Application of Concretes Made with Glass Powder Binder at High Replacement Rates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Aggregates
2.1.2. Cement
2.2. Sample Preparation
2.3. Characterization Tests
- Consistency test
- 2.
- Air content
- 3.
- Apparent density
- 4.
- Workability
- 5.
- Permeability test
2.4. Slab Manufacturing
3. Results and Discussion
3.1. Results of the Characterization of Concrete
3.2. Mechanical Properties of Concrete
3.3. Results of Permeability Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- David, A.; Thangavel, Y.D.; Sankriti, R. Recover, recycle and reuse: An efficient way to reduce the waste. Int. J. Mech. Prod. Eng. Res. Dev. 2019, 9, 31–42. [Google Scholar] [CrossRef]
- Ginga, C.P.; Ongpeng, J.M.C.; Daly, M.K.M. Circular economy on construction and demolition waste: A literature review on material recovery and production. Materials 2020, 13, 2970. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Cheng, S.T.; Ho, W.C.; Chang, Y.H. Measuring the sustainability of construction projects throughout their lifecycle: A Taiwan Lesson. Sustainability 2018, 10, 1523. [Google Scholar] [CrossRef] [Green Version]
- Chau, K.W. Incorporation of sustainability concepts into a civil engineering curriculum. J. Prof. Issues Eng. Educ. Pract. 2007, 133, 188–191. [Google Scholar] [CrossRef]
- Pade, C.; Guimaraes, M. The CO2 uptake of concrete in a 100 year perspective. Cem. Concr. Res. 2007, 37, 1348–1356. [Google Scholar] [CrossRef]
- Hendrik, G.; van Amy, C. Padovani Cement Manufacture and the Environment Part II: Environmental Challenges and OpportunitiesNo Title. J. Ind. Ecol. 2008, 7, 93–126. [Google Scholar]
- Jani, Y.; Hogland, W. Waste glass in the production of cement and concrete—A review. J. Environ. Chem. Eng. 2014, 2, 1767–1775. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Properties of high volume glass powder concrete. Cem. Concr. Compos. 2017, 75, 22–29. [Google Scholar] [CrossRef]
- Ahmad, S.; Xu, A. Performance of glass powder as a pozzolanic material in concrete: A field trial on concrete slabs. Cem. Concr. Res. 2007, 36, 457–468. [Google Scholar]
