Van der Waals Heterostructures—Recent Progress in Electrode Materials for Clean Energy Applications
Abstract
:1. Introduction
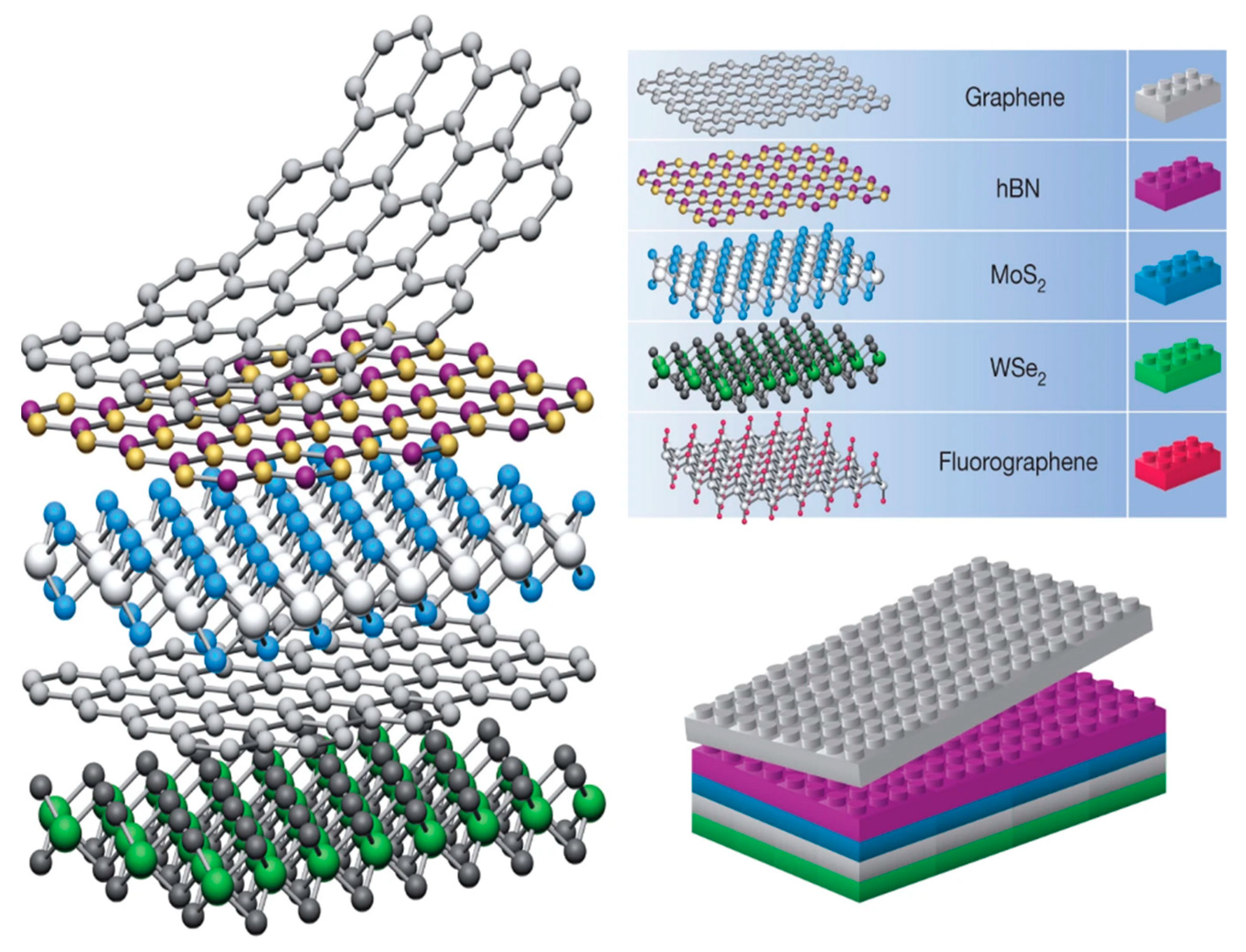
2. Van der Waals Heterostructures
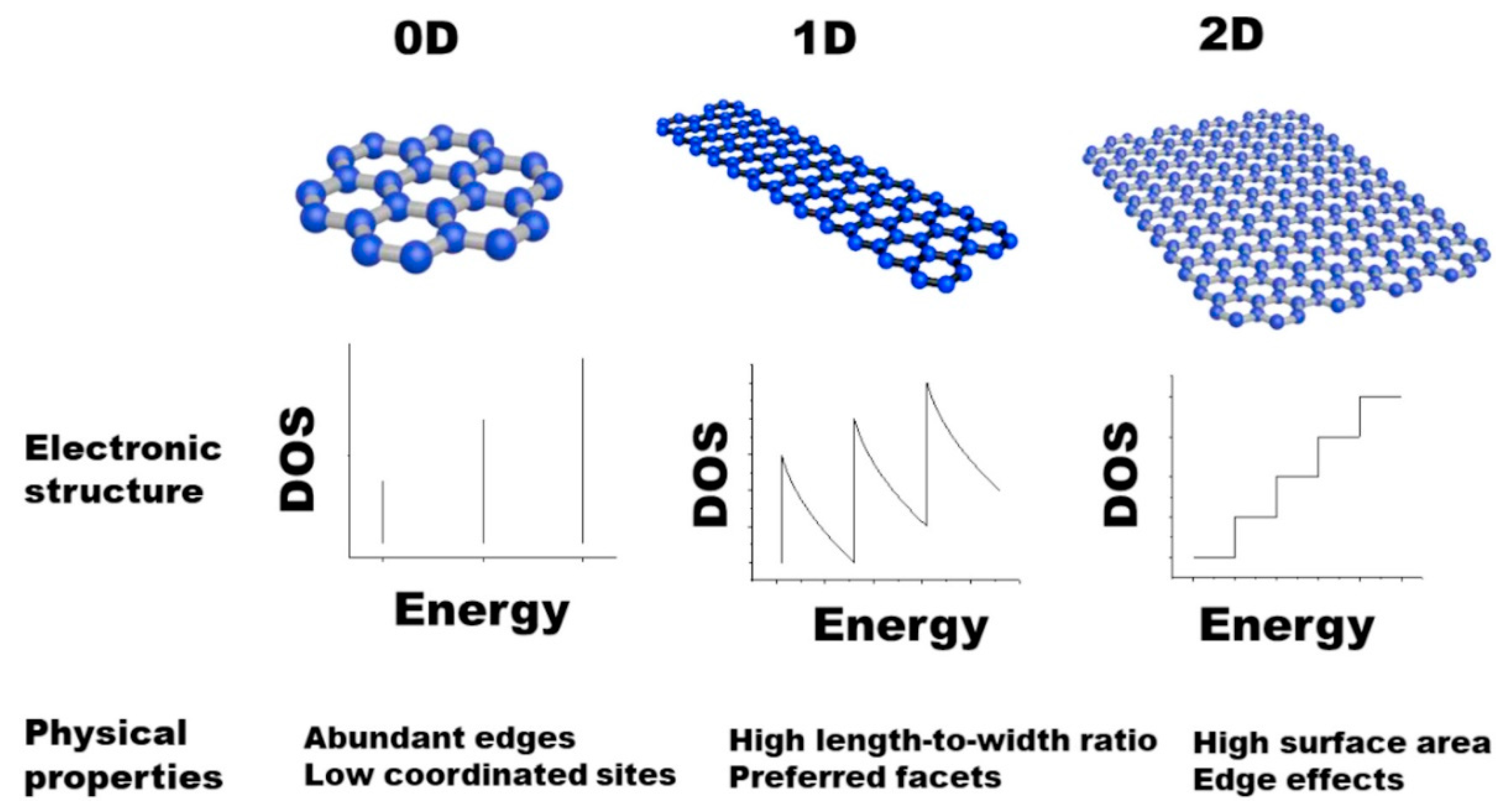
2.1. Dimensional Materials
2.2. Confined Catalysis
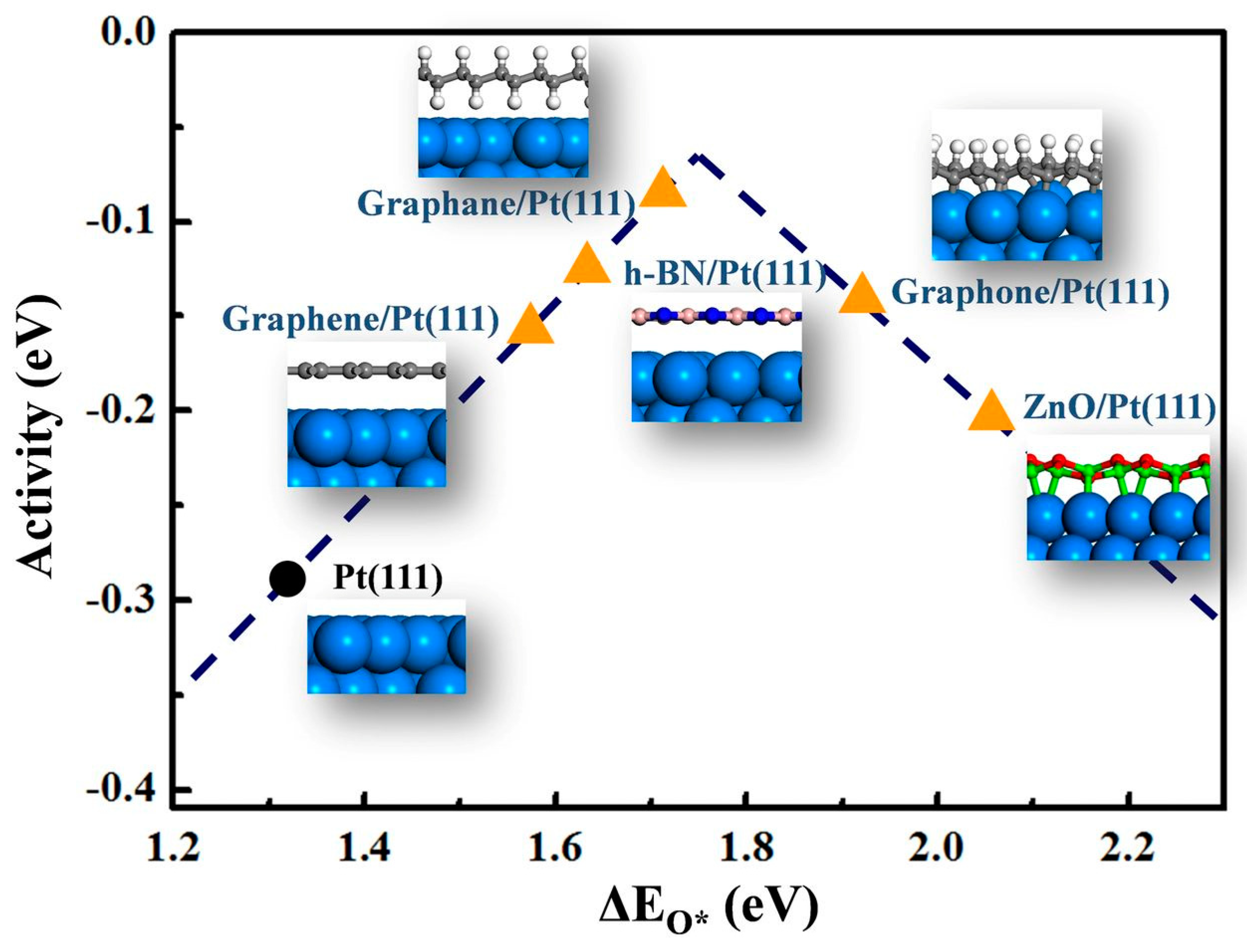
