The Influence of the Hydrophobic Polymeric Coating on 5-ASA Release from the Bipolymeric Milibeads with Amidated Pectin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Coated Pectin Beads
2.2. Morphological Study
2.3. Carrier Stability Study
2.4. Release Study of 5-ASA from Coated Pectin Beads
2.5. Statistical Analysis
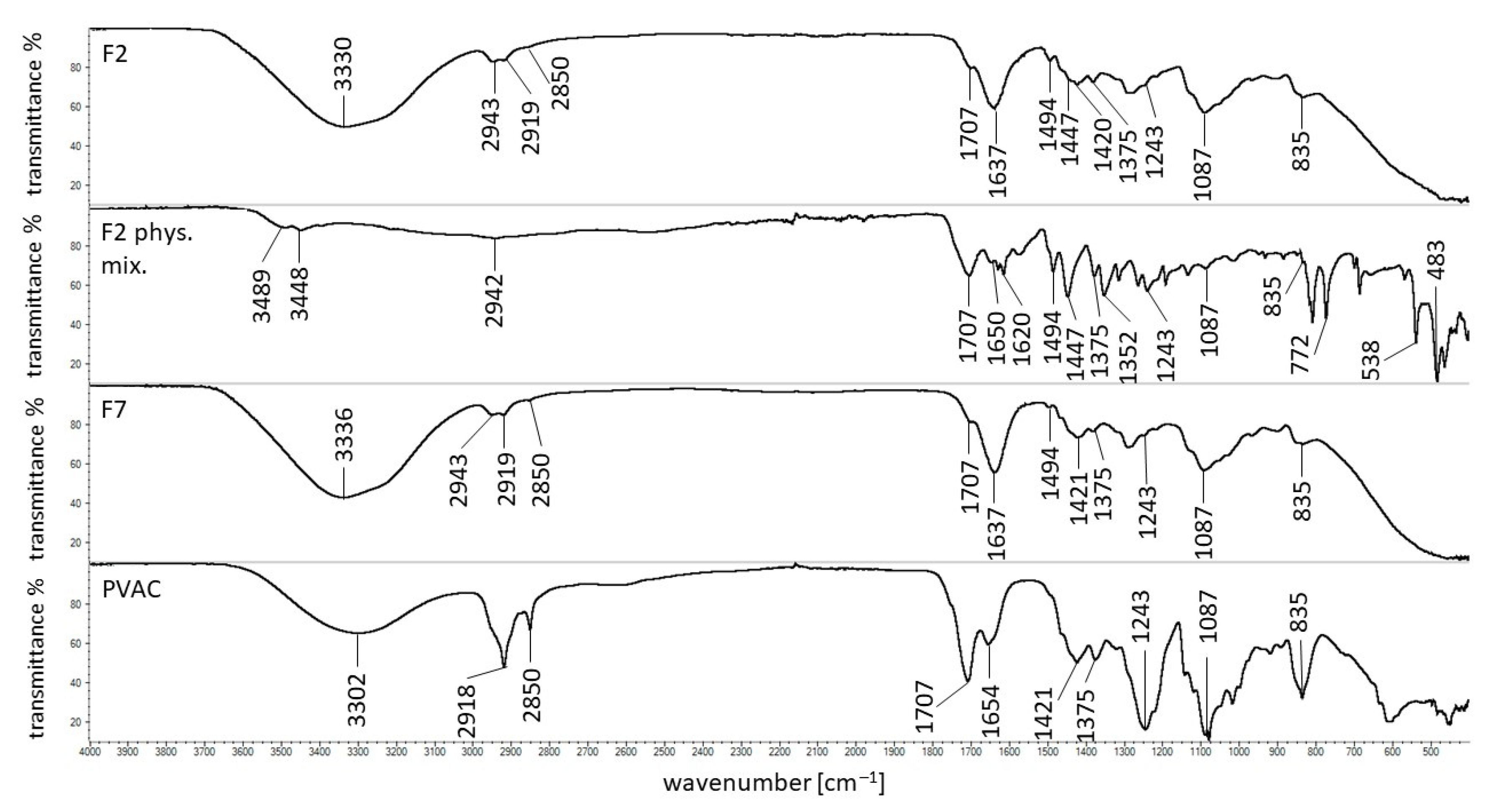
2.6. FTIR Study
3. Results and Discussion
3.1. Morphological Study
3.2. Carrier Stability Study
3.3. Release Study of 5-ASA at Acidic Conditions
3.4. Kinetics of 5-ASA Release at pH = 7.4 with Pectinase
3.5. The Difference Factor f1 and the Similarity Factor f2
3.6. Statistical Analysis
3.7. FTIR Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in theBiomedical and Food Sector-Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Bidhendi, A.J.; Geitmann, A. Relating the mechanics of the primary plant cell wall to morphogenesis. J. Exp. Bot. 2016, 67, 449–461. [Google Scholar] [CrossRef] [Green Version]
- Kameshwar, A.K.S.; Qin, W. Structural and functional properties of pectin and lignin–carbohydrate complexes de-esterases: A review. Bioresour. Bioprocess. 2018, 5, 1–16. [Google Scholar] [CrossRef]
- Liang, R.H.; Chen, J.; Liu, W.; Liu, C.M.; Yu, W.; Yuan, M.; Zhou, X.Q. Extraction, characterization and spontaneous gel-forming property of pectin from creeping fig (Ficus pumila Linn.) seeds. Carbohydr. Polym. 2012, 87, 76–83. [Google Scholar] [CrossRef]
