Applying Mixture of Municipal Incinerator Bottom Ash and Sewage Sludge Ash for Ceramic Tile Manufacturing
Abstract
1. Introduction
2. Materials
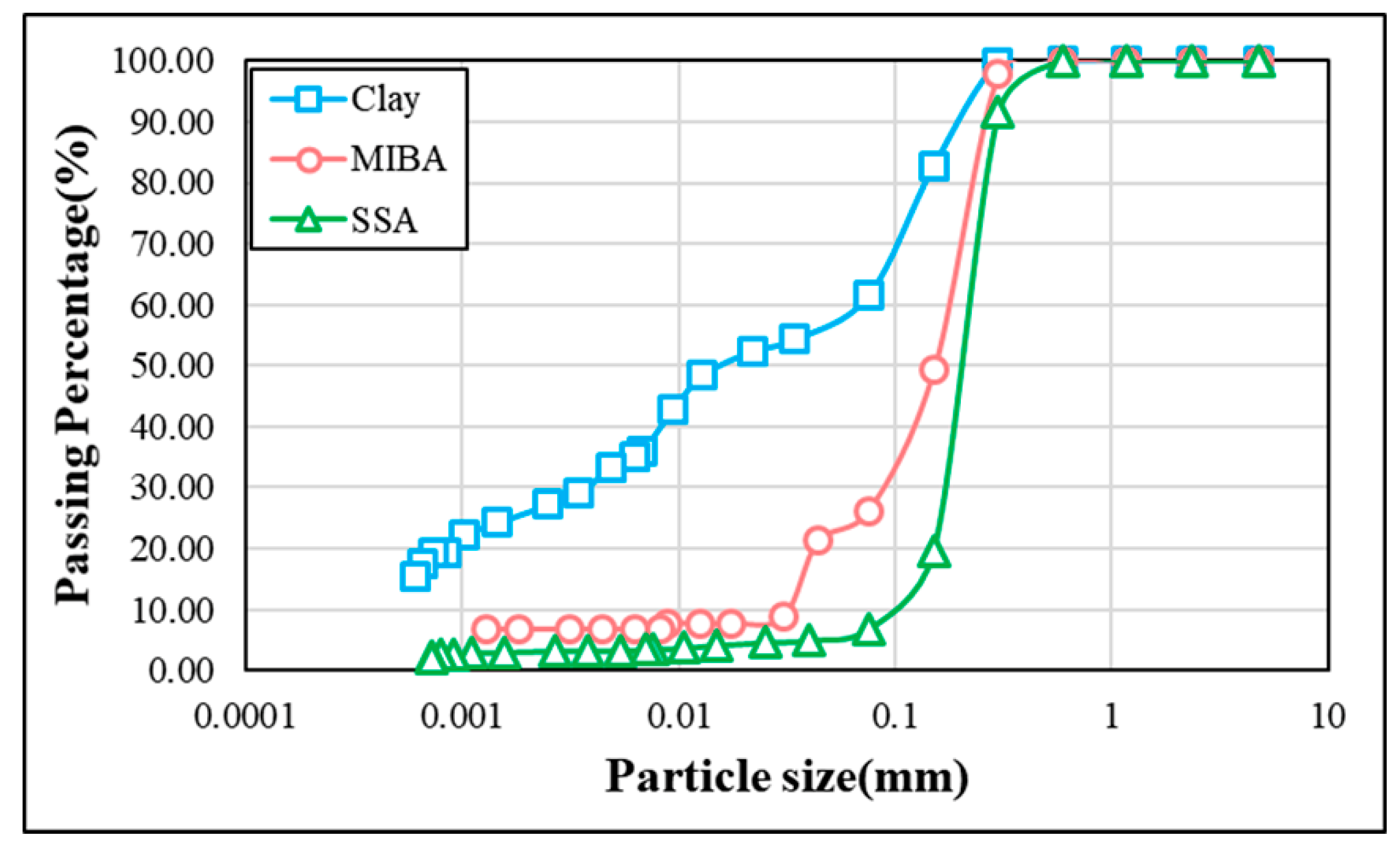
2.1. Raw Material Properties
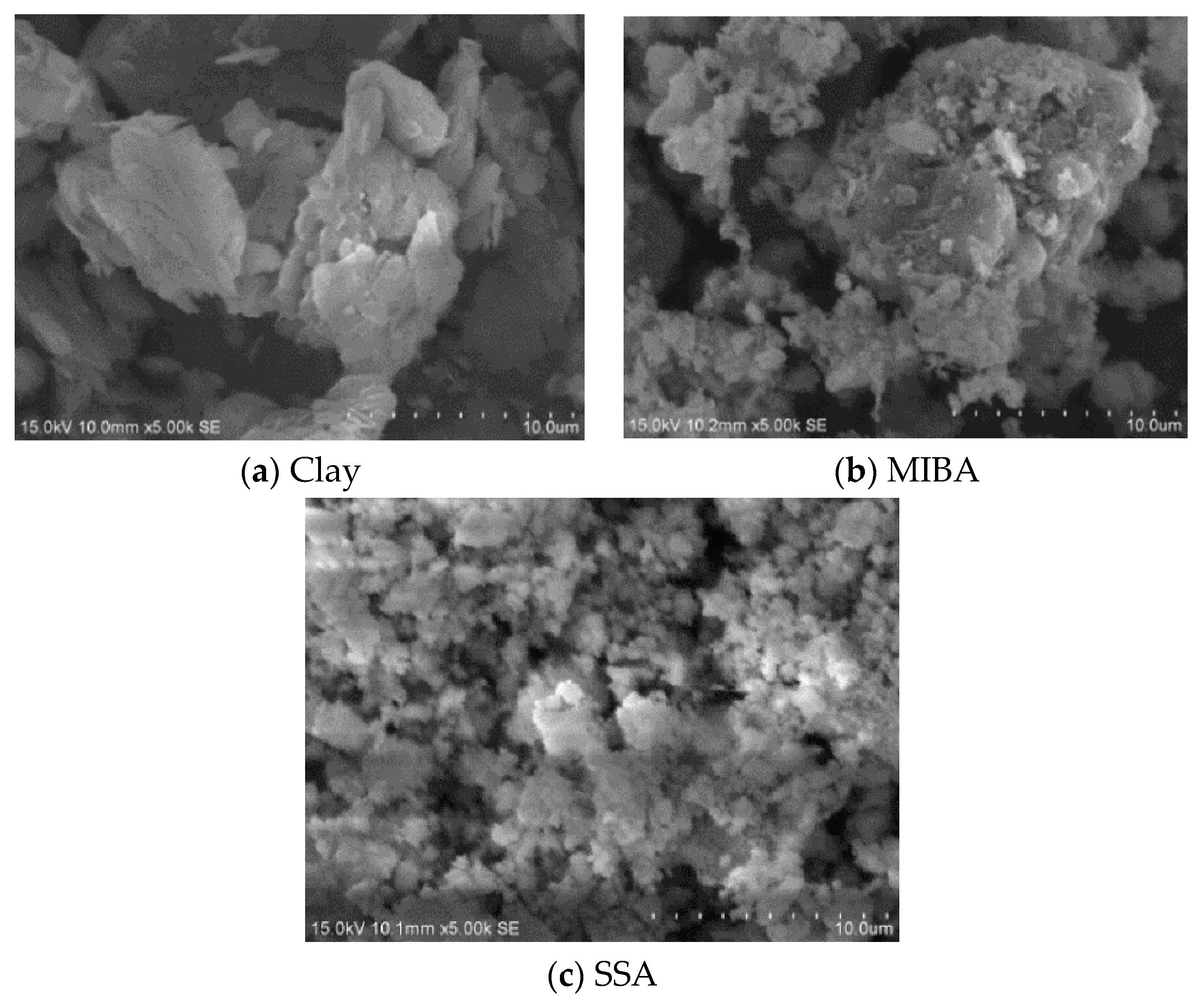
2.2. SEM Images for Raw Materials
2.3. EDS Analysis
2.4. TCLP Test
3. Results and Discussion
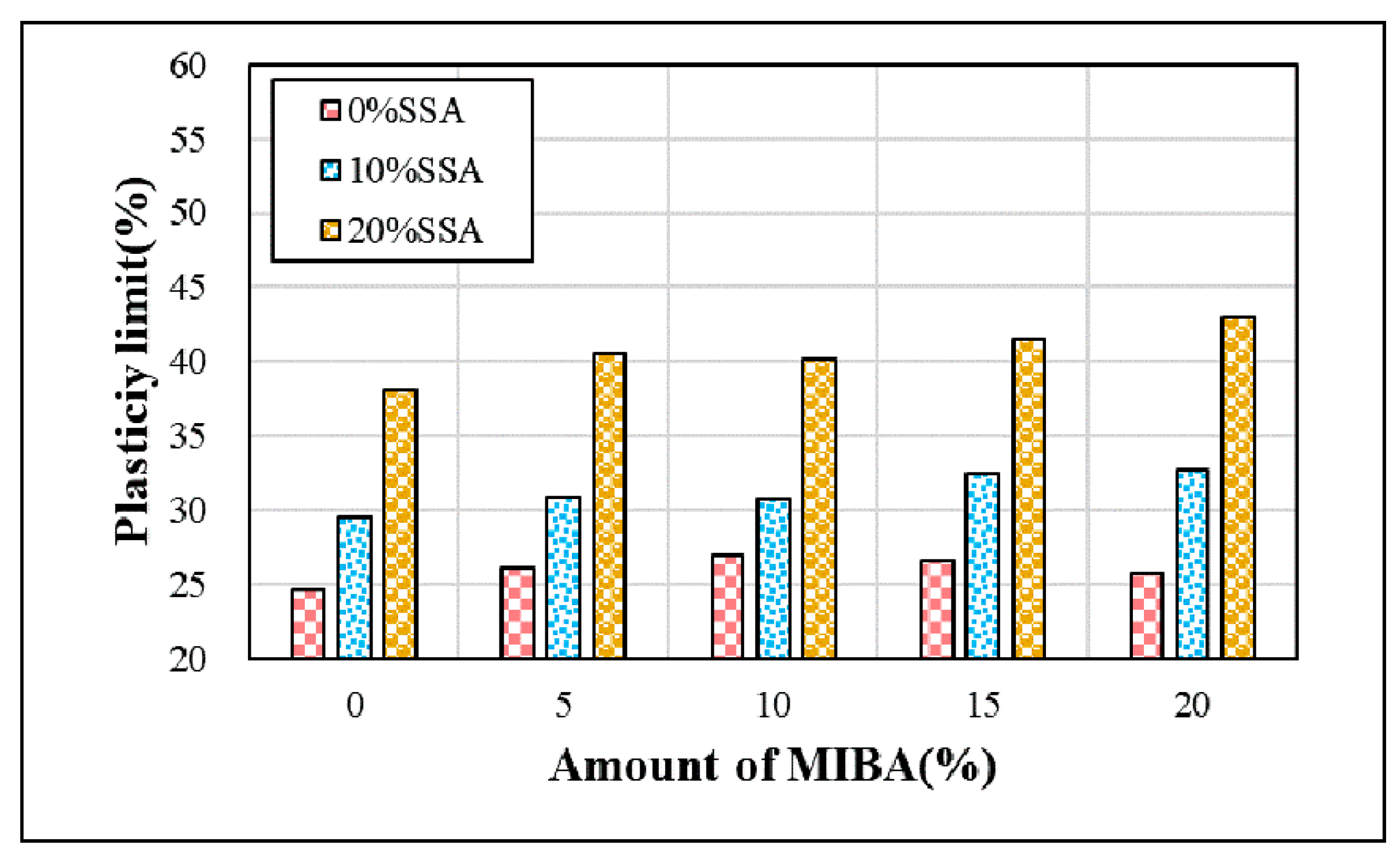
3.1. Atterberg Limits
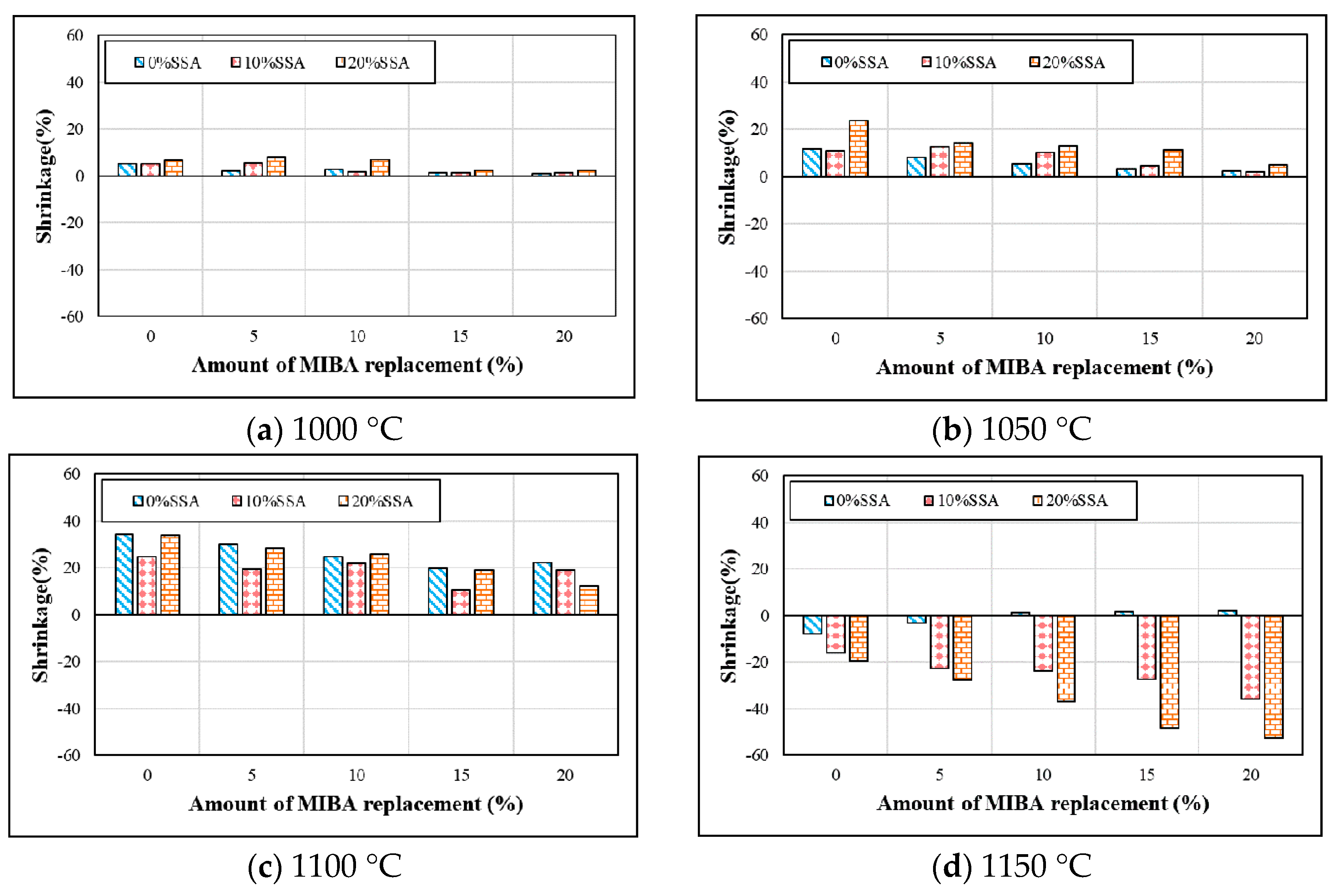
3.2. Shrinkage
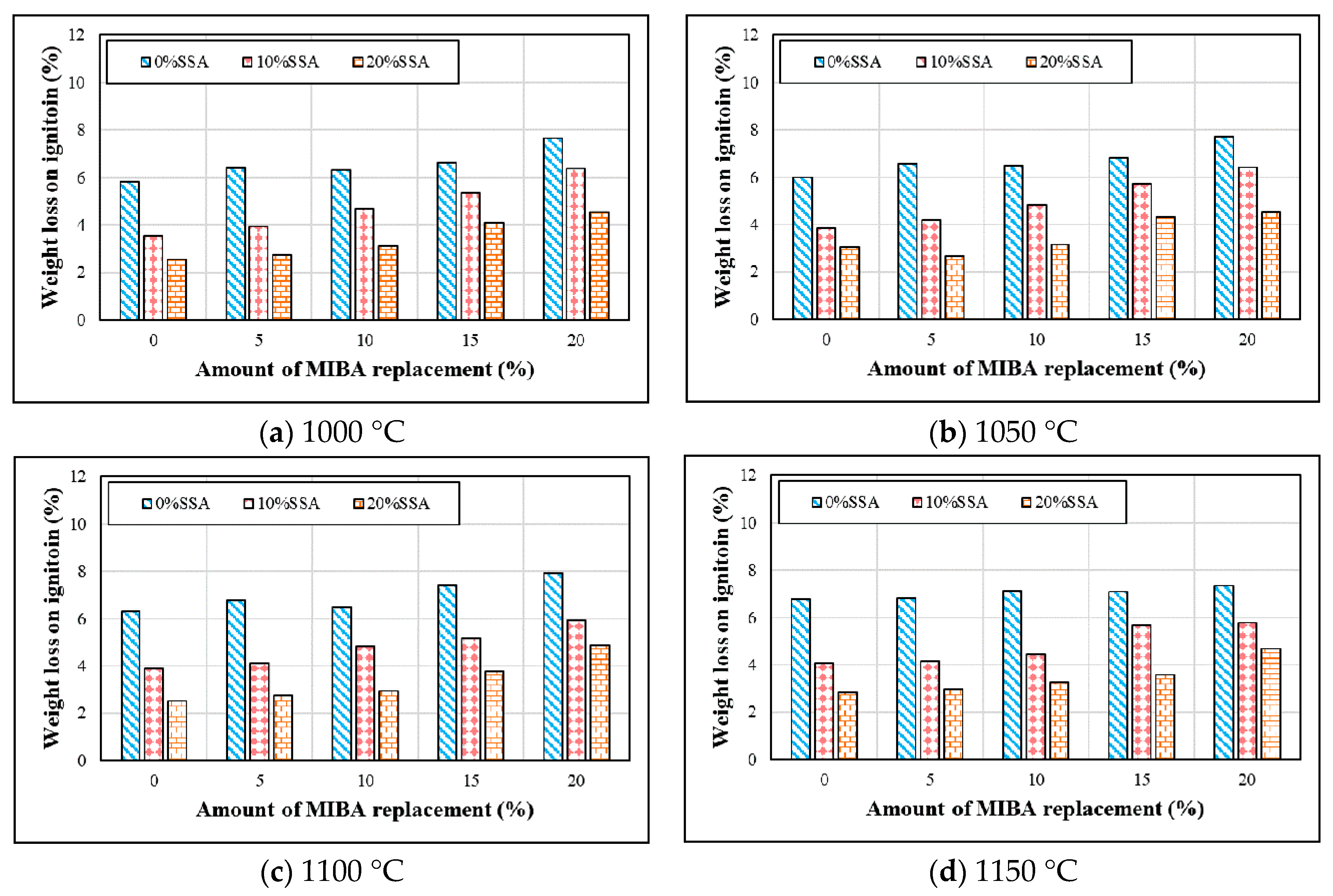
3.3. Weight Loss on Ignition
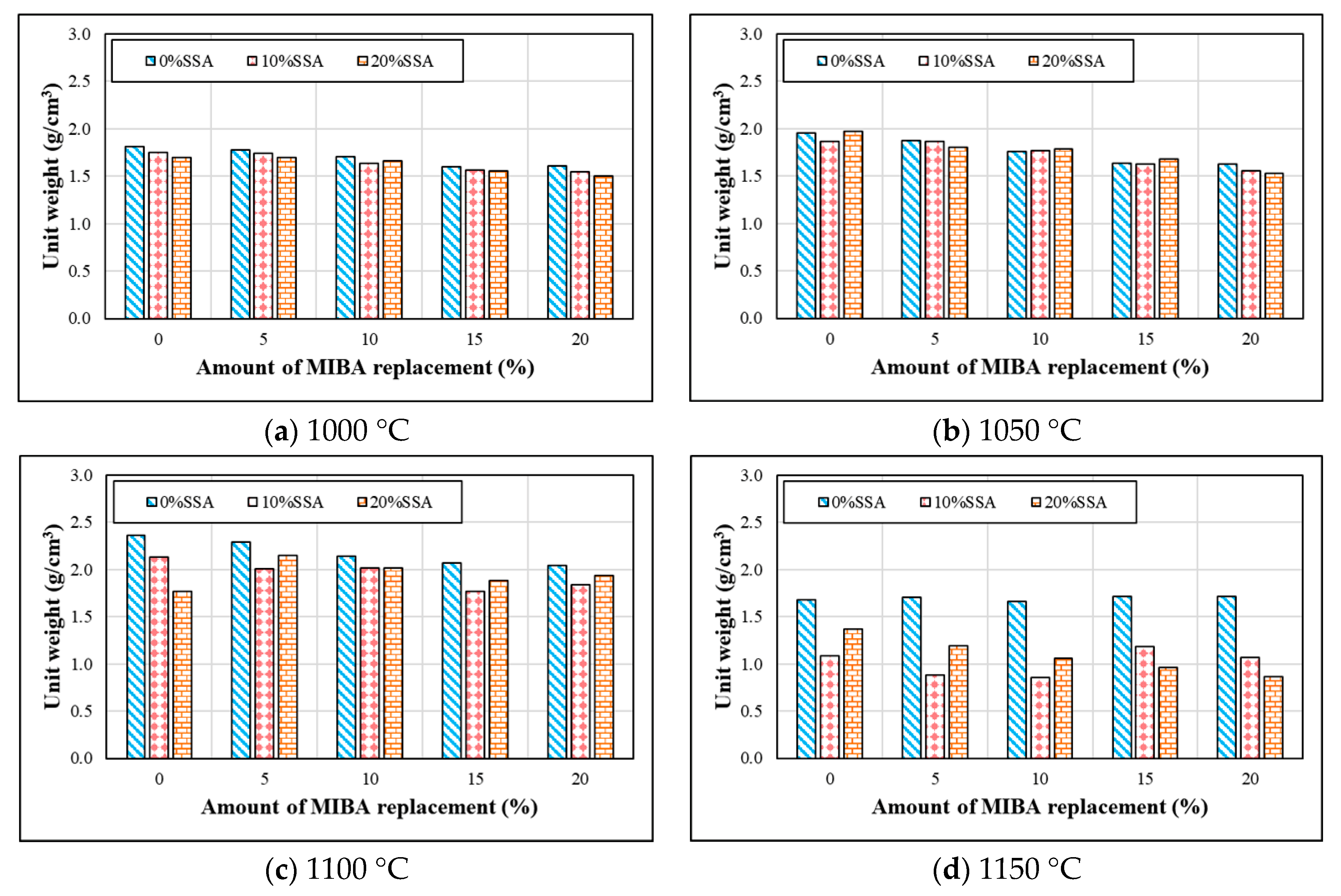
3.4. Specific Gravity
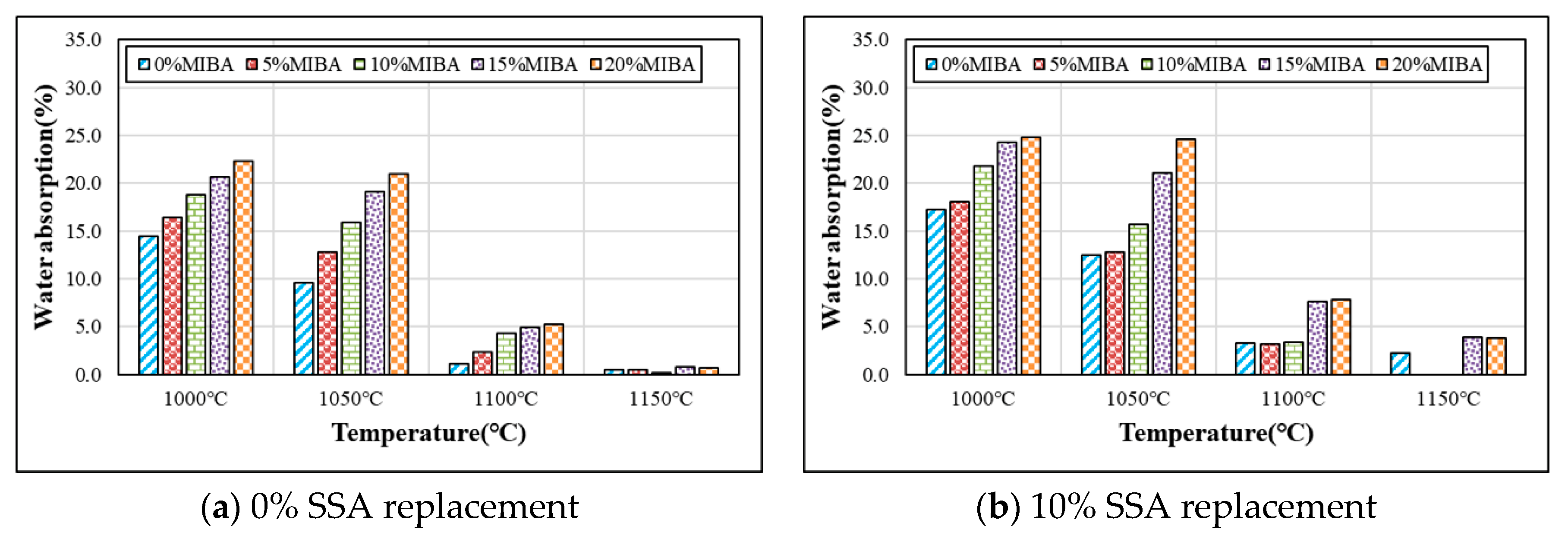
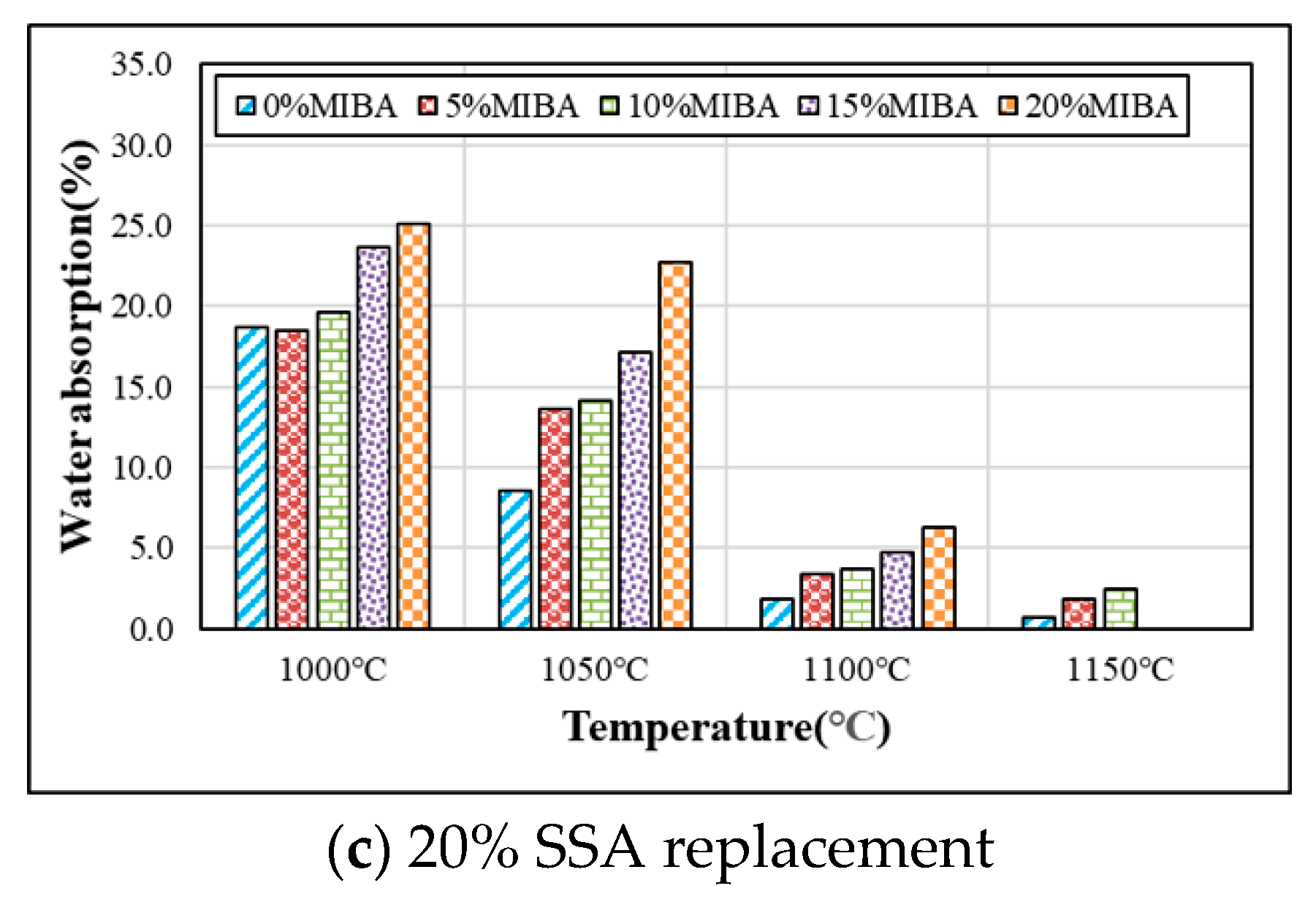
3.5. Water Absorption
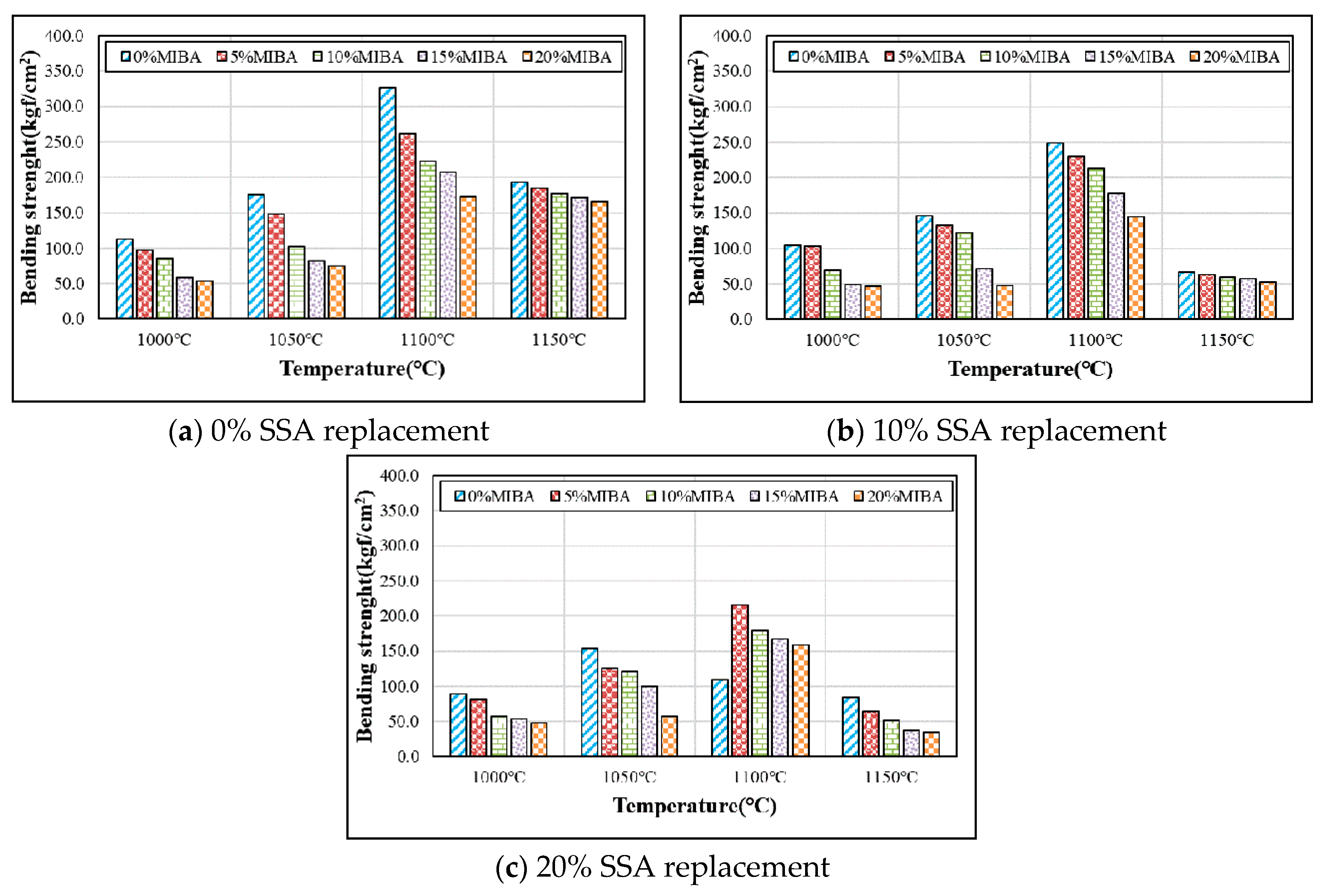
3.6. Bending Strength
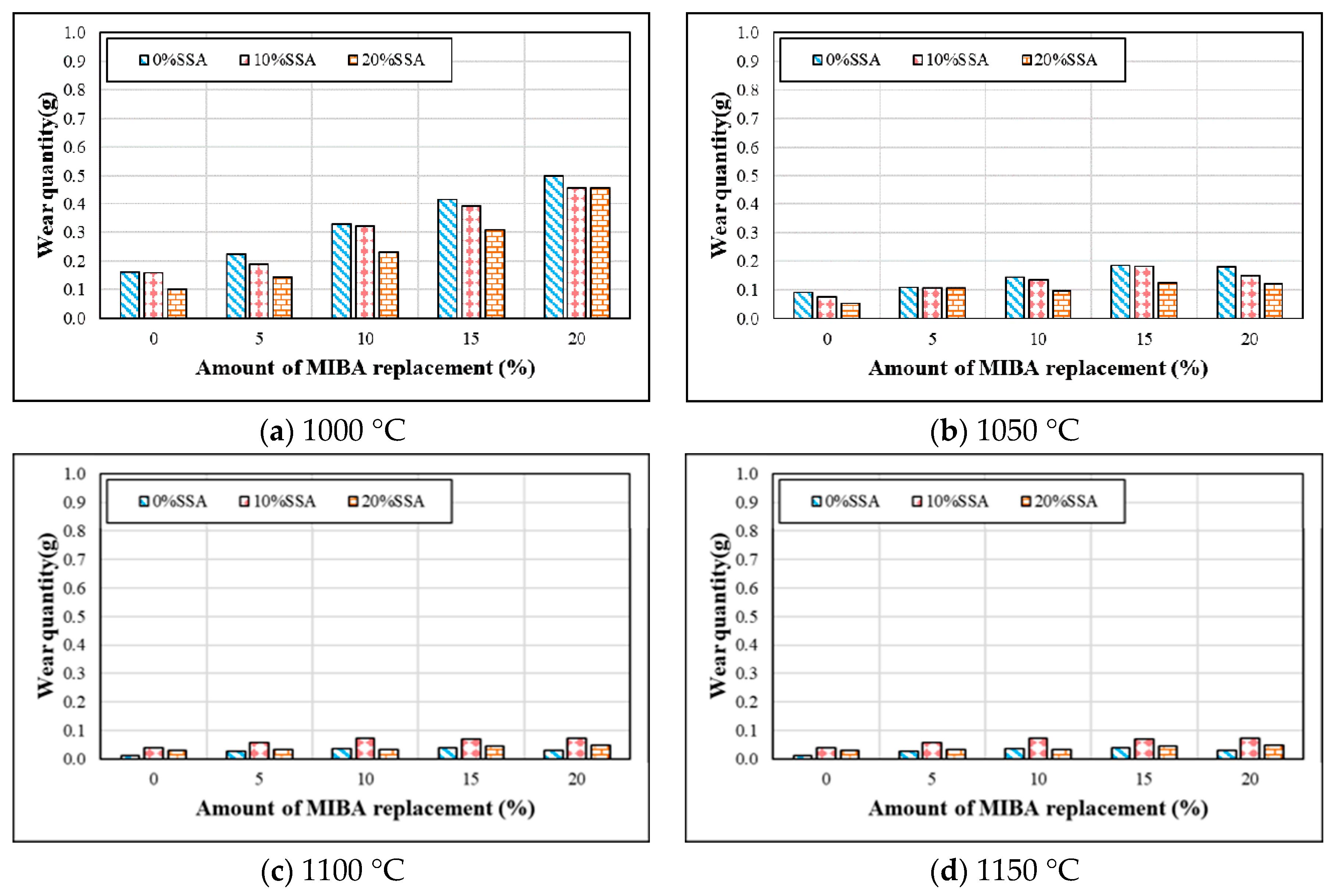
3.7. Wear Resistance