3. Electrochemical Oxygen Reduction Reaction (ORR) and Evolution (OER): vdW Cathode Materials for a Metal–Air Battery and Fuel Cell
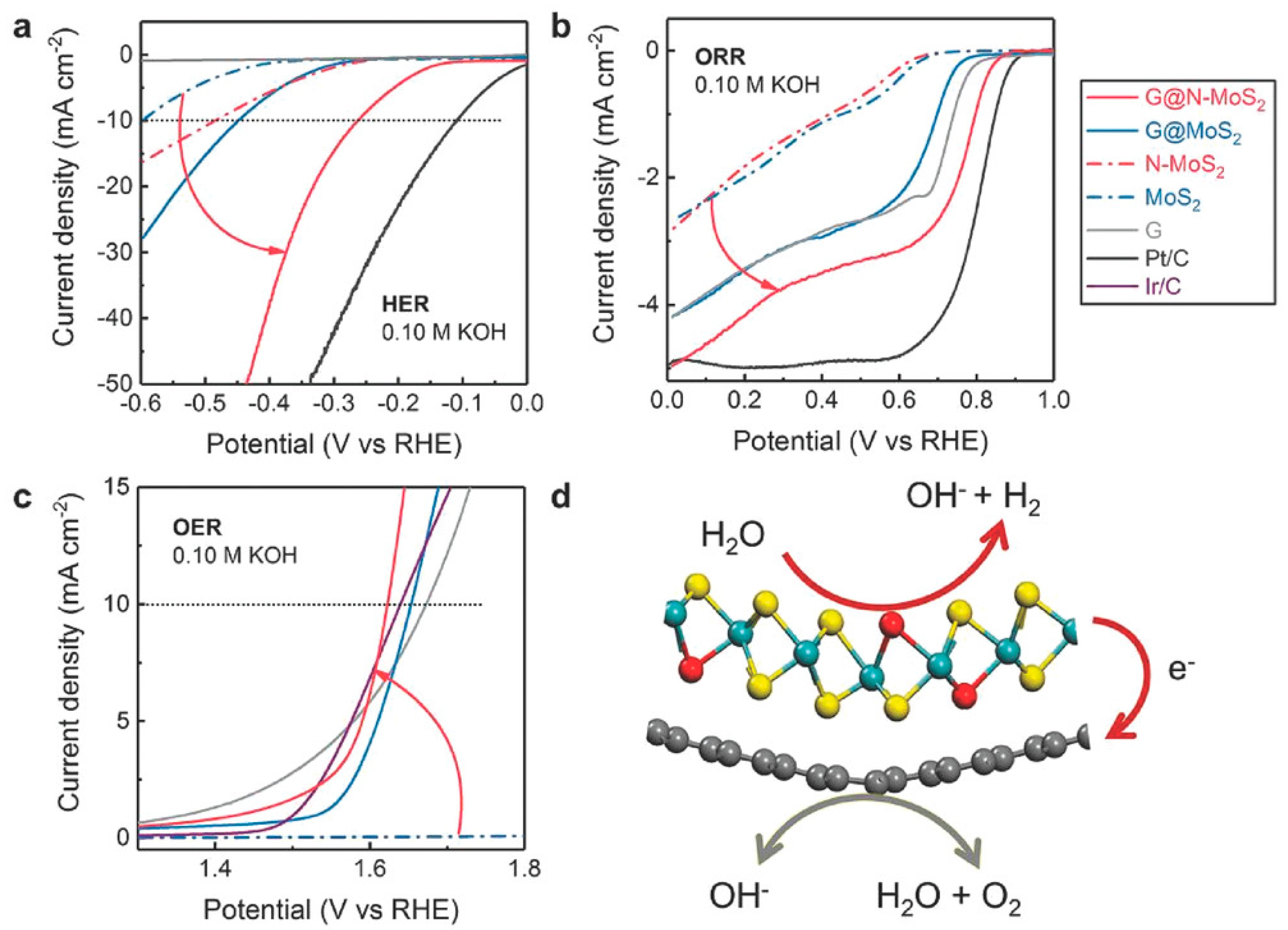
Prospective of van der Waals Heterostructures as ORR/OER Catalyst for Metal–Air and Fuel Cell Cathodes
- (1)
- Establishing a relationship between the electrode catalyst support and the vdWs is important to realize the effective utilization of vdWs from their distribution on the support. Possible approaches include incorporating the support in the formation of the vdWs during the synthesis or combining the support with the as-synthesized heterostructures. It is well-known that optimizing the catalyst loading and the catalyst/support interaction can further improve the electrochemical performance.
