Physical Mechanism of Photoinduced Charge Transfer in One- and Two-Photon Absorption in D-D-π-A Systems
Abstract
:1. Introduction
2. Methodology
2.1. Calculation Details
2.2. One-Photon Absorption
2.3. Two-Photon Absorption
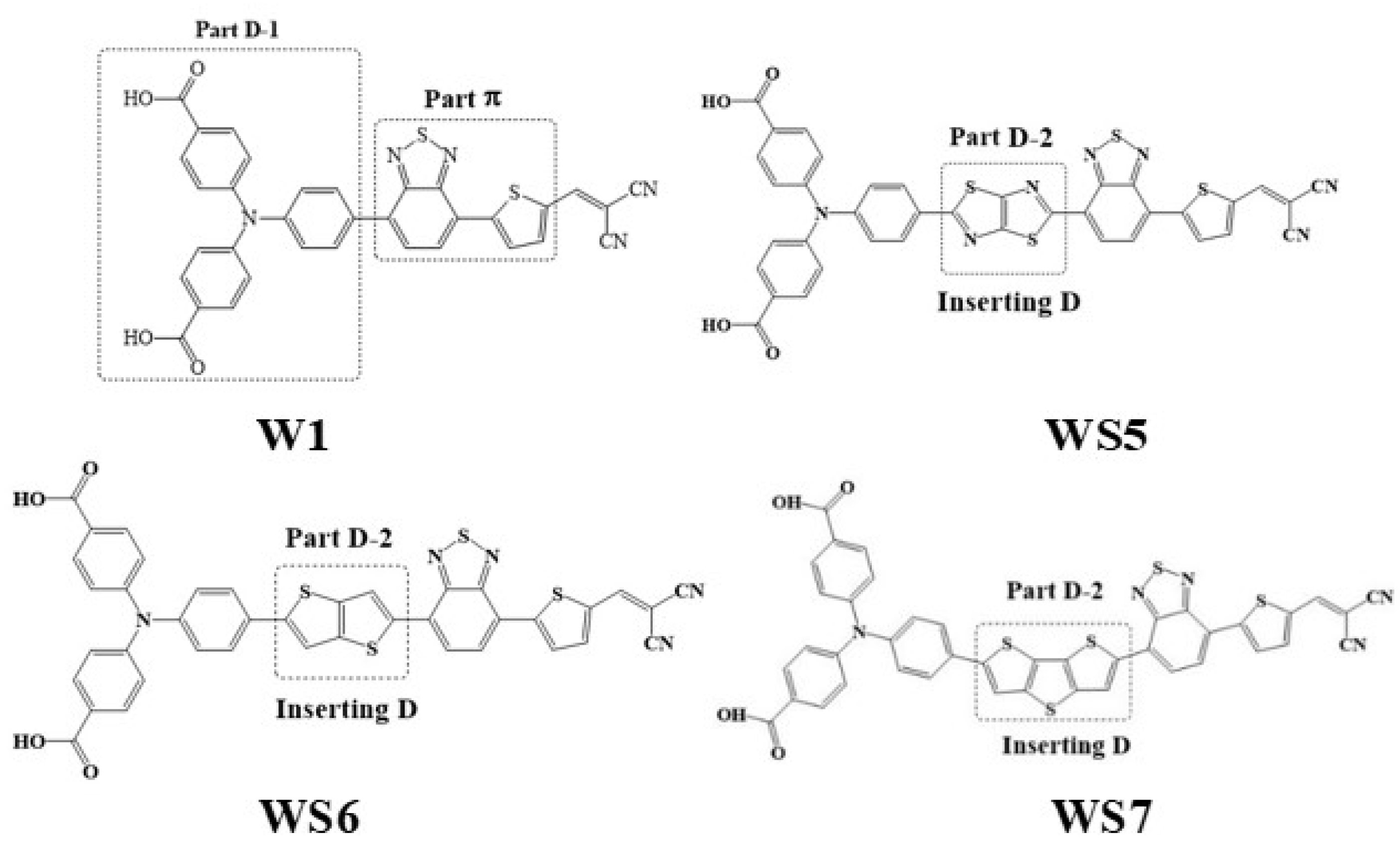
3. Results and Discussion
3.1. OPA and TPA Spectra
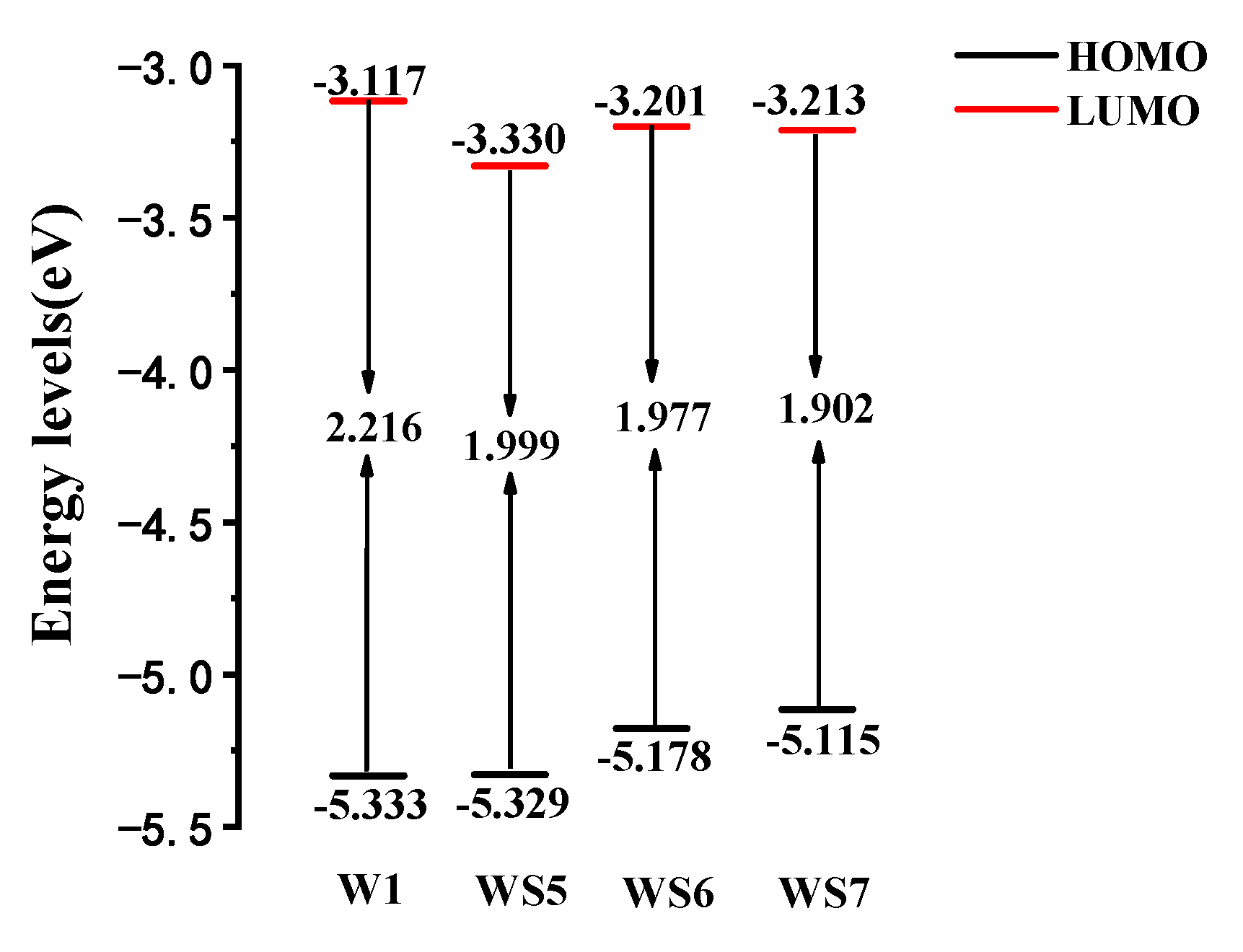
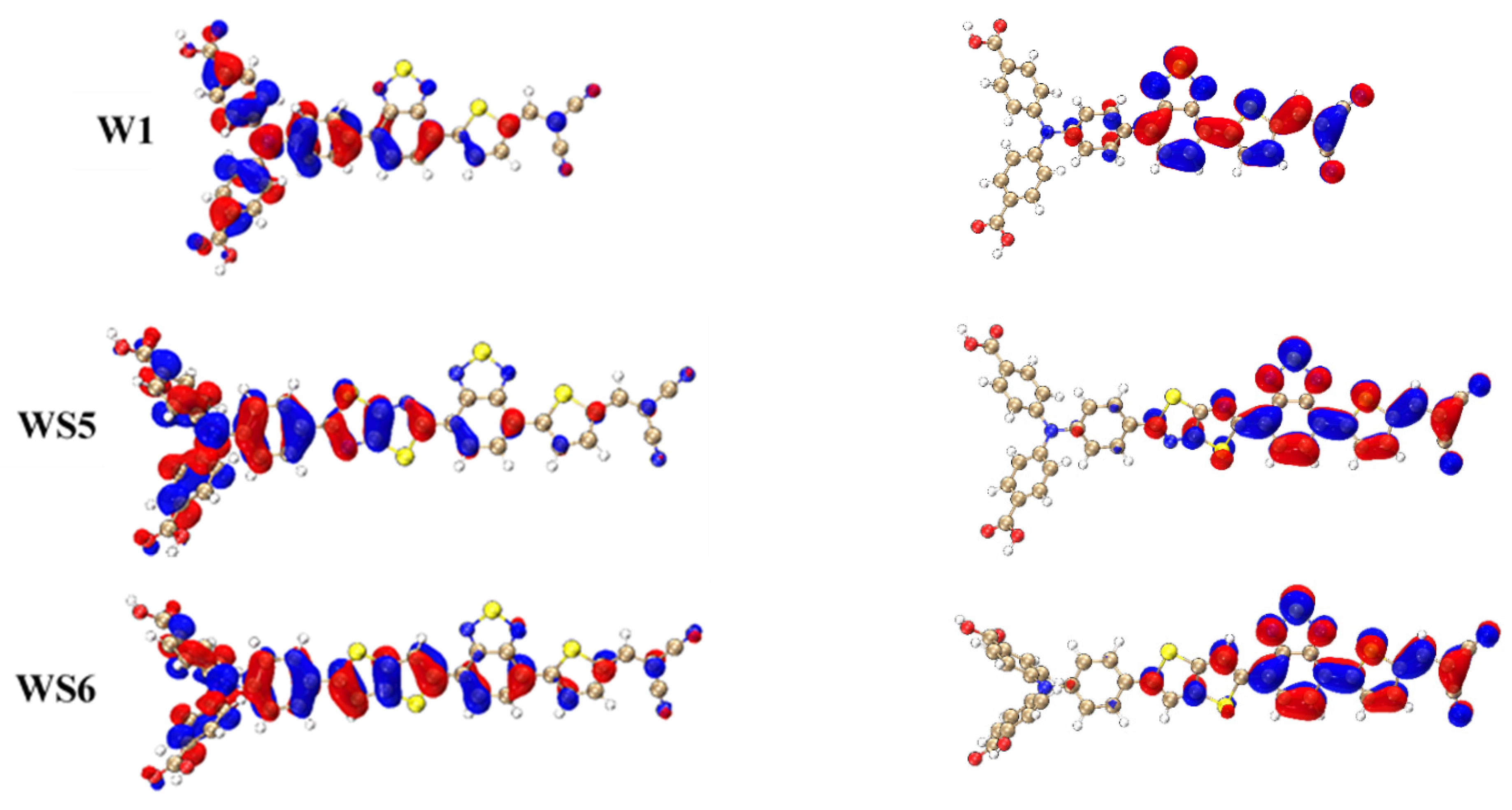
3.2. Frontier Molecular Orbitals
3.3. One-Photon Absorption
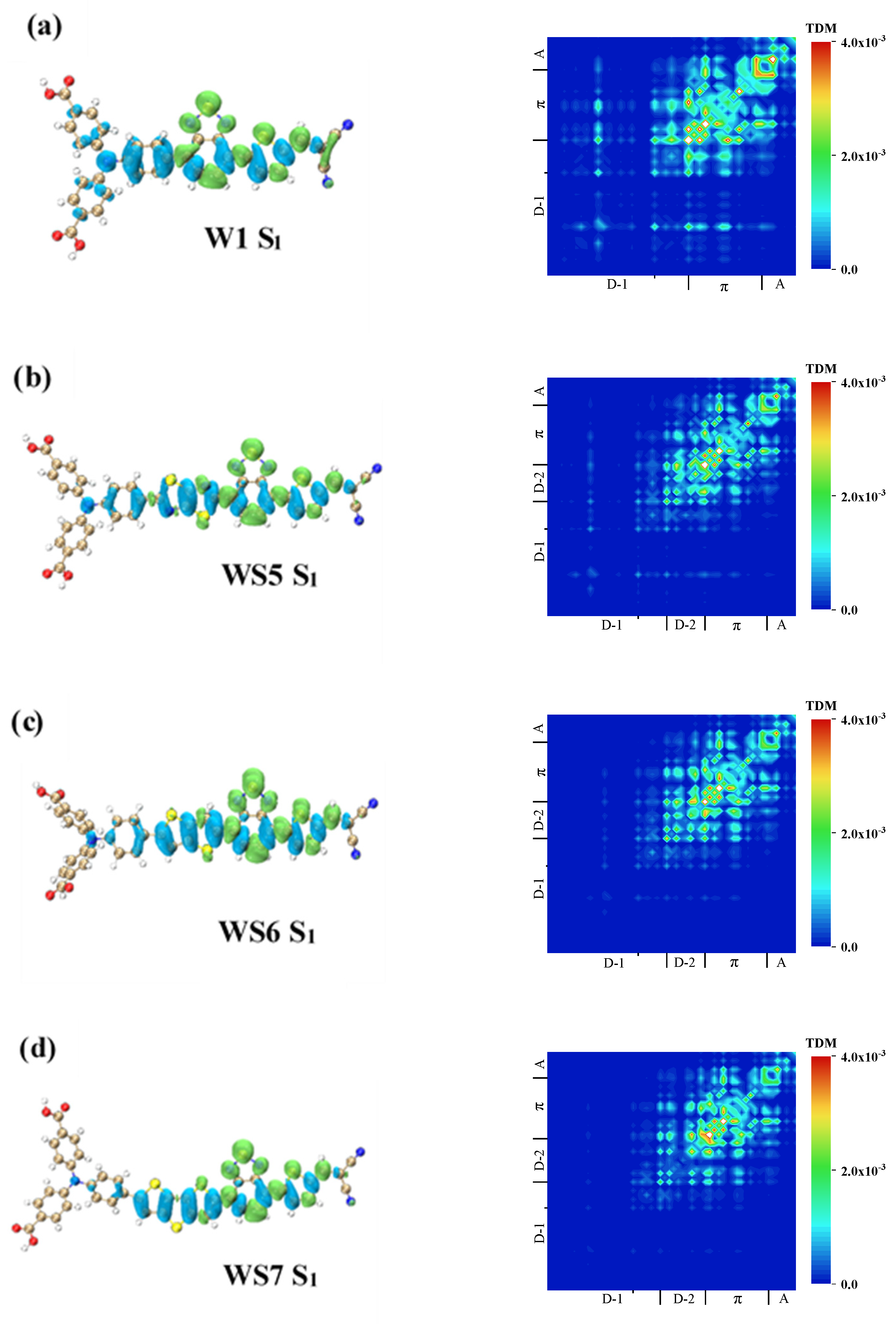
3.4. Transition Dipole Moments
3.5. Two-Photon Absorption
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, G.S.; Tan, L.S.; Zheng, Q.D.; Prasad, P.N. Multiphoton absorbing materials: Molecular designs, characterizations, and applications. Chem. Rev. 2008, 108, 1245–1330. [Google Scholar] [CrossRef]
- Göppert-Mayer, M. Über elementaraktemitzwei quantensprüngen. Ann. Phys. 1931, 401, 273–294. [Google Scholar] [CrossRef]
- Kaiser, W.K.; Garrett, C. Two-photon excitation in CaF2: Eu2+. Phys. Rev. Lett. 1961, 7, 229–231. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.J.; Xu, X.F.; Zhou, Y.; Chen, M.D.; Sun, M.T. Optical characterizations of two-dimensional materials using nonlinear optical microscopies of CARS, TPEF, and SHG. Nanophotonics 2018, 7, 873–881. [Google Scholar] [CrossRef]
- Mi, X.H.; Wang, Y.Y.; Li, R.; Sun, M.T.; Zhang, Z.L.; Zhong, H. Multiple surface plasmon resonances enhanced nonlinear optical microscopy. Nanophotonics 2019, 8, 487–493. [Google Scholar] [CrossRef]