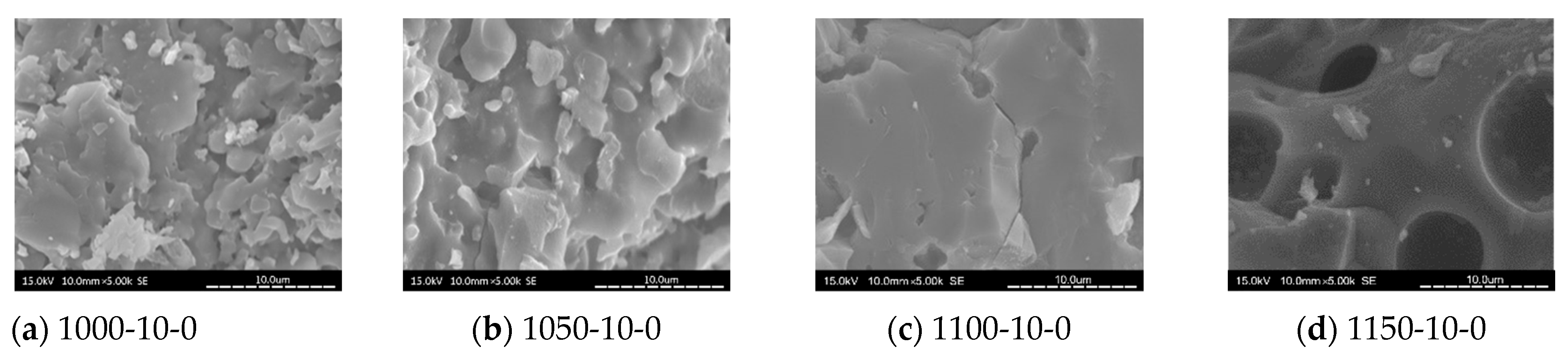
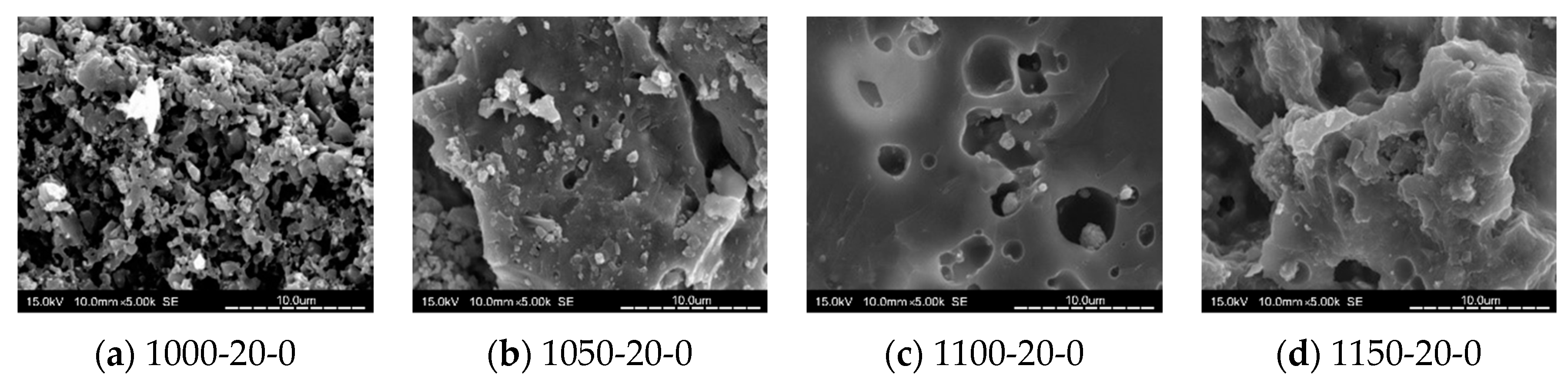
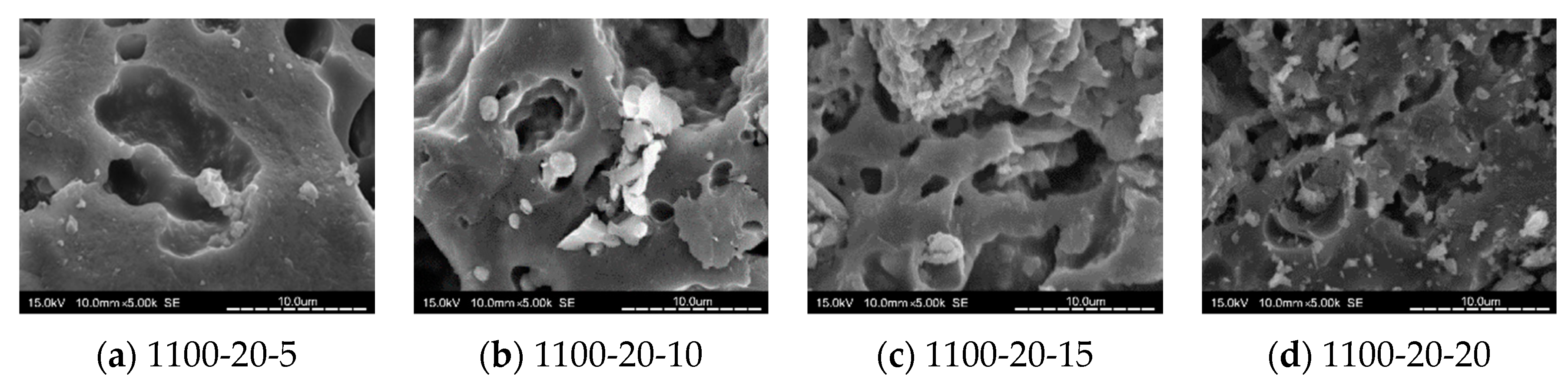
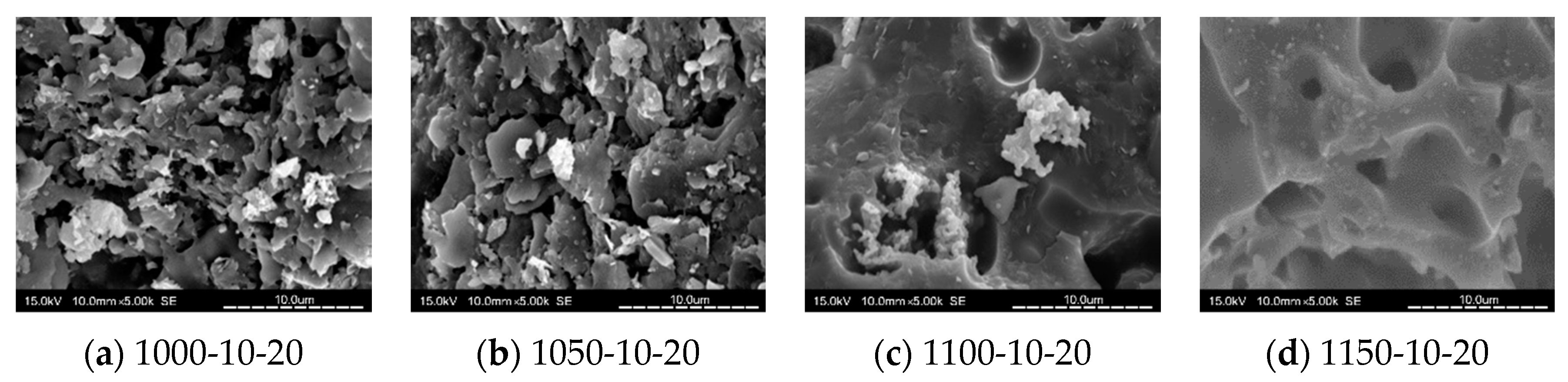
3.8. SEM Analysis
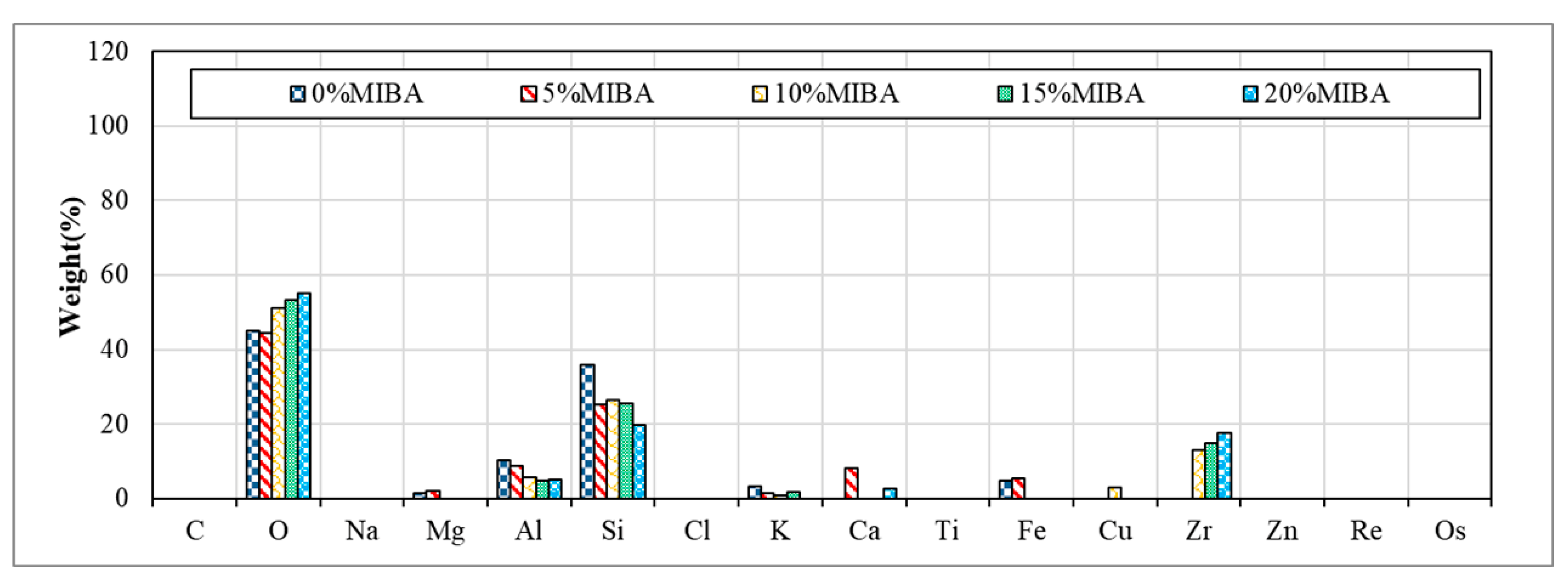
3.9. EDS Analysis
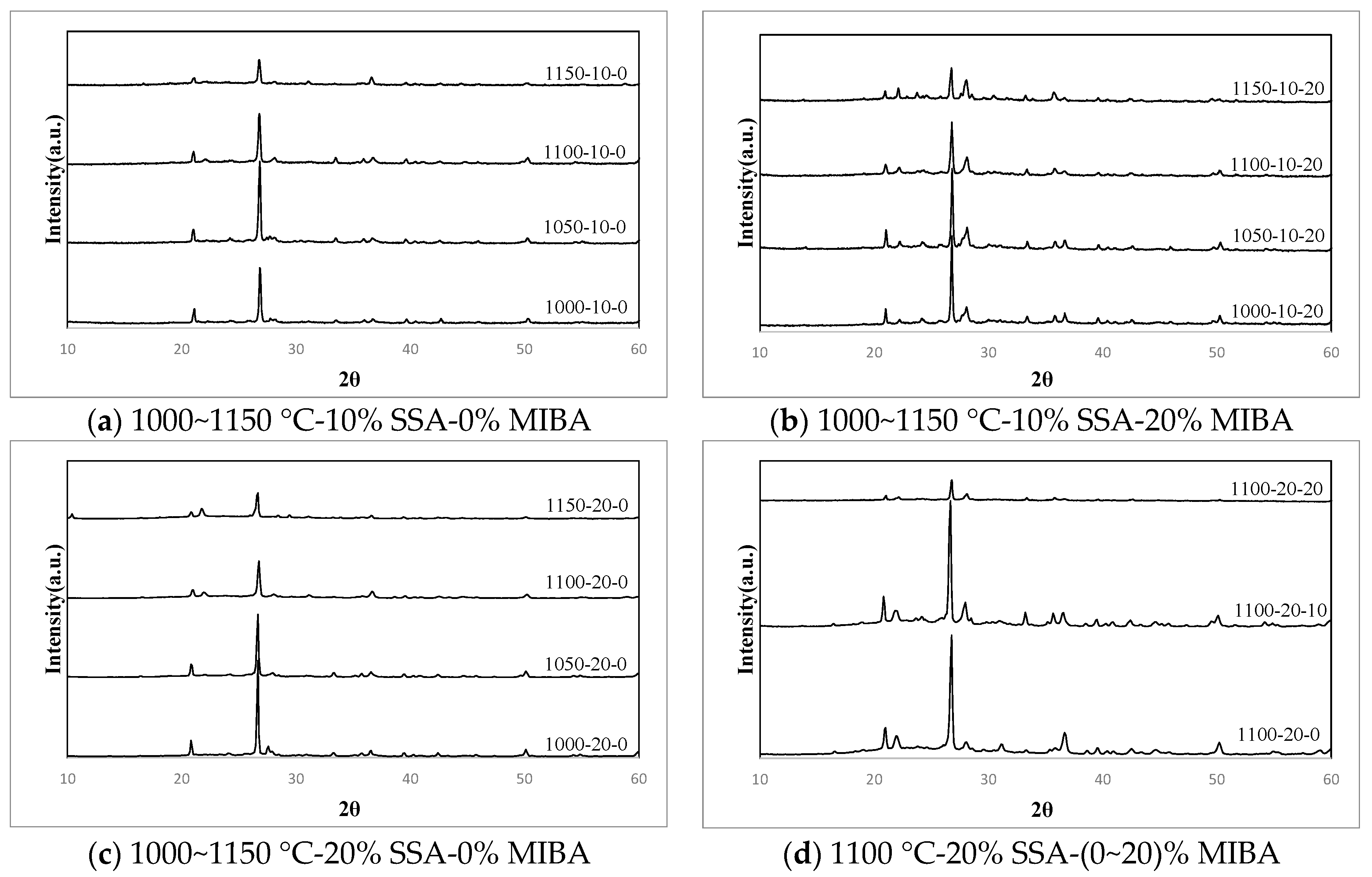
3.10. XRD Analysis
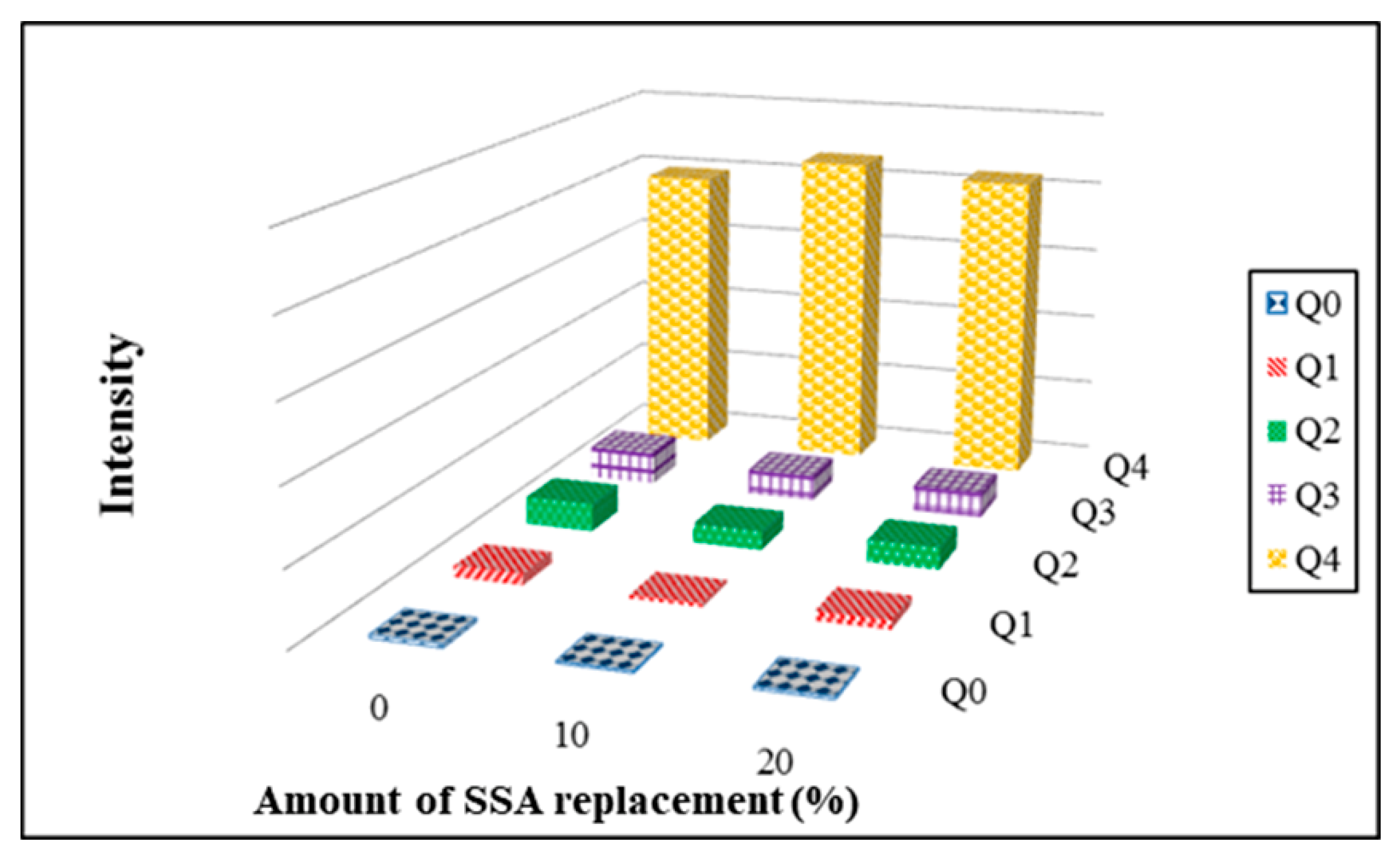
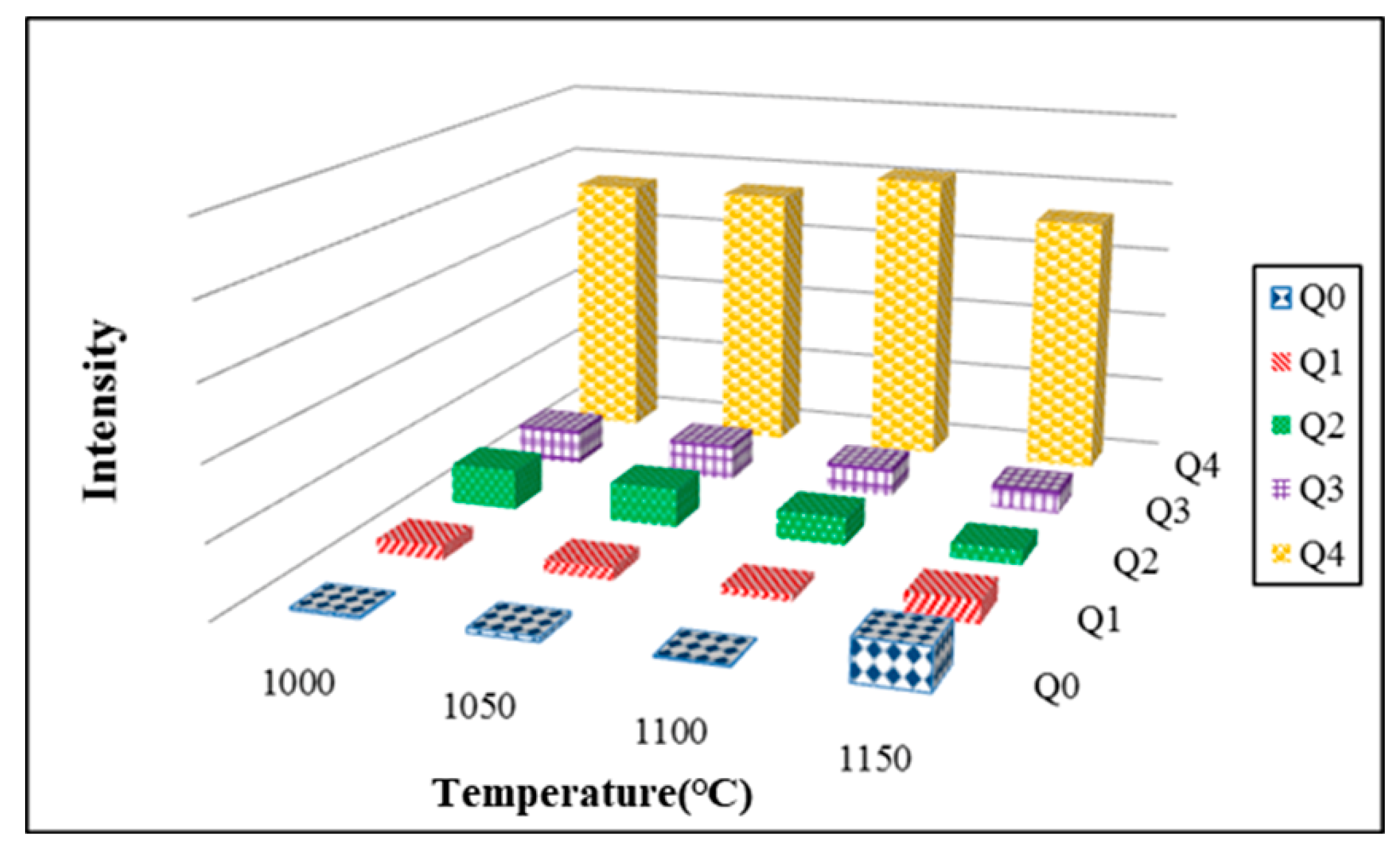
3.11. Si-NMR Analysis
3.12. Quality Classification
4. Conclusions
- The application of MIBA could reduce the shrinkage rate of floor tile specimens. On the other hand, due to the fact that the SSA contained SiO2, the use of SSA could increase the shrinkage rate of floor tile specimens within a kiln temperature of 1000–1050 °C. However, when kiln temperature reached 1150 °C, the glass phase surface formed, the tile bodies were expanded, and the shrinkage rate was reversed.