- (2)
- Both bottom-up and top-down synthesis procedures must be more scalable and more energy efficient in order to make the commercialization of these catalysts viable and benefit the corresponding energy systems.
- (3)
- Structural stability under the ORR/OER conditions is an emerging area of research that must be completed. Particularly, the chemical instability of vdWs in alkaline solution, surface segregation, Kirkendall effect, vdW aggregation between layers, and its connection to supporting material must be ensured to maintain a contact activity during long-life electrochemical processes.
- (4)
- Lastly, the vdW-based catalyst layer of the commercial electrodes, such as air cathode or membrane electrode assembly (MEA), should be developed and optimized. In particular, more research is needed to ensure the best choice for the catalyst support material resulting in the highest utilization of vdWs, and thus sufficient/or improved performance when compared to commercialized fuel cells and metal–air batteries.
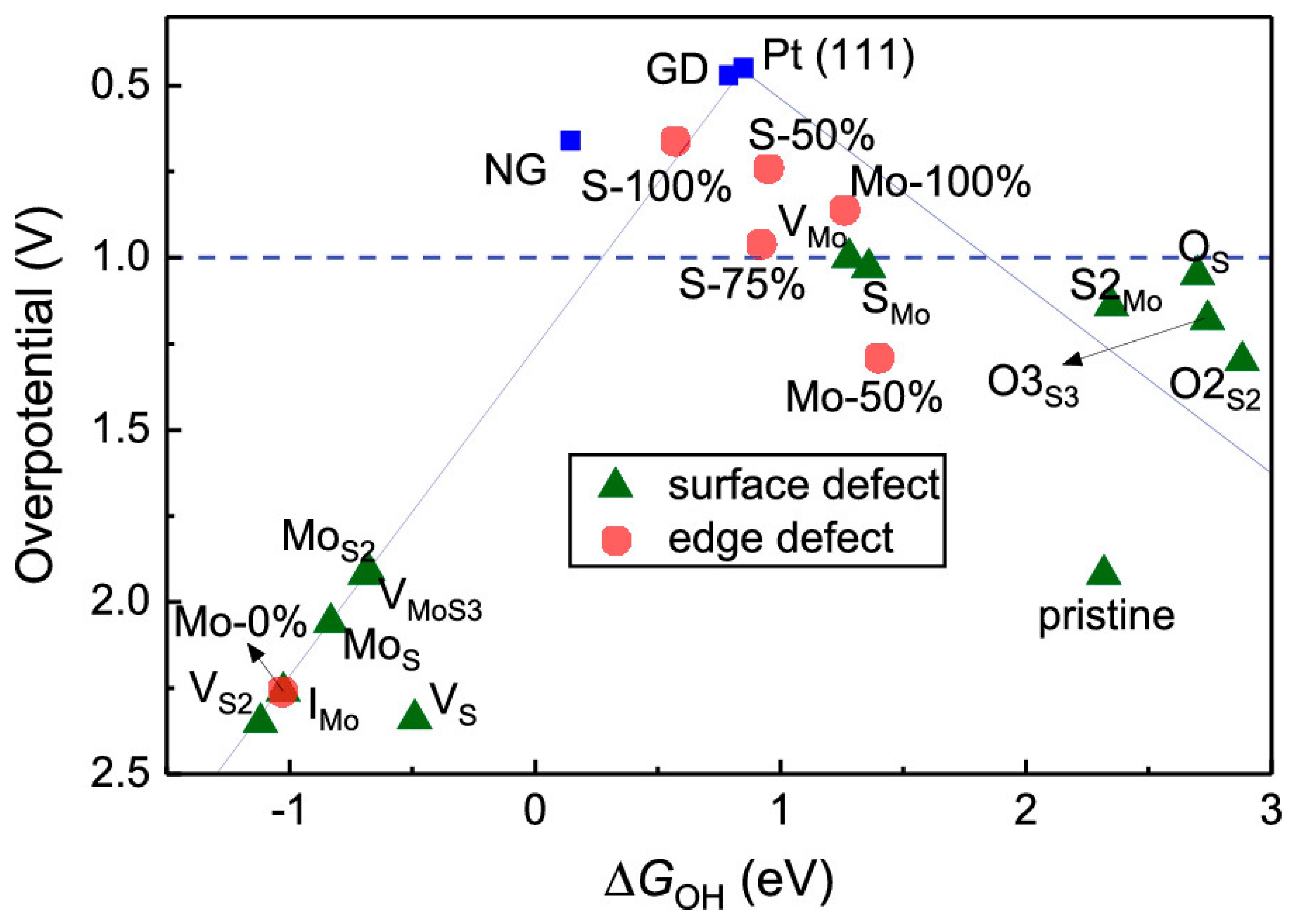
4. Electrochemical Hydrogen Evolution (HER): vdW Electrodes for Water Splitting
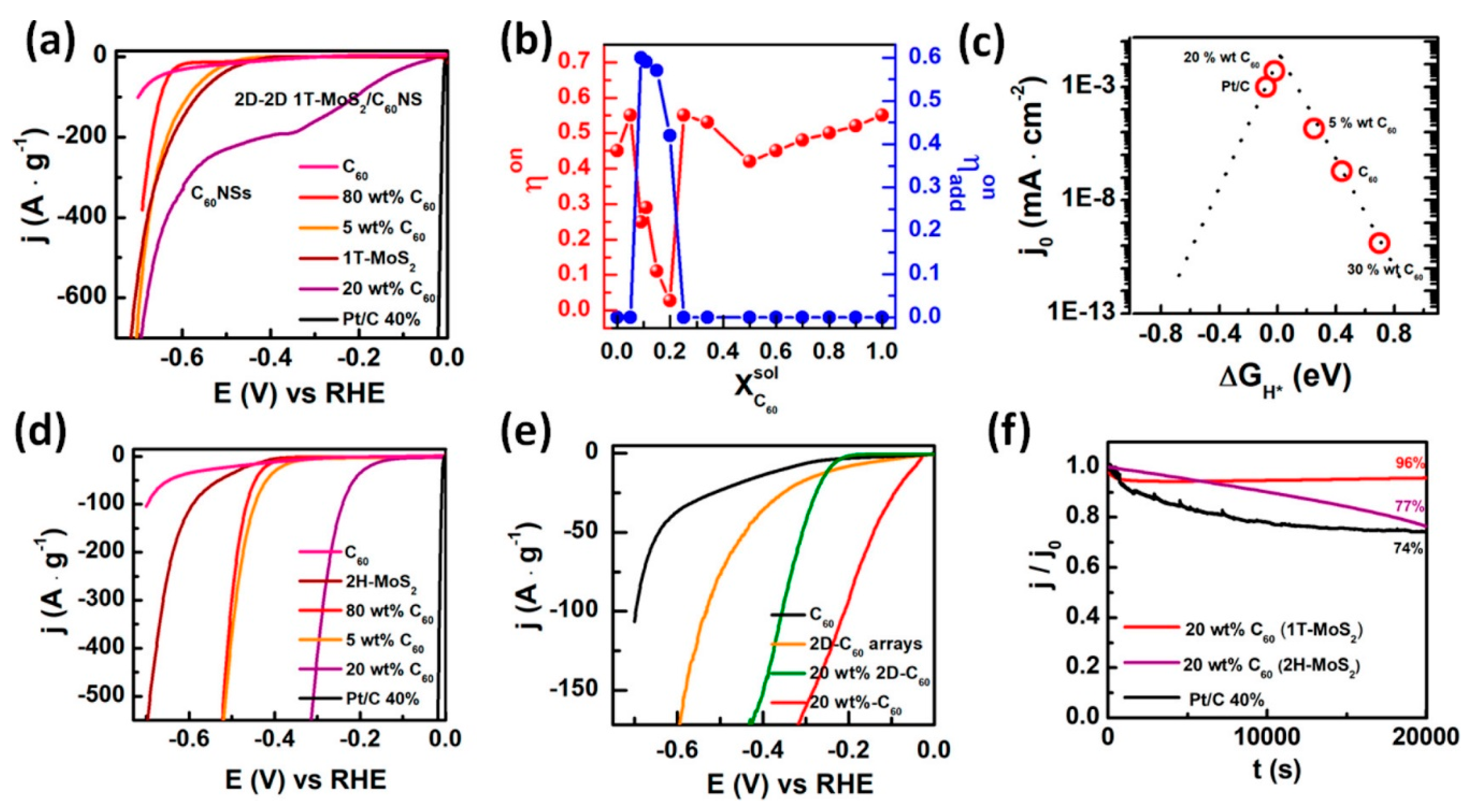
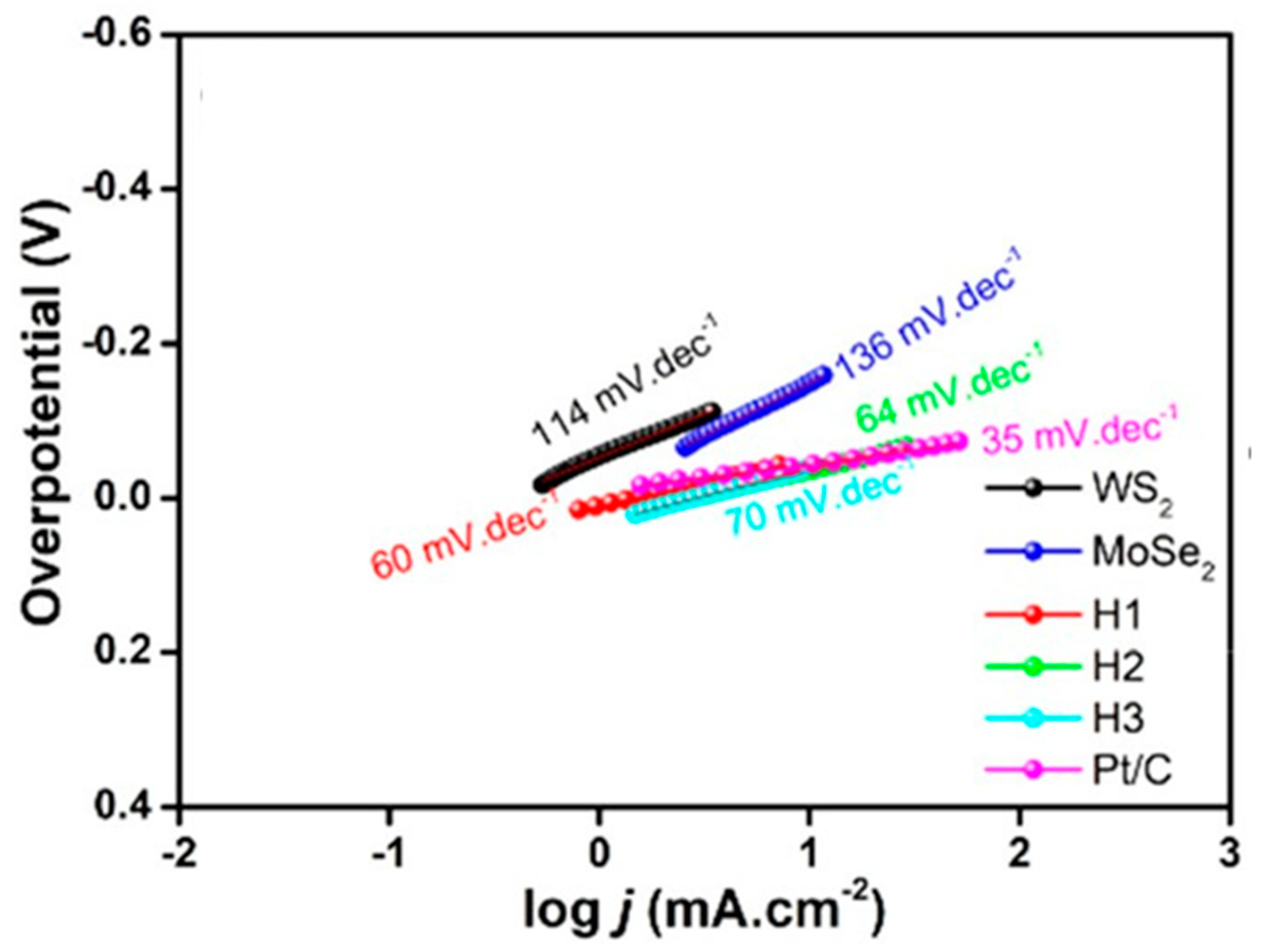
Prospective of van der Waals Heterostructures as the HER Electrocatalysts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walther, G.; Post, E.; Convey, P.; Menzel, A.; Parmesank, C.; Beebee, T.J.C.; Fromentin, J.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef] [Green Version]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Torabi, F.; Ahmadi, P. Simulation of Battery Systems, 1st ed.; Elsevier: San Diego, CA, USA, 2020; pp. 1–54. ISBN 9780128162125. [Google Scholar]
- Vikraman, D.; Hussain, S.; Patil, S.A.; Truong, L.; Arbab, A.A.; Jeong, S.H.; Chun, S.H.; Jung, J.; Kim, H.S. Engineering MoSe2/WS2 hybrids to replace the scarce platinum electrode for hydrogen evolution reactions and dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2021, 13, 5061–5072. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Pollet, B.G.; Kocha, S.S.; Staffell, I. Current status of automotive fuel cells for sustainable transport. Curr. Opin. Electrochem. 2019, 16, 90–95. [Google Scholar] [CrossRef]
- Greim, P.; Solomon, A.A.; Breyer, C. Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat. Commun. 2020, 11, 4570. [Google Scholar] [CrossRef]
- Platinum Metal Reserves Worldwide as of 2020, by Country. Available online: https://www.statista.com/statistics/273624/platinum-metal-reserves-by-country/ (accessed on 2 July 2021).
