Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review
Abstract
:1. Introduction
Research Approach
2. Advanced Oxidation Process (AOP)
2.1. TiO2 Photocatalyst
2.1.1. TiO2 Modification Supports
- Cationic doping of TiO2
- Anionic doping of TiO2
- Other supporting metallic oxides
2.1.2. Mechanism of TiO2 Photocatalysis
2.2. Operational Parameters in Photocatalysis
- pH
- Catalyst loading
- Light intensity
- Catalyst type and temperature
- Dissolved oxygen and contaminant concentration
3. Integrated Photocatalytic Membrane (IPM) Reactors
3.1. Types of IPM Reactors
3.2. Freestanding Pure TiO2-Based Membrane Reactors
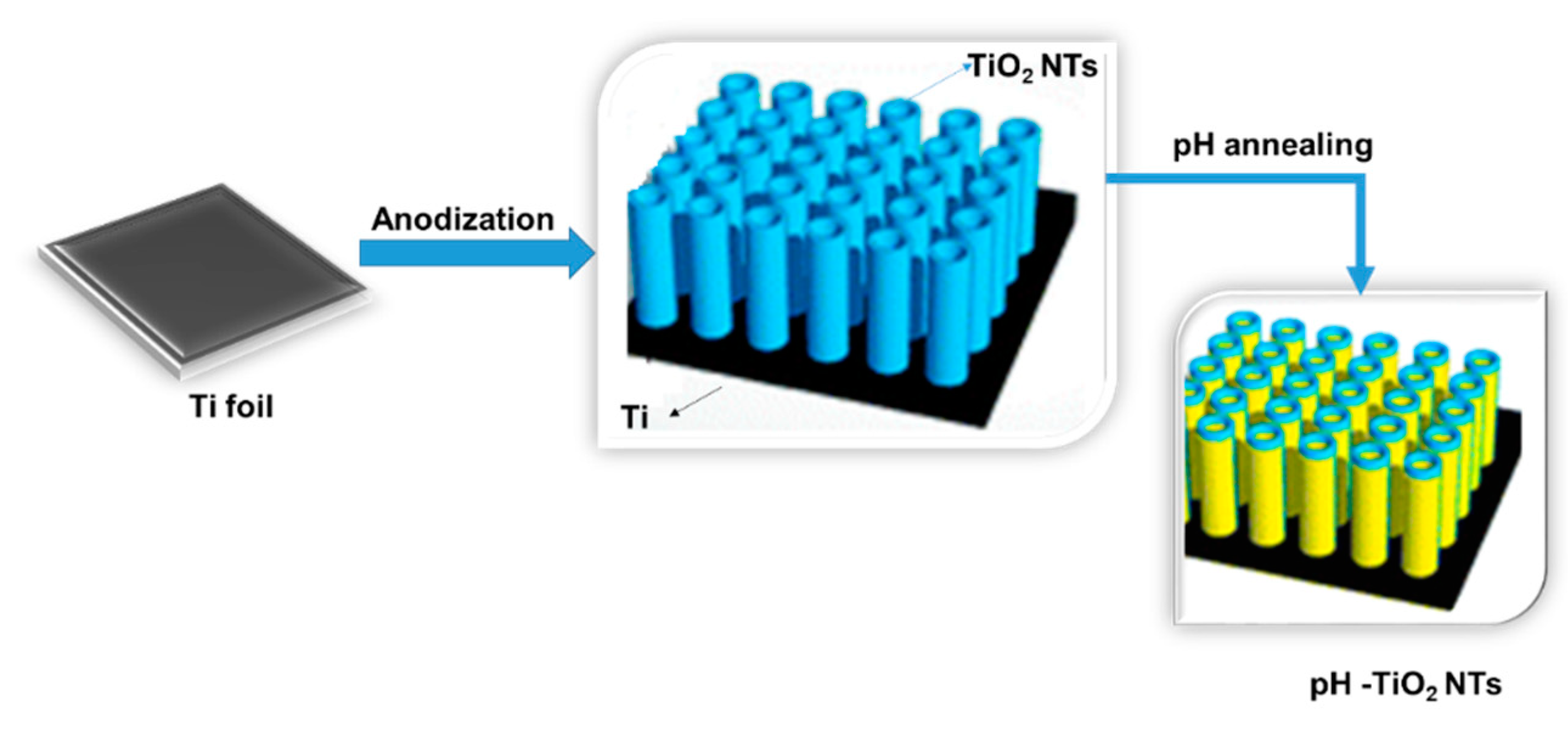
3.2.1. TiO2 Nanotube-Based Reactors
3.2.2. TiO2 Nanofiber-Based Membranes
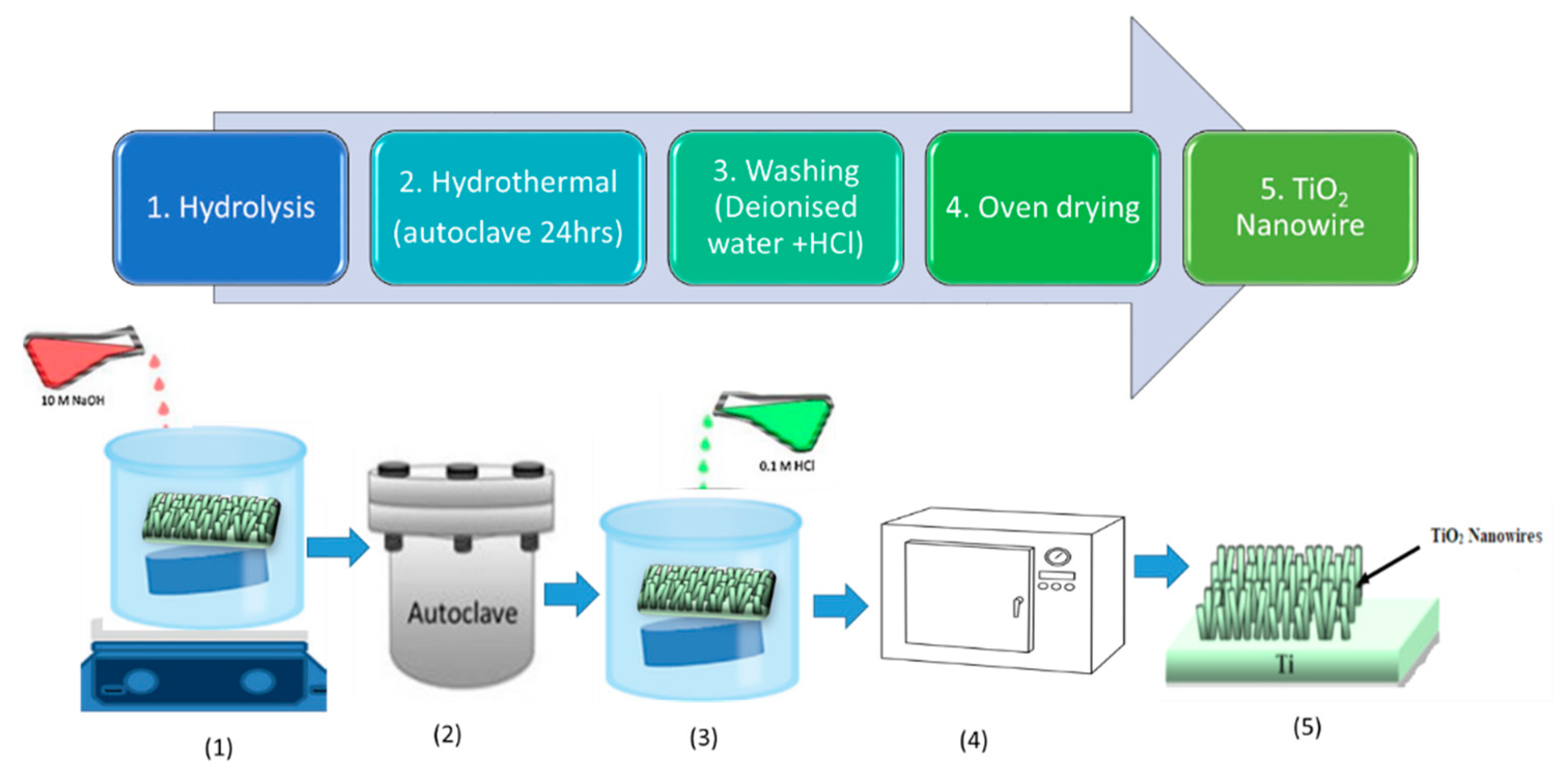
3.2.3. TiO2 Nanowires
3.3. Composite TiO2 Photocatalytic Membrane Reactors
3.3.1. TiO2 Nanoparticle-Coated Polymer Membranes
3.3.2. TiO2-Based Polymeric Membranes
3.3.3. TiO2 Ceramic Membranes
3.4. TiO2-Based Membrane Modification Techniques
3.5. Characteristics of Photocatalytic Membranes
3.6. Application of TiO2-Based Membranes
3.6.1. Wastewater Treatment
3.6.2. Other Industrial Applications
4. Challenges and Future Prospects of Photocatalytic Membranes
4.1. Photocatalytic Membranes
4.2. Magnetized TiO2 Photocatalytic Membranes
4.3. Life Cycle Assessment (LCA)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tetteh, E.K.; Amankwa, M.O.; Armah, E.K.; Rathilal, S. Fate of covid-19 occurrences in wastewater systems: Emerging detection and treatment technologies—A review. Water 2020, 12, 2680. [Google Scholar] [CrossRef]
- Zhang, J.; Tong, H.; Pei, W.; Liu, W.; Shi, F.; Li, Y.; Huo, Y. Integrated photocatalysis-adsorption-membrane separation in rotating reactor for synergistic removal of RhB. Chemosphere 2021, 270. [Google Scholar] [CrossRef]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710. [Google Scholar] [CrossRef]
- Schutte, C.F.; Focke, W. Evaluation of Nanotechnology for Application in Water and Wastewater Treatment and Related Aspects in South Africa; Water Research Commission: Pretoria, South Africa, 2007; p. 28. [Google Scholar]
- Chollom, M.N.; Rathilal, S.; Swalaha, F.M.; Bakare, B.F.; Tetteh, E.K. Removal of antibiotics during the anaerobic digestion of slaughterhouse wastewater. Int. J. Sustain. Dev. Plan. 2020, 15. [Google Scholar] [CrossRef]
- Anvari, A.; Amoli-Diva, M.; Sadighi-Bonabi, R. Concurrent photocatalytic degradation and filtration with bi-plasmonic TiO2 for wastewater treatment. Micro Nano Lett. 2021, 16. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Amo-Duodu, G. Desalination of municipal wastewater using forward osmosis. Membranes 2021, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Chollom, M.N.; Rathilal, S.; Swalaha, F.M.; Bakare, B.F.; Tetteh, E.K. Anaerobic treatment of slaughterhouse wastewater: Evaluating operating conditions. WIT Trans. Ecol. Environ. 2019, 239. [Google Scholar] [CrossRef]
- della Rocca, D.G.; Peralta, R.M.; Peralta, R.A.; Moreira, R.d.P.M. Recent development on Ag2MoO4-based advanced oxidation processes: A review. React. Kinet. Mech. Catal. 2021, 132. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, X.; Zhang, Y.; Zhu, Y.; Long, C.; Su, L.; Liu, S.; Tang, Z. Applications of Nanomaterials in Asymmetric Photocatalysis: Recent Progress, Challenges, and Opportunities. Adv. Mater. 2021, 33. [Google Scholar] [CrossRef]