- Since a large amount of organic, non-organic matters, and heavy metals in MIBA were easily burned at high kiln temperatures, the weight loss on ignition of floor tile specimens increased with more MIBA. In contrast, a large amount of SiO2 in SSA could improve the melting temperature of tile specimens and was hard to be burnt out; the weight loss on ignition of tile specimens increased with more SSA.
- In general, the water absorption of tile specimens increased with increasing amounts of MIBA and SSA, but the water absorption reduced with the increasing kiln temperature. When the kiln temperature reached 1150 °C, the tile surface became vitreous and shiny, and it prevented water penetration, so that the water absorption was reduced to close zero.
- The bending strength of floor tile specimens reduced with more MIBA and SSA replacements. Moreover, the tile bending strength increased with increasing kiln temperature within the range of 1000–1100 °C and would achieve the maximum strength at 1100 °C. However, when the kiln temperature reached 1150 °C, foam was produced in the tile specimens and so forth, resulting in the reduction of bending strength.
- Tile wear increased with more MIBA replacement, while the tile wear decreased with more SSA replacement at kiln temperatures of 1000 and 1050 °C. The amount of wear increased first and then reduced with more SSA replacement at 1100 °C. However, when the kiln temperature reached 1150 °C, the amount of tile wear increased. The amount of tile wear for tile specimens increased with more SSA and MIBA replacements at 1150 °C.
