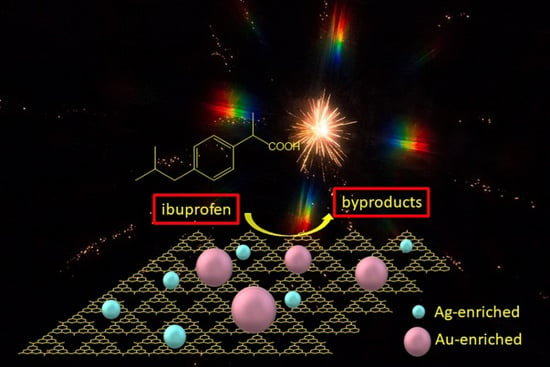
Combination of Au-Ag Plasmonic Nanoparticles of Varied Compositions with Carbon Nitride for Enhanced Photocatalytic Degradation of Ibuprofen under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Photocatalysts
2.2.1. Synthesis of g-C3N4 Nanosheets (1)
2.2.2. Synthesis of 0.5% Au-Ag-g-C3N4 Nanohybrid (2)
2.2.3. Synthesis of 1% Au-Ag-g-C3N4 Nanohybrid (3)
2.3. Characterization
2.4. Photocatalytic Activity Measurement
2.5. Photodegradation Analysis
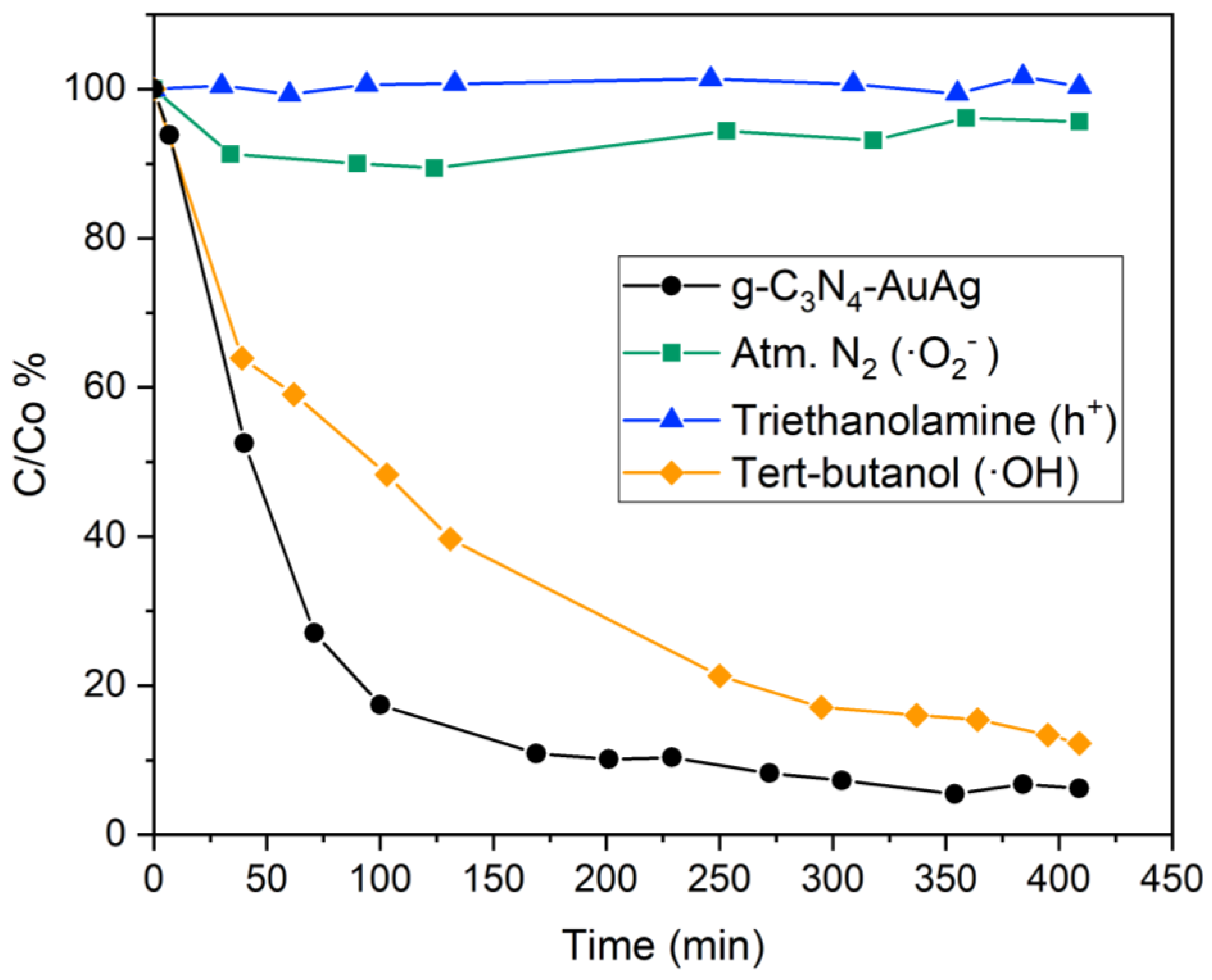
2.6. Detection of Active Species in the Photocatalysis
3. Results and Discussion
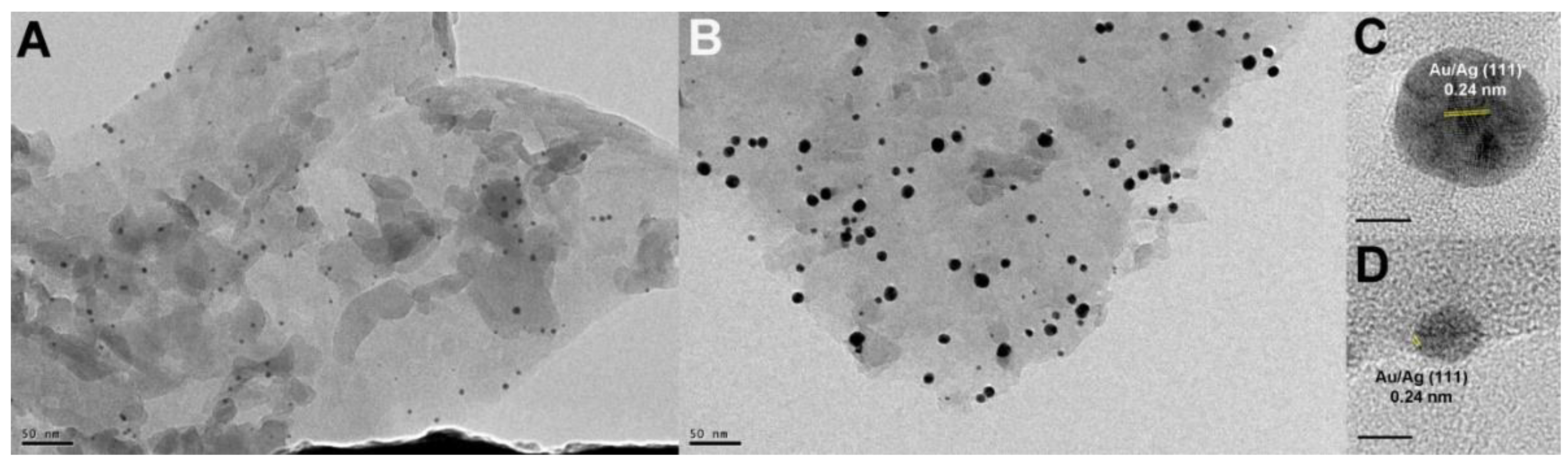
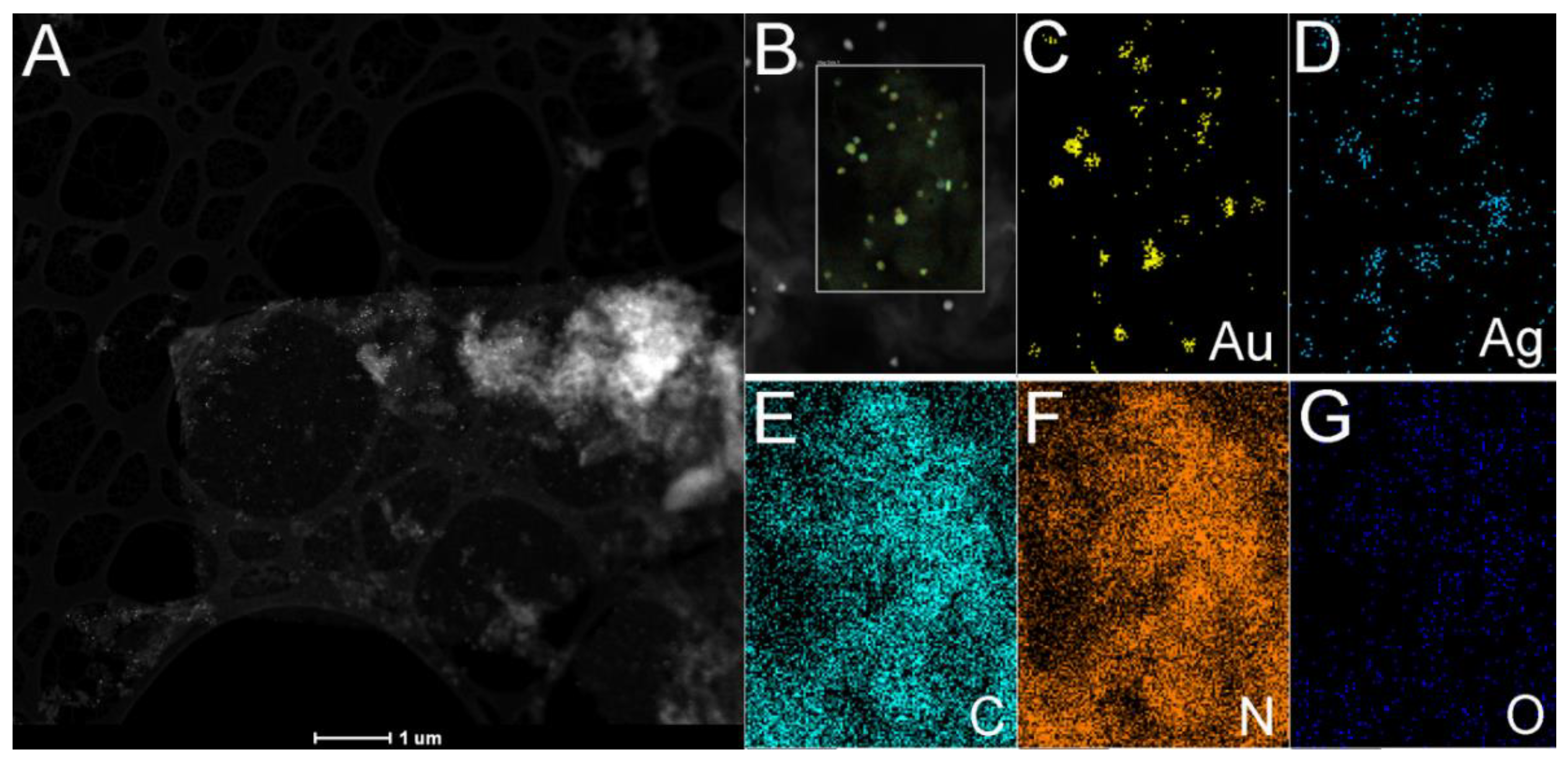
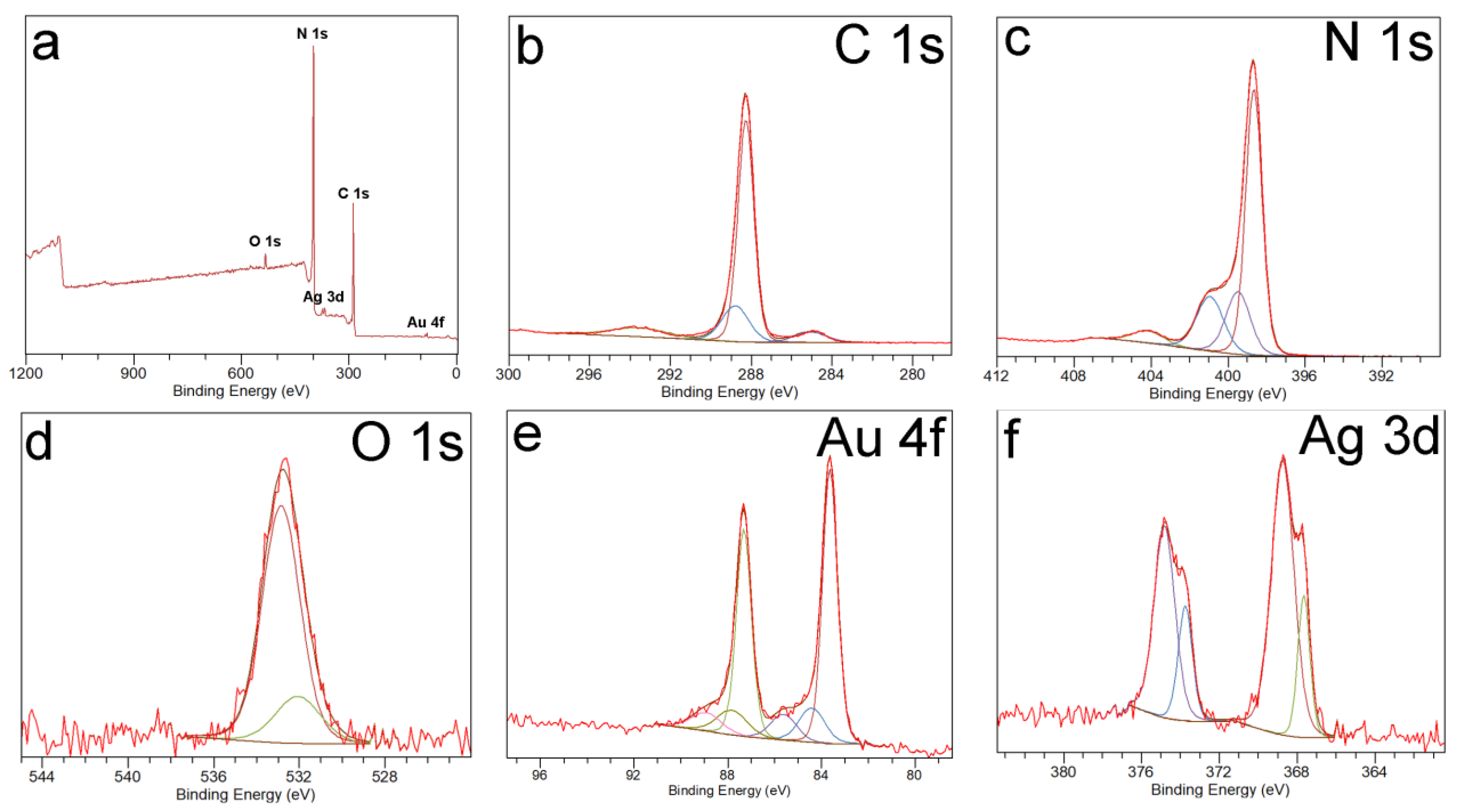
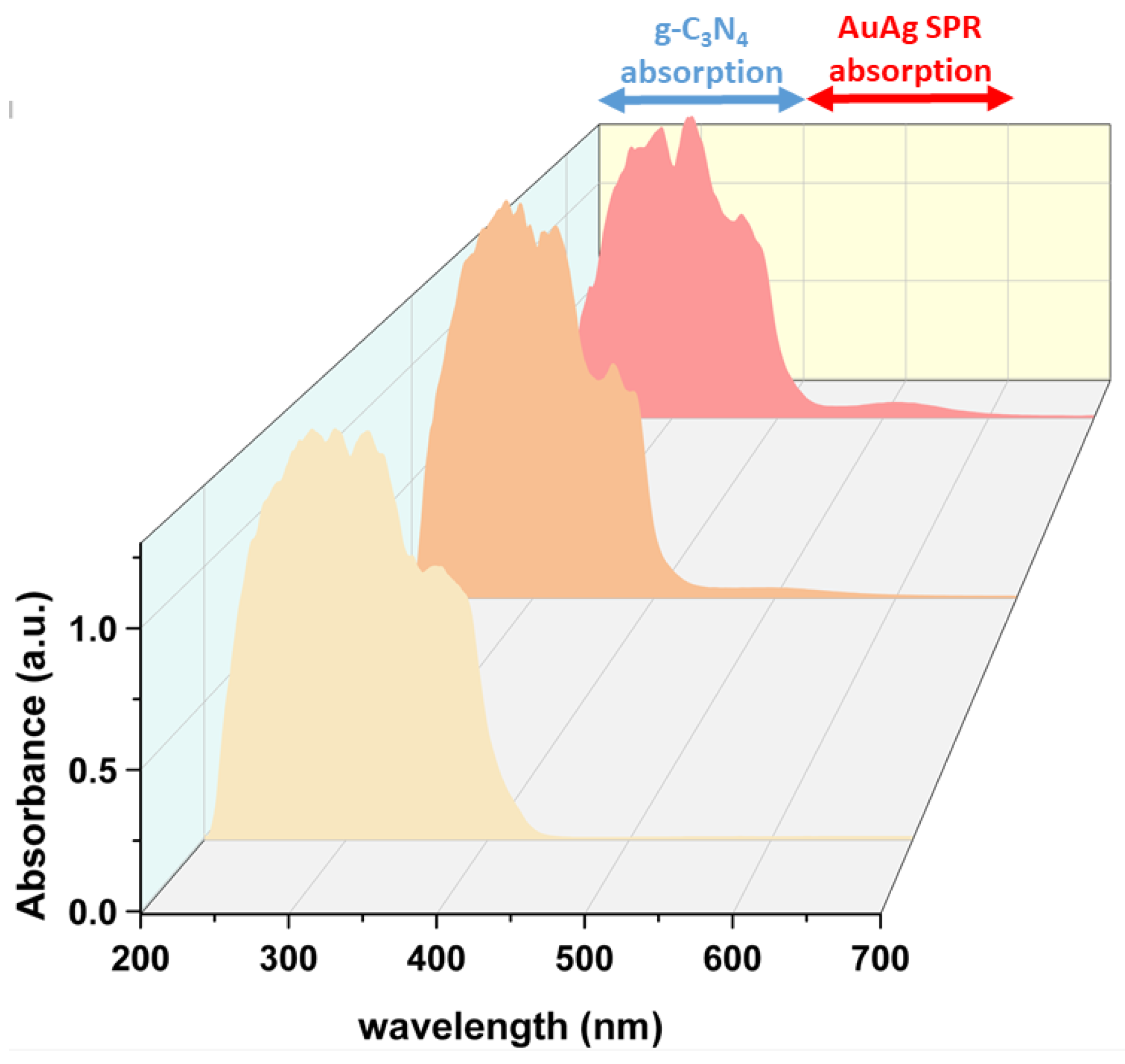
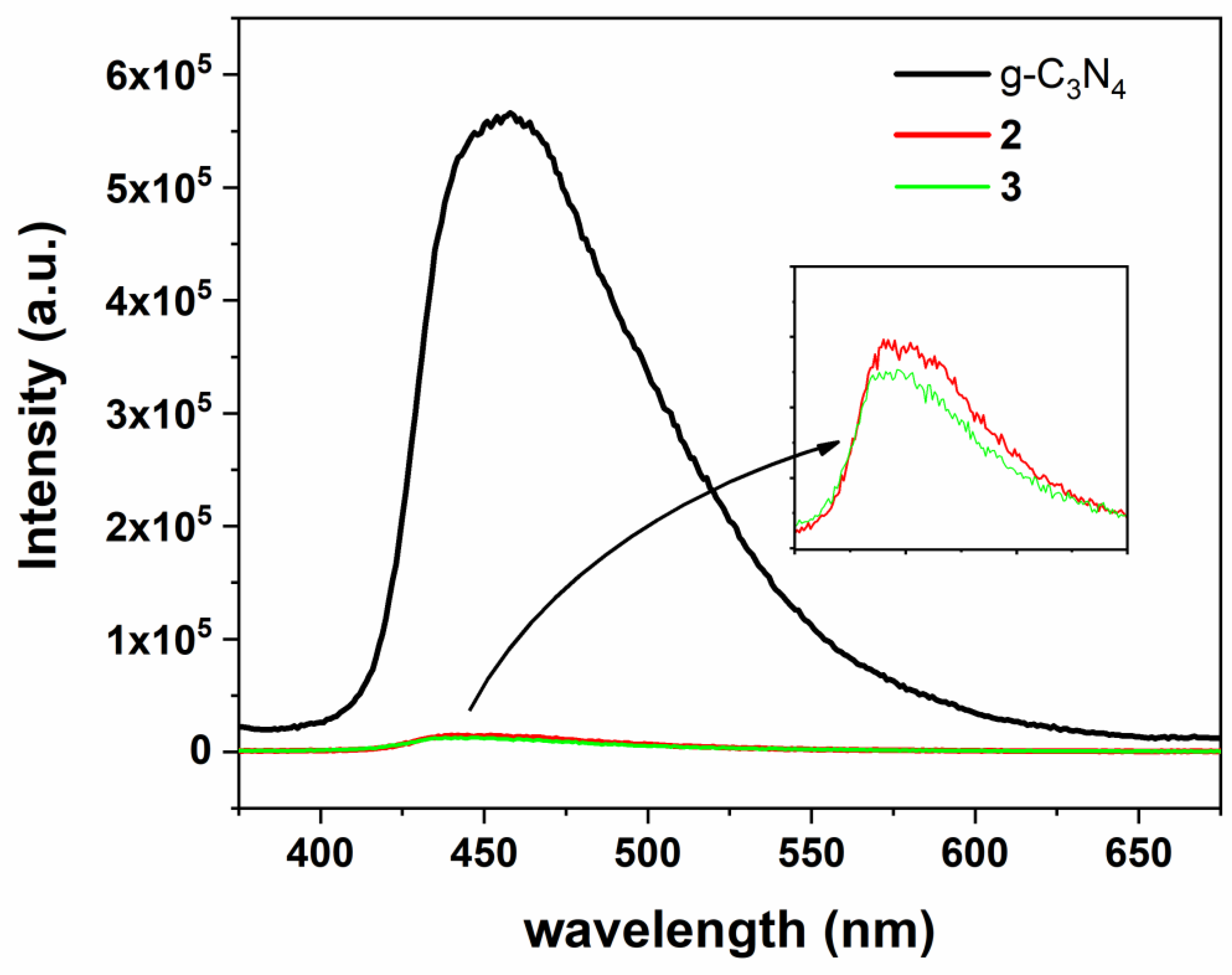
3.1. Synthesis and Characterization of Photocatalysts
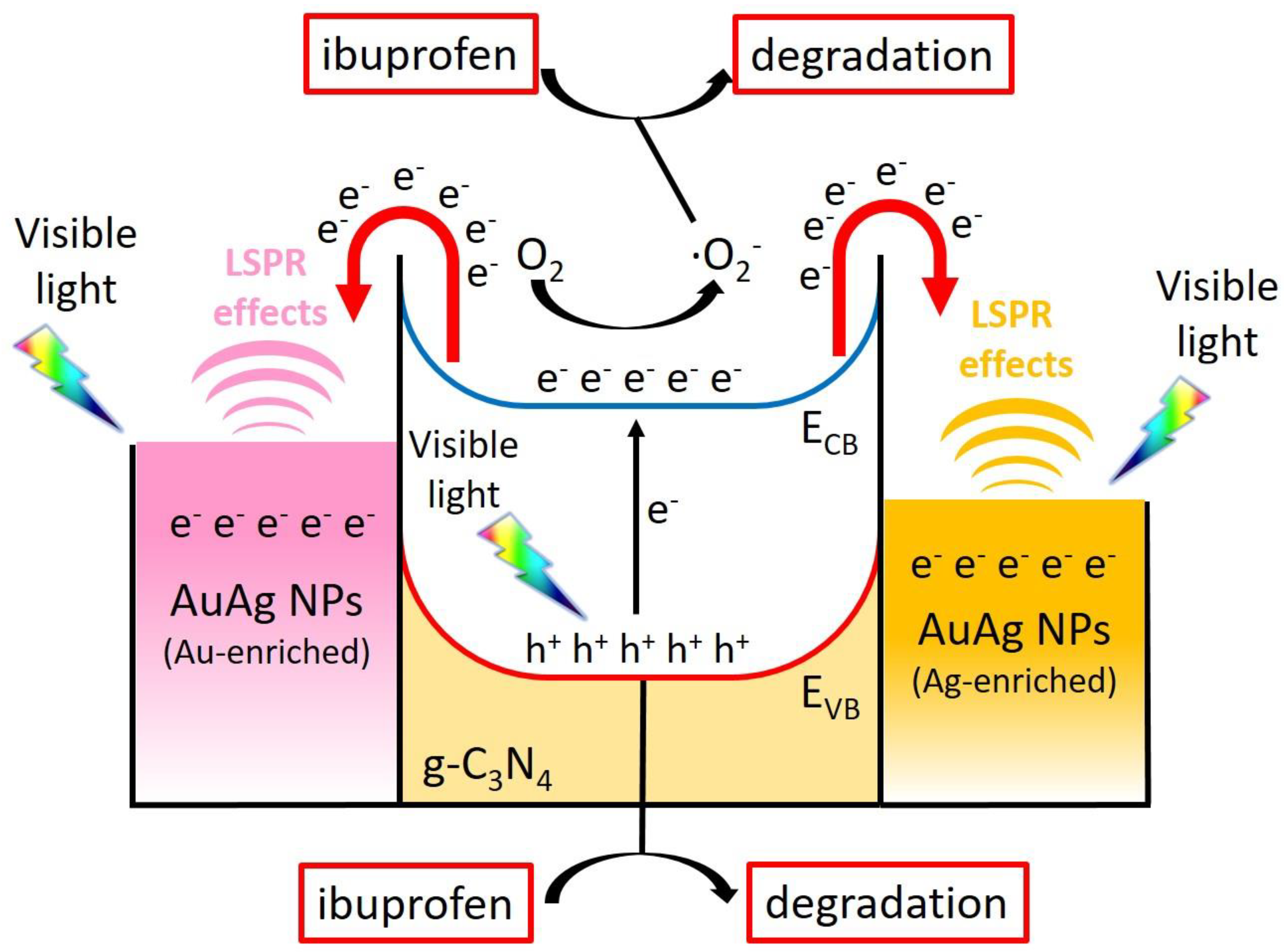
3.2. Mechanism of Formation of Photocatalysts
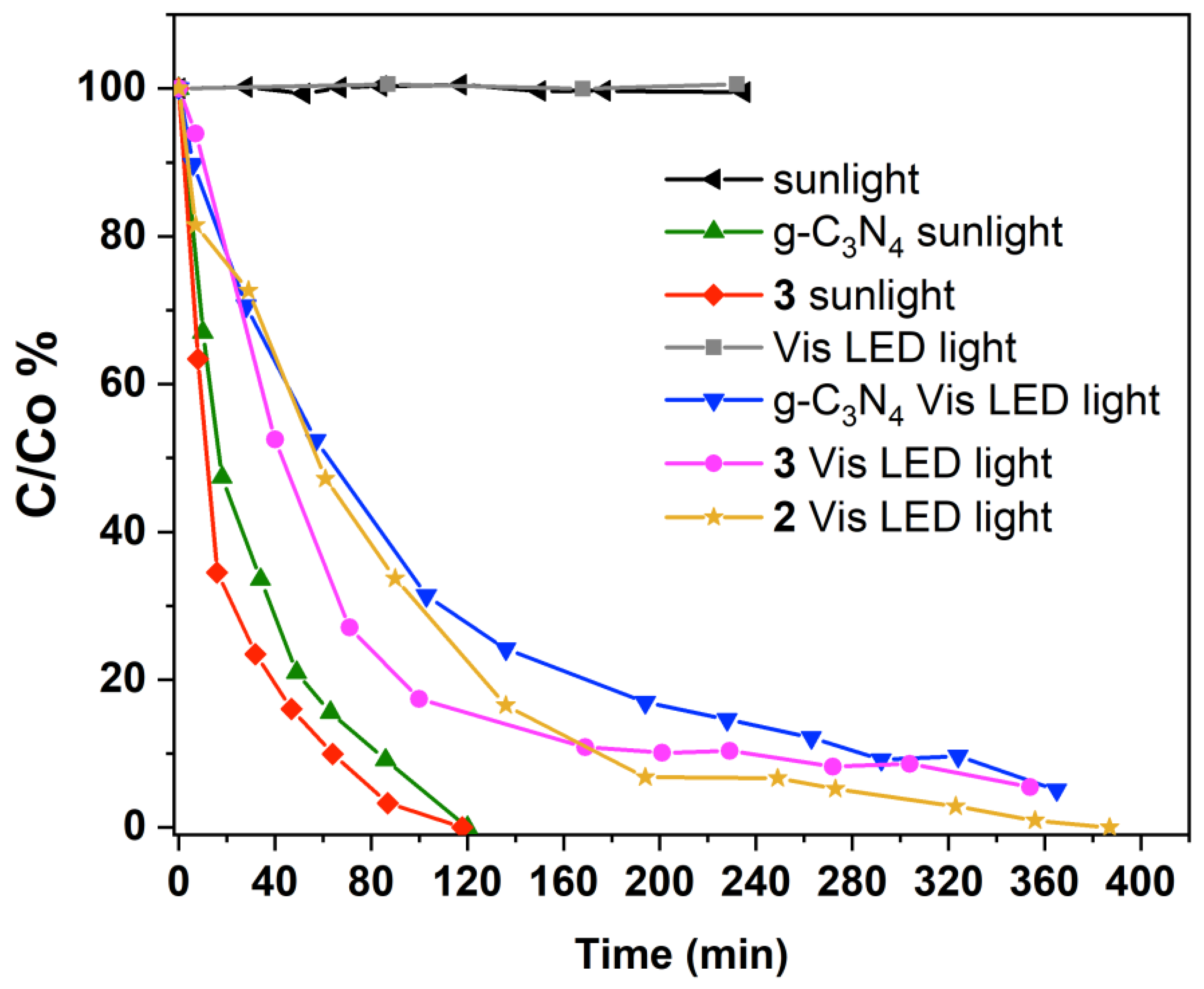
3.3. Photodegradation of Ibuprofen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boukherroub, R.; Ogale, S.B.; Robertson, N. Nanostructured Photocatalyst. From Materials to Applications in Solar Fuels and Environmental