- Yu, Q.; Luo, Y.; Mahmood, A.; Liu, B.; Cheng, H.-M. Engineering two-dimensional materials and their heterostructures as high-performance electrocatalysts. Electrochem. Energy Rev. 2019, 2, 373–394. [Google Scholar] [CrossRef]
- Bartolomeo, A. Di Emerging 2d materials and their van der waals heterostructures. Nanomaterials 2020, 10, 579. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Liu, Q.; Chou, S.L.; Dou, S.X. Two-dimensional material-based heterostructures for rechargeable batteries. Cell Rep. Phys. Sci. 2021, 2, 100286. [Google Scholar] [CrossRef]
- Lee, M.K.; Shokouhimehr, M.; Kim, S.Y.; Jang, H.W. Two-dimensional metal–organic frameworks and covalent–organic frameworks for electrocatalysis: Distinct merits by the reduced dimension. Adv. Energy Mater. 2021, 2003990. [Google Scholar] [CrossRef]
- Yan, Y.; Zeng, Z.; Huang, M.; Chen, P. Van der waals heterojunctions for catalysis. Mater. Today Adv. 2020, 6, 100059. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhan, X.; Rawach, D.; Chen, Z.; Zhang, G.; Sun, S. Low-dimensional catalysts for oxygen reduction reaction. Prog. Nat. Sci. Mater. Int. 2020, 30, 787–795. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Jiao, Y.; Shi, B.; Liu, J.; Xie, Z.; Chen, X.; Zhang, Q.; Qiao, S. Coordination tunes selectivity: Two-electron oxygen reduction on high-loading molybdenum single-atom catalysts. Angew. Chem. 2020, 132, 9256–9261. [Google Scholar] [CrossRef]
- Siahrostami, S.; Tsai, C.; Karamad, M.; Koitz, R.; García-Melchor, M.; Bajdich, M.; Vojvodic, A.; Abild-Pedersen, F.; Nørskov, J.K.; Studt, F. Two-dimensional materials as catalysts for energy conversion. Catal. Lett. 2016, 146, 1917–1921. [Google Scholar] [CrossRef]
- Murdachaew, G.; Laasonen, K. Oxygen evolution reaction on nitrogen-doped defective carbon nanotubes and graphene. J. Phys. Chem. C 2018, 122, 25882–25892. [Google Scholar] [CrossRef]
- Hao, Y.; Gong, P.L.; Xu, L.C.; Pu, J.; Wang, L.; Huang, L.F. Contrasting oxygen reduction reactions on zero- and one-dimensional defects of MoS2 for versatile applications. ACS Appl. Mater. Interfaces 2019, 11, 46327–46336. [Google Scholar] [CrossRef]
- Koitz, R.; Nørskov, J.K.; Studt, F. A systematic study of metal-supported boron nitride materials for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2015, 17, 12722–12727. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, P.; Ellis, P.; French, S.; Kelly, G.; Lok, C.M. Brønsted-Evans-Polanyi relation of multistep reactions and volcano curve in heterogeneous catalysis. J. Phys. Chem. C 2008, 112, 1308–1311. [Google Scholar] [CrossRef]
- Li, H.; Xiao, J.; Fu, Q.; Bao, X. Confined catalysis under two-dimensional materials. Proc. Natl. Acad. Sci. USA 2017, 114, 5930–5934. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Xia, Z.; Li, H.; Yu, S.; Wang, S.; She, G. Mechanism and activity of oxygen reduction reaction on single atom doped graphene: A DFT method. RSC Adv. 2019, 9, 7086–7093. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, Z.; Qiu, H.; Yang, W.; Zhao, Z.; Zhao, J.; Cui, G. Pursuit of reversible Zn electrochemistry: A time-honored challenge towards low-cost and green energy storage. NPG Asia Mater. 2020, 12, 1–24. [Google Scholar] [CrossRef]
- Garche, J.; Dyer, C.K.; Moseley, P.T.; Ogumi, Z.; Rand, D.A.J.; Scrosati, B. Encylopedia of Electrochemical Power Sources, 1st ed.; Newnes: Boston, MA, USA, 2009; pp. 356–375. ISBN 9780444527455. [Google Scholar]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Dincer, I. Green methods for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 1954–1971. [Google Scholar] [CrossRef]
- Çelik, D.; Yıldız, M. Investigation of hydrogen production methods in accordance with green chemistry principles. Int. J. Hydrogen Energy 2017, 42, 23395–23401. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Chen, R.; Xu, X.; Peng, S.; Chen, J.; Yu, D.; Xiao, C.; Li, Y.; Chen, Y.; Hu, X.; Liu, M.; et al. A flexible and safe aqueous zinc-air battery with a wide operating temperature range from −20 to 70 °C. ACS Sustain. Chem. Eng. 2020, 8, 11501–11511. [Google Scholar] [CrossRef]
- Chen, B.; He, X.; Yin, F.; Wang, H.; Liu, D.-J.; Shi, R.; Chen, J.; Yin, H. MO-Co@N-doped carbon (M = Zn or Co): Vital roles of inactive ZN and highly efficient activity toward oxygen reduction/evolution reactions for rechargable Zn-air battery. Adv. Funct. Mater. 2017, 27, 1700795. [Google Scholar] [CrossRef]