- Moreira, H.R.; Munarin, F.; Gentilini, R.; Visai, L.; Granja, P.L.; Tanzi, M.C.; Petrini, P. Injectable pectin hydrogels produced by internal gelation: PH dependence of gelling and rheological properties. Carbohydr. Polym. 2014, 103, 339–347. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives. EFSA J. 2017, 15, 1–62. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit Softening: Revisiting the Role of Pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Formulation and characterization of hydrogel based on pectin and brea gum. Int. J. Biol. Macromol. 2019, 123, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Ahirrao, S.P.; Gide, P.S.; Shrivastav, B.; Sharma, P. Ionotropic Gelation: A Promising Cross Linking Technique for Hydrogels. J. Pharm. Nanotechnol. 2014, 2, 1–6. [Google Scholar]
- Wójcik-Pastuszka, D.; Potempa, A.; Musiał, W. Bipolymeric Pectin Millibeads Doped with Functional Polymers as Matrices for the Controlled and Targeted Release of Mesalazine. Molecules 2020, 25, 5711. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 10.0; European Directorate for the Quality of Medicines Council of Europe: Strasbourg, France, 2018; p. 5099.
- European Pharmacopoeia 9.0; European Directorate for the Quality of Medicines Council of Europe: Strasbourg, France, 2017; pp. 302–309.
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles Paulo. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Guidance for Industry Dissolution Testing of Immediate Release Solid Oral Dosage Forms. 1997; Volume 4, pp. 15–22. Available online: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070246.pdf (accessed on 15 December 2015).
