Preparation and Characterization of Physicochemical Properties of Spruce Cone Biochars Activated by CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Biochars
- One-stage pyrolysis: raw material was heated from room temperature to 800 °C (heating rate: 5 °C min−1) in the nitrogen atmosphere (gas flow: 20 dm3 h−1). The material was kept at this temperature for 3 h. The obtained carbon was designated as BC-1.
- Three-step pyrolysis: the material was heated from room temperature to 180 °C (heating rate: 2 °C min−1). The precursor was kept at the temperature of 180 °C for 5 h. Then with the temperature rise rate of 5 °C min−1, the material was heated to 400 °C and kept for 1 h. The final temperature (obtained at the heating rate: 5 °C min−1), at which the material was soaked for 3 h, was 850 °C. The process proceeded in the nitrogen atmosphere at the rate of 20 dm3 h−1. The obtained carbon was designated as BC-2.
- The quartz boats with the sample BC-1 were placed in the furnace and heated from room temperature to 850 °C (heating rate: 10 °C min−1) in the nitrogen atmosphere (20 dm3 h−1). Then the activation process was conducted at this temperature by CO2 (99.998%) for 6 h (CO2 flow rate: 6 dm3 h−1). The cooling process was conducted in the nitrogen atmosphere (20 dm3 h−1). The obtained carbon was designated as BC-1-CO2-6h.
- The quartz boats with the sample BC-1 were placed in the furnace and heated from room temperature to 850 °C (heating rate: 10 °C min−1) in the nitrogen atmosphere (20 dm3 h−1). Then the activation process was carried out at this temperature by CO2 (99.995%) for 6 h or 12 h (CO2 flow rate: 6 dm3 h−1). The cooling process took place in the nitrogen atmosphere (20 dm3 h−1). The obtained carbons were designated as BC-1-CO2-6h* and BC-1-CO2-12h*.
- The quartz boats with the sample BC-2 were placed in the furnace and heated from room temperature to 850 °C (heating rate: 10 °C min−1) in the nitrogen atmosphere (20 dm3 h−1). Then the activation process was conducted at this temperature by CO2 (99.998%) for 6 h (CO2 flow rate: 6 dm3 h−1). The cooling process was conducted in the nitrogen atmosphere (20 dm3 h−1). The obtained carbon was designated as BC-2-CO2-6h.
2.3. Characterization of Biochars
2.4. Pulse Chemisorption and Temperature-Programmed Desorption (TPD) of Ammonia
3. Results and Discussion
3.1. Characterization of Biochars
3.1.1. Porous Structure of Biochars
3.1.2. SEM/EDS Analysis
3.1.3. ED-XRF Analysis
3.1.4. Thermal Analysis