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K. Two-photon absorbing molecules as potential materials for 3D optical memory. Appl. Sci. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, P.; Hu, Y.L.; Lin, G.; Wu, Y.Q.; Huang, W.H.; Zhao, Q.Z. Novel carbazole derivatives with quinoline ring: Synthesis, electronic transition, and two-photon absorption three-dimensional optical data storage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 139, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.Y.S.; Mccord-Maughon, D.; et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 31, 51–54. [Google Scholar] [CrossRef]
- Ogawa, K.; Kobuke, Y. Recent advances in two-photon photodynamic therapy. Anti Cancer Agents Med. Chem. 2008, 8, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Pandey, R.K.; Prasad, P.N. Organically modified silica nanoparticles co-encapsulating photosensitizing drug and aggregation-enhanced two-photon absorbing fluorescent dye aggregates for two-photon photodynamic therapy. J. Am. Chem. Soc. 2007, 129, 2669–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.Y.; Zhang, L.P.; Wu, F.P.; Zhao, Y.X. Photosensitizers for two-photon excited photodynamic therapy. Adv. Funct. Mater. 2017, 27, 1704079. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Shuhendler, A.J.; Ye, D.J.; Xu, J.J.; Chen, H.Y. Two-photon excitation nanoparticles for photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef]