Remediation; Boukherroub, R., Ogale, S.B., Robertson, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Dionysiou, D.D.; Puma, G.L.; Ye, J.; Schneider, J.; Bahnemann, D. Photocatalysis: Applications; Dionysiou, D.D., Puma, G.L., Ye, J., Schneider, J., Bahnemann, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Khan, M.M.; Pradhan, D.; Sohn, Y. Nanocomposites for Visible Light-induced Photocatalysis; Khan, M.M., Pradhan, D., Sohn, Y., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Ghosh, S. Visible-Light-Active Photocatalysis: Nanostructured Catalyst Design, Mechanisms, and Applications; Ghosh, S., Ed.; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Marcelino, R.B.P.; Amorim, C.C. Towards visible-light photocatalysis for environmental applications: Band-gap engineering versus photons absorption—A review. Environ. Sci. Pollut. Res. 2019, 26, 4155–4170. [Google Scholar] [CrossRef] [PubMed]
- Peiris, S.; McMurtrie, J.; Zhu, H.-Y. Metal nanoparticle photocatalysts: Emerging processes for green organic synthesis. Catal. Sci. Technol. 2016, 6, 320–338. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Teixeira, I.F.; Barbosa, E.C.M.; Tsang, S.C.E.; Camargo, P.H.C. Carbon nitrides and metal nanoparticles: From controlled synthesis to design principles for improved photocatalysis. Chem. Soc. Rev. 2018, 47, 7783–7817. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, R.; Nithya, P.M.; Girish Kumar, S. Noble metal deposited graphitic carbon nitride based heterojunction photocatalysts. App. Surf. Sci. 2020, 508, 145142. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring Surface Plasmons through the Morphology and Assembly of Metal Nanoparticles. Langmuir 2005, 22, 32–41. [Google Scholar] [CrossRef]
- Noguez, C. Surface Plasmons on Metal Nanoparticles: The Influence of Shape and Physical Environment. J. Phys. Chem. C 2007, 111, 3806–3819. [Google Scholar] [CrossRef]
- Polavarapu, L.; Perez-Juste, J.; Xu, Q.-H.; Liz-Marzan, L.M. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J. Mater. Chem. C 2014, 2, 7460–7476. [Google Scholar] [CrossRef]
- Guan, Z.; Polavarapu, L.; Xu, Q.-H. Enhanced Two-Photon Emission in Coupled Metal Nanoparticles Induced by Conjugated Polymers. Langmuir 2010, 26, 18020–18023. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2002, 107, 668–677. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.-H.; Chen, F.-C. Plasmonic nanostructures for light trapping in organic photovoltaic devices. Nanoscale 2014, 6, 8444–8458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Lopez-Puente, V.; Wang, Q.; Polavarapu, L.; Pastoriza-Santos, I.; Xu, Q.