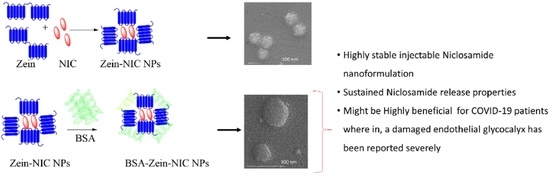
Bovine Serum Albumin-Coated Niclosamide-Zein Nanoparticles as Potential Injectable Medicine against COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Zein-NIC NPs
2.3. Preparation of BSA-Coated Zein-NIC NPs
2.4. Drug Content Analysis and InVitro Drug Release Studies
2.5. Colloidal Stability
2.6. Characterizations
2.7. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Injectable Formulation
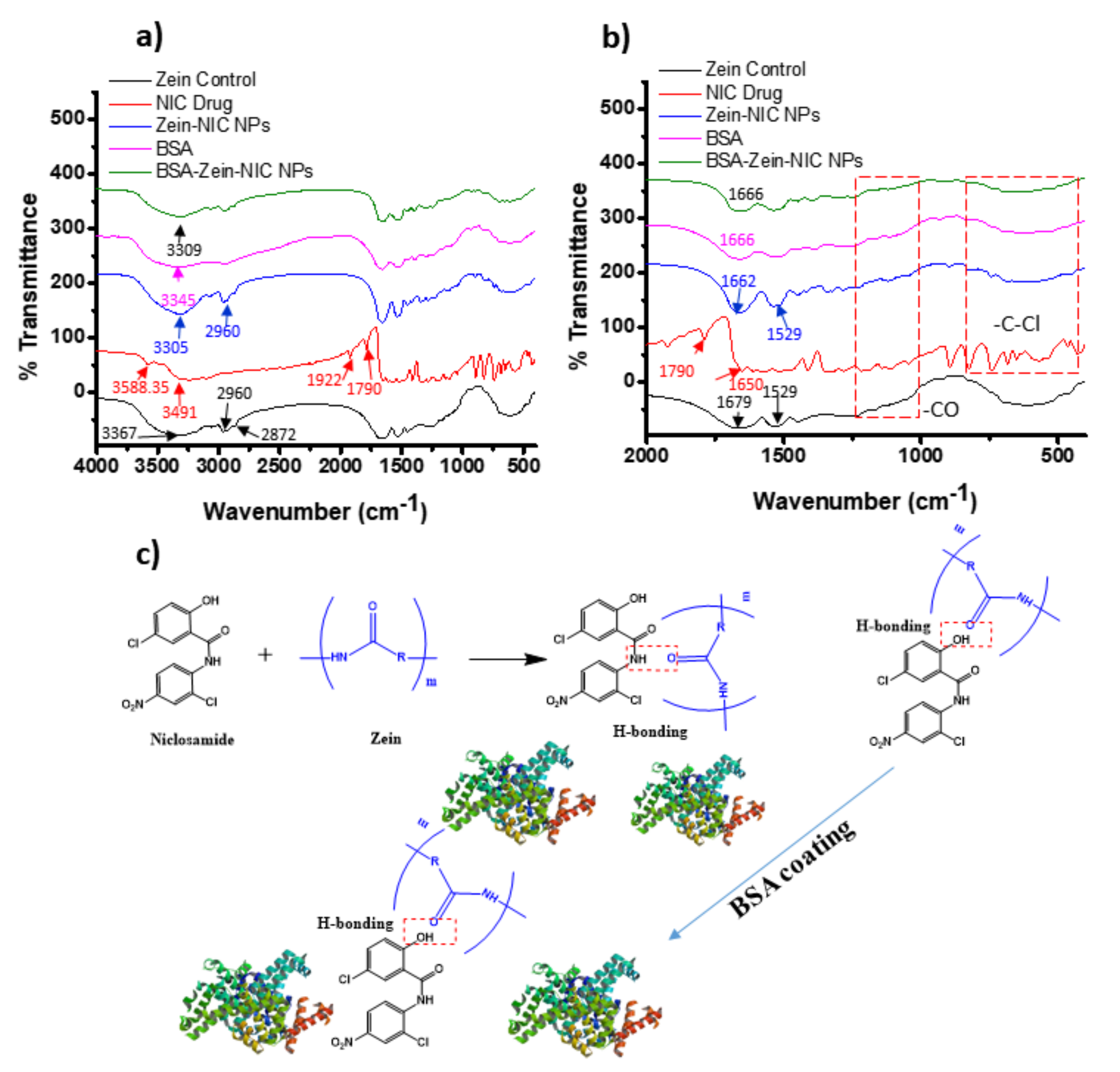
3.1.1. XRD, FT-IR, and NMR Analysis
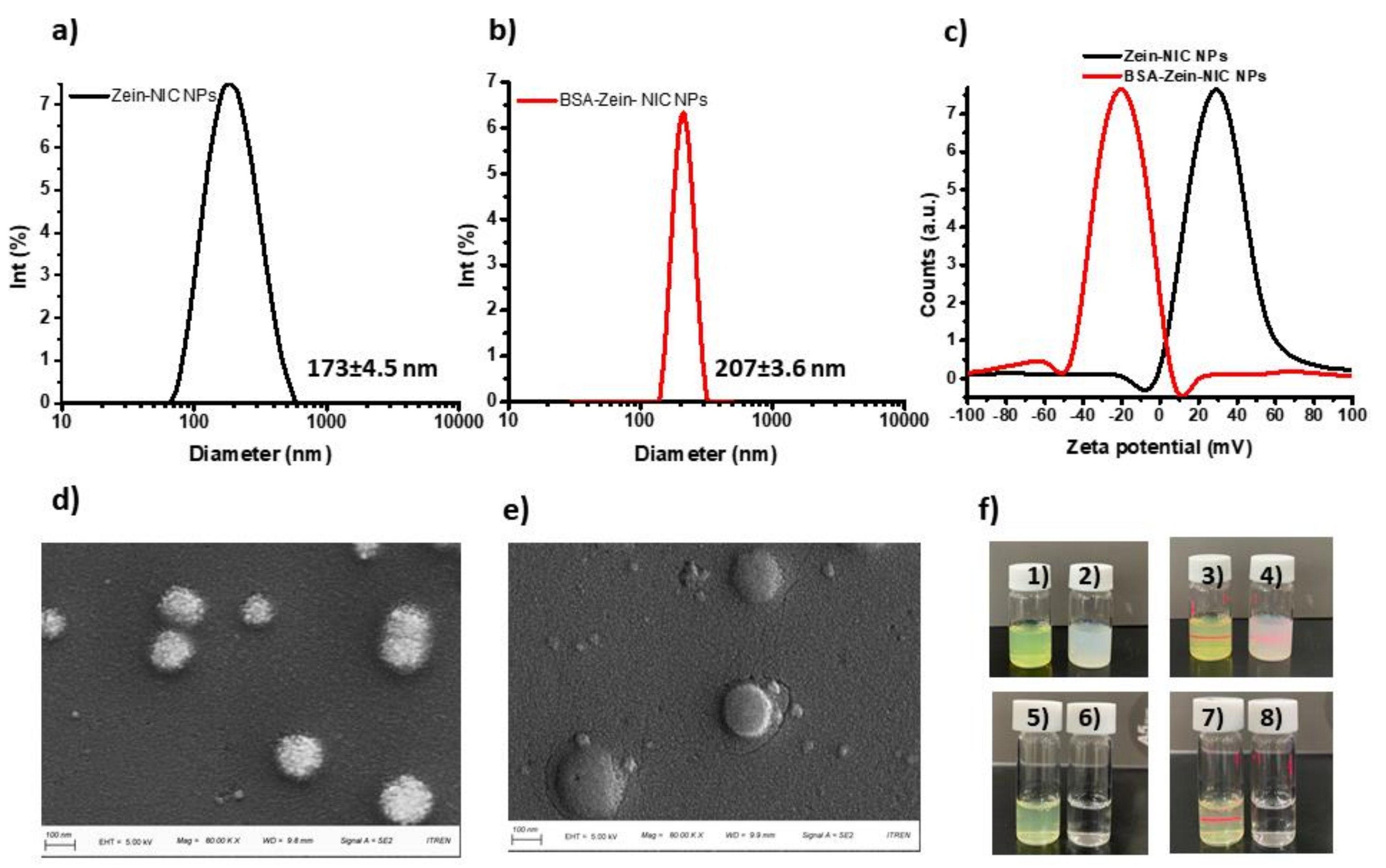
3.2. Particle Size, Zeta, and Surface Morphology Analyses
3.3. In Vitro Drug Release Studies
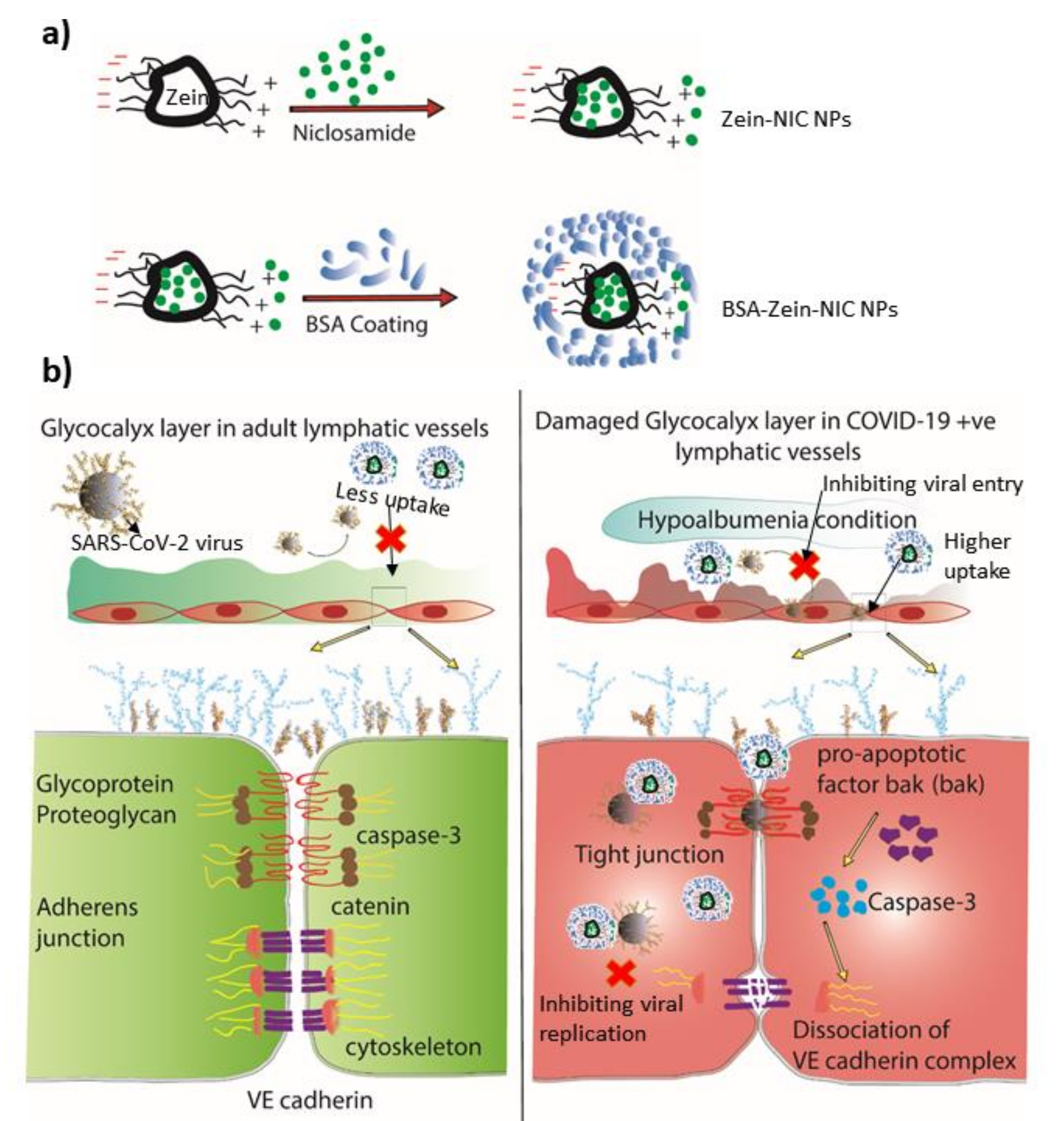
4. Clinical Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tracy, M.F. Understanding the Impact of COVID-19: Now and for the Future. AACN Adv. Crit. Care 2021, 32, 157–158. [Google Scholar] [CrossRef]
- Rossen, L.M.; Gold, J.A.; Ahmad, F.B.; Sutton, P.D.; Branum, A.M. Trends in the distribution of COVID-19 deaths by age and race/ethnicity—United States, April 4–December 26, 2020. Ann. Epidemiology 2021. [Google Scholar] [CrossRef]
- Kearney, A.; Searl, J.; Erickson-DiRenzo, E.; Doyle, P.C. The Impact of COVID-19 on Speech-Language Pathologists Engaged in Clinical Practices With Elevated Coronavirus Transmission Risk. Am. J. Speech-Language Pathol. 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Esper, J.J.; Garg, D.; McClafferty, B.R.; Golamari, R.; Jain, R. COVID-19 Isolation and Quarantine Guidelines for Older Adults in Nursing Homes. South Dak. Med. J. South Dak. State Med Assoc. 2021, 74, 58–60. [Google Scholar]
- Amos, O.A.; Adebisi, Y.A.; Bamisaiye, A.; Olayemi, A.H.; Ilesanmi, E.B.; Micheal, A.I.; Ekpenyong, A.; Lucero-Prisno, D.E. COVID-19 and progress towards achieving universal health coverage in Africa: A case of Nigeria. Int. J. Heal. Plan. Manag. 2021, 1–6. [Google Scholar] [CrossRef]