- Mckenzie, L.K.; Bryant, H.E.; Weinstein, J.A. Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy. Coord. Chem. Rev. 2018, 379, 2–29. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Z.; Pullerits, T.; Zhao, M.Y.; Sun, M.T. Theoretical characterization of the PC60BM:PDDTT model for an organic solar cell. J. Phys. Chem. C 2011, 115, 21865–21873. [Google Scholar] [CrossRef]
- Li, Y.Z.; Xu, B.B.; Song, P.; Ma, F.C.; Sun, M.T. D–A−π–A system: Light harvesting, charge transfer, and molecular designing. J. Phys. Chem. C 2017, 121, 12546–12561. [Google Scholar] [CrossRef]
- Li, Y.Z.; Qi, D.W.; Song, P.; Ma, F.C. Fullerene-based photoactive layers for heterojunction solar cells: Structure, absorption spectra and charge transfer process. Materials 2014, 8, 42–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, P.; Li, Y.Z.; Ma, F.C.; Pullerits, T.; Sun, M.T. Photoinduced electron transfer in organic solar cells. Chem. Rec. 2016, 16, 734–753. [Google Scholar] [CrossRef]
- Albota, M.; Beljonne, D.; Bredas, J.L.; Ehrlich, J.E.; Fu, J.Y.; Heikal, A.A.; Hess, S.E.; Kogej, T.; Levin, M.D.; Marder, S.R.; et al. Design of organic molecules with large two-photon absorption cross sections. Science 1998, 281, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Zhang, Q. Recent progress on intramolecular charge-transfer compounds as photoelectric active materials. Sci. China Mater. 