Effect of Bioactive Glass-Containing Light-Curing Varnish on Enamel Remineralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enamel Specimen Preparation and Experimental Materials
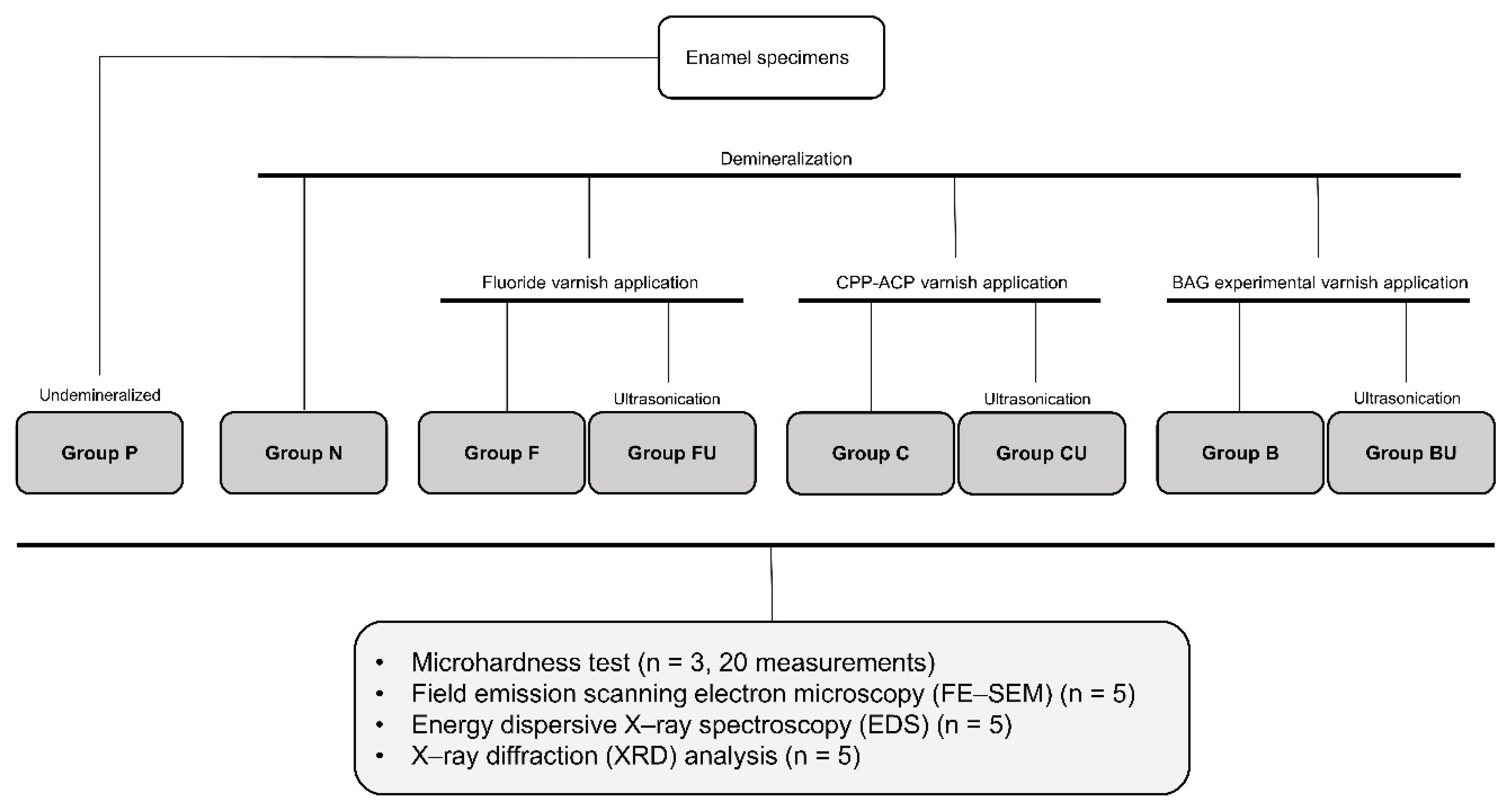
2.2. Experimental Groups
2.3. Microhardness Test
2.4. FE-SEM/EDS Analysis
2.5. XRD Analysis
2.6. Statistical Analysis
3. Results
3.1. Microhardness Test
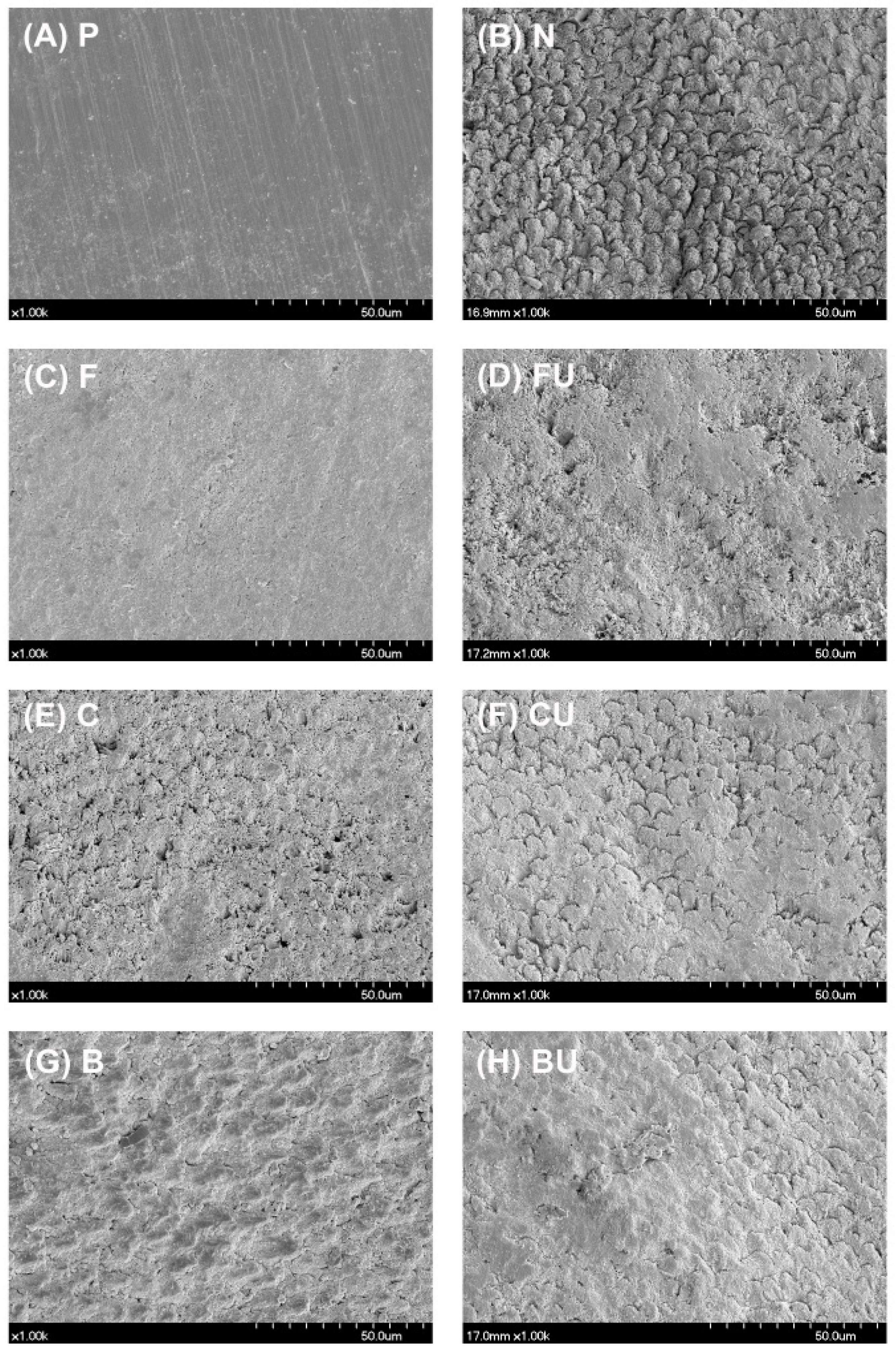
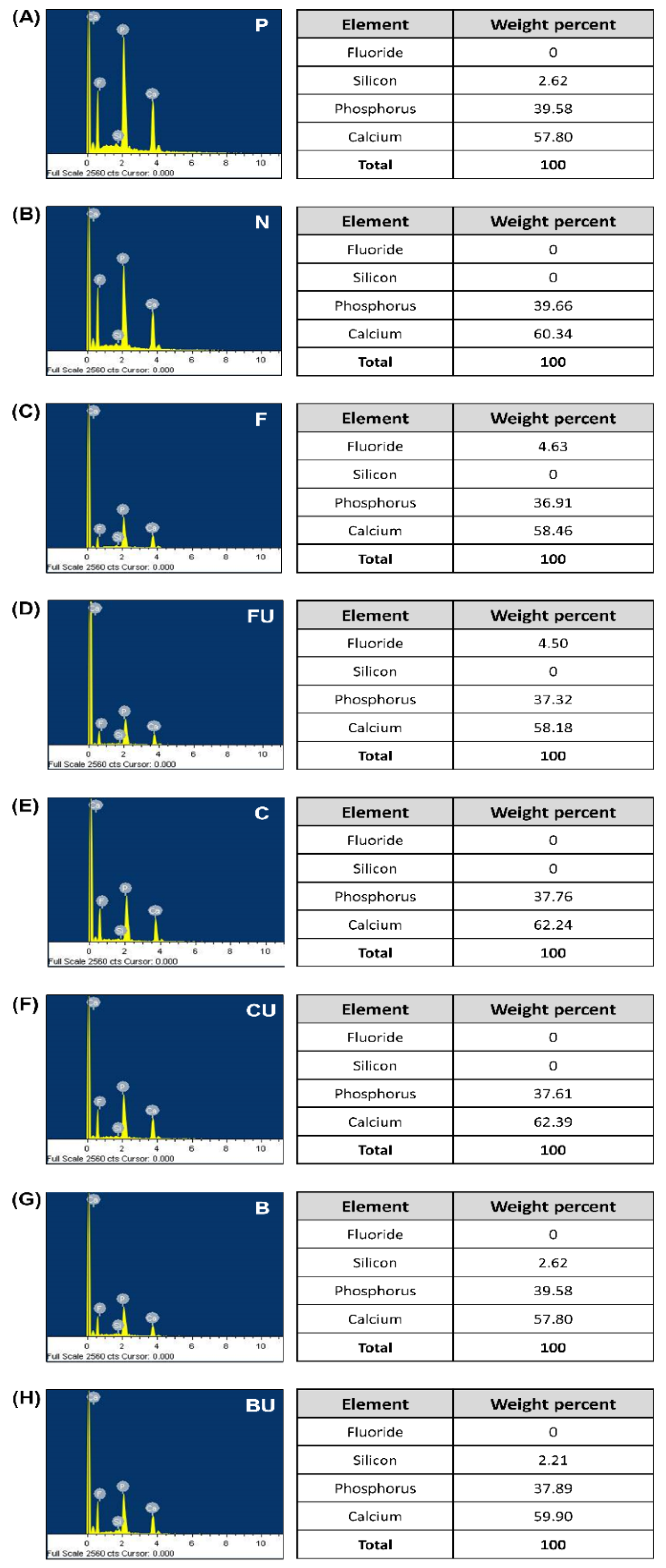
3.2. FE-SEM/EDS Analysis
3.3. XRD Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lippert, F.; Parker, D.M.; Jandt, K.D. In vitro demineralization/remineralization cycles at human tooth enamel surfaces investigated by AFM and nanoindentation. J. Colloid Interface Sci. 2004, 280, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, J.; Li, J.; Chen, L.; Tang, B.; Chen, X.; Wu, W.; Li, J. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 2013, 34, 5036–5047. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Cate, J.T. Tooth enamel remineralization. J. Cryst. Growth 1981, 53, 135–147. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, ume 11, 4743–4763. [Google Scholar] [CrossRef]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Bakry, A.S.; Marghalani, H.Y.; Amin, O.A.; Tagami, J. The effect of a bioglass paste on enamel exposed to erosive challenge. J. Dent. 2014, 42, 1458–1463. [Google Scholar] [CrossRef]
- Rošin-Grget, K.; Peroš, K.; Sutej, I. The cariostatic mechanisms of fluoride. Acta Medica Acad. 2013, 42, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Rahiotis, C.; Vougiouklakis, G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. J. Dent. 2007, 35, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.; Cai, F.; Shen, P.; Walker, G. Retention in Plaque and Remineralization of Enamel Lesions by Various Forms of Calcium in a Mouthrinse or Sugar-free Chewing Gum. J. Dent. Res. 2003, 82, 206–211. [Google Scholar] [CrossRef]
- Chen, H.; Gu, L.; Liao, B.; Zhou, X.; Cheng, L.; Ren, B. Advances of Anti-Caries Nanomaterials. Molecules 2020, 25, 5047. [Google Scholar] [CrossRef] [PubMed]