- Rafael, R.D.M.R.; Correia, L.M.; de Mello, A.S.; Prata, J.A.; Depret, D.G.; Santo, T.B.D.E.; E Silva, F.V.C.; Acioli, S. Psychological distress in the COVID-19 pandemic: prevalence and associated factors at a nursing college. Rev. Bras. Enferm. 2021, 74 Suppl. 1, e20210023. [Google Scholar] [CrossRef]
- Yasri, S.; Wiwanitkit, V. COVID-19-induced de novo nephritic syndrome. Revista da Associação Médica Brasileira 2021, 67, 6. [Google Scholar] [CrossRef]
- Pérez-De-Llano, L.; Romay-Lema, E.M.; Baloira-Villar, A.; Anchorena, C.; Torres-Durán, M.L.; Sousa, A.; Corbacho-Abelaira, D.; Paz-Ferrin, J.; Diego-Roza, C.; Vilariño-Maneiro, L.; et al. COVID-19 pneumonia in Galicia (Spain): Impact of prognostic factors and therapies on mortality and need for mechanical ventilation. PLOS ONE 2021, 16, e0253465. [Google Scholar] [CrossRef]
- Adjodah, D.; Dinakar, K.; Chinazzi, M.; Fraiberger, S.P.; Pentland, A.; Bates, S.; Staller, K.; Vespignani, A.; Bhatt, D.L. Association between COVID-19 outcomes and mask mandates, adherence, and attitudes. PLOS ONE 2021, 16, e0252315. [Google Scholar] [CrossRef]
- Yamamoto, S.; Saito, M.; Tamura, A.; Prawisuda, D.; Mizutani, T.; Yotsuyanagi, H. The human microbiome and COVID-19: A systematic review. PLOS ONE 2021, 16, e0253293. [Google Scholar] [CrossRef] [PubMed]
- Stastna, D.; Menkyova, I.; Drahota, J.; Mazouchova, A.; Adamkova, J.; Ampapa, R.; Grunermelova, M.; Peterka, M.; Recmanova, E.; Rockova, P.; et al. Multiple sclerosis, neuromyelitis optica spectrum disorder and COVID-19: A pandemic year in Czechia. Mult. Scler. Relat. Disord. 2021, 54, 103104. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wong, M.; Xing, H.; Kwan, M.-P.; Zhu, R. Assessing the Country-Level Excess All-Cause Mortality and the Impacts of Air Pollution and Human Activity during the COVID-19 Epidemic. Int. J. Environ. Res. Public Heal. 2021, 18, 6883. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, X.