- When the kiln temperature was considered as the main parameter, in general, the compactness of the tile body was improved by the increasing kiln temperature. At a kiln temperature of 1100 °C, the amount of pores increased with more MIBA and SSA replacements, and more pores were observed in the tile bodies from SEM images.
- The results obtained from Si-NMR analysis show that the peak values for Q4 became apparent with increasing kiln temperature. It suggests that the increase of kiln temperature helped Si atoms improve the development of the silicate structure. The values of Q4 after integration increased first and then reduced with increasing kiln temperature. The highest value of Q4 was obtained at 1100 °C. It suggests that the kiln temperature of 1100 °C helped improve the development of a SiO4 tetrahedral structure. This result conformed with that obtained from bending strength tests.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Huang, C.; Yang, W.; Ma, H.; Song, Y. The potential of recycling and reusing municipal solid waste incinerator ash in Taiwan. Waste Manag. 2006, 26, 979–987. [Google Scholar] [CrossRef]
- Lin, K.-L. Effects of the Basicity on the Comelting Conditions of Municipal Solid Waste Incinerator Fly Ash and Sewage Sludge Ash. J. Air Waste Manag. Assoc. 2006, 56, 1743–1749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crillesen, K.; Skaarup, J.; Bojsen, K. International Solid Waste Association: Management of Bottom ash from WTE Plants—An Overview of Management Options and Treatment Methods, ISWA. 2006. Available online: https://www.iswa.org/uploads/tx_iswaknowledgebase/Bottom_ash_from_WTE_2006_01.pdf (accessed on 2 May 2021).