- Marco, J.; Garcia, E.; Más, M.I.; Alcaraz, V.; Luizaga, A. Estudio de la resistencia a compresión de morteros fabricados con conglomerante compuesto de polvo de vidrio. Inf. Constr. 2012, 64, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Hohberg, I.; de Groot, G.J.; van der Veen, A.M.H.; Wassing, W. Development of a leaching protocol for concrete. Stud. Environ. Sci. 1997, 71, 217–228. [Google Scholar] [CrossRef]
- Głuchowski, A.; Sas, W.; Dziecioł, J.; Soból, E.; Szymaňski, A. Permeability and leaching properties of recycled concrete aggregate as an emerging material in civil engineering. Appl. Sci. 2018, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Diotti, A.; Galvin, A.P.; Piccinali, A.; Plizzari, G.; Sorlini, S. Chemical and leaching behavior of construction and demolition wastes and recycled aggregates. Sustainability 2020, 12, 326. [Google Scholar] [CrossRef]
- Marion, A.M.; De Lanève, M.; De Grauw, A. Study of the leaching behaviour of paving concretes: Quantification of heavy metal content in leachates issued from tank test using demineralized water. Cem. Concr. Res. 2005, 35, 951–957. [Google Scholar] [CrossRef]
- Mas, M.I.; García, E.M.; Marco, L.J.; De Marco, J. Análisis de la Viabilidad Ambiental de la Utilización de Morteros Fabricados con Polvo de Vidrio en la Estabilización de Suelos. Inf. Tecnol. 2016, 27, 77–86. [Google Scholar] [CrossRef]
- Shaoa, Y.; Lefort, T.; Morasa, S. Damian Rodriguez Studies on concrete containing ground waste glass. Cem. Concr. Res. 2000, 30, 91–100. [Google Scholar] [CrossRef]
- Lu, J.X.; Duan, Z.J.; Poon, C.S. Combined use of waste glass powder and cullet in architectural mortar. Cem. Concr. Compos. 2017, 82, 34–44. [Google Scholar] [CrossRef]
- Taha, B.; Nounu, G. Properties of concrete contains mixed colour waste recycled glass as sand and cement replacement. Constr. Build. Mater. 2008, 22, 713–720. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Effect of curing temperature and glass type on the pozzolanic reactivity of glass powder. Cem. Concr. Res. 2014, 58, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Maraghechi, H.; Maraghechi, M.; Rajabipour, F.; Pantano, C.G. Pozzolanic reactivity of recycled glass powder at elevated temperatures: Reaction stoichiometry, reaction products and effect of alkali activation. Cem. Concr. Compos. 2014, 53, 105–114. [Google Scholar] [CrossRef]