-P.; Bai, L.; Wang, M.; Deng, W.; Ning, N. A systematic review of etiology, epidemiology, clinical manifestations, image findings, and medication of 2019 Corona Virus Disease-19 in Wuhan, China. Medicine 2020, 99, e22688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, J.Y.; Sun, H.; Wang, L.; Ying, J.S.; Liu, H.X. Investigation of protective exposure risk events in nurses against corona virus disease 2019 in Wuhan. Beijing Da Xue Xue Bao Yi Xue Ban 2020, 52, 711–714. [Google Scholar]
- Yang, L.; Liu, J.; Zhang, R.; Li, M.; Li, Z.; Zhou, X.; Hu, C.; Tian, F.; Zhou, F.; Lei, Y. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: A descriptive study. J. Clin. Virol. 2020, 129, 104475. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Chen, J.; Zhou, Y.; Hua, L.; Yuan, J.; He, S.; Guo, Y.; Zhang, S.; Jia, Q.; Zhao, C.; et al. The effectiveness of quarantine of Wuhan city against the Corona Virus Disease 2019 (COVID-19): A well-mixed SEIR model analysis. J. Med Virol. 2020, 92, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Mallah, S.I.; Ghorab, O.K.; Al-Salmi, S.; Abdellatif, O.S.; Tharmaratnam, T.; Iskandar, M.A.; Sefen, J.A.N.; Sidhu, P.; Atallah, B.; El-Lababidi, R.; et al. COVID-19: Breaking down a global health crisis. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 1–36. [Google Scholar] [CrossRef]
- Sahoo, B.M.; Kumar, B.V.V.R.; Sruti, J.; Mahapatra, M.K.; Banik, B.K.; Borah, P. Drug Repurposing Strategy (DRS): Emerging Approach to Identify Potential Therapeutics for Treatment of Novel Coronavirus Infection. Front. Mol. Biosci. 2021, 8, 628144. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, H.; Zhang, Y.; Li, R.; Han, Y.; Lin, Y.; Jiang, J. Repurposing clinical drugs is a promising strategy to discover drugs against Zika virus infection. Front. Med. 2021, 15, 404–415. [Google Scholar] [CrossRef]