3.1.5. ATR-FTIR Analysis
3.1.6. Raman Analysis
3.1.7. Boehm’s Titration Method
- (1)
- Carboxylic-affected by NaHCO3.
- (2)
- Carboxyl + lactone-affected by Na2CO3.
- (3)
- Carboxylic + lactone + phenolic-affected by NaOH.
- (4)
- Carboxylic + lactone + phenolic + carbonyl-affected by C2H5ONa.
3.1.8. The Point of Zero Charge (pHpzc)
3.2. Pulse Chemisorption and Temperature-Programmed Desorption (TPD) of Ammonia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gwenzi, W.; Chaukura, N.; Wenga, T.; Mtisi, M. Biochars as media for air pollution control systems: Contaminant removal, applications and future research directions. Sci. Total Environ. 2021, 753, 142249–142270. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Choi, T.R.; Gurav, R.; Bhatia, S.K.; Park, J.L.; Kim, H.J.; Kan, E.; Yang, J.H. Adsorption behavior of tetracycline onto Spirulina sp. (microalgae)-derived biochars produced at different temperatures. Sci. Total Environ. 2020, 710, 136282–136292. [Google Scholar] [CrossRef]
- Choi, J.K.; Gurav, R.; Kim, H.J.; Yang, J.-H.; Bhatia, S.K. Evaluation for simultaneous removal of anionic and cationic dyes onto maple leaf-derived biochar using response surface methodology. Appl. Sci. 2020, 10, 2982. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Choi, Y.-K.; Kim, H.J.; Song, H.-S.; Lee, S.M.; Park, S.L.; Lee, H.S.; Koh, J.; et al. Application of macroalgal biomass derived biochar and bioelectrochemical system with Shewanella for the adsorptive removal and biodegradation of toxic azo dye. Chemosphere 2021, 264, 128539–128576. [Google Scholar] [CrossRef] [PubMed]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464–110491. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Kim, H.J.; Yang, S.Y.; Song, H.S.; Park, J.Y.; Park, Y.L.; Han, Y.H.; Choi, Y.K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872–122877. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Park, J.-H.; Wang, J.J.; Kim, S.-H.; Kang, S.-W.; Jeong, C.Y.; Jeon, J.-R.; Park, K.H.; Cho, J.-S.; Delaune, R.D.; Seo, D.-C. Cadmium adsorption characteristics of biochars derived using various pine tree residues and pyrolysis temperatures. J. Colloid Interface Sci. 2019, 553, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kołtowski, M.; Hilber, I.; Bucheli, T.D.; Charmas, B.; Skubiszewska-Zięba, J.; Oleszczuk, P. Activated biochars reduce the exposure of polycyclic aromatic hydrocarbons in industrially contaminated soils. Chem. Eng. J. 2017, 310, 33–40. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, Q.; Shen, B. Application of biochar and its composites in catalysis. Chemosphere 2020, 240, 124842–124852. [Google Scholar] [CrossRef]