- Han, Z.; Feng, J.J.; Yao, Y.Q.; Wang, Z.G.; Zhang, L.; Wang, A.J. Mn, N, P-tridoped bamboo-like carbon nanotubes decorated with ultrafine Co2P/FeCo nanoparticles as bifunctional oxygen electrocatalyst for long-term rechargeable Zn-air battery. J. Colloid Interface Sci. 2021, 590, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Halme, A.; Selkäinaho, J.; Noponen, T.; Kohonen, A. An alternative concept for DMFC—Combined electrolyzer and H2 PEMFC. Int. J. Hydrogen Energy 2016, 41, 2154–2164. [Google Scholar] [CrossRef]
- Chen, D.; Cao, W.; Liu, J.; Wang, J.; Li, X.; Jiang, L. Filling the in situ-generated vacancies with metal cations captured by C−N bonds of defect-rich 3D carbon nanosheet for bifunctional oxygen electrocatalysis. J. Energy Chem. 2021, 59, 47–54. [Google Scholar] [CrossRef]
- Chen, D.; Pan, L.; Pei, P.; Huang, S.; Ren, P.; Song, X. Carbon-coated oxygen vacancies-rich Co3O4 nanoarrays grow on nickel foam as efficient bifunctional electrocatalysts for rechargeable zinc-air batteries. Energy 2021, 224, 120142. [Google Scholar] [CrossRef]
- Tang, C.; Zhong, L.; Zhang, B.; Wang, H.F.; Zhang, Q. 3D Mesoporous van der Waals heterostructures for trifunctional energy electrocatalysis. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Li, H.; Chen, J.; Fei, J.; Yu, Z.; Yuan, Z.; Wang, C.; Zheng, H.; Liu, Z.; et al. One-dimensional van der Waals heterostructures as efficient metal-free oxygen electrocatalysts. ACS Nano 2021, 15, 3309–3319. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Yang, L.; Yang, X.; Yu, L.; Lv, R. Defect engineering of van der Waals solids for electrocatalytic hydrogen evolution. Chem. An Asian J. 2020, 15, 3682–3695. [Google Scholar] [CrossRef]
- Song, D.-X.; Xie, L.; Zhang, Y.-F.; Lu, Y.; An, M.; Ma, W.-G.; Zhang, X. Multilayer ion load and diffusion on TMD/MXene heterostructure anodes for alkali-ion batteries. ACS. Appl. Energy Mat. 2020, 3, 7699–7709. [Google Scholar] [CrossRef]
- King’ori, G.W.; Ouma, C.N.M.; Mishra, A.K.; Amolo, G.O.; Makaua, N.W. Two-dimensional graphene–HfS2 van der Waals heterostructure as electrode material for alkali-ion batteries. RSC Adv. 2020, 10, 30127–30138. [Google Scholar] [CrossRef]
- Barbi, G.B.; Mari, C.M. Electrochemical kinetics of the water reduction at strontium doped lanthanum chromite (SDLC)/yttria stabilized zirconia interfaces at high temperature. Solid State Ion. 1983, 9, 979–988. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, G.; Gong, S.; Liu, C.; Wu, C.; Lyu, P.; Maurin, G.; Zhang, N. Design of MoS2/graphene van der Waals heterostructure as highly efficient and stable electrocatalyst for hydrogen evolution in acidic and alkaline media. ACS Appl. Mater. Interfaces 2020, 12, 24777–24785. [Google Scholar] [CrossRef]
- Puente Santiago, A.R.; He, T.; Eraso, O.; Ahsan, M.A.; Nair, A.N.; Chava, V.S.N.; Zheng, T.; Pilla, S.; Fernandez-Delgado, O.; Du, A.; et al. Tailoring the interfacial interactions of van der Waals 1T-MoS2/C60 heterostructures for high-performance hydrogen evolution reaction electrocatalysis. J. Am. Chem. Soc. 2020, 142, 17923–17927. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.R.; Chan, M.K.Y.; Sun, Y. Edge-terminated molybdenum disulfide with a 9.4-Å interlayer spacing for electrochemical hydrogen production. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Obligacion, J.K.A.; Putungan, D.B. 2D 1T′-MoS2-WS2van der Waals heterostructure for hydrogen evolution reaction: Dispersion-corrected density functional theory calculations. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Norskov, J.K.; Bligaard, T.; Rossmeil, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blackstone, C.; Ignaszak, A. Van der Waals Heterostructures—Recent Progress in Electrode Materials for Clean Energy Applications. Materials 2021, 14, 3754. https://doi.org/10.3390/ma14133754
Blackstone C, Ignaszak A. Van der Waals Heterostructures—Recent Progress in Electrode Materials for Clean Energy Applications. Materials. 2021; 14(13):3754. https://doi.org/10.3390/ma14133754
Chicago/Turabian StyleBlackstone, Chance, and Anna Ignaszak. 2021. "Van der Waals Heterostructures—Recent Progress in Electrode Materials for Clean Energy Applications" Materials 14, no. 13: 3754. https://doi.org/10.3390/ma14133754
APA StyleBlackstone, C., & Ignaszak, A. (2021). Van der Waals Heterostructures—Recent Progress in Electrode Materials for Clean Energy Applications. Materials, 14(13), 3754. https://doi.org/10.3390/ma14133754