- Strength, C. Chemical Reactions of Glass Cullet as cement component. J. Mater. 2001, 13, 412–417. [Google Scholar]
- Rashidian-Dezfouli, H.; Afshinnia, K.; Rangaraju, P.R. Efficiency of Ground Glass Fiber as a cementitious material, in mitigation of alkali-silica reaction of glass aggregates in mortars and concrete. J. Build. Eng. 2018, 15, 171–180. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Más-López, M.I.; del Toro, E.M.G.; Patiño, A.L.; García, L.J.M. Eco-friendly pavements manufactured with glass waste: Physical and mechanical characterization and its applicability in soil stabilization. Materials 2020, 13, 3727. [Google Scholar] [CrossRef] [PubMed]
- Lasalle, E. Hormigones desactivados: Hormigones con árido visto en pavimentos y hormigones decorativos en prefabricados industriales. In Jornadas Sobre hormigones Especiales; Escuela Técnica Superior de Ingeniería de Caminos Canales y Puertos, Ed.; Universidad Politécnica de Cataluña: Barcelona, Spain, 2008; pp. 29–42. ISBN 9788487691560. [Google Scholar]
- AENOR UNE-EN 123501-1. Ensayos de Hormigón Fresco; Instituto Español de Normalización: Madrid, Spain, 2006; pp. 1–2. [Google Scholar]
- AENOR. Ensayos de Hormigón;Rotura por Comprensión: Norma Española: UNE 83-304-84; Instituto Español de Normalización: Madrid, Spain, 1985; pp. 1–3. [Google Scholar]
- UNE 12350-2. Ensayos de Hormigón Fresco. Ensayo de Asentamiento; Instituto Español de Normalización: Madrid, Spain, 2006. [Google Scholar]
- Comité Técnico CEN/TC 104 Hormigón y productos Relacionados UNE-EN 12350-7: Ensayos de hormigón fresco. Parte 7: Contenido de Aire Métodos de Presión; Instituto Español de Normalización: Madrid, Spain, 2020.
- CTN 83—HORMIGÓN UNE-EN 1015-6:1999: Densidad Aparente del Hormigón Fresco; Instituto Español de Normalización: Madrid, Spain, 1999.
- ANEFHOP UNE-EN 12350-5:2020. Ensayos de Hormigón Fresco. Parte 5: Ensayo de la Mesa de Sacudidas; Instituto Esapñol de Normalización: Madrid, Spain, 2020; pp. 1–15. [Google Scholar]
- UNE-EN. Norma Española Parte 8: Profundidad de Penetración de Agua Bajo Presión; Instituto Español de Normalización: Madrid, Sapain, 2020; pp. 6–8. [Google Scholar]