- Cho, D.W.; Kim, S.; Tsang, Y.F.; Song, H. Preparation of nitrogen-doped Cu-biochar and its application into catalytic reduction of p-nitrophenol. Environ. Geochem. Health 2019, 41, 1729–1737. [Google Scholar] [CrossRef]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Břendová, K.; Száková, J.; Lhotka, M.; Krulikovská, T.; Punčochář, M.; Tlustoš, P. Biochar physicochemical parameters as a result of feedstock material and pyrolysis temperature: Predictable for the fate of biochar in soil? Environ. Geochem. Health 2017, 39, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Kanouo, B.M.D.; Allaire, S.E.; Munson, A.D. Quality of biochars made from eucalyptus tree bark and corncob using a pilot-scale retort kiln. Waste Biomass Valor. 2018, 9, 899–909. [Google Scholar] [CrossRef]
- Qadeer, S.; Anjum, M.; Khalid, A.; Waqas, M.; Batool, A.; Mahmood, T. A dialogue on perspectives of biochar applications and its environmental risks. Water Air Soil Pollut. 2017, 228, 281–306. [Google Scholar] [CrossRef]
- Saletnik, B.; Zaguła, G.; Grabek-Lejko, D.; Kasprzyk, I.; Bajcar, M.; Czernicka, M.; Puchalski, C. Biosorption of cadmium(II), lead(II) and cobalt(II) from aqueous solution by biochar from cones of larch (Larix decidua Mill. subsp. decidua) and spruce (Picea abies L. H. Karst). Environ. Earth Sci. 2017, 76, 574–584. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Biores. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.S.; Khim, J. Peat moss-derived biochars as effective sorbents for VOCs’ removal in groundwater. Environ. Geochem. Health 2019, 41, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Monsalvo, V.M.; Mohedano, A.F.; Rodriguez, J.J. Activated carbons from sewage sludge. Application to aqueous-phase adsorption of 4-chlorophenol. Desalination 2011, 277, 377–382. [Google Scholar] [CrossRef]
- Özhan, A.; Şahin, Ö.; Küçük, M.M.; Saka, C. Preparation and characterization of activated carbon from pine cone by microwave-induced ZnCl2 activation and its effects on the adsorption of methylene blue. Cellulose 2014, 21, 2457–2467. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Biores. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]
- Zhang, P.; O’Connor, D.; Wang, Y.; Jiang, L.; Xia, T.; Wang, L.; Tsang, D.C.W.; Ok, Y.S.; Hou, D. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286–121293. [Google Scholar] [CrossRef] [PubMed]