- Borzabadi-Farahani, A.; Borzabadi, E.; Lynch, E. Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol. Scand. 2013, 72, 413–417. [Google Scholar] [CrossRef]
- Moshaverinia, M.; Sameni, A.; Ansari, S.; Moshaverinia, A.; Borzabadi-Farahani, A. Effects of incorporation of nano-fluorapatite particles on microhardness, fluoride releasing properties, and biocompatibility of a conventional glass ionomer cement (GIC). Dent. Mater. J. 2016, 35, 817–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C 2011, 31, 1245–1256. [Google Scholar] [CrossRef] [Green Version]
- Thompson, I.D.; Hench, L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Milly, H.; Festy, F.; Watson, T.F.; Thompson, I.; Banerjee, A. Enamel white spot lesions can remineralise using bio-active glass and polyacrylic acid-modified bio-active glass powders. J. Dent. 2014, 42, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, M.; Brunner, T.J.; Knecht, S.; Grass, R.N.; Zehnder, M.; Imfeld, T.; Stark, W.J. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007, 3, 936–943. [Google Scholar] [CrossRef]
- Bakry, A.S.; Abbassy, M.A.; Alharkan, H.F.; Basuhail, S.; Al-Ghamdi, K.; Hill, R. A Novel Fluoride Containing Bioactive Glass Paste is Capable of Re-Mineralizing Early Caries Lesions. Materials 2018, 11, 1636. [Google Scholar] [CrossRef] [Green Version]
- Salehi, S.; Davis, H.B.; Ferracane, J.L.; Mitchell, J.C. Sol-gel-derived bioactive glasses demonstrate antimicrobial effects on common oral bacteria. Am. J. Dent. 2015, 28, 111–115. [Google Scholar]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Therapeutic effects of novel resin bonding systems containing bioactive glasses on mineral-depleted areas within the bonded-dentine interface. J. Mater. Sci. Mater. Med. 2012, 23, 1521–1532. [Google Scholar] [CrossRef]
- Davis, H.B.; Gwinner, F.; Mitchell, J.C.; Ferracane, J.L. Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent. Mater. 2014, 30, 1187–1194. [Google Scholar] [CrossRef] [Green Version]