- Murthy, P.K.; Sivashanmugam, K.; Kandasamy, M.; Subbiah, R.; Ravikumar, V. Repurposing of histone deacetylase inhibitors: A promising strategy to combat pulmonary fibrosis promoted by TGF-β signalling in COVID-19 survivors. Life Sci. 2021, 266, 118883. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Shi, P.-Y.; Li, H.; Zhou, J. Broad Spectrum Antiviral Agent Niclosamide and Its Therapeutic Potential. ACS Infect. Dis. 2020, 6, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Piao, H.; Rejinold, N.; Jin, G.; Choi, G.; Choy, J.-H. Niclosamide-Clay Intercalate Coated with Nonionic Polymer for Enhanced Bioavailability toward COVID-19 Treatment. Polymers 2021, 13, 1044. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeño, J.M.; Ortega-Prieto, A.M.; Bussani, R.; et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nat. Cell Biol. 2021, 594, 88–93. [Google Scholar] [CrossRef]
- Barbosa, E.J.; Löbenberg, R.; de Araujo, G.L.B.; Bou-Chacra, N.A. Niclosamide repositioning for treating cancer: Challenges and nano-based drug delivery opportunities. Eur. J. Pharm. Biopharm. 2019, 141, 58–69. [Google Scholar] [CrossRef]
- Choi, G.; Piao, H.; Rejinold, N.; Yu, S.; Kim, K.-Y.; Jin, G.-W.; Choy, J.-H. Hydrotalcite-Niclosamide Nanohybrid as Oral Formulation towards SARS-CoV-2 Viral Infections. Pharmaceuticals 2021, 14, 486. [Google Scholar] [CrossRef]
- Potje, S.R.; Costa, T.J.; Fraga-Silva, T.F.; Martins, R.B.; Benatti, M.N.; Almado, C.E.; de Sá, K.S.; Bonato, V.L.; Arruda, E.; Louzada-Junior, P.; et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021, 276, 119376. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Jilma, B.; Kopp, C.W.; Ertl, S.; Gremmel, T.; Koppensteiner, R. Glycocalyx as Possible Limiting Factor in COVID-19. Front. Immunol. 2021, 12, 607306. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka-Tojo, M. Vascular Endothelial Glycocalyx Damage in COVID-19. Int. J. Mol. Sci. 2020, 21, 9712. [Google Scholar] [CrossRef]