- O’Hara, T.; Dunne, A.; Butler, J.; Devane, J. A review of methods used to compare dissolution profile data. Pharm. Sci. Technol. Today 1998, 1, 214–223. [Google Scholar] [CrossRef]
- Aguirre Calvo, T.; Santagapita, P. Physicochemical Characterization of Alginate Beads Containing Sugars and Biopolymers. J. Qual. Reliab. Eng. 2016, 2016. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Mazurek, K.L.; Szumny, A.J.; Alagöz, F.; Musiał, W.S. Properties of pectin based polymeric matrices for targeted drug delivery. Acta Pol. Pharm. Drug Res. 2017, 74, 1875–1885. [Google Scholar]
- French, D.L.; Mauger, J.W. Evaluation of the Physicochemical Properties and Dissolution Characteristics of Mesalamine: Relevance to Controlled Intestinal Drug Delivery. Pharm. Res. An Off. J. Am. Assoc. Pharm. Sci. 1993, 10, 1285–1290. [Google Scholar] [CrossRef]
- Aunis, J.G.; Southard, M.Z.; Myers, R.A.; Himmelstein, K.J.; Stella, V.J. Dissolution of Carboxylic Acids III: The Effect of Polyionizable Buffers. J. Pharm. Sci. 1985, 74, 1305–1316. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [CrossRef]
- D’Amelia, R.P.; Gentile, S.; Nirode, W.F.; Huang, L. Quantitative Analysis of Copolymers and Blends of Polyvinyl Acetate (PVAc) Using Fourier Transform Infrared Spectroscopy (FTIR) and Elemental Analysis (EA). World J. Chem. Educ. 2016, 4, 25–31. [Google Scholar] [CrossRef]