- Naddeo, V.; Balakrishnan, M.; Choo, K.-H. Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability: Proceedings of the 2nd WaterEnergyNEXUS Conference; Springer Nature: Salemo, Italy, 2019. [Google Scholar]
- Wang, Z.; Wang, L.; Yao, L.; Pei, M.; Zhang, G. Membrane separation coupled with photocatalysis for water supply and wastewater treatment. Adv. Mater. Res. 2013, 671–674, 2571–2574. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Szymański, K.; Darowna, D.; Mozia, S. Photocatalytic membrane reactors for wastewater treatment. In Current Trends and Future Developments on (Bio-) Membranes: Membrane Technology for Water and Wastewater Treatment Advances and Emerging Processes; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Cai, Z.; Dwivedi, A.D.; Lee, W.N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Jia, R.; Shao, J.; He, Y. Enhanced catalytic degradation of amoxicillin with TiO2-Fe3O4 composites: Via a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). RSC Adv. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Lavorato, C.; Argurio, P.; Molinari, R. TiO2 and Pd/TiO2 as Photocatalysts for Hydrogenation of Ketones and Perspective of Membrane Application. Int. J. Adv. Res. Chem. Sci. 2019, 6, 33–41. [Google Scholar] [CrossRef]
- Indonesia, G.B. Development of TiO2 Nanoparticles/Nanosolution for Photocatalytic Activity. Ph.D. Thesis, Universiti Sains Malaysia, Penang, Malaysia, 2015. [Google Scholar]
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A review on UV/TiO 2 photocatalytic oxidation process. Korean J. Chem. Eng. 2008, 25, 64–72. [Google Scholar] [CrossRef]
- Zheng, Z. Synthesis and Modifications of Metal Oxide Nanostructures and Their Applications. Ph.D. Thesis, Queensland University of Technology Brisbane Australia, Brisbane, Australia, 2009; p. 27. [Google Scholar]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2015, 4, 582–598. [Google Scholar] [CrossRef]
- Luo, H.; Yan, M.; Wu, Y.; Lin, X.; Yan, Y. Facile synthesis of PVDF photocatalytic membrane based on NCQDs/BiOBr/TiO2 heterojunction for effective removal of tetracycline. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 265. [Google Scholar] [CrossRef]
- Choi, H.; Sofranko, A.C.; Dionysiou, D.D. Nanocrystalline TiO2 photocatalytic membranes with a hierarchical mesoporous multilayer structure: Synthesis, characterization, and multifunction. Adv. Funct. Mater. 2006, 16. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Das, A.P. Modified titanium oxide (TiO2) nanocomposites and its array of applications: A review. Toxicol. Environ. Chem. 2015, 97. [Google Scholar] [CrossRef]
- Fua, J.; Wanga, X.; Mac, Z.; Wenmingd, H.; Lie, J.; Wanga, Z.; Wanga, L. Photocatalytic ultrafiltration membranes based on visible light responsive photocatalyst: A review. Desalin. Water Treat. 2019, 168. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Ghaedi, M.; Karimi, H. Photocatalytic activity based on electrospun nanofibers. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 32, pp. 625–672. [Google Scholar]
- Umar, M.; Aziz, H.A. Photocatalytic degradation of organic pollutants in water. Organic pollutants-monitoring, risk and treatment. Org. Pollut.-Monit. Risk Treat. 2013, 30, 196–197. [Google Scholar]
