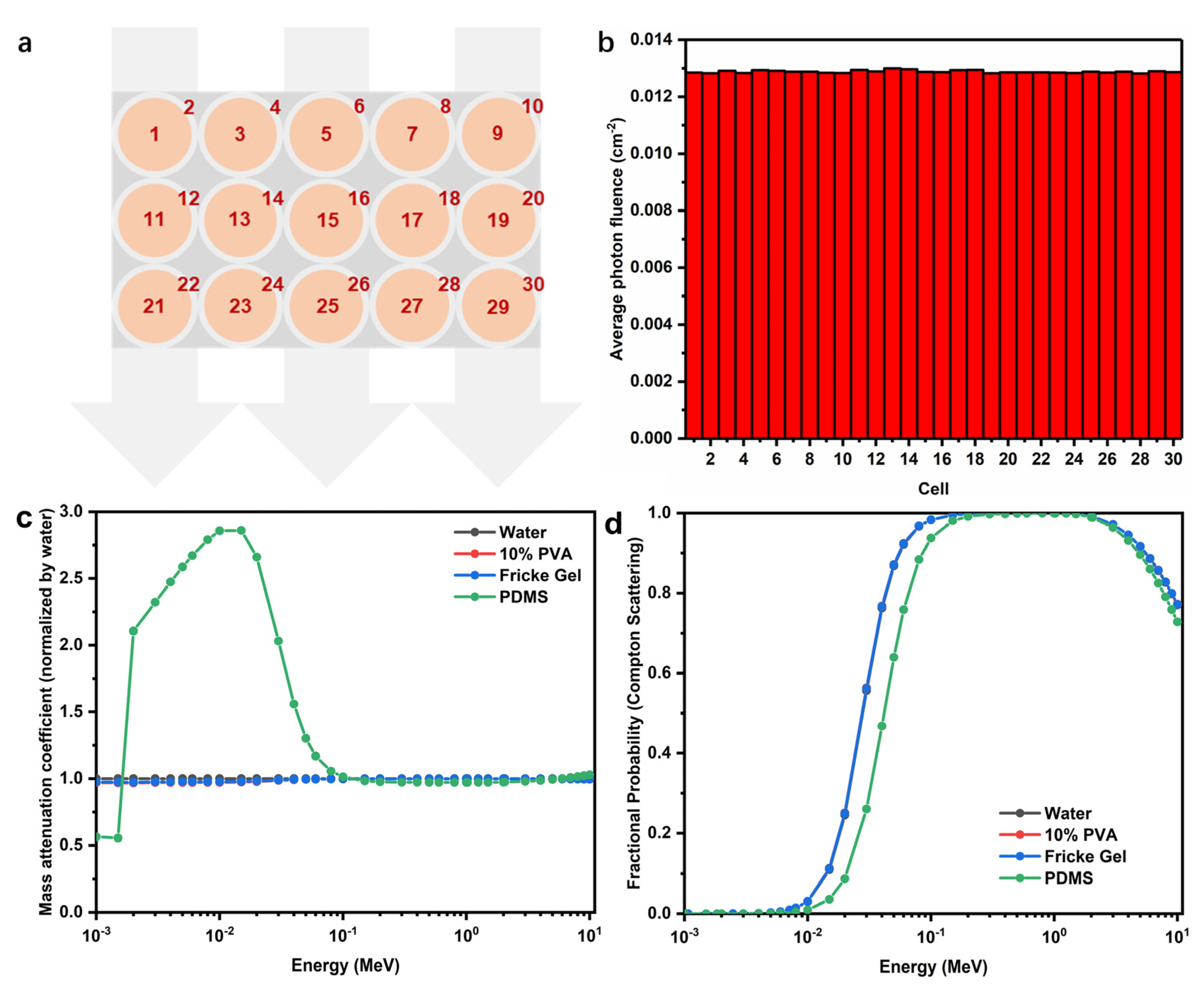
2.9. Dosimetry of FPX@PDMS Dosimeter
3. Results and Discussion
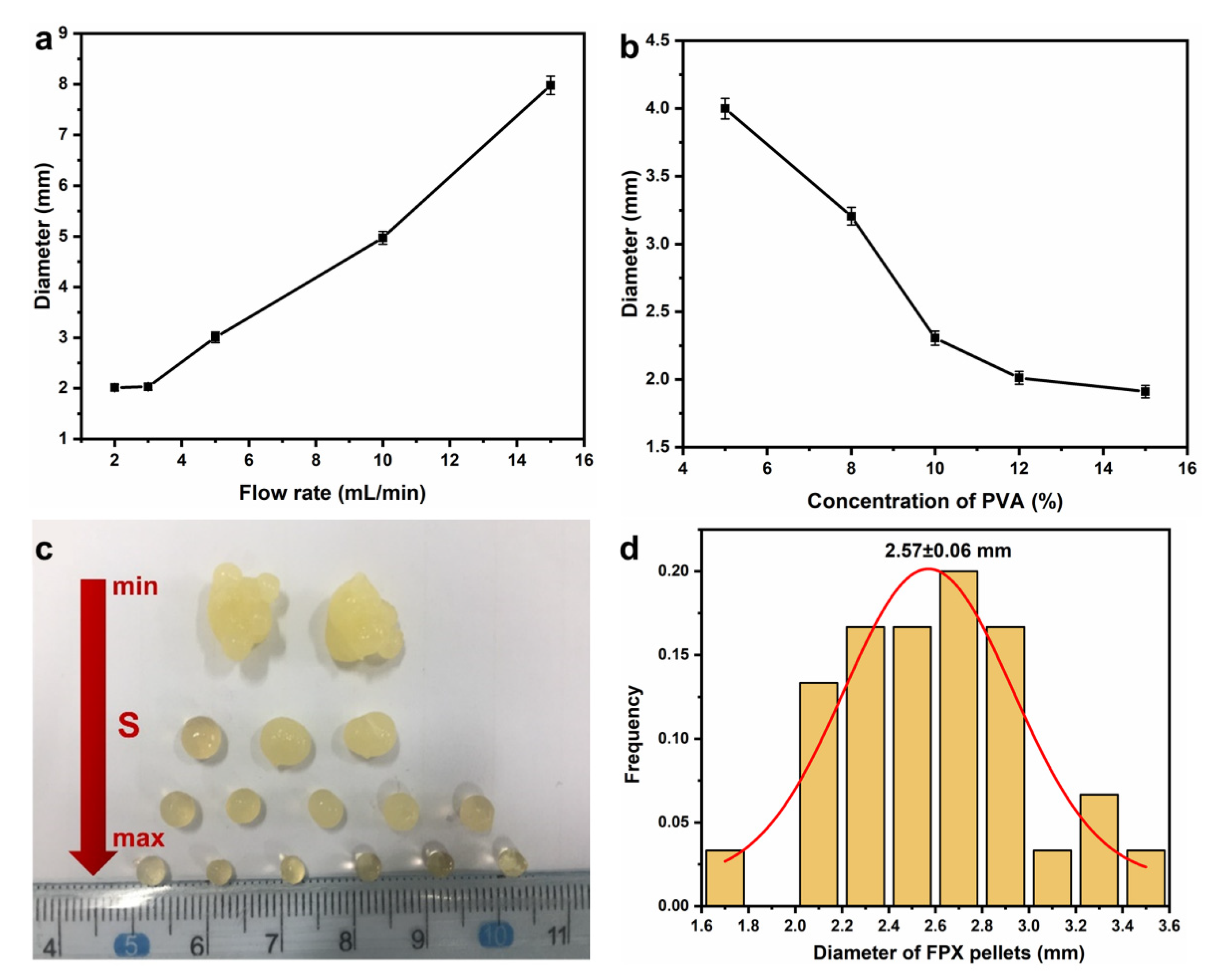
3.1. Optimization and Characterization of FPX Pellets
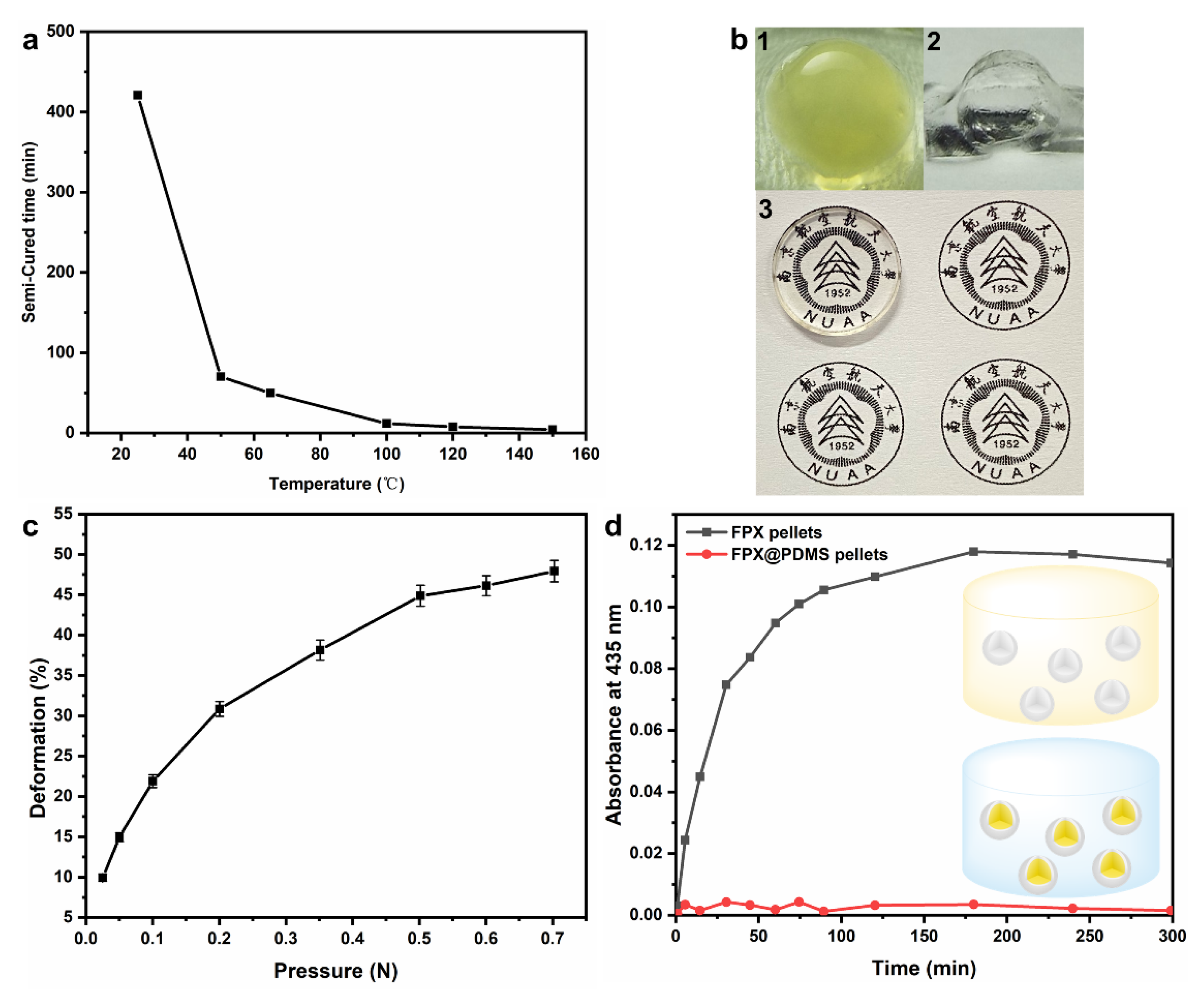
3.2. Optimization and Characterization of Core-Shell FPX@PDMS Pellets
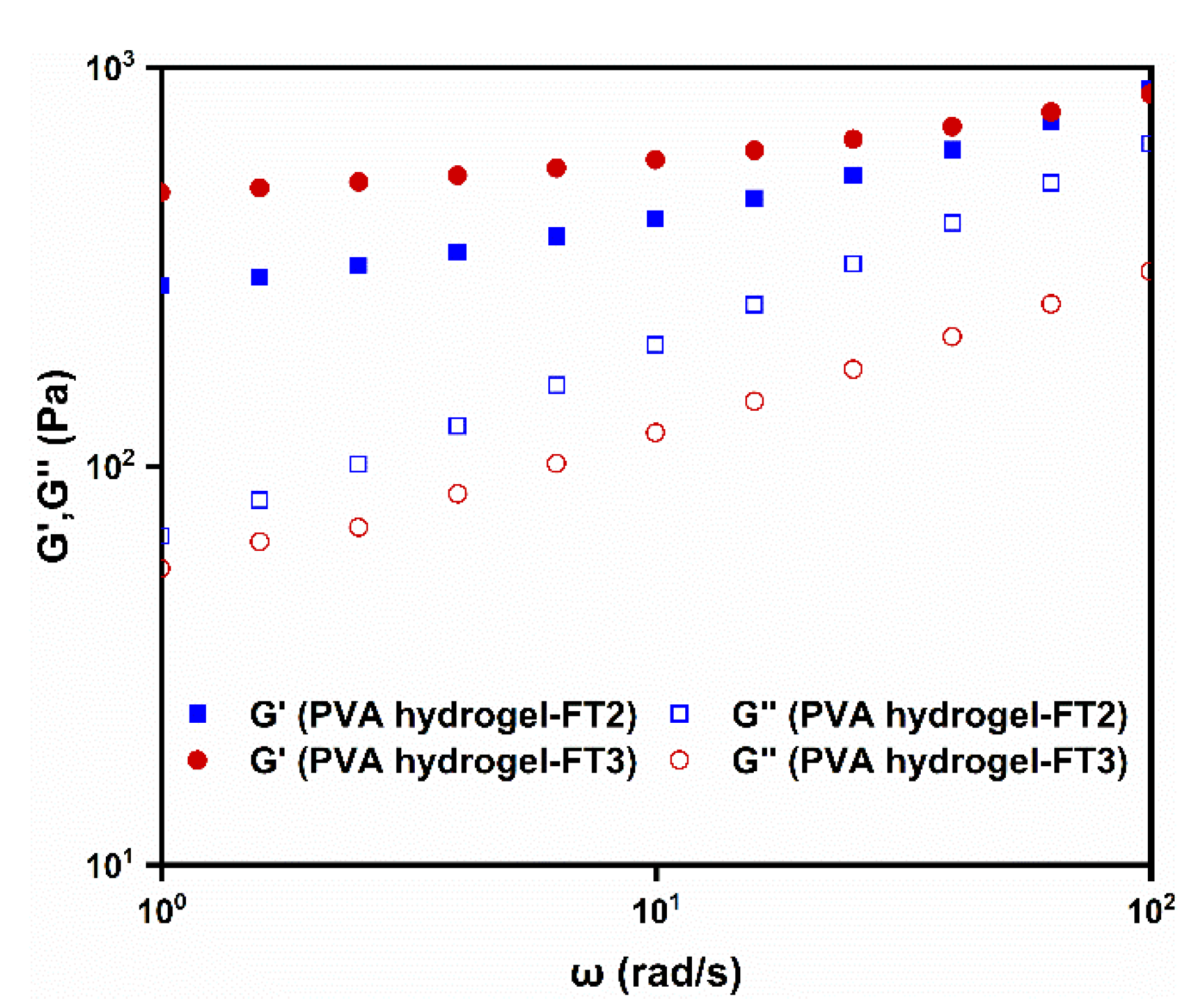
3.3. Rheological Behavior of PVA Gels
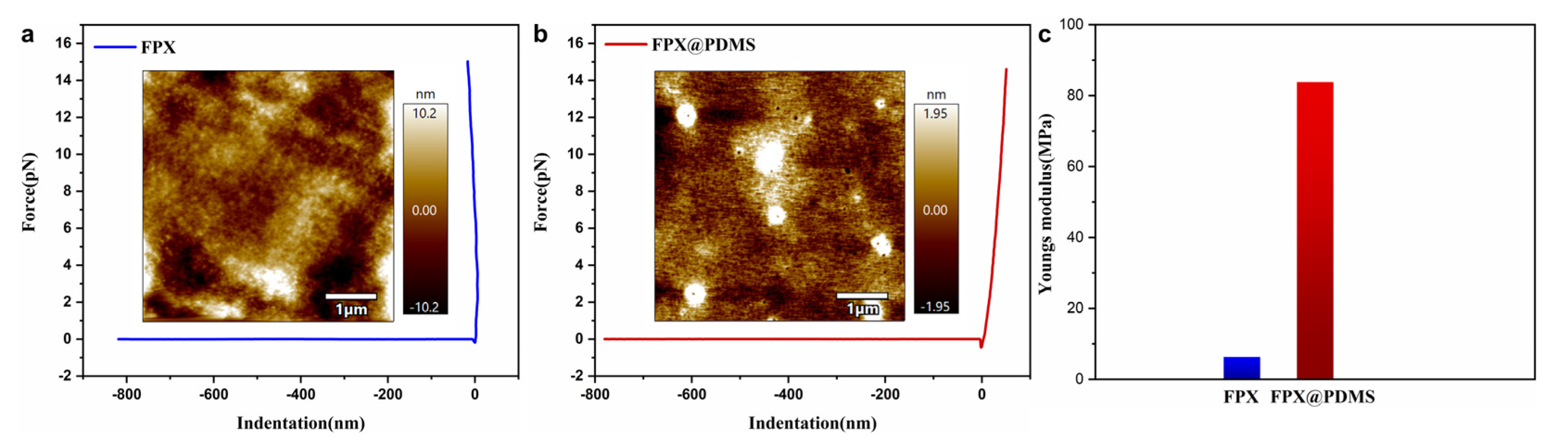
3.4. Micro-Mechanical Properties of FPX and FPX@PDMS Pellets
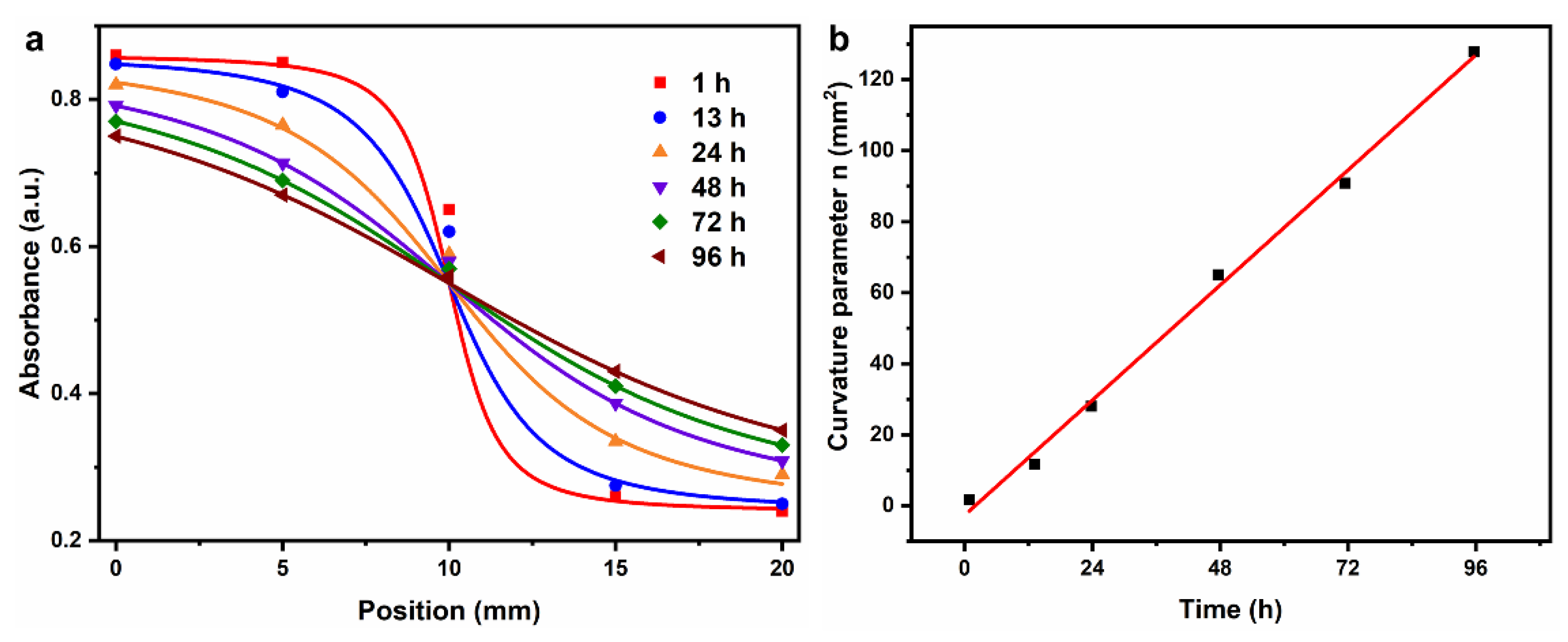
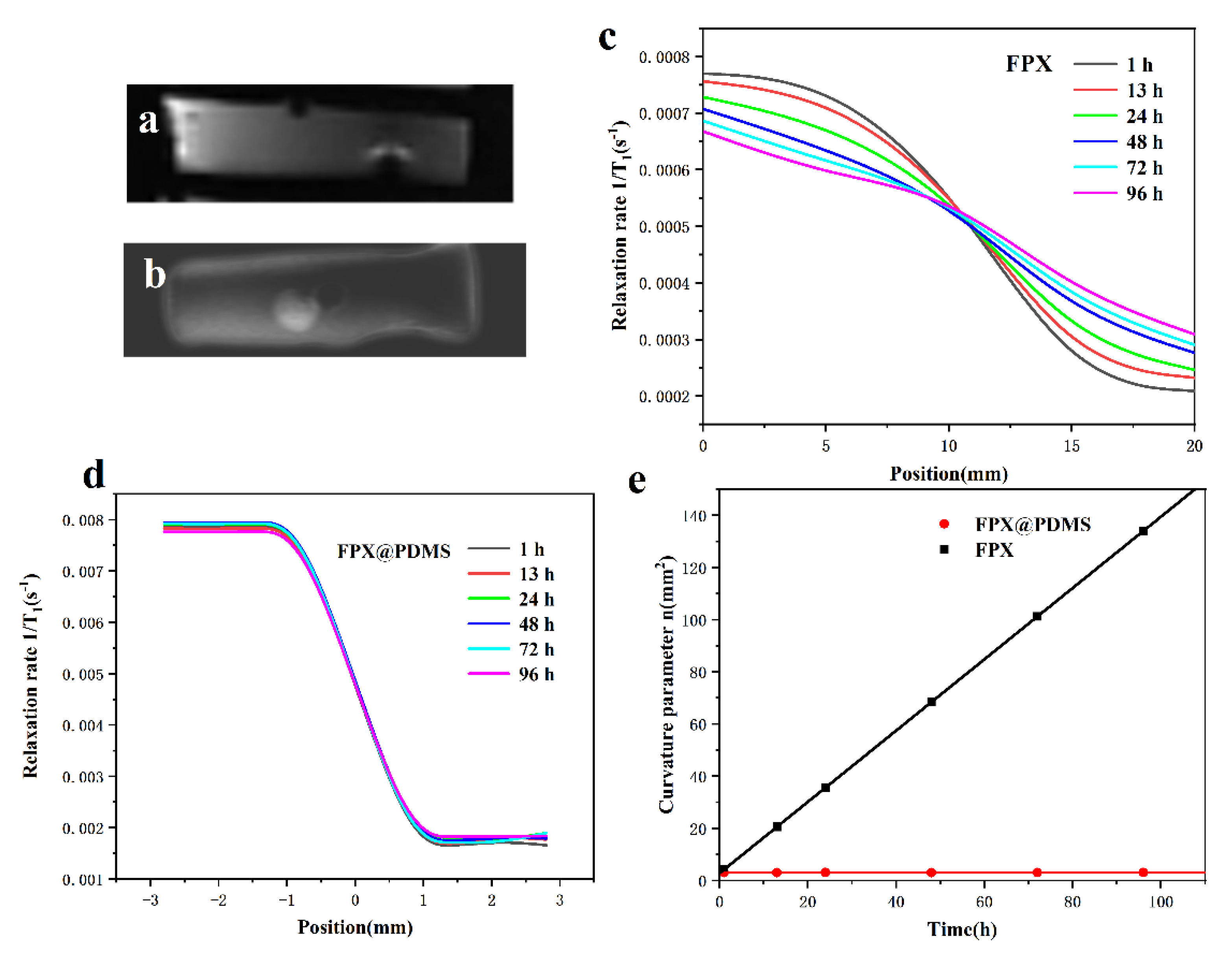
3.5. Diffusion Behavior
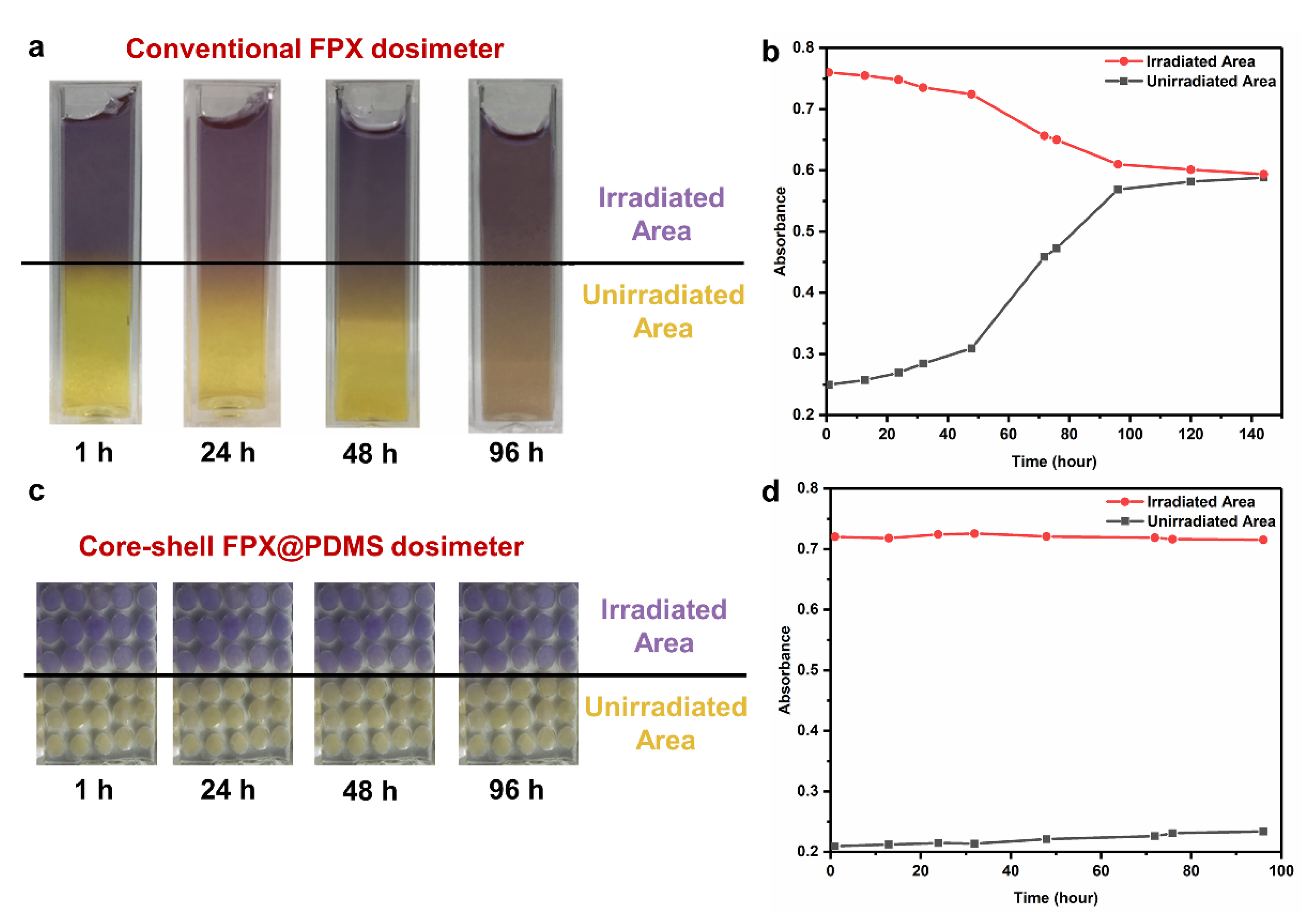
3.6. Dosimetry of 3D Fricke Gel Dosimeters with Core-Shell Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDMS | polydimethylsiloxane |
| 3D | three-dimensional |
| FPX | Fricke-PVA-xylenol orange |
| PVA | poly(vinyl alcohol) |
| XO | xylenol orange |
| AFM | atomic force microscopy |
| MRI | magnetic resonance imaging |
| TE | echo time |
| TR | repetition time |
| TI | inversion time |
References
- Rosenblatt, E.; Izewska, J.; Anacak, Y.; Pynda, Y.; Scalliet, P.; Boniol, M.; Autier, P. Radiotherapy capacity in European countries: An analysis of the Directory of Radiotherapy Centres (DIRAC) database. Lancet Oncol. 2013, 14, e79–e86. [Google Scholar] [CrossRef]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation oncology in the era of precision medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef]