- Khvostenko, D.; Hilton, T.; Ferracane, J.; Mitchell, J.; Kruzic, J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Khvostenko, D.; Mitchell, J.; Hilton, T.; Ferracane, J.; Kruzic, J. Mechanical performance of novel bioactive glass containing dental restorative composites. Dent. Mater. 2013, 29, 1139–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.-H.; Lee, M.G.; Ferracane, J.L.; Davis, H.; Bae, H.E.; Choi, D.; Kim, D.-S. Effect of bioactive glass-containing resin composite on dentin remineralization. J. Dent. 2018, 75, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Watson, T.F.; Thompson, I.; Toledano, M.; Nucci, C.; Banerjee, A. Influence of air-abrasion executed with polyacrylic acid-Bioglass 45S5 on the bonding performance of a resin-modified glass ionomer cement. Eur. J. Oral Sci. 2012, 120, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Bae, H.E.; Lee, J.-E.; Park, I.-S.; Kim, H.-G.; Kwon, J.; Kim, D.-S. Effects of bioactive glass incorporation into glass ionomer cement on demineralized dentin. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Mehta, A.; Paramshivam, G.; Chugh, V.K.; Singh, S.; Halkai, S.; Kumar, S. Effect of light-curable fluoride varnish on enamel demineralization adjacent to orthodontic brackets: An in-vivo study. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.B.; Gao, S.S.; Yu, H.Y. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mater. 2009, 4, 034104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cengiz, B.; Gokce, Y.; Yildiz, N.; Aktas, Z.; Calimli, A. Synthesis and characterization of hydroxyapatite nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 29–33. [Google Scholar] [CrossRef]
- Aljabo, A.; Neel, E.A.A.; Knowles, J.C.; Young, A.M. Development of dental composites with reactive fillers that promote precipitation of antibacterial-hydroxyapatite layers. Mater. Sci. Eng. C 2016, 60, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Brundavanam, R.K.; Poinern, G.E.J.; Fawcett, D. Modelling the crystal structure of a 30 nm sized particle based