-H. Plasmon-enhanced light harvesting: Applications in enhanced photocatalysis, photodynamic therapy and photovoltaics. RSC Adv. 2015, 5, 29076–29097. [Google Scholar] [CrossRef]
- Baffou, G.; Quidant, R. Nanoplasmonics for chemistry. Chem. Soc. Rev. 2014, 43, 3898–3907. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Crespo, J.; Falqui, A.; García-Barrasa, J.; López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Rodríguez-Castillo, M.; Sestu, M.; Soulantica, K. Synthesis and plasmonic properties of monodisperse Au–Ag alloy nanoparticles of different compositions from a single-source organometallic precursor. J. Mater. Chem. C 2014, 2, 2975–2984. [Google Scholar] [CrossRef]
- Crespo, J.; García-Barrasa, J.; López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Sáenz, Y.; Torres, C. Organometallic approach to polymer-protected antibacterial silver nanoparticles: Optimal nanoparticle size-selection for bacteria interaction. J. Nanoparticle Res. 2012, 14, 1281. [Google Scholar] [CrossRef]
- Fernández, E.J.; López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Puelles, R.C.; Laguna, A.; Mohamed, A.A.; Fackler, J.P., Jr. Vapochromic Behavior of {Ag2(Et2O)2[Au(C6F5)2]2}n with Volatile Organic Compounds. Inorg. Chem. 2008, 47, 8069–8076. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Zhao, Y.; Mao, Z.; Agrawal, D.K.; Wang, D. Microwave modification of surface hydroxyl density for g-C3N4 with enhanced photocatalytic activity. Mater. Res. Express 2018, 5, 035502. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Muller, J.-O.; Schlogl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Fina, F.; Ménard, H.; Irvine, J.T.S. The effect of Pt NPs crystallinity and distributionon the photocatalytic activity of Pt–g-C3N4. Phys. Chem. Chem. Phys. 2015, 17, 13929–13936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caux, M.; Menard, H.; AlSalik, Y.M.; Irvine, J.T.S.; Idriss, H. Photo-catalytic hydrogen production over Au/g-C3N4: Effect of gold particle dispersion and morphology. Phys. Chem. Chem. Phys. 2019, 21, 15974–15987. [Google Scholar] [CrossRef] [Green Version]
- Fujigaya, T.; Kim, C.; Hamasaki, Y.; Nakashima, N. Growth and Deposition of Au Nanoclusters on Polymer-wrapped Graphene and Their Oxygen Reduction Activity. Sci. Rep. 2016, 6, 21314. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Goswami, N.L.; Bright, R.; Kopecki, Z.; Cowin, A.J.; Garga, S.; Vasilev, K. The interplay between size and valence state on the antibacterial activity of sub-10 nm silver nanoparticles. Nanoscale Adv. 2019, 1, 2365–2371. [Google Scholar] [CrossRef] [Green Version]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: Berlin, Germany, 1995. [Google Scholar]