- Kron, T.; Lehmann, J.; Greer, P.B. Dosimetry of ionising radiation in modern radiation oncology. Phys. Med. Biol. 2016, 61, R167–R205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Zhang, W.; Zhang, X.; Hu, X.; Chang, S.; Zhang, H. Highly Sensitive Gold Nanoparticles–DNA Nanosensor for γ-Radiation Detection. ACS Appl. Mater. Interfaces 2020, 12, 42403–42409. [Google Scholar] [CrossRef] [PubMed]
- Low, D.A.; Moran, J.M.; Dempsey, J.F.; Dong, L.; Oldham, M. Dosimetry tools and techniques for IMRT. Med Phys. 2011, 38, 1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiner, L.J. Review of Fricke gel dosimeters. J. Physics: Conf. Ser. 2004, 3, 9–21. [Google Scholar] [CrossRef]
- Moussous, O. Characterization of ferrous-agarose-xylenol gel dosimeter at (60)Co gamma-rays beam therapy unit. Radiol. Phys. Technol. 2021, 14, 105–112. [Google Scholar] [CrossRef]
- Gallo, S.; Artuso, E.; Brambilla, M.G.; Gambarini, G.; Lenardi, C.; Monti, A.F.; Torresin, A.; Pignoli, E.; Veronese, I. Characterization of radiochromic poly(vinyl-alcohol)–glutaraldehyde Fricke gels for dosimetry in external x-ray radiation therapy. J. Phys. D Appl. Phys. 2019, 52, 225601. [Google Scholar] [CrossRef]
- Choosin, P.; Tippayamontri, T.; Ninlaphruk, S.; Pungkun, V. Study on characteristic of Fricke xylenol gel dosimeter: Application for dose evaluation in radiotherapy. J. Phys. Conf. Ser. 2019, 1285, 012029. [Google Scholar] [CrossRef] [Green Version]