2017, 60, 1093–1101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.T.; Guo, J.X.; Li, X.R.; Zhao, M.Y.; Wei, Q.; Song, P. One- and two-photon absorption properties of quadrupolar A–π–D–π–A dyes with donors of varying strengths. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2020, 230, 118015. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-photon absorption and the design of two-photon dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Elisei, F.; Foggi, P.; Marrocchi, A.; Spalletti, A.; Carlotti, B. Photobehavior and nonlinear optical properties of pushpull, symmetrical and highly fluorescent benzothiadiazole derivatives. J. Phys. Chem. C 2016, 120, 23726–23739. [Google Scholar] [CrossRef]
- Dereka, B.; Koch, M.; Vauthey, E. Looking at photoinduced charge transfer processes in the IR: Answers to several long-standing questions. Acc. Chem. Res. 2017, 50, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, K.E.; Schlawin, F.; Mukamel, S. Nonlinear optical signals and spectroscopy with quantum light. Rev. Mod. Phys. 2016, 88, 045008. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.T.; Chen, J.N.; Xu, H.X. Visualizations of transition dipoles, charge transfer, and electron-hole coherence on, electronic state transitions between excited states for two-photon absorption. J. Chem. Phys. 2008, 128, 064106. [Google Scholar] [CrossRef]