hydroxyapatite powder synthesised under the influence of ultrasound irradiation from X-ray powder diffraction data. Am. J. Mater. Sci. 2013, 3, 84–90. [Google Scholar]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Gavic, L.; Gorseta, K.; Borzabadi-Farahani, A.; Tadin, A.; Glavina, D.; Gavic, L.; Gorseta, K.; Borzabadi-Farahani, A.; Tadin, A.; Glavina, D. Influence of toothpaste pH on its capacity to prevent enamel demineralization. Contemp. Clin. Dent. 2018, 9, 554–559. [Google Scholar] [CrossRef] [PubMed]
- González-Cabezas, C.; Fernandez, C.E. Recent Advances in Remineralization Therapies for Caries Lesions. Adv. Dent. Res. 2018, 29, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; Van Loveren, C. Fluorides and non-fluoride remineralization systems. Toothpastes 2013, 23, 15–26. [Google Scholar]
- Fernández, C.E.; Fontana, M.; Samarian, D.; Cury, J.A.; Rickard, A.H.; González-Cabezas, C. Effect of Fluoride-Containing Toothpastes on Enamel Demineralization and Streptococcus mutans Biofilm Architecture. Caries Res. 2016, 50, 151–158. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Cai, F.; Cochrane, N.J.; Shen, P.; Walker, G.D.; Morgan, M.V. Fluoride and Casein Phosphopeptide-Amorphous Calcium Phosphate. J. Dent. Res. 2008, 87, 344–348. [Google Scholar] [CrossRef]
- Lagerweij, M.; Cate, J.T. Remineralisation of Enamel Lesions with Daily Applications of a High-Concentration Fluoride Gel and a Fluoridated Toothpaste: An in situ Study. Caries Res. 2002, 36, 270–274. [Google Scholar] [CrossRef]
- Haugejorden, O.; Birkeland, J.M. Ecological time-trend analysis of caries experience at 12 years of age and caries incidence from age 12 to 18 years: Norway 1985–2004. Acta Odontol. Scand. 2006, 64, 368–375. [Google Scholar] [CrossRef]
- Li, J.; Xie, X.; Wang, Y.; Yin, W.; Antoun, J.S.; Farella, M.; Mei, L. Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in vivo: A systematic review. J. Dent. 2014, 42, 769–777. [Google Scholar] [CrossRef]