- Padmanabhan, N.T.; John, H. Titanium dioxide based self-cleaning smart surfaces: A short review. J. Environ. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379. [Google Scholar] [CrossRef]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, A.; Pomilla, F.R.; Marcì, G.; Garcia-Lopez, E.I.; Fontananova, E.; Palmisano, L.; Barbieri, G. CO2 reduction by C3N4-TiO2 Nafion photocatalytic membrane reactor as a promising environmental pathway to solar fuels. Appl. Catal. B Environ. 2019, 255. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Ezugbe, E.O.; Rathilal, S.; Asante-Sackey, D. Removal of COD and SO4 from oil refinery wastewater using a photo-catalytic system-comparing TiO2 and zeolite efficiencies. Water 2020, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Li, J.; Shen, W.; Corriou, J.P.; Chen, X.; Xi, H. Different photocatalytic levels of organics in papermaking wastewater by flocculation-photocatalysis and SBR-photocatalysis: Degradation and GC–MS experiments, adsorption and photocatalysis simulations. Chem. Eng. J. 2021, 412. [Google Scholar] [CrossRef]
- Salmerón, I.; Sharma, P.K.; Polo-López, M.I.; Tolosana, A.; McMichael, S.; Oller, I.; Byrne, J.A.; Fernández-Ibáñez, P. Electrochemically assisted photocatalysis for the simultaneous degradation of organic micro-contaminants and inactivation of microorganisms in water. Process Saf. Environ. Prot. 2021, 147. [Google Scholar] [CrossRef]
- Dominguez, S.; Huebra, M.; Han, C.; Campo, P.; Nadagouda, M.N.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Magnetically recoverable TiO 2 -WO 3 photocatalyst to oxidize bisphenol A from model wastewater under simulated solar light. Environ. Sci. Pollut. Res. 2017, 24, 12589–12598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otieno, O.V.; Csáki, E.; Kéri, O.; Simon, L.; Lukács, I.E.; Szécsényi, K.M.; Szilágyi, I.M. Synthesis of TiO2 nanofibers by electrospinning using water-soluble Ti-precursor. J. Therm. Anal. Calorim. 2020, 139. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Zhao, J. Preparation and photocatalytic properties of a novel kind of loaded photocatalyst of TiO2/SiO2/γ-Fe2O3. Catal. Letters 1999, 58. [Google Scholar] [CrossRef]
- Faraldos, M.; Bahamonde, A. Environmental applications of titania-graphene photocatalysts. Catal. Today 2017, 285. [Google Scholar] [CrossRef]
- Esfandiari, N.; Kashefi, M.; Afsharnezhad, S.; Mirjalili, M. Insight into enhanced visible light photocatalytic activity of Fe3O4–SiO2–TiO2 core-multishell nanoparticles on the elimination of Escherichia coli. Mater. Chem. Phys. 2020, 244. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Li, P.; Shao, J.; He, Y. Photo-Fenton degradation of amoxicillin via magnetic TiO2-graphene oxide-Fe3O4 composite with a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). J. Hazard. Mater. 2019, 373. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Y.; Shen, L.; Xu, Y.; Li, R.; Huang, L.; Lin, H. Magnetic field assisted arrangement of photocatalytic TiO2 particles on membrane surface to enhance membrane antifouling performance for water treatment. J. Colloid Interface Sci. 2020, 570. [Google Scholar] [CrossRef]
- Zhao, T.; Qian, R.; Zhou, G.; Wang, Y.; Lee, W.I.; Pan, J.H. Mesoporous WO3/TiO2 spheres with tailored surface properties for concurrent solar photocatalysis and membrane filtration. Chemosphere 2021, 263. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Ghalamchi, L.; Vatanpour, V.; Khataee, A. Photocatalytic-membrane technology: A critical review for membrane fouling mitigation. J. Ind. Eng. Chem. 2021, 93. [Google Scholar] [CrossRef]