- Farhood, B.; Geraily, G.; Abtahi, S.M.M. A systematic review of clinical applications of polymer gel dosimeters in radiotherapy. Appl. Radiat. Isotopes 2019, 143, 47–59. [Google Scholar] [CrossRef]
- Abtahi, S.M.M.; Langaroodi, R.K.S.; Akbari, M.E. Dose distribution verification in intraoperative radiation therapy using an N-isopropyl acrylamide-based polymer gel dosimeter. J. Radioanal. Nucl. Chem. 2020, 324, 481–488. [Google Scholar] [CrossRef]
- Khan, M.; Heilemann, G.; Lechner, W.; Georg, D.; Berg, A.G. Basic Properties of a New Polymer Gel for 3D-Dosimetry at High Dose-Rates Typical for FFF Irradiation Based on Dithiothreitol and Methacrylic Acid (MAGADIT): Sensitivity, Range, Reproducibility, Accuracy, Dose Rate Effect and Impact of Oxygen Scavenger. Polymer 2019, 11, 1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khezerloo, D.; Nedaie, H.A.; Takavar, A.; Zirak, A.; Farhood, B.; Movahedinejhad, H.; Banaee, N.; Ahmadalidokht, I.; Knuap, C. PRESAGE (R) as a solid 3-D radiation dosimeter: A review article. Radiat. Phys. Chem. 2017, 141, 88–97. [Google Scholar] [CrossRef]
- Jaszczak, M.; Sasiadek, E.; Kadlubowski, S.; Dudek, M.; Kozicki, M. Preliminary study on a new 3D radiochromic KI-Pluronic F-127 gel dosimeter for radiotherapy. Radiat. Phys. Chem. 2021, 185, 109507. [Google Scholar] [CrossRef]