- Wu, G.; Liu, X.; Hou, Y. Analysis of the effect of CPP-ACP tooth mousse on enamel remineralization by circularly polarized images. Angle Orthod. 2010, 80, 933–938. [Google Scholar] [CrossRef]
- ZERO, D.T. Recaldent™—Evidence for clinical activity. Adv. Dent. Res. 2009, 21, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Andersson, Ö.H.; Kangasniemi, I. Calcium phosphate formation at the surface of bioactive glass in vitro. J. Biomed. Mater. Res. 1991, 25, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Burwell, A.K.; Litkowski, L.J.; Greenspan, D.C. Calcium Sodium Phosphosilicate (NovaMin®): Remineralization Potential. Adv. Dent. Res. 2009, 21, 35–39. [Google Scholar] [CrossRef]
- Bakry, A.S.; Takahashi, H.; Otsuki, M.; Sadr, A.; Yamashita, K.; Tagami, J. CO2 Laser Improves 45S5 Bioglass Interaction with Dentin. J. Dent. Res. 2010, 90, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Girot, A.; Mezahi, F.Z.; Mami, M.; Oudadesse, H.; Harabi, A.; Le Floch, M. Sol–gel synthesis of a new composition of bioactive glass in the quaternary system SiO2–CaO–Na2O–P2O5: Comparison with melting method. J. Non-Cryst. Solids 2011, 357, 3322–3327. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Lukowiak, A.; Lao, J.; Lacroix, J.; Nedelec, J.-M. Bioactive glass nanoparticles obtained through sol–gel chemistry. Chem. Commun. 2013, 49, 6620–6622. [Google Scholar] [CrossRef]
- Bakry, A.; Takahashi, H.; Otsuki, M.; Tagami, J. Evaluation of new treatment for incipient enamel demineralization using 45S5 bioglass. Dent. Mater. 2014, 30, 314–320. [Google Scholar] [CrossRef]
- Golpayegani, M.V.; Sohrabi, A.; Biria, M.; Ansari, G. Remineralization Effect of Topical NovaMin Versus Sodium Fluoride (1.1%) on Caries-Like Lesions in Permanent Teeth. J. Dent. 2012, 9, 68–75. [Google Scholar]
- Benjasuwantep, P.; Rirattanapong, P.; Vongsavan, K. The remineralization effect of bioactive glass on enamel caries-like lesions in primary teeth. Southeast Asian J. Trop. Med. 2017, 48, 1127–1132. [Google Scholar]