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochem. 2021, 71. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 Nanotube Photocatalysts for Solar Water Splitting. Adv. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Mamba, B.B. Photocatalytic Membranes for Efficient Water Treatment. In Semiconductor Photocatalysis Materials, Mechanisms and Applications; IntechOpen: London, UK, 2016. [Google Scholar]
- di Paola, A.; Bellardita, M.; Palmisano, L. Brookite the least known TiO2 photocatalyst. Catalysts 2013, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Shayegan, Z.; Lee, C.S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334. [Google Scholar] [CrossRef] [Green Version]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, S.; Zbořil, R.; Schmuki, P. Photocatalysis with reduced TiO 2: From Black TiO 2 to cocatalyst-free hydrogen production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Vaiano, V.; Sacco, O.; Sannino, D.; Stoller, M.; Ciambelli, P.; Chianese, A. Photocatalytic removal of phenol by ferromagnetic N-TiO2/SiO2/Fe3O4 nanoparticles in presence of visible light irradiation. Chem. Eng. Trans. 2016, 47, 235–240. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Shende, T.P.; Sonawane, S.H. A review on graphene–TiO2 and doped graphene–TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Technol. Rev. 2017, 6. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19. [Google Scholar] [CrossRef]
- Sharma, A.; Liu, N.; Ma, Q.; Zheng, H.; Kawazoe, N.; Chen, G.; Yang, Y. PEG assisted P/Ag/Ag2O/Ag3PO4/TiO2 photocatalyst with enhanced elimination of emerging organic pollutants in salinity condition under solar light illumination. Chem. Eng. J. 2020, 385. [Google Scholar] [CrossRef]
- Izadpanah, M.; Sarrafzadeh, M.H.; Rezaei-Dashtarzhandi, M.; Vojoudi, H. Aquatic center sewage reclamation and water reuse, using an integrated system combining adsorption, RO membrane system, and TiO2/Fe3O4photocatalytic oxidation. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Li, S.; Liu, Y. Enhanced photocatalytic degradation of organic dyes by ultrasonic-assisted electrospray TiO2/graphene oxide on polyacrylonitrile/β-cyclodextrin nanofibrous membranes. Ultrason. Sonochem. 2021, 70. [Google Scholar] [CrossRef]
- Lakshmanan, R. Application of Magnetic Nanoparticles and Reactive Filter Materials for Wastewater Treatment Ramnath Lakshmanan. Ph.D. Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2013. [Google Scholar]
- Ahangar, L.E.; Movassaghi, K.; Emadi, M.; Yaghoobi, F. Photocatalytic application of TiO2 / SiO2-based magnetic nanocomposite (Fe3O4 @ SiO2 / TiO2) for reusing of textile wastewater. Nano Chem. Res. 2016, 1, 33–39. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Y.; Zheng, J. An efficient, stable and reusable polymer/TiO2 photocatalytic membrane for aqueous pollution treatment. J. Mater. Sci. 2021. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Venkatesan, U.; Marthala, V.R.; Hartmann, M.; Winter, B.; Spiecker, E.; Osvet, A.; Zolnhofer, E.M.; et al. Hydrogenated anatase: Strong photocatalytic dihydrogen evolution without the use of a co-catalyst. Angew. Chemie Int. Ed. 2014, 53, 14201–14205. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, S.; Smith, K.; Wang, Y.; Hu, H. Light-driven breakdown of 1,4-Dioxane for potable reuse: A review. Chem. Eng. J. 2019, 373. [Google Scholar] [CrossRef]
- Pirilä, M. Adsorption and Photocatalysis in Water Treatment: Active, Abundant and Inexpensive Materials and Methods; Acta Universitatis Ouluensis, University of Oulu: Oulu, Finland, 2015. [Google Scholar]