- Mu, X.J.; Wang, J.G.; Sun, M.T. Visualization of photoinduced charge transfer and electron-hole coherence in two-photon absorption. J. Phys. Chem. C 2019, 123, 14132–14143. [Google Scholar] [CrossRef]
- Sun, J.J.; Cao, J.; Cheng, H.L.; Wang, J.C.; Wang, S.X. Fluorescent turn-on probe based on napthalimide fused triphenylamine unit for quickly detecting thiophenol in aqueous solution. Chem. Res. Chin. Univ. 2019, 35, 990–996. [Google Scholar] [CrossRef]
- Dumat, B.; Bordeau, G.; Aranda, A.I.; Mahuteau-Betzer, F.; El Harfouch, Y.; Metgé, G.; Charra, F.; Fiorini-Debuisschert, C.; Teulade-Fichou, M.P. Vinyl-triphenylamine dyes, a new family of switchable fluorescent probes for targeted two-photon cellular imaging: From DNA to protein labeling. Org. Biomol. Chem. 2012, 10, 6054–6061. [Google Scholar] [CrossRef]
- Dumat, B.; Bordeau, G.; Faurel-Paul, E.; Mahuteau-Betzer, F.; Saettel, N.; Metge, G.; Fiorini-Debuisschert, C.; Charra, F.; Teulade-Fichou, M.P. DNA Switches on the two-photon efficiency of an ultrabright triphenylamine fluorescent probe specific of AT regions. J. Am. Chem. Soc. 2013, 135, 12697–12706. [Google Scholar] [CrossRef]
- Chennoufi, R.; Trinh, N.D.; Simon, F.; Bordeau, G.; Naud-Martin, D.; Moussaron, A.; Cinquin, B.; Bougherara, H.; Rambaud, B.; Tauc, P.; et al. Interplay between cellular uptake, intracellular localization and the cell death mechanism in triphenylamine-mediated photoinduced cell death. Sci. Rep. 2020, 10, 6881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, P.; Lan, X.Y.; Sun, J.H.; Huang, J.H.; Zhang, Y. Enhancing performance of quantum-dot light-emitting diodes based on poly(indenofluorene-co-triphenylamine) copolymer as hole-transporting layer. J. Mater. Sci. Mater. Electron. 