- Al-Khafaji, T.J.; Wong, F.; Fleming, P.S.; Karpukhina, N.; Hill, R. Novel fluoride and strontium-containing bioactive glasses for dental varnishes-design and bioactivity in Tris buffer solution. J. Non-Cryst. Solids 2019, 503–504, 120–130. [Google Scholar] [CrossRef]
- Almeida, A.P.G.; Braz, D.; Colaço, M.V.; Barroso, R.C.; Porto, I.M.; Gerlach, R.F.; Droppa, R. Analysis of synchrotron X-ray diffraction patterns from fluorotic enamel samples. In Proceedings of the International Nuclear Atlantic Conference, Rio de Janeiro, RJ, Brazil, 27 September–2 October 2009. [Google Scholar]
- Mneimne, M.; Hill, R.G.; Bushby, A.J.; Brauer, D.S.; Mneimne, M.; Hill, R.G.; Bushby, A.J.; Brauer, D.S. High phosphate content significantly increases apatite formation of fluoride-containing bioactive glasses. Acta Biomater. 2011, 7, 1827–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Materials | Composition | Applications |
|---|---|---|
| Fluoride varnish (Nupro White; Dentsply international, York, PA, USA) | Urethane Dimethacrylate Resin 30–40 wt% 2-Propanol 20–30 wt% Sodium Fluoride 5 wt% Titanium Dioxide <1 wt% | Apply once (2 h) |
| CPP-ACP Varnish (Tooth Mousse; GC Corporation Tokyo, Japan) | CPP-ACP 10 wt% Glycerol 20 wt% D-Sorbitol 5 wt% Silicon dioxide 2 wt% Propylene glycol 2 wt% Titanium dioxide 2 wt% Ethyl-p-hydroxybenzoate <0.1 wt% Butyl-p-hydroxybenzoate <0.1 wt% Propyl-p-hydroxybenzoate <0.1 wt% | Apply daily for 7 days (30 min per day) |
| BAG-containing Varnish (Experimentally manufactured) | 63S Bioglass 30 wt% UDMA (Diurethane dimethacrylate) 31.36 wt% HEMA (Ethylene glycol methacrylate) 9.24 wt% CQ (Camphorquinone) 0.35 wt% EDMAB (ethyl-4-dimethylaminobenzoate) 0.7 wt% BHT (2,6-di-tert-butyl-4-methylphenol) 0.175 wt% TP (2,2-(P-Tolylimino)-diethanol) 0.175 wt% EtOH 28 wt% | Indirect application; Light curing (20 s) after applying the varnish on composite resin block and fixing the applied surface and enamel surface in tight contact |
| Groups | Description | Procedures |
|---|---|---|
| Group P | Positive control (Undemineralized enamel) | Untreated |
| Group N | Negative control (Demineralized enamel) | Demineralization (37% phosphoric acid, 20 min) |