- Barakat, N.A.M.; Erfan, N.A.; Mohammed, A.A.; Mohamed, S.E.I. Ag-decorated TiO2 nanofibers as Arrhenius equation-incompatible and effective photocatalyst for water splitting under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.D.; Luo, S.; Liu, Z.; Gamalski, A.D.; Tao, J.; Xu, W.; Stach, E.A.; Polyansky, D.E.; Senanayake, S.D.; Fujita, E.; et al. Striving Toward Noble-Metal-Free Photocatalytic Water Splitting: The Hydrogenated-Graphene − TiO 2 Prototype. Chem. Mater. 2015, 27, 6282–6296. [Google Scholar] [CrossRef]
- Chen, F.; Xie, Y.; Zhao, J.; Lu, G. Photocatalytic degradation of dyes on a magnetically separated photocatalyst under visible and UV irradiation. Chemosphere 2001, 44. [Google Scholar] [CrossRef]
- Zhang, A.-P.; Sun, Y.-P. Photocataiytic killing effect of Fe3O4-TiO2nanopartic1es on hepatoma carcinoma cells for targeting photodynamic therapy. Chinese J. Inorg. Chem. 2017, 32. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumran, S.; Jegatheesan, V. Factors affecting the degradation of remazol turquoise blue (RTB) dye by titanium dioxide (TiO2) entrapped photocatalytic membrane. J. Environ. Manag. 2020, 272. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Rathilal, S. Kinetics and nanoparticle catalytic enhancement of biogas production from wastewater using a magnetized biochemical methane potential (Mbmp) system. Catalysts 2020, 10, 1200. [Google Scholar] [CrossRef]
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-doped electrospun nanofibrous membrane for photocatalytic water treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef] [Green Version]
- Ghernaout, D. Magnetic field generation in the water treatment perspectives: An overview. Int. J. Adv. Appl. Sci. 2017, 5, 193–203. [Google Scholar] [CrossRef]
- Štefušová, K.; Václavíková, M.; Lovás, M.; Hredzák, S. Use of magnetic filtration in wastewater treatment. Acta Montan. Slovaca 2012, 17, 81–84. [Google Scholar]
- Yao, H.; Fan, M.; Wang, Y.; Luo, G.; Fei, W. Magnetic titanium dioxide-based nanomaterials: Synthesis, characteristics, and photocatalytic application in pollutant degradation. J. Mater. Chem. A 2015, 3, 17511–17524. [Google Scholar] [CrossRef]
- Marinko, Ž.; Suhadolnik, L.; Samardžija, Z.; Kovač, J.; Čeh, M. The influence of a surface treatment of metallic titanium on the photocatalytic properties of TiO2 nanotubes grown by anodic oxidation. Catalysts 2020, 10, 803. [Google Scholar] [CrossRef]
- Tetteh, E.K.; Naidoo, D.B.; Rathilal, S. Optimization of photo-catalytic degradation of oil refinery wastewater using Box-Behnken design. Environ. Eng. Res. 2019, 24. [Google Scholar] [CrossRef]
- Heng, J.Z.X.; Tang, K.Y.; Regulacio, M.D.; Lin, M.; Loh, X.J.; Li, Z.; Ye, E. Solar-powered photodegradation of pollutant dyes using silver-embedded porous TiO2 nanofibers. Nanomaterials 2021, 11, 856. [Google Scholar] [CrossRef]
- Sharotri, N.; Sharma, D.; Sud, D. Experimental and theoretical investigations of Mn-N-co-doped TiO2 photocatalyst for visible light induced degradation of organic pollutants. J. Mater. Res. Technol. 2019, 8. [Google Scholar] [CrossRef]
- Liu, H.; Dao, A.Q.; Fu, C. Activities of combined TiO2 semiconductor nanocatalysts under solar light on the reduction of CO2. J. Nanosci. Nanotechnol. 2016, 16. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Huang, C.W. In situ DRIFTS study of photocatalytic CO 2 reduction under UV irradiation. Front. Chem. Eng. China 2010, 4, 120–126. [Google Scholar] [CrossRef]