- Hayashi, S.-I.; Ono, K.; Fujino, K.; Ikeda, S.; Tanaka, K. Novel radiochromic gel dosimeter based on a polyvinyl alcohol–Iodide complex. Radiat. Meas. 2020, 131, 106226. [Google Scholar] [CrossRef]
- Eyadeh, M.M.; Rabaeh, K.A.; Aldweri, F.M.; Shorman, M.Y.A.-; Alheet, S.M.; Awad, S.I.; Hailat, T.F. Nuclear magnetic resonance analysis of a chemically cross-linked ferrous–methylthymol blue–polyvinyl alcohol radiochromic gel dosimeter. Appl. Radiat. Isot. 2019, 153, 108812. [Google Scholar] [CrossRef]
- Penev, K.I.; Mequanint, K. The effect of mixed dopants on the stability of Fricke gel dosimeters. J. Phys. Conf. Ser. 2013, 444, 012105. [Google Scholar] [CrossRef]
- Rae, W.I.D.; Willemse, C.A.; Lötter, M.G.; Engelbrecht, J.S.; Swarts, J.C. Chelator effect on ion diffusion in ferrous-sulfate-doped gelatin gel dosimeters as analyzed by MRI. Med. Phys. 1996, 23, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.C.; Jordan, K.J.; Battista, J.; Van Dyk, J.; Rutt, B.K. Polyvinyl alcohol-Fricke hydrogel and cryogel: Two new gel dosimetry systems with low Fe3+ diffusion. Phys. Med. Biol. 2000, 45, 955–969. [Google Scholar] [CrossRef]
- Smith, S.T.; Boase, N.; Masters, K.S.; Hosokawa, K.; Asena, A.; Crowe, S.B.; Kairn, T.; Trapp, J.V. A very low diffusion Fricke gel dosimeter with functionalised xylenol orange-PVA (XOPVA). Phys. Med. Biol. 2019, 64, 205017. [Google Scholar] [CrossRef]
- Lazzaroni, S.; Liosi, G.; Mariani, M.; Dondi, D. An innovative Fe3+ selective ligand for Fricke-gel dosimeter. Radiat. Phys. Chem. 2020, 171, 108733. [Google Scholar] [CrossRef]