- Wei, S.; Pintus, V.; Schreiner, M. Photochemical degradation study of polyvinyl acetate paints used in artworks by Py-GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 158–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahariah, B.; Sarma, B.K. Relative orientation of the carbonyl groups determines the nature of orbital interactions in carbonyl-carbonyl short contacts. Chem. Sci. 2019, 10, 909–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayzullin, R.R.; Shteingolts, S.A.; Lodochnikova, O.A.; Mamedova, V.L.; Korshin, D.E.; Mamedov, V.A. Intermolecular head-to-head interaction of carbonyl groups in bicyclic hydrogen-bonded synthon based on β-hydroxy ketones. CrystEngComm 2019, 21, 1587–1599. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, L.D.; Pan, H.C.; He, L.; Lin, C.T.; Tan, M.C. Fabrication and interfacial characteristics of surface modified Ag nanoparticle based conductive composites. RSC Adv. 2017, 7, 29702–29712. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gavilan, C.M.; Lopez-Vega, C.; Mendez-Sanchez, I.M. Ulcerative colitis with gastric and duodenal involvement. Rev. Esp. Enferm. Dig. 2017, 109, 535–536. [Google Scholar] [CrossRef]
- Sasaki, M.; Okada, K.; Koyama, S.; Yoshioka, U.; Inoue, H.; Fujiyama, Y.; Bamba, T. Ulcerative colitis complicated by gastroduodenal lesions. J. Gastroenterol. 1996, 31, 585–589. [Google Scholar] [CrossRef]
- Horje, C.S.H.T.; Meijer, J.; Rovers, L.; Van Lochem, E.G.; Groenen, M.J.M.; Wahab, P.J. Prevalence of Upper Gastrointestinal Lesions at Primary Diagnosis in Adults with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, E33–E34. [Google Scholar] [CrossRef]