- Goei, R.; Lim, T.T. Ag-decorated TiO2 photocatalytic membrane with hierarchical architecture: Photocatalytic and anti-bacterial activities. Water Res. 2014, 59. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Zhou, X.; He, X.; Zhou, M.; Jia, K.; Liu, X. Design of polymer composite-based porous membrane for in-situ photocatalytic degradation of adsorbed organic dyes. J. Phys. Chem. Solids 2021, 154. [Google Scholar] [CrossRef]
- Jia, Y.; Cai, F.; Lu, S.; Yang, F.; Zhao, Y. Research on the preparation and application of through-hole TiO2 nanotube arrays membranes. Cailiao Daobao/Materials Rev. 2016, 30. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44. [Google Scholar] [CrossRef]
- Penboon, L.; Khrueakham, A.; Sairiam, S. TiO2 coated on PVDF membrane for dye wastewater treatment by a photocatalytic membrane. Water Sci. Technol. 2019, 79. [Google Scholar] [CrossRef]
- Bergamasco, R.; Coldebella, P.F.; Camacho, F.P.; Rezende, D.; Yamaguchi, N.U.; Klen, M.R.F.; Tavares, C.J.M.; Amorim, M.T.S.P. Self-assembly modification of polyamide membrane by coating titanium dioxide nanoparticles for water treatment applications. Rev. Ambient. E Agua 2019, 14. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Łożyńska, M.; Barszcz, W.; Sowa, S.; Wieciński, P.; Woskowicz, E. Microfiltration membranes modified with composition of titanium oxide and silver oxide by magnetron sputtering. Polymers 2021, 13, 141. [Google Scholar] [CrossRef]
- Ou, W.; Pan, J.; Liu, Y.; Li, S.; Li, H.; Zhao, W.; Wang, J.; Song, C.; Zheng, Y.; Li, C. Two-dimensional ultrathin MoS2-modified black Ti3+–TiO2 nanotubes for enhanced photocatalytic water splitting hydrogen production. J. Energy Chem. 2020, 43. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Shi, M.; Xu, F.; Qiu, Y.; Zhang, P.; Shen, K.; Zhao, Q.; Yu, J.; Zhang, Y. Graphdiyne-modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, Y.; Guo, J.; Wu, S.; Chen, N.; Fan, H.; Gao, M.; Yang, J.; Sheng, Z.; Lang, J. One-step hydrothermal synthesis of the modified carbon cloth membrane: Towards visible light driven and self-cleaning for efficient oil-water separation. Surf. Coatings Technol. 2021, 409. [Google Scholar] [CrossRef]
- Arifin, K.; Yunus, R.M.; Minggu, L.J.; Kassim, M.B. Improvement of TiO2 nanotubes for photoelectrochemical water splitting: Review. Int. J. Hydrogen Energy 2021, 46. [Google Scholar] [CrossRef]
- Fu, F.; Cha, G.; Denisov, N.; Chen, Y.; Zhang, Y.; Schmuki, P. Water Annealing of TiO2 Nanotubes for Photocatalysis Revisited. ChemElectroChem 2020, 7. [Google Scholar] [CrossRef]
- Mansouri, S.; Abbaspour-Fard, M.H.; Meshkini, A. Lily (Iris Persica) pigments as new sensitizer and TiO2 nanofibers as photoanode electrode in dye sensitized solar cells. Optik 2020, 202. [Google Scholar] [CrossRef]
- Gao, J.; Jia, S.; Liu, J.; Sun, Z.; Yang, X.; Tang, D. Enhanced effect of adsorption and photocatalytics by TiO2 nanoparticles embedded porous PVDF nanofiber scaffolds. J. Mater. Res. 2021. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Yan, J.; Yu, J.; Sun, G.; Ding, B. Soft Zr-doped TiO2 Nanofibrous Membranes with Enhanced Photocatalytic Activity for Water Purification. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Joseph, E.; Madhusudanan, S.P.; Mohanta, K.; Karthega, M.; Batabyal, S.K. Multiple negative differential resistance in perovskite (CH3NH3PbI3) decorated electrospun TiO2 nanofibers. Appl. Phys. A Mater. Sci. Process. 2020, 126. [Google Scholar] [CrossRef]