- Liosi, G.; Dondi, D.; Griend, D.V.; Lazzaroni, S.; D’Agostino, G.; Mariani, M. Fricke-gel dosimeter: Overview of Xylenol Orange chemical behavior. Radiat. Phys. Chem. 2017, 140, 74–77. [Google Scholar] [CrossRef]
- Del Lama, L.S.; Petchevist, P.C.D.; De Almeida, A. Fricke Xylenol Gel characterization at megavoltage radiation energy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 394, 89–96. [Google Scholar] [CrossRef]
- Alves, A.V.S.; De Almeida, W.S.; Sussuchi, E.M.; Lazzeri, L.; D’Errico, F.; De Souza, S.O. Investigation of chelating agents/ligands for Fricke gel dosimeters. Radiat. Phys. Chem. 2018, 150, 151–156. [Google Scholar] [CrossRef]
- Lazzeri, L.; Marini, A.; Cascone, M.G.; D’Errico, F. Dosimetric and chemical characteristics of Fricke gels based on PVA matrices cross-linked with glutaraldehyde. Phys. Med. Biol. 2019, 64, 085015. [Google Scholar] [CrossRef]
- Marrale, M.; Collura, G.; Gallo, S.; Nici, S.; Tranchina, L.; Abbate, B.F.; Marineo, S.; Caracappa, S.; D’Errico, F. Analysis of spatial diffusion of ferric ions in PVA-GTA gel dosimeters through magnetic resonance imaging. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 396, 50–55. [Google Scholar] [CrossRef]
- Maeyama, T.; Fukunishi, N.; Ishikawa, K.L.; Fukasaku, K.; Furuta, T.; Takagi, S.; Noda, S.; Himeno, R. Diffusion Suppression in Gel Dosimetry by Addition of Nanoclay; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1183–1186. [Google Scholar]