- Wathukarage, A.; Herath, I.; Iqbal, M.C.M.; Vithanage, M. Mechanistic understanding of crystal violet dye sorption by woody biochar: Implications for wastewater treatment. Environ. Geochem. Health 2019, 41, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Sallam, A.; Zhang, M.; Vithanage, M.; Ahmad, M.; Al-Farraj, A.; Ok, Y.S.; Abduljabbar, A.; Al-Wabel, M. Sorption process of date palm biochar for aqueous Cd (II) removal: Efficiency and mechanisms. Water Air Soil Pollut. 2016, 227, 449–464. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Utilization of oil palm biodiesel solid residue as renewable sources for preparation of granular activated carbon by microwave induced KOH activation. Biores. Technol. 2013, 130, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Biores. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Xiahou, G.; Zhang, C.; Li, L.; Cen, X.; Li, Z.; Zhou, J. Study on the Kinetics of Barks of Trees Pyrolysis by TG/DTG Analysis. In Challenges of Power Engineering and Environment; Cen, K., Chi, Y., Wang, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 1, pp. 1148–1151. [Google Scholar]
- Kuznetsov, B.N.; Chesnokov, N.V.; Ivanov, I.P.; Kuznetsova, S.A.; Ivanchenko, N.M. Production of porous carbon materials from bark. Solid Fuel Chem. 2015, 49, 278–288. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.H.; Ahn, Y.T.; Chung, J.W. Comparison of heavy metal adsorption by peat moss and peat moss-derived biochar produced under different carbonization conditions. Water Air Soil Pollut. 2015, 226, 9–19. [Google Scholar] [CrossRef]
- Onishchenko, D.V.; Reva, V.P. High-energy treatment of sphagnum moss to produce carbon sorbents. Coke Chem. 2012, 55, 396–398. [Google Scholar] [CrossRef]
- Toscano, G.; Cimino, G. New carbon from low cost vegetal precursors: Acorn and cypress cone. Cent. Eur. J. Chem. 2013, 11, 2012–2021. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, P.; Kuszyńska, I.; Przepiórski, J.; Pietrzak, R. The effect of chemical activation method on properties of activated carbons obtained from pine cones. Cent. Eur. J. Chem. 2013, 11, 78–85. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiate. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, H.; Wang, Y.; Huang, H.; Chen, Y. Characteristic of heavy metals in biochar derived from sewage sludge. J. Mater. Cycles Waste Manag. 2015, 18, 725–733. [Google Scholar] [CrossRef]