- Lin, J.; McKenna, B.J.; Appelman, H.D. Morphologic findings in upper gastrointestinal biopsies of patients with ulcerative colitis: A controlled study. Am. J. Surg. Pathol. 2010, 34, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Zheng, C.-Q.; Sang, L.-X. Mucosal lesions of the upper gastrointestinal tract in patients with ulcerative colitis: A review. World J. Gastroenterol. 2021, 27, 2963–2978. [Google Scholar] [CrossRef] [PubMed]
- San Aye, K.; Lin Htun, L.; Win, T.M.; Aye, M.T.; Tin, D.; Aye, T.T. Gastroduodenal Ulcerative Colitis in Association with Ulcerative Pancolitis. Case Rep. Gastrointest. Med. 2021, 2021, 1–5. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.Q.; Chen, W.J.; Ma, Z.H.; Liu, G. Gastroduodenitis associated with ulcerative colitis: A case report. World J. Clin. Cases 2020, 8, 3847–3852. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Cui, J.; Qin, H.; Shi, Y.; Zhang, S.; Zhao, Y. Ulcerative colitis with mucosal lesions in Duodenum: Two case reports. Medicine 2019, 98, 1–5. [Google Scholar] [CrossRef]
- Nomura, Y.; Moriichi, K.; Fujiya, M.; Okumura, T. The endoscopic findings of the upper gastrointestinal tract in patients with Crohn’s disease. Clin. J. Gastroenterol. 2017, 10, 289–296. [Google Scholar] [CrossRef] [Green Version]

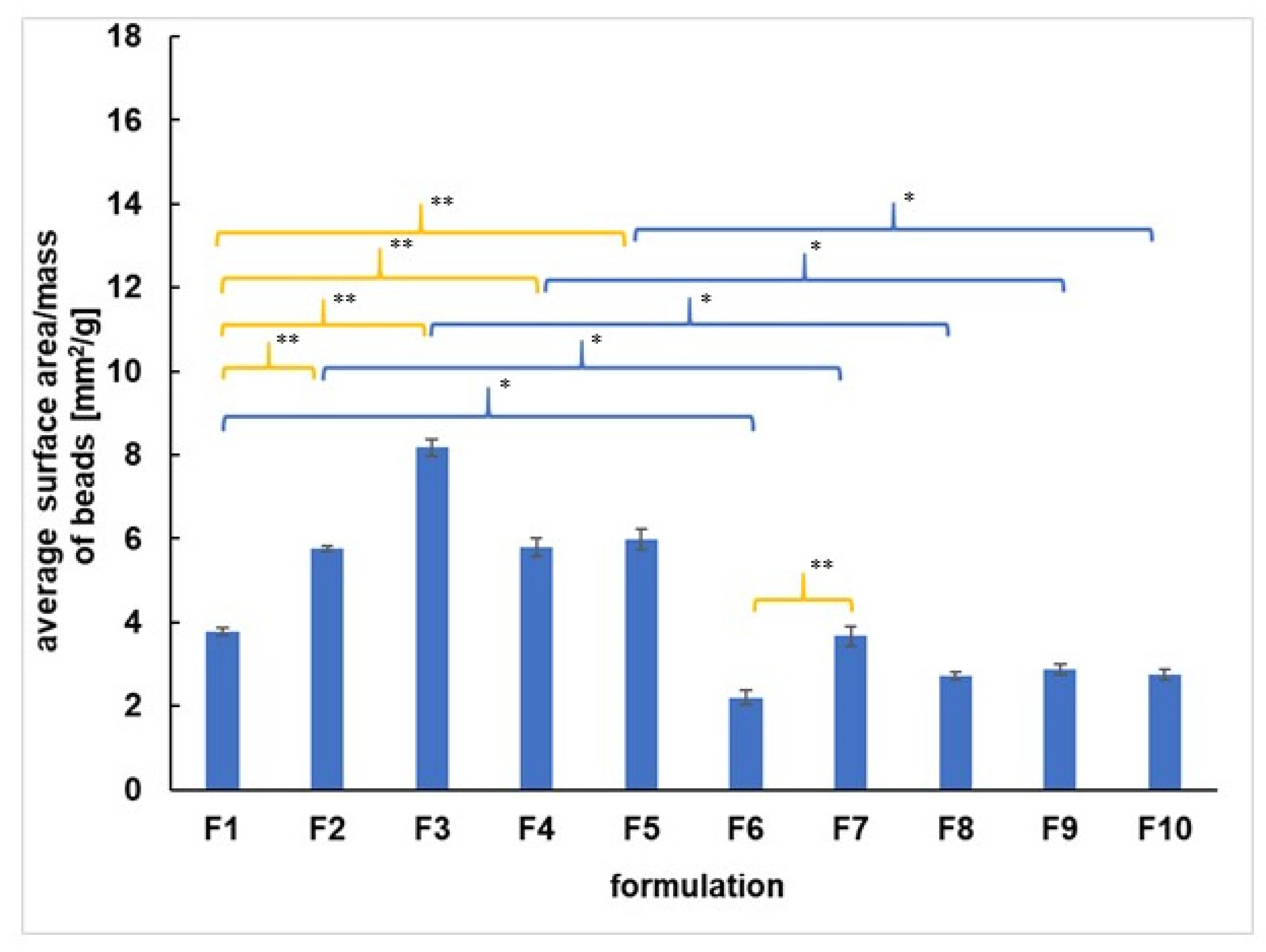
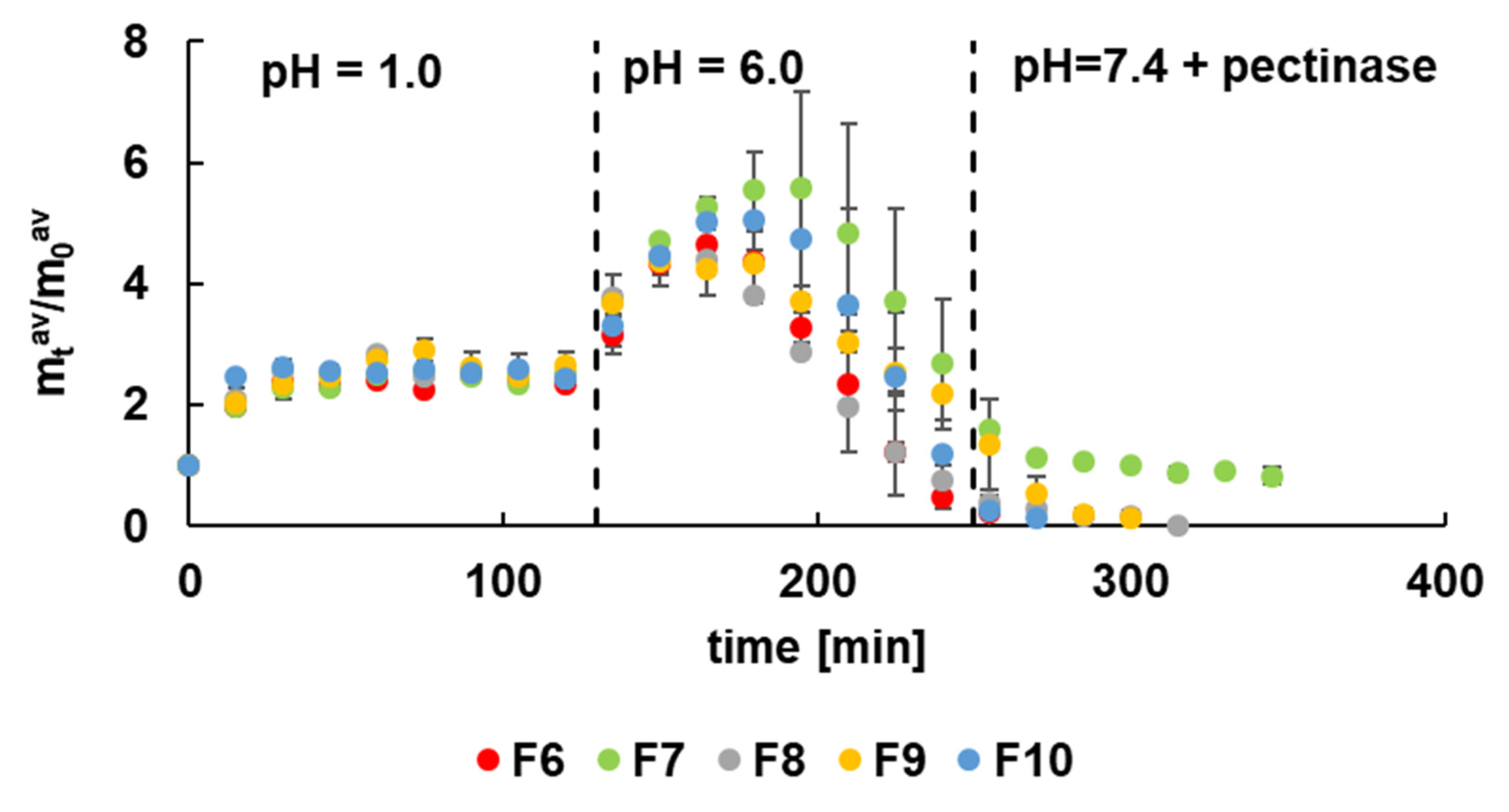
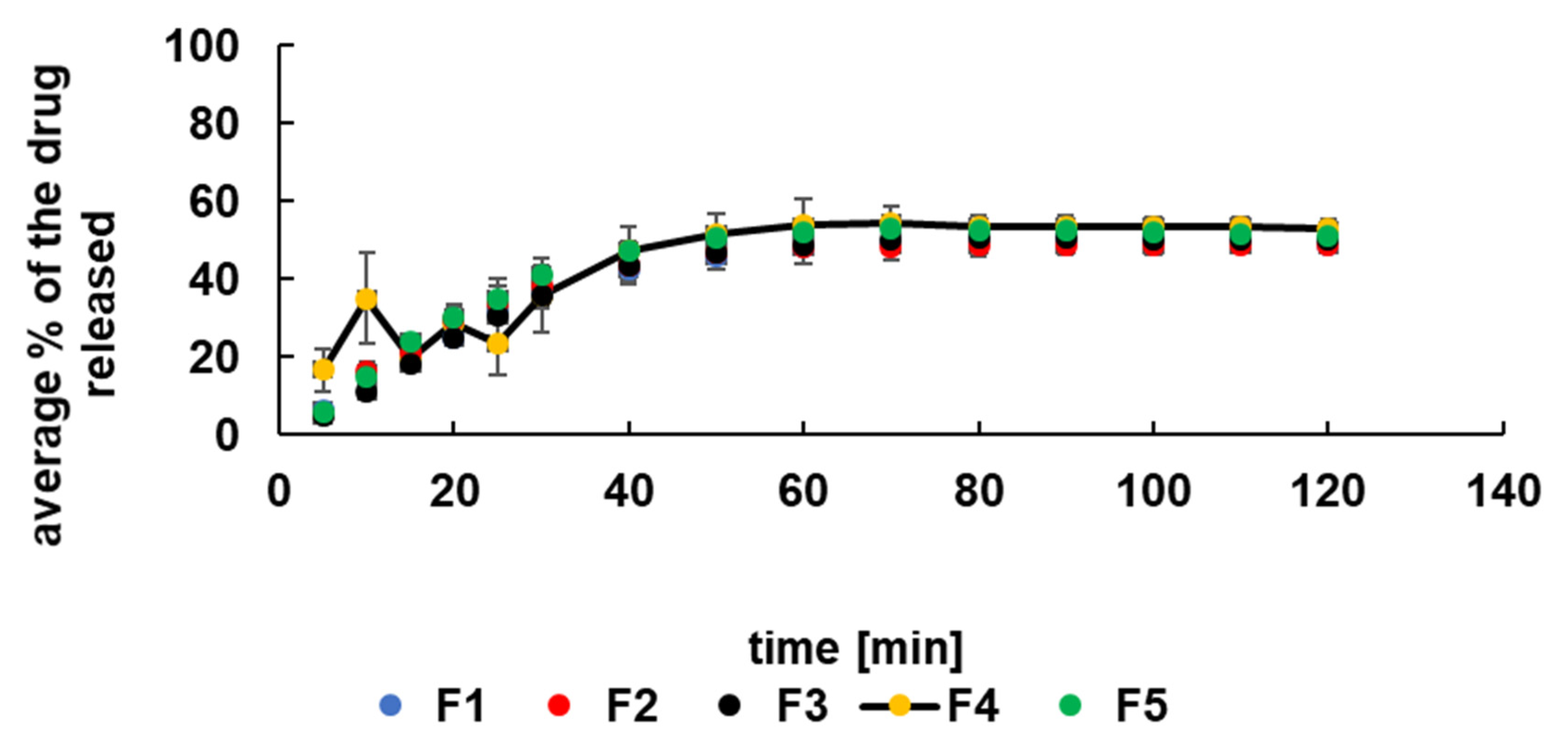
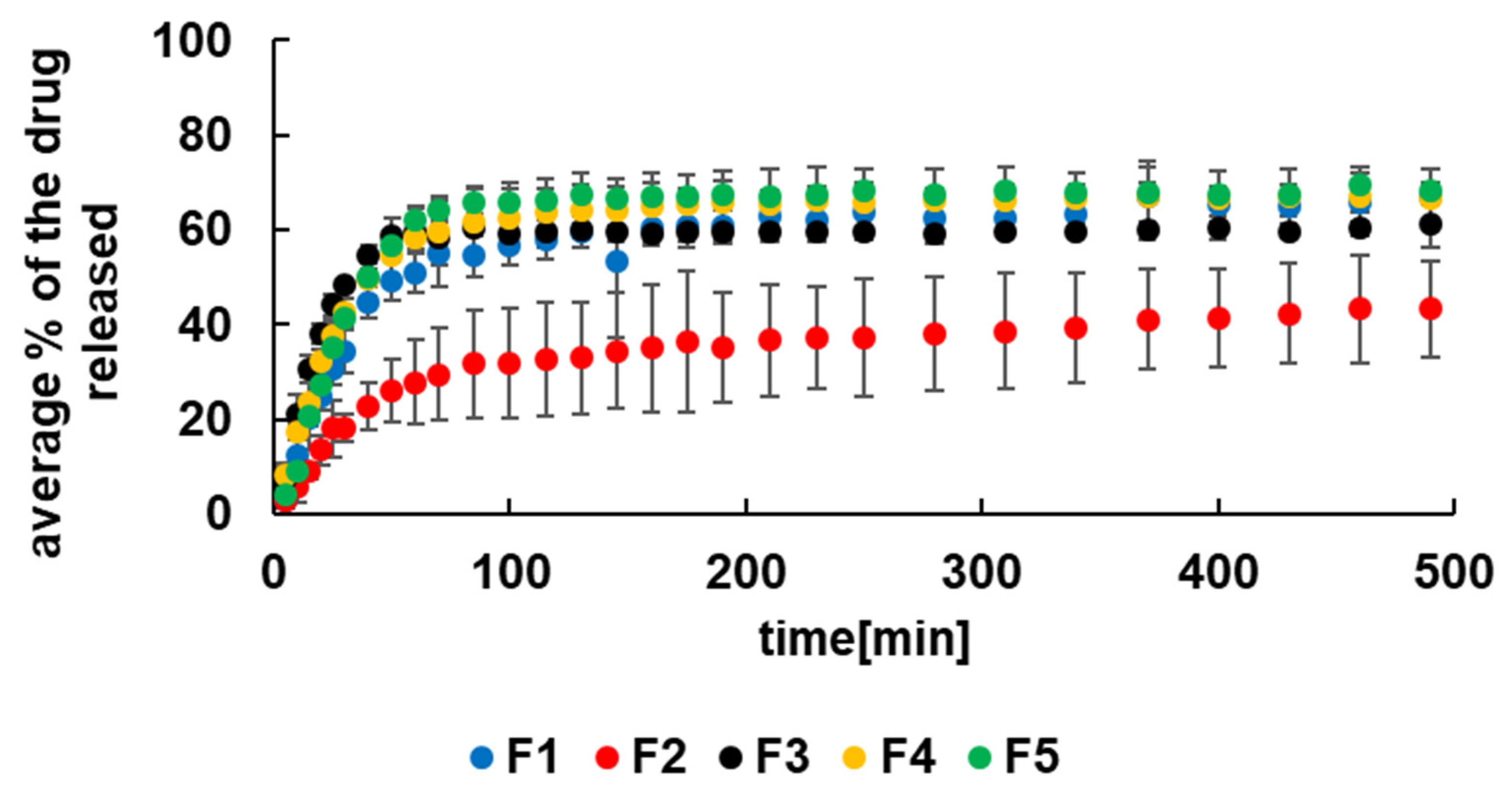

| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 5-ASA [g] | 3 | 3 | 3 | 3 | 3 | ― | ― | ― | ― | ― |
| APN [g] | 6 | 5 | 5 | 5 | 5 | 6 | 5 | 5 | 5 | 5 |
| PA [g] | ― | 1 | ― | ― | ― | ― | 1 | ― | ― | ― |