- Del Toro, E.G.; Alcala-Gonzalez, D.; Más-Lópe, M.I.; García-Salgado, S.; Pindado, S. Use of ecofriendly glass powder concrete in construction of wind farms. Appl. Sci. 2021, 11, 3050. [Google Scholar] [CrossRef]



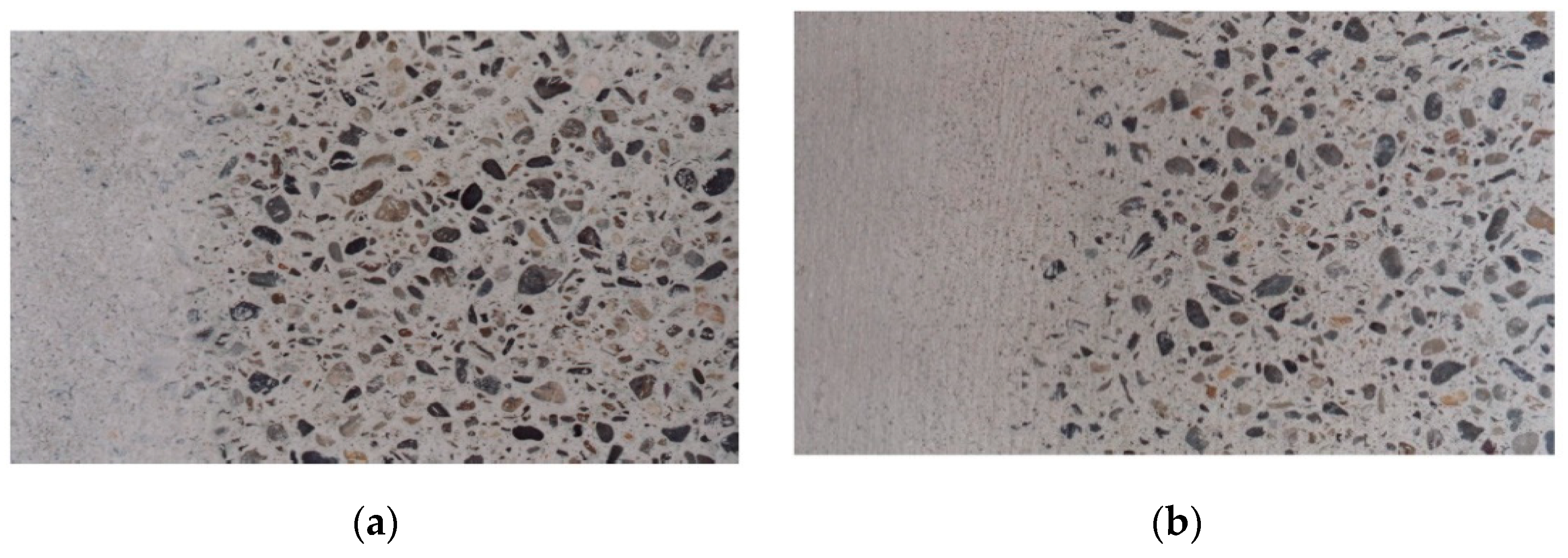
| Glass Powder Used | ||
|---|---|---|
| d10 | d50 | d90 |
| 20 ± 0.01 µm | 21 ± 1 µm | 76 ± 3 µm |
| Sample ID | |||
|---|---|---|---|
| Concrete Composition | G70 | G80 | Control |
| Cement substitution rate for glass powder (%) | 70 | 80 | 0 |
| Cement CEM I 52,5 R (kg/m3) | 99 | 66 | 330 |
| Glass powder (kg/m3) | 231 | 264 | 0 |
| Equivalent binder (kg/m3) | 330 | ||
| w/c ratio (%) | 0.52 | ||
| Arid < 4 mm | 740 | ||
| Gravel 4–12 mm | 310 | ||
| Gravel 12–20 mm | 850 | ||
| G70 | G80 | Control | |
|---|---|---|---|
| Consistency (mm) | 0 | 0 | 11 |
| Air content (%) | 7.1 | 7.1 | 2.8 |
| Apparent density (kg/m3) | 2098 | 2051 | 2390 |
| Workability (easy/difficult) | Difficult | Easy enough | Easy |
| Days | Compressive Strength (MPa) | Control | |
|---|---|---|---|
| G70 | G80 | 0 | |
| 7 | 5.6 | 1.5 | 29.9 |
| 28 | 10.0 | 3.7 | 34.5 |
| 90 | 14.2 | 8.6 | 39.6 |
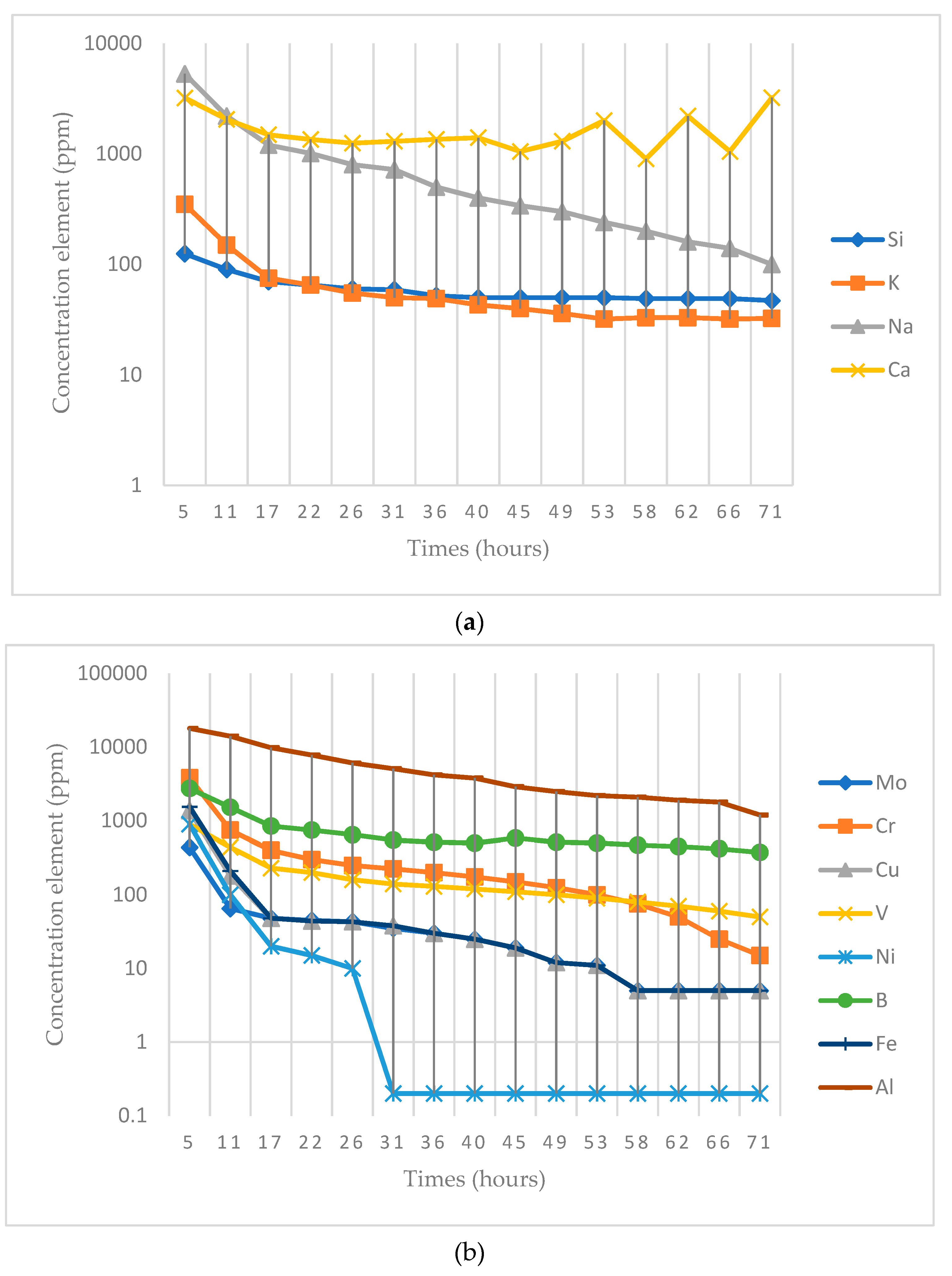
| Element | Experimental Value (ppm) | Results Obtained by Mas et al. [16] (ppm) | ||
|---|---|---|---|---|
| Na | 97 ± 3 | 100 ± 3 | 9.46 | 1.35 |
| Si | 45 ± 3 | 47 ± 4 | 13.11 | 0.65 |
| K | 31 ± 2 | 32 ± 2 | 3.71 | 0.70 |
| Ca | 3.2 ± 0.3 | 3.2 ± 0.5 | 0.17 | 0.15 |
| Al | 1.36 ± 0.09 | 1.2 ± 0.2 | 0.02 | 1.28 |
| B | 0.30 ±0.02 | 0.38 ± 0.05 | 0.002 | 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Más-López, M.I.; García del Toro, E.M.; García-Salgado, S.; Alcala-Gonzalez, D.; Pindado, S. Application of Concretes Made with Glass Powder Binder at High Replacement Rates. Materials 2021, 14, 3796. https://doi.org/10.3390/ma14143796
Más-López MI, García del Toro EM, García-Salgado S, Alcala-Gonzalez D, Pindado S. Application of Concretes Made with Glass Powder Binder at High Replacement Rates. Materials. 2021; 14(14):3796. https://doi.org/10.3390/ma14143796
Chicago/Turabian StyleMás-López, M. Isabel, Eva M. García del Toro, Sara García-Salgado, Daniel Alcala-Gonzalez, and Santiago Pindado. 2021. "Application of Concretes Made with Glass Powder Binder at High Replacement Rates" Materials 14, no. 14: 3796. https://doi.org/10.3390/ma14143796