- Abdelmaksoud, M.; Mohamed, A.; Sayed, A.; Khairy, S. Physical properties of PVDF-GO/black-TiO2 nanofibers and its photocatalytic degradation of methylene blue and malachite green dyes. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.H.; Li, R.; Cui, Y.Q.; Wang, X.X.; Yu, J.Q.; Long, Y.Z. Efficient under visible catalysts from electrospun flexible Ag2S/TiO2 composite fiber membrane. J. Mater. Sci. 2021, 56. [Google Scholar] [CrossRef]
- Huang, G.; Liu, X.; Shi, S.; Li, S.; Xiao, Z.; Zhen, W.; Liu, S.; Wong, P. Hydrogen producing water treatment through mesoporous TiO2 nanofibers with oriented nanocrystals. Chinese J. Catal. 2020, 41. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46. [Google Scholar] [CrossRef]
- Al-Hajji, L.A.; Ismail, A.A.; Alsaidi, M.; Ahmed, S.A.; Almutawa, F.; Bumajdad, A. Comparison of TiO2 nanowires and TiO2 nanoparticles for photodegradation of resorcinol as endocrine model. J. Nanoparticle Res. 2020, 22. [Google Scholar] [CrossRef]
- Zhang, X.; Du, A.J.; Lee, P.; Sun, D.D.; Leckie, J.O. TiO2 nanowire membrane for concurrent filtration and photocatalytic oxidation of humic acid in water. J. Memb. Sci. 2008, 313, 44–51. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11. [Google Scholar] [CrossRef]
- Qi, H.; Wu, Y. Synaptic plasticity of TiO2 nanowire transistor. Microelectron. Int. 2020, 37. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Koyuncu, I. Fabrication and application areas of mixed matrix flat-sheet membranes. In Application of Nanotechnology in Membranes for Water Treatment; CRC Press: Boca Raton, FL, USA, 2017; pp. 49–66. [Google Scholar]
- Yang, C.; Wang, P.; Li, J.; Wang, Q.; Xu, P.; You, S.; Zheng, Q.; Zhang, G. Photocatalytic PVDF ultrafiltration membrane blended with visible-light responsive Fe (III)-TiO2 catalyst: Degradation kinetics, catalytic performance and reusability. Chem. Eng. J. 2021, 417. [Google Scholar] [CrossRef]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Tailoring the effects of titanium dioxide (Tio2) and polyvinyl alcohol (pva) in the separation and antifouling performance of thin-film composite polyvinylidene fluoride (pvdf) membrane. Membranes 2021, 11, 241. [Google Scholar] [CrossRef]
- Hamed, S.M.; Abdollah, F.S.; Nosratollah, M. Preparation, characterization, and performance study of PVDF nanocomposite contained hybrid nanostructure TiO2-POM used as a photocatalytic membrane. Iran. J. Chem. Chem. Eng. 2021, 40. [Google Scholar] [CrossRef]
- Choi, H.; Antoniou, M.G.; de la Cruz, A.A.; Stathatos, E.; Dionysiou, D.D. Photocatalytic TiO2 films and membranes for the development of efficient wastewater treatment and reuse systems. Desalination 2007, 202, 199–206. [Google Scholar] [CrossRef]
- Corredor, J.; Perez-Peña, E.; Rivero, M.J.; Ortiz, I. Performance of rgo/tio2 photocatalytic membranes for hydrogen production. Membranes 2020, 10, 218. [Google Scholar] [CrossRef]
- Yan, M.; Wu, Y.; Liu, X. Photocatalytic nanocomposite membranes for high-efficiency degradation of tetracycline under visible light: An imitated core-shell Au-TiO2-based design. J. Alloys Compd. 2021, 855. [Google Scholar] [CrossRef]
- Sun, J.; Li, S.; Ran, Z.; Xiang, Y. Preparation of Fe3O4@TiO2 blended PVDF membrane by magnetic coagulation bath and its permeability and pollution resistance. J. Mater. Res. Technol. 2020. [Google Scholar] [CrossRef]