- Maeyama, T.; Fukunishi, N.; Ishikawa, K.; Furuta, T.; Fukasaku, K.; Takagi, S.; Noda, S.; Himeno, R.; Fukuda, S. A diffusion-free and linear-energy-transfer-independent nanocomposite Fricke gel dosimeter. Radiat. Phys. Chem. 2014, 96, 92–96. [Google Scholar] [CrossRef]
- Silva, N.A.; Nicolucci, P.; Baffa, O. Spatial resolution of magnetic resonance imaging Fricke-gel dosimetry is improved with a honeycomb phantom. Med. Phys. 2002, 30, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, L.; Ju, J.; Li, C.; Tian, Y.; Jiang, L.; Liu, M. Superhydrophobic Diffusion Barriers for Hydrogels via Confined Interfacial Modification. Adv. Mater. 2016, 28, 7383–7389. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Hu, X.; Zhang, X.; Chang, S.; Zhang, H. Preparation of W 1 /O/ W 2 emulsion to limit the diffusion of Fe3+ in the Fricke gel 3D dosimeter. Polym. Adv. Technol. 2020, 31, 2127–2135. [Google Scholar] [CrossRef]
- Schweiger, S.; Neubauer, C.; Fraser, S.D.; Klein, T.; Schennach, R.; Reichmann, A.; Gruber-Woelfler, H. Coating of glass substrates to prevent alkali ion diffusion into pharmaceutical solutions. Surf. Coatings Technol. 2014, 258, 1249–1255. [Google Scholar] [CrossRef]
- Cao, Z.-F.; Wang, J.; Qiu, P.; Yang, F.; Wang, S.; Liu, G.; Zhong, H. Hydrophobic coatings for improving corrosion resistance of manganese substrate. Surf. Coatings Technol. 2018, 347, 235–244. [Google Scholar] [CrossRef]