- Yamaoka-Tojo, M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed. J. 2020, 43, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Stahl, K.; Gronski, P.A.; Kiyan, Y.; Seeliger, B.; Bertram, A.; Pape, T.; Welte, T.; Hoeper, M.M.; Haller, H.; David, S. Injury to the Endothelial Glycocalyx in Critically Ill Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 202, 1178–1181. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem. Toxicol. 2020, 145, 111694. [Google Scholar] [CrossRef]
- Naqvi, S.; Mohiyuddin, S.; Gopinath, P. Niclosamide loaded biodegradable chitosan nanocargoes: An in vitro study for potential application in cancer therapy. R. Soc. Open Sci. 2017, 4, 170611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, M.; Mahmoodzadeh, F.; Maroufi, L.Y.; Nezhad-Mokhtari, P. Electrospun tetracycline hydrochloride loaded zein/gum tragacanth/poly lactic acid nanofibers for biomedical application. Int. J. Biol. Macromol. 2020, 165, 1312–1322. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.; Ramos-Rivera, L.; Silva, R.; Nazhat, S.N.; Boccaccini, A.R. Zein-based composites in biomedical applications. J. Biomed. Mater. Res. Part A 2017, 105, 1656–1665. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Li, F.; Shi, N.; Li, C.; Yu, X.; Chen, Y.; Kong, W. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int. J. Pharm. 2016, 513, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Joshi, M.K.; Tiwari, A.P.; Park, C.-H.; Kim, C.S. Fabrication, characterization and biomedical application of two-nozzle electrospun polycaprolactone/zein-calcium lactate composite nonwoven mat. J. Mech. Behav. Biomed. Mater. 2016, 60, 312–323. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent Advances in Food-Packing, Pharmaceutical and Biomedical Applications of Zein and Zein-Based Materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahatheeswaran, D.; Mathew, A.; Aswathy, R.G.; Nagaoka, Y.; Venugopal, K.; Yoshida, Y.; Maekawa, T.; Kumar, S. Hybrid fluorescent curcumin loaded zein electrospun nanofibrous scaffold for biomedical applications. Biomed. Mater. 2012, 7, 045001. [Google Scholar] [CrossRef]