| PEG400 [g] | ― | ― | 1 | ― | ― | ― | ― | 1 | ― | ― |
| AX [g] | ― | ― | ― | 1 | ― | ― | ― | ― | 1 | ― |
| PVAC-P [g] | ― | ― | ― | ― | 1 | ― | ― | ― | ― | 1 |
| PVAC-D [g] | 1.5 | 1.3 | 1.3 | 1.6 | 1.6 | 1.5 | 1.5 | 1.4 | 1.4 | 1.3 |
| Kinetic Model | Kinetic Parameters | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|---|
| Z-O | k0 [mg × min−1] | 4.7 ± 0.6 | 2.5 ± 0.3 | 6.2 ± 1.2 | 5.4 ± 0.8 | 5.4 ± 0.6 |
| t0.5 [min] | 58.8 ± 6.8 | 106.6 ± 13.7 | 44.3 ± 8.6 | 47.2 ± 7.0 | 46.2 ± 5.1 | |
| R2 | 0.96 ± 0.01 | 0.97 ± 0.02 | 0.92 ± 0.02 | 0.95 ± 0.02 | 0.97 ± 0.01 | |
| F-O | k1 × 102 [min−1] | 1.2 ± 0.2 | 0.5 ± 0.1 | 1.2 ± 0.4 | 1.3 ± 0.2 | 1.6 ± 0.2 |
| t0.5 [min] | 60.1 ± 10.9 | 155.7 ± 34.9 | 56.5 ± 16.9 | 55.2 ± 10.0 | 43.6 ± 5.4 | |
| R2 | 0.94 ± 0.03 | 0.92 ± 0.05 | 0.85 ± 0.03 | 0.94 ± 0.04 | 0.97 ± 0.01 | |
| S-O | k2 × 105 [mg−1 × min−1] | 3.8 ± 0.6 | 1.5 ± 0.2 | 4.2 ± 1.0 | 5.3 ± 0.5 | 5.8 ± 0.4 |
| t0.5 [min] | 55.1 ± 8.4 | 187.3 ± 39.1 | 110.1 ± 27.0 | 46.2 ± 6.2 | 35.1 ± 2.6 | |
| R2 | 0.95 ± 0.03 | 0.93 ± 0.05 | 0.89 ± 0.03 | 0.97 ± 0.02 | 0.99 ± 0.01 | |
| H | kH [mg × min−1/2] | 32.1 ± 3.4 | 17.3 ± 1.8 | 43.1 ± 3.6 | 5.4 ± 0.8 | 36.3 ± 4.4 |
| t0.5 [min] | 63.0 ± 13.4 | 258.4 ± 49.8 | 40.06 ± 6.6 | 2238.7 ± 673.6 | 47.0 ± 11.6 | |
| R2 | 0.97 ± 0.02 | 0.98 ± 0.01 | 0.98 ± 0.004 | 0.95 ± 0.02 | 0.97 ± 0.01 | |
| K–P | kK-P × 102 [min−n] | 1.8 ± 1.1 | 2.0 ± 0.2 | 5.6 ± 2.3 | 3.6 ± 1.2 | 1.4 ± 0.7 |
| t0.5 [min] | 33.0 ± 30.1 | 40.1 ± 25.7 | 20.3 ± 14.7 | 26.5 ± 17.0 | 30.2 ± 25.3 | |
| R2 | 0.92 ± 0.03 | 0.95 ± 0.02 | 0.90 ± 0.09 | 0.95 ± 0.03 | 0.92 ± 0.1 | |