- Welzel, P.B.; Friedrichs, J.; Grimmer, M.; Vogler, S.; Freudenberg, U.; Werner, C. Cryogel Micromechanics Unraveled by Atomic Force Microscopy-Based Nanoindentation. Adv. Health Mater. 2014, 3, 1849–1853. [Google Scholar] [CrossRef]
- Hertz, H. On the contact of elastic solids. J. Reine Angew. Math. 1881, 92, 156–171. [Google Scholar]
- De Oliveira, L.N.; Zimmerman, R.L.; Moreira, M.V.; Ila, D.; De Almeida, A. Determination of diffusion coefficient in Fricke Xylenol gel dosimeter after electron beam bombardment. Surf. Coatings Technol. 2009, 203, 2367–2369. [Google Scholar] [CrossRef]
- Dridi, W.; Daoudi, M.; Farah, K.; Hosni, F. Monte Carlo validation of dose mapping for the Tunisian Gamma Irradiation Facility using the MCNP6 code. Radiat. Phys. Chem. 2020, 173, 108942. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces; John Wiley & Sons, Inc.: New York, NY, USA, 1997. [Google Scholar]
- Adliene, D.; Urbonavicius, B.G.; Laurikaitiene, J.; Puiso, J. New application of polymer gels in medical radiation dosimetry: Plasmonic sensors. Radiat. Phys. Chem. 2020, 168, 108609. [Google Scholar] [CrossRef]
- Kron, T.; Jonas, D.; Pope, J.M. Fast T1 imaging of dual gel samples for diffusion measurements in NMR dosimetry gels. Magn. Reson. Imaging 1997, 15, 211–221. [Google Scholar] [CrossRef]