- Acceptabilité Environnementale de Matériaux Alternatifs Entechnique Routière—Les Mâchefers D’incinération de Déchets non Dangereux (MIDND), Sétra, Ministère de l’Ecologie. 2006. Available online: http://www.bretagne.developpement-durable.gouv.fr/IMG/pdf/2016-01_guide_setra_materiaux_de_deonstruction_du_btp.pdf (accessed on 2 May 2021).
- Ciarán, J.L.; Ravindra, K.D.; Gurmel, S. Ghataora, Sewage sludge ash characteristics and potential for use in bricks, tiles and glass ceramics. Water Sci. Technol. 2016, 74, 17–29. Available online: https://iwaponline.com/wst/article/74/1/17/19134/Sewage-sludge-ash-characteristics-and-potential (accessed on 2 May 2021).
- Collivignarelli, M.C.; Cillari, G.; Ricciardi, P.; Carnevale Miino, M.; Torretta, V.; Rada, E.C.; Abbà, A. The Production of Sustainable Concrete with the Use of Alternative Aggregates: A Review. Sustainability 2020, 12, 7903. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Cillari, G.; Ricciardi, R. A review on alternative binders, admixtures and water for the production of sustainable concrete. J. Clean. Prod. 2021, 295, 126408. [Google Scholar] [CrossRef]
- Saikia, N.; Mertens, G.; Van Balen, K.; Elsen, J.; Van Gerven, T.; Vandecasteele, C. Pre-Treatment of municipal solid waste incineration (MSWI) bottom ash for utilisation in cement mortar. Constr. Build. Mater. 2015, 96, 76–85. [Google Scholar] [CrossRef]
- Cheng, A. Effect of incinerator bottom ash properties on mechanical and pore size of blended cement mortars. Mater. Des. 2012, 36, 859–864. [Google Scholar] [CrossRef]
- Sorlini, S.; Abbà, A.; Collivignarelli, C. Recovery of MSWI and soil washing residues as concrete aggregates. Waste Manag. 2011, 31, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Manufacture of artificial aggregate using MSWI bottom ash. Waste Manag. 2011, 31, 281–288. [Google Scholar] [CrossRef]
- Van der Wegen, G.; Hofstra, U.; Speerstra, J. Upgraded MSWI Bottom Ash as Aggregate in Concrete. Waste Biomass Valoriz. 2013, 4, 737–743. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Sorlini, S.; Bruggi, M. Evaluation of concrete production with solid residues obtained from fluidized-bed incineration of MSW-derived solid recovered fuel (SRF). J. Mater. Cycles Waste Manag. 2017, 19, 1374–1383. [Google Scholar] [CrossRef]
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S.; West, R.P. Sewage sludge ash characteristics and potential for use in concrete. Constr. Build. Mater. 2015, 98, 767–779. [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. The potential of raw sewage sludge in construction industry—A review. J. Clean. Prod. 2018, 200, 342–356. [Google Scholar] [CrossRef]
- Cheeseman, C.R.; Rocha, S.; Monteiro, D.; Sollars, C.; Bethanis, S.; Boccaccini, A.R. Ceramic processing of incinerator bottom ash. Waste Manag. 2003, 23, 907–916. [Google Scholar] [CrossRef]
- Holmes, N.; O’Malley, C.P.; Mullen, H.; Keane, G. Performance of masonry blocks containing different proportions of incinerator bottom ash. Sustain. Mater. Technol. 2016, 8, 14–19. [Google Scholar] [CrossRef]
- Bourtsalas, A.; Vandeperre, L.J.; Grimes, S.M.; Themelis, N.; Cheeseman, C.R. Production of pyroxene ceramics from the fine fraction of incinerator bottom ash. Waste Manag. 2014, 45, 217–225. [Google Scholar] [CrossRef]
- Schabbach, L.M.; Andreola, F.; Barbieri, L.; Lancellotti, I.; Karamanova, E.; Ranguelov, B.; Karamanov, A. Post-treated incinerator bottom ash as alternative raw material for ceramic manufacturing. J. Eur. Ceram. Soc. 2012, 32, 2843–2852. [Google Scholar] [CrossRef]
- Endo, H.; Nagayoshi, Y.; Suzuki, K. Production of glass ceramics from sewage sludge. Water Sci. Technol. 1997, 36, 235–241. [Google Scholar] [CrossRef]
- Lin, D.F.; Weng, C.H. Use of sewage sludge ash as brick material. J. Environ. Eng. 2001, 127, 922–927. [Google Scholar] [CrossRef]
- Anderson, M. Encouraging prospects for recycling incinerated sewage sludge ash (ISSA) into clay-based building products. J. Chem. Technol. Biotechnol. 2002, 77, 352–360. [Google Scholar] [CrossRef]
- Lin, D.F.; Luo, H.L.; Sheen, Y.N. Glazed tiles manufactured from incinerated sludge ash and clay. J. Air Waste Manag. Assoc. 2005, 55, 163–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, D.F.; Chang, W.C.; Yuan, C.; Luo, H.L. Production and characterization of glazed tiles containing incinerated sewage sludge. Waste Manag. 2008, 28, 502–508. [Google Scholar] [CrossRef]
- Dayalan, J.; Beulah, M. Glazed sludge tile. Int. J. Eng. Res. Appl. 2014, 4, 201–204. [Google Scholar]
- Wong, C.-Y. Sludge Ash Utilized in Fired Bricks and Its Sintering Properties. Master’s Thesis, Department of Resources Engineering, National Cheng-Kung University, Tainan, Taiwan, 2015. [Google Scholar]
- Amin, S.K.; Abdel Hamid, E.M.; El-Sherbiny, S.A.; SiIBAk, H.A.; AIBAdir, M.F. The use of sewage sludge in the production of ceramic floor tiles. Hbrc J. 2018, 14, 309–315. [Google Scholar] [CrossRef]
- Lin, D.-F.; Luo, H.-L.; Zhang, S.-W. Effects of Nano-SiO2 on tiles manufactured with clay and incinerated sewage sludge ash. J. Mater. Civ. Eng. 2007, 19, 801–808. [Google Scholar] [CrossRef]
- Lin, K.L.; Cheng, C.J. Utilization of Solar Panel Waste Glass and Clay Co-Sintered as Eco-Tile Materials. Taiwan Min. Ind. 2012, 64, 38–48. [Google Scholar]
- Kim, K.; Kim, K.; Hwang, J. Characterization of ceramic tiles containing LCD waste glass. Ceram. Int. 2016, 42, 7626–7631. [Google Scholar] [CrossRef]
- Rozenstrauha, I.; Sosins, G.; Petersone, L.; Krage, L.; Drille, M.; Filipenkov, V. Production of glass-ceramics from sewage sludge and waste glass. In Proceedings of the IOP Conference Series Materials Science and Engineering, Kerala, India, 4–6 August 2011. [Google Scholar]
- Esmeray, E.; Atıs, M. Utilization of sewage sludge, oven slag and fly ash in clay brick production. Constr. Build. Mater. 2019, 194, 110–121. [Google Scholar] [CrossRef]
- CNS (Chinese National Standard, Taiwan). Available online: http://cns-standards.org/ (accessed on 1 May 2021).
- Bethanis, S.; Cheeseman, C.R.; Sollars, C.J. Properties and microstructure of sintered incinerator bottom ash. Ceram. Int. 2002, 28, 881–886. [Google Scholar] [CrossRef]
- Barbieri, L.; Karamanov, A.; Corradi, A.; Lancellotti, I.; Pelino, M.; Rincon, J.M. Structure, chemical durability and crystallization behavior of incinerator-based glassy systems. J. Non Cryst. Solids 2008, 354, 521–528. [Google Scholar] [CrossRef]
- Appendino, P.; Ferraris, M.; Matekovits, I.; Salvo, M. Production of glass–ceramic bodies from the bottom ashes of municipal solid waste incinerators. J. Eur. Ceram. Soc. 2004, 24, 803–810. [Google Scholar] [CrossRef]
- Ferber, N.L.; Minh, D.P.; Falcoz, Q.; Meffre, N.; Tessier-Doyen, N.; Nzihou, A.; Goet, V. Ceramics from municipal waste incinerator bottom ash and wasted clay for sensible heat storage at high temperature. Waste Biomass Valorization 2020, 11, 3107–3120. [Google Scholar] [CrossRef]
- Piribauer, C.; Knodt, R.; Link, S.; Engels, M.; Stern, B.; Braun, N. Humic Substances as Additives in Ceramic Clays and Bodies. Ceram. Forum Int. 2020, 97, 36–42. [Google Scholar]
- Amin, M.; Supriyatna, Y.I.; Hendro, Y.; Birawidha, D.C. Addition of feldspart minerals as a substitution material on ceramics clay manufacture. Iop Conf. Ser. Mater. Sci. Eng. 2019, 478, 012038. [Google Scholar] [CrossRef]
- Simić, V.; Jović, V.; Djurić, S. Geology and Mineralogy of Ceramic Clay Deposits in Western Serbia. Geolegica Carpathica Clays 1997, 6, 57–60. [Google Scholar]
- Kobayashi, K.; Koyanagi, W. 17-Year-Long Sewage Sludge Ash Concrete Exposure Test. Infrastructures 2021, 6, 74. Available online: https://www.researchgate.net/publication/351508159_17-Year-Long_Sewage_Sludge_Ash_Concrete_Exposure_Test (accessed on 30 April 2021). [CrossRef]
- Chin, S.C.; Ing, D.S.; Kusbiantoro, A.; Wan Ahmad, S. Characterization of sewage sludge ASH (SSA) in cement mortar. J. Eng. Appl. Sci. 2016, 11, 2242–2247. [Google Scholar]
- Tantawy, M.A.; El-Roudi, A.M.; Abdalla, E.M.; Abdelzaher, M.A. Evaluation of the Pozzolanic Activity of Sewage Sludge Ash. Isrn Chem. Eng. 2012, 8. Available online: https://www.researchgate.net/publication/258403899_Evaluation_of_the_Pozzolanic_Activity_of_Sewage_Sludge_Ash (accessed on 30 April 2021). [CrossRef]
- Chen, C.-H.; Chiou, I.-J.; Wang, K.-S. Sintering effect on cement bonded sewage sludge ash. Cem. Concr. Compos. 2006, 28, 26–32. [Google Scholar] [CrossRef]
- Environmental Protection Administration (Taiwan), Standards for Defining Hazardous Industrial Waste. 2021. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=O0050023 (accessed on 30 April 2021).