| n | 0.95 ± 0.20 | 0.87 ± 0.1 | 0.74 ± 0.2 | 0.80 ± 0.1 | 0.99 ± 0.2 | |
| H–C | kH-C × 102 [mg1/3 × min−1] | 2.7 ± 0.5 | 1.3 ± 0.2 | 2.8 ± 0.9 | 2.9 ± 0.5 | 3.5 ± 0.5 |
| t0.5 [min] | 61.9 ± 12.3 | 147.7 ± 34.1 | 60.6 ± 19.6 | 58.6 ± 11.9 | 46.6 ± 7.1 | |
| R2 | 0.93 ± 0.03 | 0.91 ± 0.05 | 0.83 ± 0.04 | 0.93 ± 0.04 | 0.95 ± 0.02 | |
| best fit | H | H | H | S-O | S-O | |
| Kinetic Model | kinetic Parameters | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|---|
| Z-O | k0 [mg × min−1] | 0.12 ± 0.02 | 1.6 ± 0.02 | 0.08 ± 0.02 | 0.08 ± 0.01 | 0.06 ± 0.08 |
| t0.5 [min] | 1262 ± 258 | 525 ± 94 | 3246 ± 784 | 2186 ± 366 | 3019 ± 488 | |
| R2 | 0.86 ± 0.04 | 0.89 ± 0.05 | 0.82 ± 0.08 | 0.88 ± 0.05 | 0.91 ± 0.05 | |
| F-O | k1 × 104 [min−1] | 6.3 ± 1.1 | 4.9 ± 0.8 | 4.0 ± 0.8 | 4.7 ± 0.7 | 3.9 ± 0.5 |
| t0.5 [min] | 1168 ± 224 | 1431 ± 223 | 3077 ± 735 | 1562 ± 261 | 2070 ± 328 | |
| R2 | 0.87 ± 0.04 | 0.90 ± 0.05 | 0.82 ± 0.09 | 0.89 ± 0.04 | 0.91 ± 0.05 | |
| S-O | k2 × 106 [mg−1 × min−1] | 3.3 ± 0.6 | 1.8 ± 0.3 | 2.1 ± 0.4 | 3.4 ± 0.5 | 2.5 ± 0.3 |
| t0.5 [min] | 1423 ± 263 | 1790 ± 275 | 4152 ± 986 | 1990 ± 319 | 2715 ± 426 | |
| R2 | 0.88 ± 0.04 | 0.90 ± 0.05 | 0.82 ± 0.09 | 0.90 ± 0.03 | 0.92 ± 0.05 | |
| H | kH [mg × min−1/2] | 3.9 ± 0.6 | 5.2 ± 0.8 | 2.4 ± 0.6 | 2.6 ± 0.4 | 1.9 ± 0.2 |
| t0.5 [min] | 1422 ± 535 | 234 ± 83 | 15,552 ± 7072 | 4739 ± 1415 | 9304 ± 2776 | |
| R2 | 0.89 ± 0.04 | 0.90 ± 0.06 | 0.80 ± 0.01 | 0.91 ± 0.03 | 0.92 ± 0.04 | |