- Nayar, S.; Mir, A.; Ashok, A.; Guha, A.; Sharma, V. Bovine serum albumin binding and drug delivery studies with PVA-ferrofluid. J. Bionic Eng. 2010, 7, 29–34. [Google Scholar] [CrossRef]
- Müller, V.; Piai, J.F.; Fajardo, A.R.; Fávaro, S.L.; Rubira, A.; Muniz, E. Preparation and Characterization of Zein and Zein-Chitosan Microspheres with Great Prospective of Application in Controlled Drug Release. J. Nanomater. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Yin, H.; Lu, T.; Liu, L.; Lu, C. Preparation, characterization and application of a novel biodegradable macromolecule: Carboxymethyl zein. Int. J. Biol. Macromol. 2015, 72, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Targosz-Korecka, M.; Kubisiak, A.; Kloska, D.; Kopacz, A.; Grochot-Przeczek, A.; Szymonski, M. Endothelial glycocalyx shields the interaction of SARS-CoV-2 spike protein with ACE2 receptors. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Herting, G.; Wallinder, I.O.; Blomberg, E. Adsorption of bovine serum albumin on silver surfaces enhances the release of silver at pH neutral conditions. Phys. Chem. Chem. Phys. 2015, 17, 18524–18534. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Uversky, V.N.; Nandi, C.K. Serum albumin-mediated strategy for the effective targeting of SARS-CoV-2. Med Hypotheses 2020, 140, 109790. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Shimomura, M.; Ikeda, M.; Yamaguchi, R.; Tanishita, K. Effect of glycocalyx on shear-dependent albumin uptake in endothelial cells. Am. J. Physiol. Circ. Physiol. 2004, 287, H2287–H2294. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.; Fatemi, R.; Winlow, W. SARS-CoV-2 Bound Human Serum Albumin and Systemic Septic Shock. Front. Cardiovasc. Med. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Ma, Z.; Gao, J.; Tai, Y.; Li, L.; Zhu, H.; Li, L.; Dong, D.; Sun, Z. Injectable pegylated niclosamide (polyethylene glycol-modified niclosamide) for cancer therapy. J. Biomed. Mater. Res. Part A 2020, 108, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Labib, G. Overview on zein protein: a promising pharmaceutical excipient in drug delivery systems and tissue engineering. Expert Opin. Drug Deliv. 2018, 15, 65–75. [Google Scholar] [CrossRef]
- Berardi, A.; Rahim, S.A.; Bisharat, L.; Cespi, M.; Rahim, A. Swelling of Zein Matrix Tablets Benchmarked against HPMC and Ethylcellulose: Challenging the Matrix Performance by the Addition of Co-Excipients. Pharmaceutics 2019, 11, 513. [Google Scholar] [CrossRef] [Green Version]

| Samples | PDI (a.u.) |
|---|---|
| NIC NPs | 0.385 ± 0.026 |
| Zein-NIC NPs | 0.314 ± 0.013 |
| BSA-Zein-NIC NPs | 0.032 ± 0.005 ** |
| Samples | Drug Content (%) |
|---|---|
| Zein-NIC NPs | 10.08 ± 0.95 |
| BSA-Zein-NIC NPs | 3.31 ± 0.041 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rejinold N, S.; Choi, G.; Piao, H.; Choy, J.-H. Bovine Serum Albumin-Coated Niclosamide-Zein Nanoparticles as Potential Injectable Medicine against COVID-19. Materials 2021, 14, 3792. https://doi.org/10.3390/ma14143792
Rejinold N S, Choi G, Piao H, Choy J-H. Bovine Serum Albumin-Coated Niclosamide-Zein Nanoparticles as Potential Injectable Medicine against COVID-19. Materials. 2021; 14(14):3792. https://doi.org/10.3390/ma14143792
Chicago/Turabian StyleRejinold N, Sanoj, Goeun Choi, Huiyan Piao, and Jin-Ho Choy. 2021. "Bovine Serum Albumin-Coated Niclosamide-Zein Nanoparticles as Potential Injectable Medicine against COVID-19" Materials 14, no. 14: 3792. https://doi.org/10.3390/ma14143792
APA StyleRejinold N, S., Choi, G., Piao, H., & Choy, J.-H. (2021). Bovine Serum Albumin-Coated Niclosamide-Zein Nanoparticles as Potential Injectable Medicine against COVID-19. Materials, 14(14), 3792. https://doi.org/10.3390/ma14143792