- Vedelago, J.; Quiroga, A.; Triviño, S.; Mattea, F.; Valente, M. Parameter estimation and mathematical modeling of the diffusion process of a benzoic acid infused Fricke gel dosimeter. Appl. Radiat. Isot. 2019, 151, 89–95. [Google Scholar] [CrossRef]
- Passenger Port of Saint-Petersburg, Marine Façade. Available online: https://www.portspb.ru/ (accessed on 11 February 2021).

| Material | Weight Percentage of Elements (%, w/w) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Fe | Na | Si | |
| PVA substrate | 5.453 | 10.967 | 83.508 | \ | 0.072 | \ | \ | \ |
| FPX core | 4.545 | 11.000 | 84.381 | 9.338 × 10−5 | 0.074 | 9.307 × 10−5 | 1.5338 × 10−5 | \ |
| PDMS shell | 32.394 | 8.156 | 21.575 | \ | \ | \ | \ | 37.875 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, K.; Zeng, Y.; Hu, X.; Zhang, X.; Chang, S.; Zhang, H. Low-Diffusion Fricke Gel Dosimeters with Core-Shell Structure Based on Spatial Confinement. Materials 2021, 14, 3932. https://doi.org/10.3390/ma14143932
Zhang W, Wang K, Zeng Y, Hu X, Zhang X, Chang S, Zhang H. Low-Diffusion Fricke Gel Dosimeters with Core-Shell Structure Based on Spatial Confinement. Materials. 2021; 14(14):3932. https://doi.org/10.3390/ma14143932
Chicago/Turabian StyleZhang, Wei, Kaikai Wang, Yufeng Zeng, Xiaodan Hu, Xiaohong Zhang, Shuquan Chang, and Haiqian Zhang. 2021. "Low-Diffusion Fricke Gel Dosimeters with Core-Shell Structure Based on Spatial Confinement" Materials 14, no. 14: 3932. https://doi.org/10.3390/ma14143932