| H–C | kH-C × 103 [mg1/3 × min−1] | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| t0.5 [min] | 1090 ± 212 | 1337 ± 210 | 2720 ± 657 | 1352 ± 234 | 1928 ± 309 | |
| R2 | 0.87 ± 0.04 | 0.90 ± 0.05 | 0.82 ± 0.07 | 0.89 ± 0.04 | 0.91 ± 0.05 | |
| best fit | H | F-O, S-O, H, H–C | Z-O, F-O, S-O, H–C | H | S-O, H | |
| f1 | f2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Formulation | F2 | F3 | F4 | F5 | F2 | F3 | F4 | F5 |
| F1 | 41.0 | 8.2 | 9.5 | 11.2 | 32.7 | 60.4 | 64.1 | 59.9 |
| F2 | ― | 79.3 | 85.9 | 88.0 | ― | 30.3 | 28.5 | 27.5 |
| F3 | ― | ― | 8.48 | 12.3 | ― | ― | 65.2 | 57.6 |
| F4 | ― | ― | ― | 4.1 | ― | ― | ― | 76.2 |
| Formulation | F2 | F3 | F4 | F5 |
|---|---|---|---|---|
| F1 | 21.2 | 2.9 | 4.9 | 5.6 |
| F2 | ― | 24.1 | 26.1 | 26.8 |
| F3 | ― | ― | 2.0 | 2.6 |
| F4 | ― | ― | ― | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Pastuszka, D.; Barczyszyn, K.; Musiał, W. The Influence of the Hydrophobic Polymeric Coating on 5-ASA Release from the Bipolymeric Milibeads with Amidated Pectin. Materials 2021, 14, 3924. https://doi.org/10.3390/ma14143924
Wójcik-Pastuszka D, Barczyszyn K, Musiał W. The Influence of the Hydrophobic Polymeric Coating on 5-ASA Release from the Bipolymeric Milibeads with Amidated Pectin. Materials. 2021; 14(14):3924. https://doi.org/10.3390/ma14143924
Chicago/Turabian StyleWójcik-Pastuszka, Dorota, Kinga Barczyszyn, and Witold Musiał. 2021. "The Influence of the Hydrophobic Polymeric Coating on 5-ASA Release from the Bipolymeric Milibeads with Amidated Pectin" Materials 14, no. 14: 3924. https://doi.org/10.3390/ma14143924