2020, 31, 2551–2556. [Google Scholar] [CrossRef]
- Delgado-Montiel, T.; Soto-Rojo, R.; Baldenebro-López, J.; Glossman-Mitnik, D. Theoretical study of the effect of different π bridges including an azomethine group in triphenylamine-based dye for dye-sensitized solar cells. Molecules 2019, 24, 3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.X.; He, M.F.; Yan, W.; Zhang, C.S.; Ju, X.H. Theoretical studies on triaryamine-based p-type D-D-π-A sensitizer. J. Chin. Chem. Soc. 2019, 66, 1257–1262. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, D.A. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1998, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Furche, F.; Ahlrichs, R. Adiabatic time-dependent density functional methods for excited state properties. J. Chem. Phys. 2002, 117, 7433–7447. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11 − 1. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Mu, X.J.; Wang, X.X.; Quan, J.; Sun, M.T. Photoinduced charge transfer in donor-bridge-acceptor in one- and two-photon absorption: Sequential and super-exchange mechanisms. J. Phys. Chem. C 2020, 124, 4968–4981. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Zong, H.; Wang, X.X.; Quan, J.; Tian, C.H.; Sun, M.T. Photoinduced charge transfer by one and two-photon absorptions: Physical mechanisms and applications. Phys. Chem. Chem. Phys. PCCP 2018, 20, 19720–19743. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.T.; Ding, Y.; Zhao, L.L.; Ma, F.C. Photoexcitation mechanisms of centrosymmetric and asymmetric fluorene derivatives in two-photon absorption. Chem. Phys. 2009, 359, 166–172. [Google Scholar] [CrossRef]



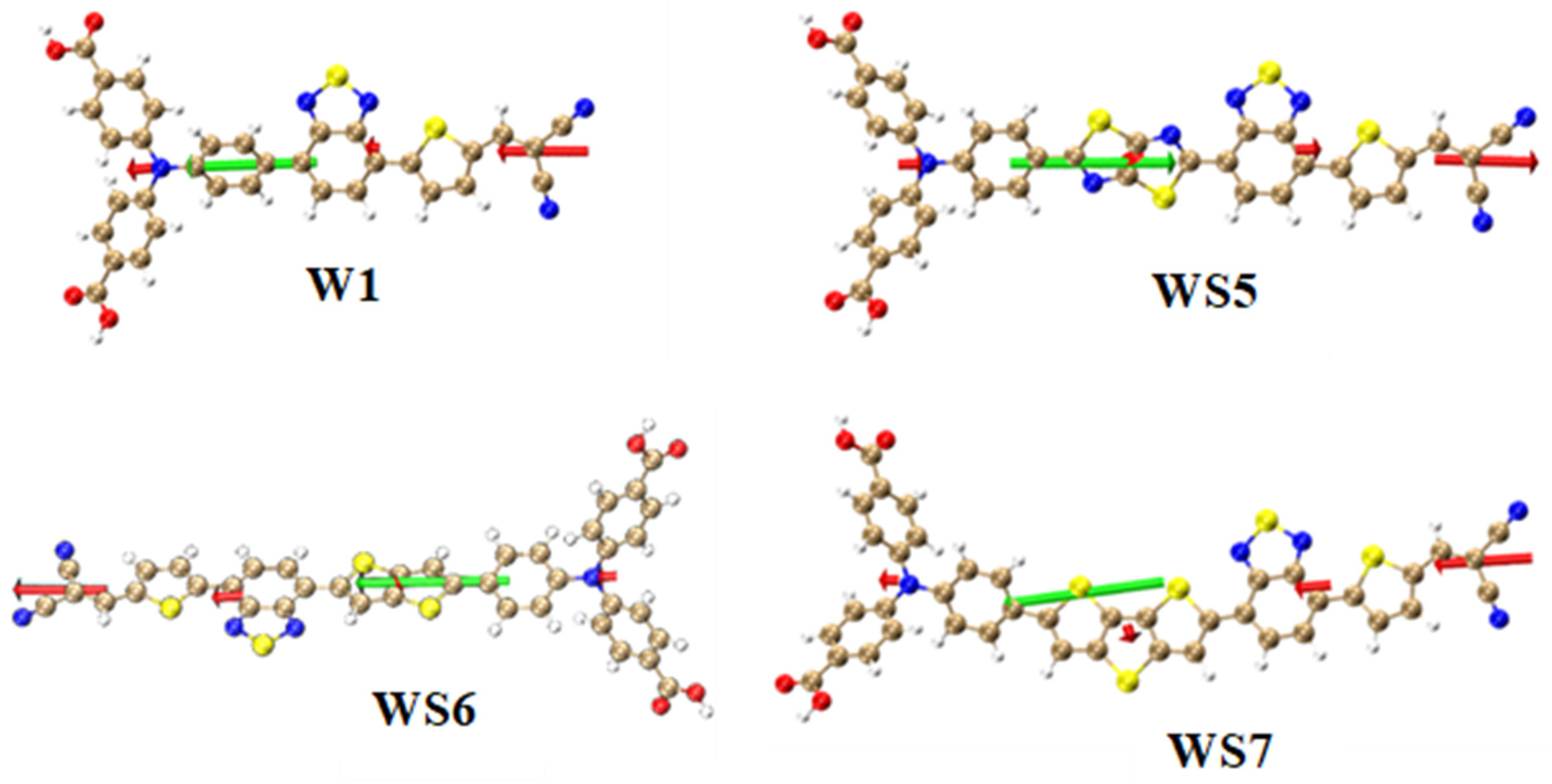
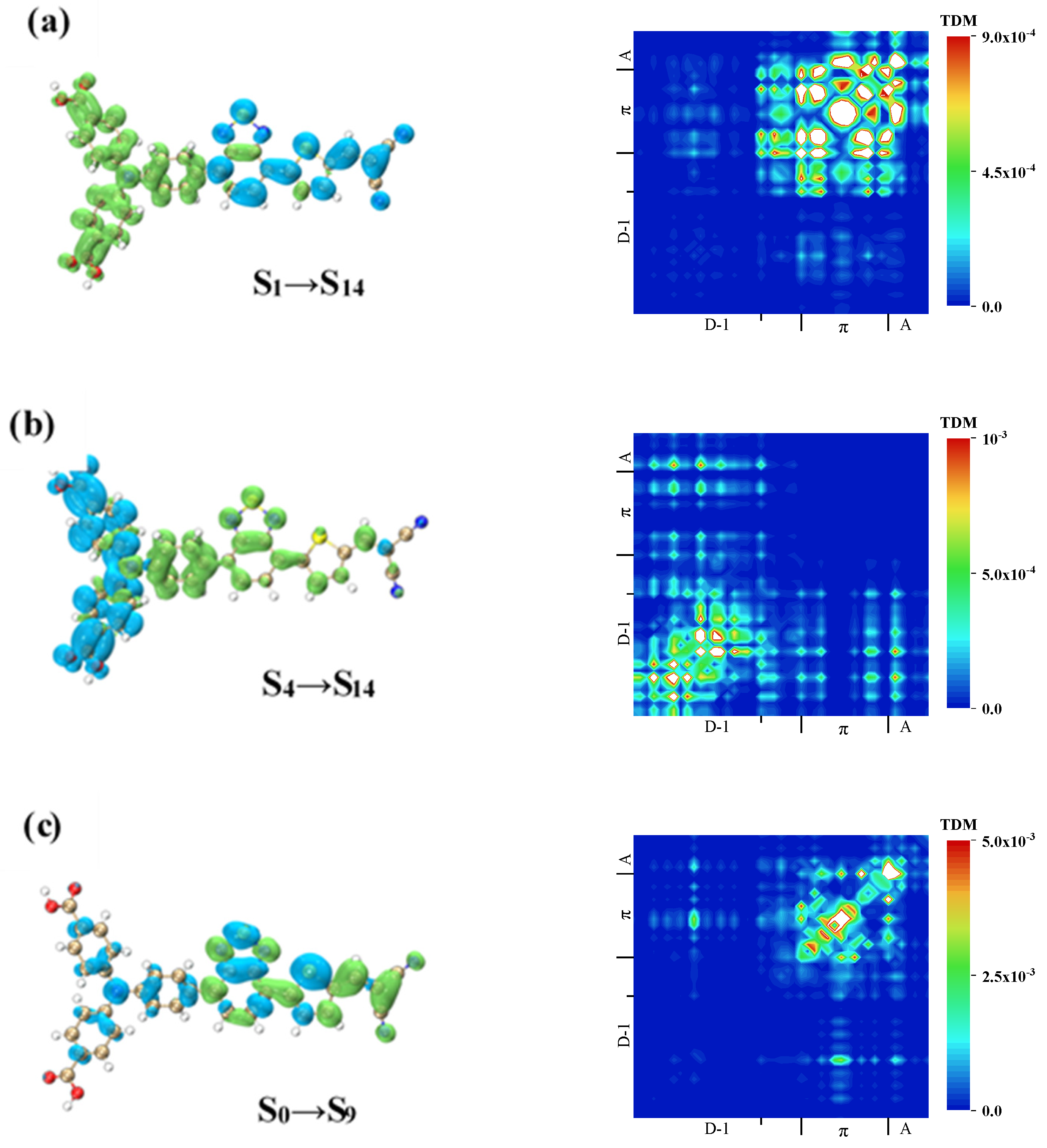

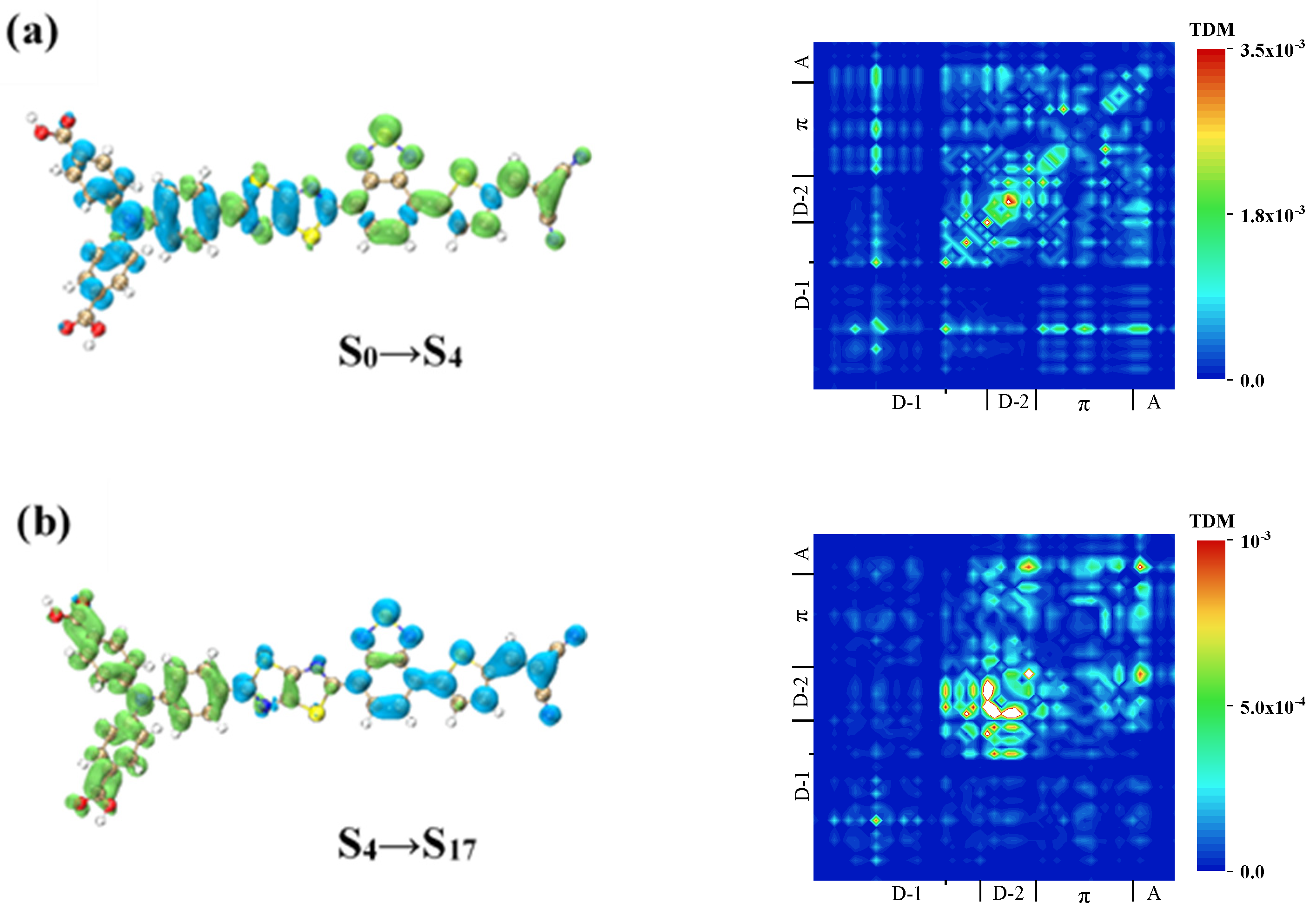


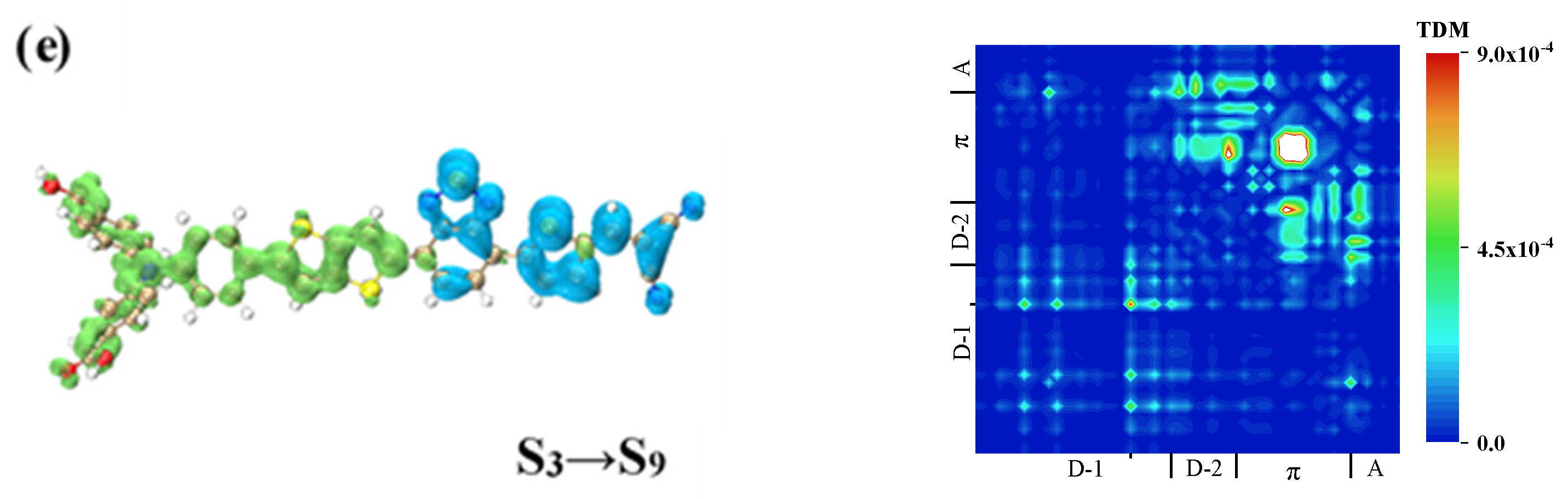


| Molecule | OPA States | E/ev | λ/nm | f |
|---|---|---|---|---|
| W1 | S1 | 2.54660 | 486.86 | 1.59124 |
| WS5 | S1 | 2.39420 | 517.86 | 2.18762 |
| WS6 | S1 | 2.24780 | 551.58 | 1.98662 |
| WS7 | S1 | 2.17120 | 571.03 | 2.02813 |
| Molecule | x | y | z | Norm |
|---|---|---|---|---|
| W1 | −5.047 | −0.152 | −0.006 | 5.050 |
| WS5 | 6.105 | 0.135 | −0.065 | 6.106 |
| WS6 | −6.003 | 0.130 | −0.103 | 6.006 |
| WS7 | −6.129 | −0.732 | −0.157 | 6.174 |
| Part (D-1) | Part (D-2) | |||||
| x | y | z | x | y | z | |
| W1 | −0.728 | −0.024 | −0.009 | |||
| WS5 | 0.753 | −0.023 | −0.003 | −0.028 | −0.069 | −0.058 |
| WS6 | −0.580 | 0.001 | −0.014 | 0.065 | 0.022 | −0.002 |
| WS7 | −0.580 | 0.026 | −0.001 | 0.116 | −0.419 | −0.079 |
| Part (π) | Part (A) | |||||
| x | y | z | x | y | z | |
| W1 | −0.277 | −0.037 | −0.000 | −3.377 | −0.228 | −0.017 |