- Jina, J.; Lia, Y.; Zhanga, J.; Wua, S.; Caoa, Y.; Lianga, P.; Zhanga, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Khoshandam, B.; Jahanshahi, M. A comparative study of CO2 and CH4 adsorption using activated carbon prepared from pine cone by phosphoric acid activation. Korean J. Chem. Eng. 2016, 33, 2943–2952. [Google Scholar] [CrossRef]
- Sivakumar, P.; Palanisamy, P.N. Adsorption studies of Basic Red 29 by a non-conventional activated carbon prepared from Euphorbia antiquorum L. Int. J. ChemTech Res. 2009, 1, 502–510. [Google Scholar]
- Ding, S.; Liu, Y. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars. Fuel 2020, 260, 116382–116391. [Google Scholar] [CrossRef]
- Patnukao, P.; Pavasant, P. Activated carbon from Eucalyptus camaldulensis Dehn bark using phosphoric acid activation. Biores. Technol. 2008, 99, 8540–8543. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterisation of novel-activated carbon from waste biomass pine cone and its application in the removal of congo red dye from aqueous solution by adsorption. Water Air Soil Pollut. 2014, 225, 1818–1833. [Google Scholar] [CrossRef]
- Adinata, D.; Daud, W.M.A.W.; Aroua, M.K. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Biores. Technol. 2007, 98, 145–149. [Google Scholar] [CrossRef]
- Rodrigues, C.C.; Moraes, D., Jr.; Nóbrega, S.W.; Barboza, M.G. Ammonia adsorption in a fixed bed of activated carbon. Biores. Technol. 2007, 98, 886–891. [Google Scholar] [CrossRef]
- Huang, C.C.; Li, H.S.; Chen, C.H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia. J. Hazard. Mater. 2008, 159, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.; Sánchez-García, L.; Oliveira-Jardim, E.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Ammonia removal using activated carbons: Effect of the surface chemistry in dry and moist conditions. Environ. Sci. Technol. 2011, 45, 10605–10610. [Google Scholar] [CrossRef] [Green Version]
- Yeom, C.; Kim, Y. Adsorption of ammonia using mesoporous alumina prepared by a templating method. Environ. Eng. Res. 2017, 22, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas Adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT Adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. Carbon slit pore model incorporating surface energetical heterogeneity and geometrical corrugation. Adsorption 2013, 19, 777–783. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Lim, C.K.; Bay, H.H.; Noeh, C.H.; Aris, A.; Majid, Z.A.; Ibrahim, Z. Application of zeolite-activated carbon macrocomposite for the adsorption of Acid Orange 7: Isotherm. kinetic and thermodynamic studies. Environ. Sci. Pollut. Res. 2013, 20, 7243–7255. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-García, M.A.; Moreno-Castilla, C. Activated carbon surface modifications by adsorption of bacteria and their effect on aqueous lead adsorption. J. Chem. Technol. Biotechnol. 2001, 76, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Choma, J.; Jaroniec, M. Characterization of nanoporous active carbons by using gas adsorption isotherms. Interface Sci. Technol. 2006, 7, 107–158. [Google Scholar]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Biochar derived from anaerobically digested sugar beet tailings: Characterization and phosphate removal potential. Biores. Technol. 2011, 102, 6273–6278. [Google Scholar] [CrossRef] [PubMed]
- Ofomaja, A.E.; Naidoo, E.B. Biosorption of copper from aqueous solution by chemically activated pine cone: A kinetic study. Chem. Eng. J. 2011, 175, 260–270. [Google Scholar] [CrossRef]
- Fu, S.; Chen, H.; Yang, J.; Yang, Z. Kinetics of thermal pyrolysis of Eucalyptus bark by using thermogravimetric-Fourier transform infrared spectrometry technique. J. Therm. Anal. Calorim. 2019, 139, 3527–3535. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Thompson, A.R.; Peterson, S.C. Comparison of biochars derived from wood pellets and pelletized wheat straw as replacements for peat in potting substrates. Indust. Crops Prod. 2013, 51, 437–443. [Google Scholar] [CrossRef]
- Ma, R.; Ma, Y.; Gao, Y.; Cao, J. Preparation of micro-mesoporous carbon from seawater-impregnated sawdust by low temperature one-step CO2 activation for adsorption of oxytetracycline. SN Appl. Sci. 2020, 2, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Fang, C.; Yang, M.; Cheng, Y.; Wang, Z.; Huang, Z.; You, C. A controllable soft-templating approach to synthesize mesoporous carbon microspheres derived from d-xylose via hydrothermal method. J. Mater. Sci. Technol. 2020, 38, 183–188. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigm. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Liu, C.Y.; Aika, K. Effect of surface oxidation of active carbon on ammonia adsorption. Bull. Chem. Soc. Jpn. 2003, 76, 1463–1468. [Google Scholar] [CrossRef]