- Cheeseman, C.R.; Sollars, C.J.; McEntee, S. Properties, microstructure and leaching of sintered sewage sludge ash. Resour. Conserv. Recycl. 2003, 40, 13–25. [Google Scholar] [CrossRef]
- Bijen, J.M. Manufacturing processes of artificial lightweight aggregates from fly ash. Int. J. Cem. Compos. Lightweight Concr. 1986, 8, 191–199. [Google Scholar] [CrossRef]
- Adell, V.; Cheeseman, C.R.; Ferraris, M.; Salvo, M.; Smeacetto, F.; Boccacini, A.R. Characterising the sintering behaviour of pulverised fuel ash using heating stage microscopy. Mater. Charact. 2007, 58, 980–988. [Google Scholar] [CrossRef]
- Acar, I.; Atalay, M.U. Characterization of sintered class F fly ashes. Fuel 2013, 106, 195–203. [Google Scholar] [CrossRef]
- CNS Online (Chinese National Standards, Taiwan). Available online: https://www.cnsonline.com.tw/ (accessed on 1 May 2021).

| Clay | MIBA | SSA | Standard * | |
|---|---|---|---|---|
| Specific gravity | 2.65 | 2.41 | 2.00 | CNS 5090 |
| Unit weight (kg/m3) | 1251.83 | 1004.62 | 698.45 | CNS 1183 |
| Pore ratio (%) | 52.84 | 58.33 | 65.14 | CNS 1163 |
| Specific surface area (cm2/g) | 2562.48 | 1276.41 | 3441.27 | CNS 2924 |
| Element | C | O | Na | Al | Si | P | S | Cl | K | Ca | Fe | Br | Zr | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clay% | - | 49.5 | - | 10.2 | 27.2 | - | - | - | 3.43 | - | 4.25 | - | 5.47 | - |
| MIBA% | 8.66 | 43.5 | 8.67 | - | 1.34 | 11.6 | 1.77 | 1.45 | - | 21.2 | - | 1.88 | - | - |
| SSA% | - | 43 | - | 9.28 | 36.9 | - | - | - | - | - | - | - | 6.79 | 3.41 |
| Element | Cu | Ba | Cd | As | Pb | Cr | Hg | Cr6+ | Se |
|---|---|---|---|---|---|---|---|---|---|
| SSA(mg/L) | 4 | <0.2 | ND | ND | ND | ND | ND | ND | ND |
| MIBA(mg/L) | 1.57 | 0.658 | <0.100 | ND | ND | ND | ND | ND | ND |
| Standard | ≤12.0 | ≤10.0 | ≤4.0 | ≤0.4 | ≤4.0 | ≤0.8 | ≤0.016 | ≤0.2 | ≤0.8 |
| SSA% | Temperature | MIBA % | SiO2 | AlPO4 | CaSiO3 | MgSiO3 | Ca(Al2Si2O8) | K(AlSi3O8) |
|---|---|---|---|---|---|---|---|---|
| 10% | 1000 °C | 0% | 47.1 | 1.3 | 5.6 | 18.0 | 16.8 | 11.3 |
| 10% | 51.9 | 1.7 | 5.7 | 17.7 | 16.1 | 6.8 | ||
| 20% | 40.7 | 0.5 | 6.3 | 27.6 | 19.6 | 5.2 | ||
| 1050 °C | 0% | 56.3 | 0.3 | 8.1 | 8.7 | 21.3 | 5.2 | |
| 10% | 53.4 | 1.2 | - | 26.1 | 19.3 | - | ||
| 20% | 42.9 | 1.0 | - | 16.7 | 33.7 | 5.8 | ||
| 1100 °C | 0% | 97.2 | 2.8 | - | - | - | - | |
| 10% | 62.0 | - | - | - | 38.0 | - | ||
| 20% | 51.3 | - | - | - | 48.7 | - | ||
| 1150 °C | 0% | 54.5 | 2.4 | - | 22.1 | 11.5 | 9.5 | |
| 10% | 45.1 | 0.4 | - | 30.9 | 15.9 | 7.7 | ||
| 20% | 33.9 | 16.5 | - | - | 49.6 | - | ||
| 20% | 1000 °C | 0% | 88.3 | 0.3 | 11.0 | 0.4 | - | - |
| 10% | 48.8 | 1.0 | 13.3 | 18.2 | 18.8 | - | ||
| 20% | 57.0 | 1.2 | - | - | 41.7 | - | ||
| 1050 °C | 0% | 87.3 | 0.9 | 8.6 | 3.2 | - | - | |
| 10% | 56.6 | 0.9 | 11.6 | 11.2 | 19.7 | - | ||
| 20% | 52.5 | 0.0 | - | 15.6 | 28.0 | 3.9 | ||
| 1100 °C | 0% | 66.9 | 1.2 | - | 19.2 | 12.8 | - | |
| 10% | 48.8 | 1.4 | - | 28.2 | 21.7 | - | ||
| 20% | 32.0 | 11.0 | 6.2 | 26.2 | 24.7 | - | ||
| 1150 °C | 0% | 55.8 | 1.0 | - | 24.7 | 18.5 | - | |
| 10% | 33.4 | - | - | 33.4 | 33.2 | - | ||
| 20% | 24.1 | 0.5 | 10.6 | 38.6 | 26.2 | - |
| Material | Temperature (°C) | Judgment Criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Interior Floor Tile | Exterior Floor Tile | Water Absorption (%) | |||||||||
| Clay (%) | SSA (%) | MIBA (%) | Bending Strength | Size Shrinkage | Bending Strength | Size Shrinkage | Ia | Ib | II | III | |
| 100 | 0 | 0 | 1000 | ○ | × | × | × | × | × | × | ○ |
| 1050 | ○ | × | ○ | ○ | × | × | ○ | ○ | |||
| 1100 | ○ | × | ○ | × | × | ○ | ○ | ○ | |||
| 1150 | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | |||
| 95 | 0 | 5 | 1000 | ○ | × | × | × | × | × | × | ○ |
| 1050 | ○ | × | ○ | ○ | × | × | × | ○ | |||
| 1100 | ○ | ○ | ○ | ○ | × | ○ | ○ | ○ | |||
| 1150 | ○ | × | ○ | ○ | × | ○ | ○ | ○ | |||
| 90 | 0 | 10 | 1000 | ○ | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | × | × | × | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | ○ | ○ | ○ | ○ | ○ | ○ | |||
| 85 | 0 | 15 | 1000 | × | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | ○ | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | ○ | ○ | × | ○ | ○ | ○ | |||
| 90 | 10 | 0 | 1000 | ○ | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | × | ○ | × | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | ○ | ○ | × | × | ○ | ○ | ○ | |||
| 85 | 10 | 5 | 1000 | ○ | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | × | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | ||||||
| 80 | 10 | 10 | 1000 | × | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | × | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | ||||||
| 75 | 10 | 15 | 1000 | × | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | × | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | × | ○ | ○ | |||
| 70 | 10 | 20 | 1000 | × | × | × | ○ | × | × | × | ○ |
| 1050 | × | × | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | × | ○ | ○ | |||
| 80 | 20 | 0 | 1000 | ○ | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | ○ | ○ | ○ | × | × | ○ | ○ | |||
| 1100 | ○ | × | ○ | × | × | ○ | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | ○ | ○ | ○ | |||
| 75 | 20 | 5 | 1000 | ○ | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | × | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | ○ | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | ○ | ○ | ○ | |||
| 70 | 20 | 10 | 1000 | × | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | × | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | ○ | ○ | ○ | |||
| 65 | 20 | 15 | 1000 | × | × | × | ○ | × | × | × | ○ |
| 1050 | ○ | × | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | ||||||
| 60 | 20 | 20 | 1000 | × | × | × | ○ | × | × | × | ○ |
| 1050 | × | × | × | ○ | × | × | × | ○ | |||
| 1100 | ○ | × | ○ | × | × | × | ○ | ○ | |||
| 1150 | ○ | × | × | ○ | × | ||||||
| Material | Temperature (°C) | Applications (Interior Floor Tile/Exterior Floor Tile) | Water Absorption (Ia, Ib, II, III) | ||
|---|---|---|---|---|---|
| Clay (%) | SSA (%) | MIBA (%) | |||
| 100 | 0 | 0 | 1050 | Exterior floor tile | II, III |
| 1150 | Exterior floor tile | Ia, Ib, II, III | |||
| 95 | 0 | 5 | 1050 | Exterior floor tile | III |
| 1100 | Interior floor tile/Exterior floor tile | Ib, II, III | |||
| 1150 | Exterior floor tile | Ib, II, III | |||
| 90 | 0 | 10 | 1150 | Exterior floor tile | Ia, Ib, II, III |
| 85 | 0 | 15 | 1050 | Interior floor tile | III |
| 1150 | Exterior floor tile | Ib, II, III | |||
| 90 | 10 | 0 | 1150 | Interior floor tile | Ib, II, III |
| 80 | 20 | 0 | 1050 | Interior floor tile/Exterior floor tile | II, III |
| 75 | 20 | 5 | 1100 | Interior floor tile | II, III |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.-F.; Wang, W.-J.; Chen, C.-W.; Lin, K.-L. Applying Mixture of Municipal Incinerator Bottom Ash and Sewage Sludge Ash for Ceramic Tile Manufacturing. Materials 2021, 14, 3863. https://doi.org/10.3390/ma14143863
Lin D-F, Wang W-J, Chen C-W, Lin K-L. Applying Mixture of Municipal Incinerator Bottom Ash and Sewage Sludge Ash for Ceramic Tile Manufacturing. Materials. 2021; 14(14):3863. https://doi.org/10.3390/ma14143863
Chicago/Turabian StyleLin, Deng-Fong, Wei-Jhu Wang, Chia-Wen Chen, and Kuo-Liang Lin. 2021. "Applying Mixture of Municipal Incinerator Bottom Ash and Sewage Sludge Ash for Ceramic Tile Manufacturing" Materials 14, no. 14: 3863. https://doi.org/10.3390/ma14143863
APA StyleLin, D.-F., Wang, W.-J., Chen, C.-W., & Lin, K.-L. (2021). Applying Mixture of Municipal Incinerator Bottom Ash and Sewage Sludge Ash for Ceramic Tile Manufacturing. Materials, 14(14), 3863. https://doi.org/10.3390/ma14143863