| WS5 | 0.707 | −0.000 | −0.007 | 3.700 | 0.193 | 0.001 |
| WS6 | −1.010 | 0.021 | 0.042 | −3.560 | 0.118 | −0.130 |
| WS7 | −1.167 | 0.080 | −0.024 | −3.629 | 0.160 | −0.033 |
| Molecule | TPA States | Process | Integral Value |
|---|---|---|---|
| W1 | S14 | 25.50–1.91 | |
| 6.41–2.48 | |||
| S16 | 1.52–8.02 | ||
| WS5 | S17 | 1.90–10.14 | |
| S18 | 6.29–5.47 | ||
| WS6 | S4 | 36.07–33.15 | |
| 5.94–8.79 | |||
| S9 | 5.94–8.87 | ||
| S12 | 5.94–21.04 | ||
| WS7 | S12 | 6.69–2.23 | |
| 5.23–1.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, D.; Li, J.; Zhang, M.; Song, P. Physical Mechanism of Photoinduced Charge Transfer in One- and Two-Photon Absorption in D-D-π-A Systems. Materials 2021, 14, 3925. https://doi.org/10.3390/ma14143925
Wang X, Wang D, Li J, Zhang M, Song P. Physical Mechanism of Photoinduced Charge Transfer in One- and Two-Photon Absorption in D-D-π-A Systems. Materials. 2021; 14(14):3925. https://doi.org/10.3390/ma14143925
Chicago/Turabian StyleWang, Xinyue, Di Wang, Jia Li, Meixia Zhang, and Peng Song. 2021. "Physical Mechanism of Photoinduced Charge Transfer in One- and Two-Photon Absorption in D-D-π-A Systems" Materials 14, no. 14: 3925. https://doi.org/10.3390/ma14143925
APA StyleWang, X., Wang, D., Li, J., Zhang, M., & Song, P. (2021). Physical Mechanism of Photoinduced Charge Transfer in One- and Two-Photon Absorption in D-D-π-A Systems. Materials, 14(14), 3925. https://doi.org/10.3390/ma14143925