- Ro, K.S.; Lima, I.M.; Reddy, G.B.; Jackson, M.A.; Gao, B. Removing gaseous NH3 using biochar as an adsorbent. Agriculture 2015, 5, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Takaya, C.A.; Parmar, K.R.; Fletcher, L.A.; Ross, A.B. Biomass-derived carbonaceous adsorbents for trapping ammonia. Agriculture 2019, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Helminen, J.; Helenius, J.; Paatero, E.; Turunen, I. Adsorption equilibria of ammonia gas on inorganic and organic sorbents at 298.15 K. J. Chem. Eng. Data 2001, 46, 391–399. [Google Scholar] [CrossRef]

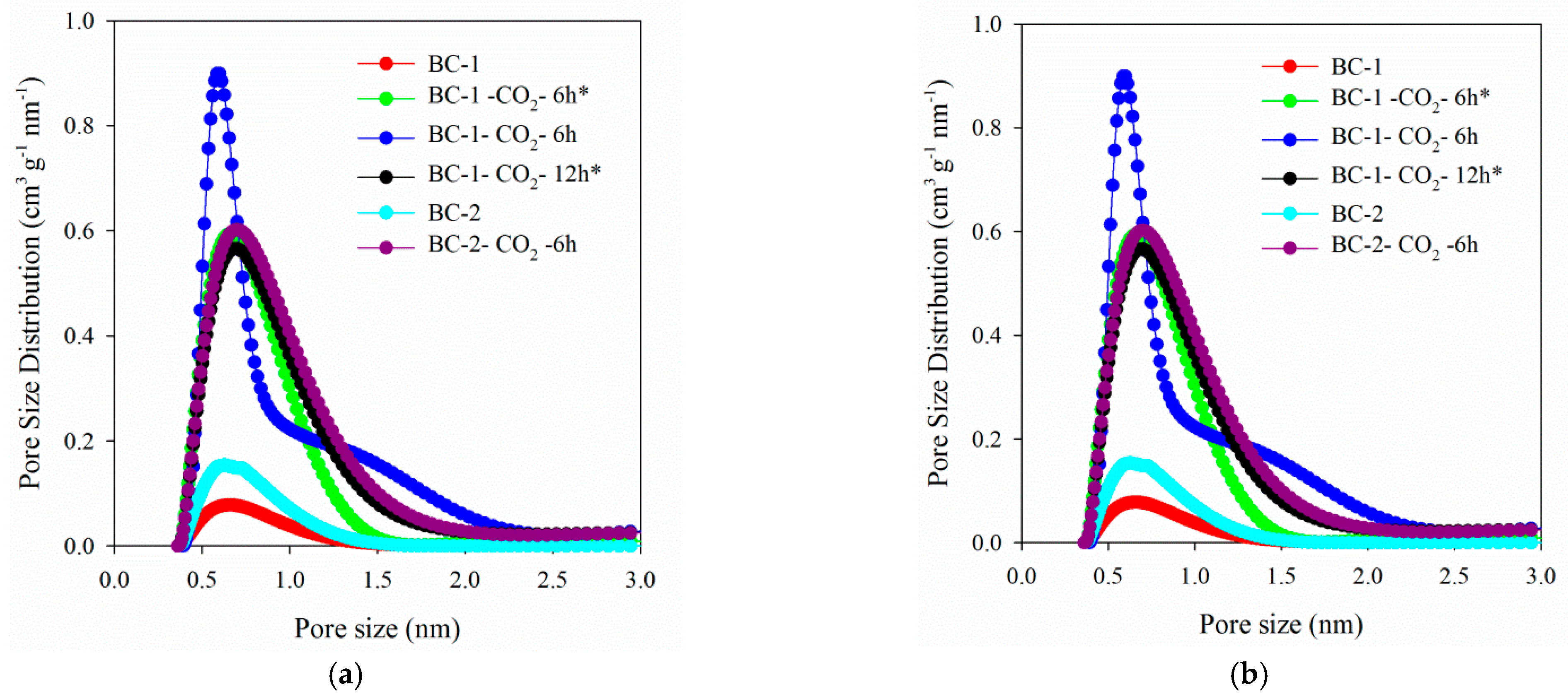


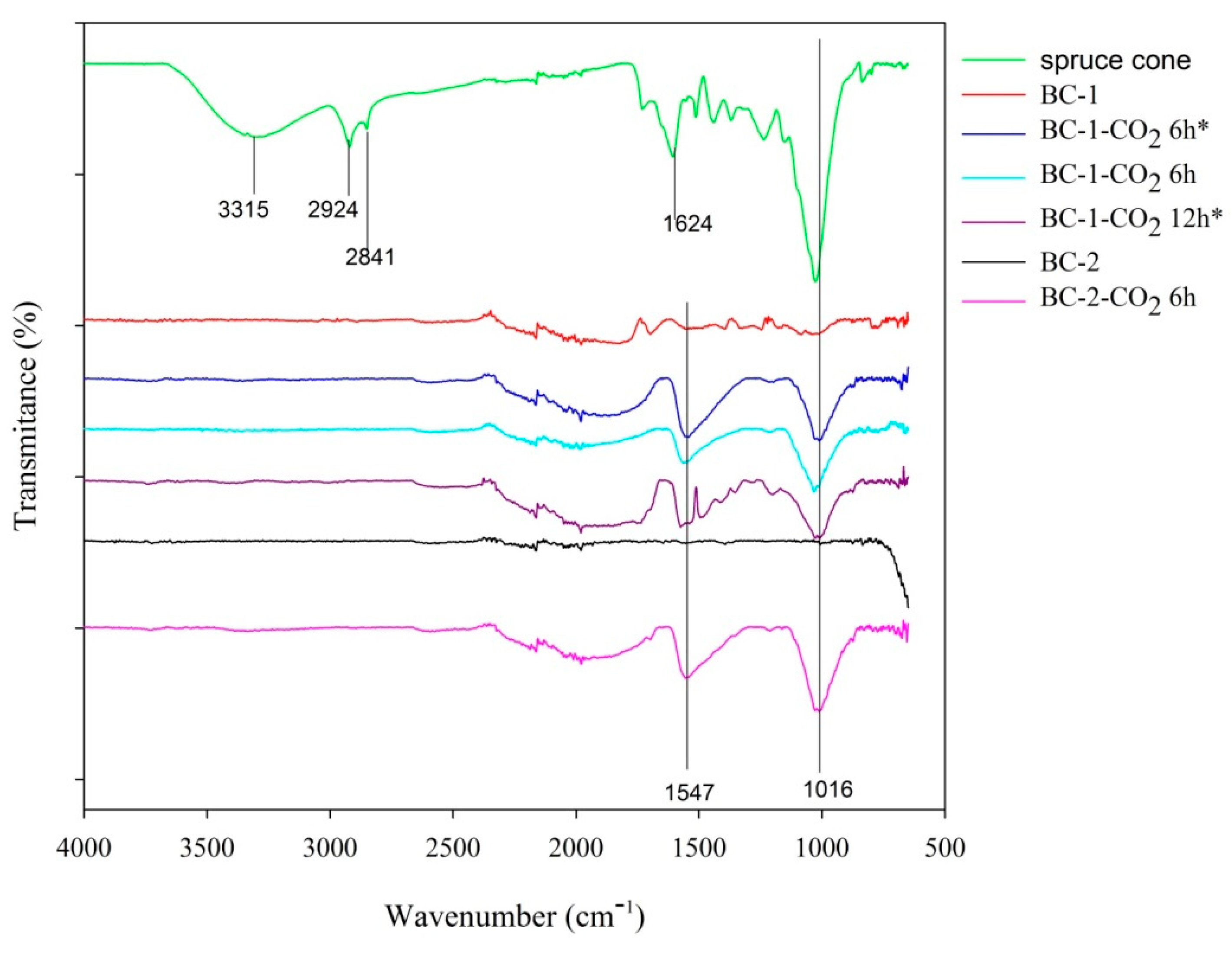
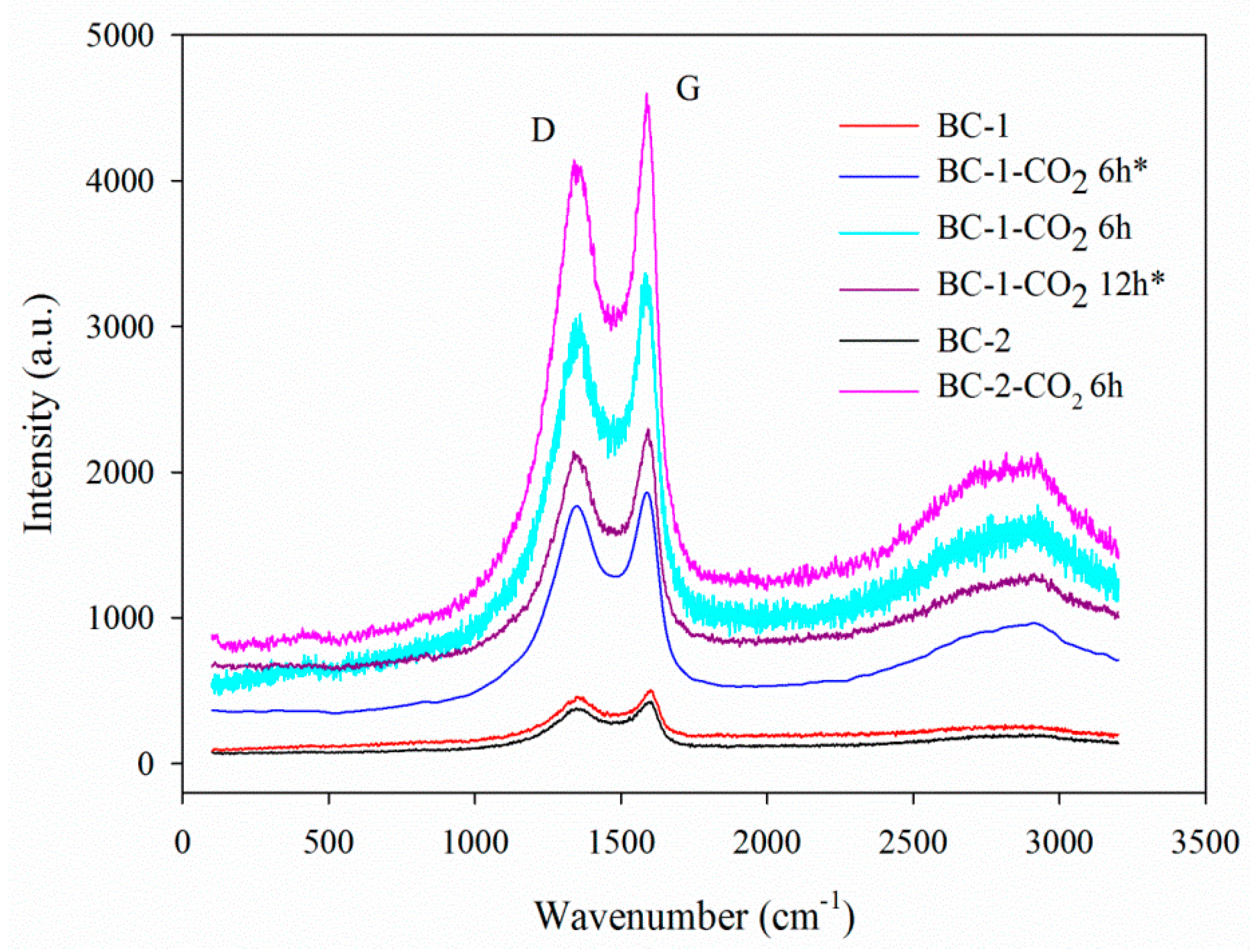
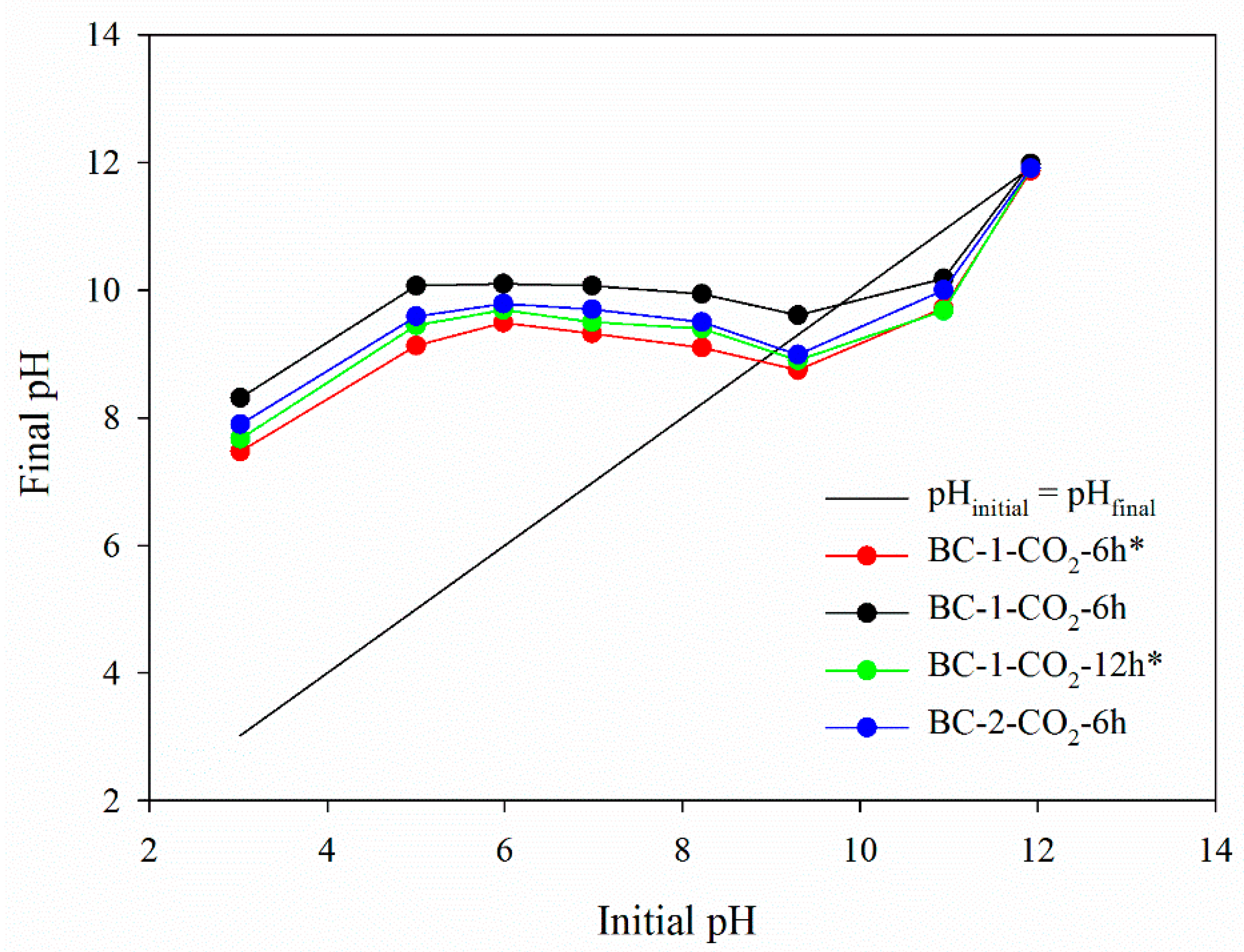
| Biochar Materials | SBET (m2 g−1) | Vt (cm3 g−1) | VultraDFT (cm3 g−1) | VmicroDFT (cm3 g−1) | Vmeso (cm3 g−1) | wmi DFT (nm) |
|---|---|---|---|---|---|---|
| BC-1 | 112 | 0.05 | 0.02 | 0.04 | 0.01 | 0.65 |
| BC-1-CO2-6h* | 884 | 0.38 | 0.13 | 0.33 | 0.05 | 0.71 |
| BC-1-CO2-6h | 1181 | 0.60 | 0.17 | 0.43 | 0.17 | 0.59 |
| BC-1-CO2-12h* | 1049 | 0.50 | 0.12 | 0.38 | 0.12 | 0.68 |
| BC-2 | 206 | 0.08 | 0.03 | 0.07 | 0.01 | 0.63 |
| BC-2-CO2-6h | 1167 | 0.56 | 0.13 | 0.42 | 0.14 | 0.70 |
| Elements | BC-1 (% w/w) | BC-1-CO2-6h* (% w/w) | BC-1-CO2-6h (% w/w) | BC-1-CO2-12h* (% w/w) | BC-2 (% w/w) | BC-2-CO2-6h (% w/w) |
|---|---|---|---|---|---|---|
| C | 85.26 | 89.05 | 84.22 | 80.09 | 82.88 | 86.82 |
| O | 10.44 | 9.67 | 10.85 | 6.56 | 10.78 | 7.91 |
| Ca | 1.37 | 0.26 | 0.97 | - | 1.08 | 0.69 |
| Mg | 0.18 | 0.11 | 0.19 | - | 0.18 | 0.15 |
| K | 2.53 | 0.16 | 2.87 | 12.61 | 0.11 | 3.86 |
| Al | - | - | - | - | 0.17 | 0.06 |
| Si | 0.22 | - | - | - | 3.92 | 0.12 |
| S | - | - | 0.33 | - | - | 0.11 |
| P | - | 0.07 | 0.57 | 0.74 | 0.11 | 0.28 |
| F | - | 0.68 | - | - | 0.77 | - |
| Elements | Spruce Cone | BC-2 |
|---|---|---|
| Ca (%) | 0.432 ± 0.02 | 1.51 ± 0.03 |
| K (%) | 1.41 ± 0.02 | 5.57 ± 0.04 |
| P (%) | 0.132 ± 0.077 | 0.50 ± 0.02 |
| S (%) | 0.20 ± 0.01 | 0.20 ± 0.01 |
| Si (%) | 0.27 ± 0.02 | 0.51 ± 0.03 |
| Cl (mg/kg) | 492 ± 20 | 312 ± 27 |
| Fe (mg/kg) | 71 ± 15 | 985 ± 35 |
| Rb (mg/kg) | 3 ± 1 | 22 ± 1 |
| Sr (mg/kg) | 6 ± 1 | 25 ± 1 |
| Zn (mg/kg) | 79 ± 5 | <15 |
| Zr (mg/kg) | 4 ± 1 | 9 ± 1 |
| Adsorbents | Total Basic Groups (mmol g−1) | Total Acidic Groups (mmol g−1) | Phenolic Groups (mmol g−1) | Lactone Groups (mmol g−1) | Carboxylic Groups (mmol g−1) | Carbonyl Groups (mmol g−1) |
|---|---|---|---|---|---|---|
| BC-1 | 0.44 | 0.22 | not determined 1 | not determined 1 | not determined 1 | 0.22 |
| BC-1-CO2-6h* | 1.04 | 0.67 | not determined 1 | not determined 1 | not determined 1 | 0.67 |