| Group F | Nupro White Varnish | Demineralization (37% phosphoric acid, 20 min) → Nupro White Varnish application (once, 2 h) → Storage (artificial saliva, 2 weeks) |
| Group FU | Nupro White Varnish + Ultrasonication | Demineralization (37% phosphoric acid, 20 min) → Nupro White Varnish application (once, 2 h) → Storage (artificial saliva, 2 weeks) → Ultrasonication (3 min) |
| Group C | Tooth mousse | Demineralization (37% phosphoric acid, 20 min) → Tooth mousse application (for 7 days, 30 min per day) → Storage (artificial saliva, 2 weeks) |
| Group CU | Tooth mousse + Ultrasonication | Demineralization (37% phosphoric acid, 20 min) → Tooth mousse application (for 7 days, 30 min per day) → Storage (artificial saliva, 2 weeks) → Ultrasonication (3 min) |
| Group B | BAG-containing varnish | Demineralization (37% phosphoric acid, 20 min) → BAG varnish application (indirectly, light curing 20 s) → Storage (artificial saliva, 2 weeks) |
| Group BU | BAG-containing varnish + Ultrasonication | Demineralization (37% phosphoric acid, 20 min) → BAG varnish application (indirectly, light curing 20 s) → Storage (artificial saliva, 2 weeks) → Ultrasonication (3 min) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Mo, S.-Y.; Kim, D.-S. Effect of Bioactive Glass-Containing Light-Curing Varnish on Enamel Remineralization. Materials 2021, 14, 3745. https://doi.org/10.3390/ma14133745
Kim H-J, Mo S-Y, Kim D-S. Effect of Bioactive Glass-Containing Light-Curing Varnish on Enamel Remineralization. Materials. 2021; 14(13):3745. https://doi.org/10.3390/ma14133745
Chicago/Turabian StyleKim, Hyun-Jung, So-Yeon Mo, and Duck-Su Kim. 2021. "Effect of Bioactive Glass-Containing Light-Curing Varnish on Enamel Remineralization" Materials 14, no. 13: 3745. https://doi.org/10.3390/ma14133745
APA StyleKim, H.-J., Mo, S.-Y., & Kim, D.-S. (2021). Effect of Bioactive Glass-Containing Light-Curing Varnish on Enamel Remineralization. Materials, 14(13), 3745. https://doi.org/10.3390/ma14133745