- Crespo, J.; López-de-Luzuriaga, J.M.; Monge, M.; Elena Olmos, M.; Rodríguez-Castillo, M.; Cormary, B.; Soulantica, K.; Sestu, M.; Falqui, A. The spontaneous formation and plasmonic properties of ultrathin gold–silver nanorods and nanowires stabilized in oleic acid. Chem. Commun. 2015, 51, 16691–16694. [Google Scholar] [CrossRef]
- Crespo, J.; Guari, Y.; Ibarra, A.; Larionova, J.; Lasanta, T.; Laurencin, D.; López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Richeter, S. Ultrasmall NHC-coated gold nanoparticles obtained through solvent free thermolysis of organometallic Au(i) complexes. Dalton Trans. 2014, 43, 15713–15718. [Google Scholar] [CrossRef]
- López-de-Luzuriaga, J.M.; Monge, M.; Quintana, J.; Rodríguez-Castillo, M. Single-step assembly of gold nanoparticles into plasmonic colloidosomes at the interface of oleic acid nanodroplets. Nanoscale Adv. 2021, 3, 198–205. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Lian, J.; Xu, H.; She, X.; Li, H. g-C3N4/TiO2 Nanocomposites for degradation of ciprofloxacin under visible light irradiation. ChemistrySelect 2016, 1, 5679–5685. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Ma, C.; Guan, Q.; Lu, Z.; Huo, P.; Yan, Y. Melamine modified P25 with heating method and enhanced the photocatalytic activity on degradation of ciprofloxacin. App. Surf. Sci. 2015, 329, 17–22. [Google Scholar] [CrossRef]
- Hao, R.; Wang, G.; Tang, H.; Sun, L.; Xu, C.; Han, D. Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojuction photocatalysts with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 47–58. [Google Scholar] [CrossRef]
- Lu, N.; Wang, P.; Su, Y.; Yu, H.; Liu, N.; Quan, X. Construction of Z-Scheme g-C3N4/RGO/WO3 with in situ photoreduced graphene oxide as electron mediator for efficient photocatalytic degradation of ciprofloxacin. Chemosphere 2019, 215, 444–453. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Meng, S.; Fu, X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalyst g-C3N4-WO3. Appl. Catal. B Environ. 2014, 150–151, 564–573. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Z.; Shi, J.; Deng, H. Fabrication of novel visible-light-driven AgI/g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Colloid Interface Sci. 2017, 496, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.P.; Behera, A.; Martha, S.; Acharya, R.; Parida, K. Facile synthesis of exfoliated graphitic carbon nitride for photocatalytic degradation of ciprofloxacin under solar irradiation. J. Mater. Sci. 2019, 54, 5726–5742. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. Combination of Au-Ag Plasmonic Nanoparticles of Varied Compositions with Carbon Nitride for Enhanced Photocatalytic Degradation of Ibuprofen under Visible Light. Materials 2021, 14, 3912. https://doi.org/10.3390/ma14143912
Jiménez-Salcedo M, Monge M, Tena MT. Combination of Au-Ag Plasmonic Nanoparticles of Varied Compositions with Carbon Nitride for Enhanced Photocatalytic Degradation of Ibuprofen under Visible Light. Materials. 2021; 14(14):3912. https://doi.org/10.3390/ma14143912
Chicago/Turabian StyleJiménez-Salcedo, Marta, Miguel Monge, and María Teresa Tena. 2021. "Combination of Au-Ag Plasmonic Nanoparticles of Varied Compositions with Carbon Nitride for Enhanced Photocatalytic Degradation of Ibuprofen under Visible Light" Materials 14, no. 14: 3912. https://doi.org/10.3390/ma14143912
APA StyleJiménez-Salcedo, M., Monge, M., & Tena, M. T. (2021). Combination of Au-Ag Plasmonic Nanoparticles of Varied Compositions with Carbon Nitride for Enhanced Photocatalytic Degradation of Ibuprofen under Visible Light. Materials, 14(14), 3912. https://doi.org/10.3390/ma14143912