| BC-1-CO2-6h | 1.33 | 0.78 | not determined 1 | not determined 1 | not determined 1 | 0.78 |
| BC-1-CO2-12h* | 1.07 | 0.70 | not determined 1 | not determined 1 | not determined 1 | 0.70 |
| BC-2 | 0.51 | 0.26 | not determined 1 | not determined 1 | not determined 1 | 0.26 |
| BC-2-CO2-6h | 1.12 | 0.75 | not determined 1 | not determined 1 | not determined 1 | 0.75 |
| Biochars | NH3 Adsorption Capacity (mmol g−1) Pressure ~750 mmHg | TPD NH3 (mmol g−1) Pressure ~750 mmHg | ||||
|---|---|---|---|---|---|---|
| T (°C) | 0 | 10 | 20 | 0–250 | 10–250 | 20–250 |
| BC-1-CO2-6h* | 1.06 | 2.33 | 2.69 | 0.36 | 0.32 | 0.23 |
| BC-1-CO2-6h | 5.18 | 3.39 | 1.13 | 0.20 | 0.24 | 0.30 |
| BC-1-CO2-12h* | 0.82 | 1.01 | 3.59 | 0.37 | 0.20 | 0.23 |
| BC-2-CO2-6h | 3.95 | 2.99 | 0.95 | 0.46 | 0.32 | 0.14 |
| Carbon Materials | SBET m2 g−1 | Adsorption Capacity (mg g−1) | Adsorption Capacity (mmol g−1) | Ref. |
|---|---|---|---|---|
| BC-1-CO2-6h* | 884 | 18.05–45.81 | 1.06–2.69 | This study |
| BC-1-CO2-6h | 1181 | 19.24–88.22 | 1.13–5.18 | This study |
| BC-1-CO2-12h* | 1049 | 13.97–61.14 | 0.82–3.59 | This study |
| BC-2-CO2-6h | 1167 | 16.18–67.27 | 0.95–3.95 | This study |
| AC 1 | 1161 | 20.27 | 1.19 | [64] |
| Na-OH-AC 2 | 1125 | 28.78 | 1.69 | [64] |
| HNO3-AC 3 | 1010 | 52.28 | 3.07 | [64] |
| H2SO4-AC 4 | 1016 | 47.34 | 2.78 | [64] |
| AA-WS250-AR 5 | 851 | 53.09 | 3.11 | [65] |
| OAK-250-KOH 6 | - | 25 | 1.47 | [66] |
| OAK-250-H2O2 7 | - | 25 | 1.47 | [66] |
| OAK-450-KOH 8 | - | 6 | 0.35 | [66] |
| OAK-450-H2O2 9 | - | 10 | 0.59 | [66] |
| AC 10 | 430 | 13.28–71.36 | 0.78–4.19 | [67] |
| AC 11 | 450 | 13.11–86.51 | 0.77–5.08 | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedynak, K.; Charmas, B. Preparation and Characterization of Physicochemical Properties of Spruce Cone Biochars Activated by CO2. Materials 2021, 14, 3859. https://doi.org/10.3390/ma14143859
Jedynak K, Charmas B. Preparation and Characterization of Physicochemical Properties of Spruce Cone Biochars Activated by CO2. Materials. 2021; 14(14):3859. https://doi.org/10.3390/ma14143859
Chicago/Turabian StyleJedynak, Katarzyna, and Barbara Charmas. 2021. "Preparation and Characterization of Physicochemical Properties of Spruce Cone Biochars Activated by CO2" Materials 14, no. 14: 3859. https://doi.org/10.3390/ma14143859
APA StyleJedynak, K., & Charmas, B. (2021). Preparation and Characterization of Physicochemical Properties of Spruce Cone Biochars Activated by CO2. Materials, 14(14), 3859. https://doi.org/10.3390/ma14143859