- Heng, Z.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C.; Koo, C.H. Novel visible-light responsive NCQDs-TiO2/PAA/PES photocatalytic membrane with enhanced antifouling properties and self-cleaning performance. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, L.; Qu, K.; Qin, L.; Zhuang, L.; Yang, H.; Xu, Z. Novel Ag-AgBr decorated composite membrane for dye rejection and photodegradation under visible light. Front. Chem. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Goei, R.; Dong, Z.; Lim, T.T. High-permeability pluronic-based TiO2 hybrid photocatalytic membrane with hierarchical porosity: Fabrication, characterizations and performances. Chem. Eng. J. 2013, 228. [Google Scholar] [CrossRef]
- Wang, L. Configurations and Membranes of Photocatalytic Membrane Reactors for Water and Wastewater Treatment. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Hong Kong, China, 2018; Volume 208. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Zinadini, S.; Feyzi, M.; Bahnemann, D.W. Preparation and characterization of a novel photocatalytic self-cleaning PES nanofiltration membrane by embedding a visible-driven photocatalyst boron doped-TiO2–SiO2/CoFe2O4 nanoparticles. Sep. Purif. Technol. 2019, 209. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symes, D.; Taylor-Cox, C.; Holyfield, L.; Al-Duri, B.; Dhir, A. Feasibility of an oxygen-getter with nickel electrodes in alkaline electrolysers. Mater. Renew. Sustain. Energy 2014, 3. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Q.; Gao, Q.; Wan, S.; Yao, P.; Zhu, X. Preparation of Ag/β-cyclodextrin co-doped TiO2 floating photocatalytic membrane for dynamic adsorption and photoactivity under visible light. Appl. Catal. B Environ. 2020, 267. [Google Scholar] [CrossRef]
- Kang, Y.; Jiao, S.; Zhao, Y.; Wang, B.; Zhang, Z.; Yin, W.; Tan, Y.; Pang, G. High-flux and high rejection TiO2 nanofibers ultrafiltration membrane with porous titanium as supporter. Sep. Purif. Technol. 2020, 248. [Google Scholar] [CrossRef]
- Baniamer, M.; Aroujalian, A.; Sharifnia, S. Photocatalytic membrane reactor for simultaneous separation and photoreduction of CO2 to methanol. Int. J. Energy Res. 2021, 45. [Google Scholar] [CrossRef]
- Baskar, D.; Nallathambi, G.; Selvam, A.K.; Kumar, P.S. Preparation of pan/lycopene-tio2 nanocomposite membrane for azo dye degradation. Desalin. Water Treat. 2021, 216. [Google Scholar] [CrossRef]
- Wafiroh, S.; Wibiarisky, A.M. Abdulloh Application of cellulose acetate-TiO2 hollow fiber photocatalytic membrane composite for profenofos degradation. Pollut. Res. 2019, 38, S183–S189. [Google Scholar]
- Manouras, T.; Koufakis, E.; Vasilaki, E.; Peraki, I.; Vamvakaki, M. Antimicrobial Hybrid Coatings Combining Enhanced Biocidal Activity under Visible-Light Irradiation with Stimuli-Renewable Properties. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef]
- Nemet, A.; Varbanov, P.S.; Klemeš, J.J. Erratum to: Cleaner production, Process Integration and intensification (Clean Techn Environ Policy, 10.1007/s10098-016-1240-x). Clean Technol. Environ. Policy 2016, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Huang, Q.; Huang, Y.; Xiao, C. Electrospun poly(tetrafluoroethylene)/TiO2 photocatalytic nanofiber membrane and its application. Fangzhi Xuebao/Journal Text. Res. 2019, 40. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.K.; Lopez, D.R.S.; da Costa, J.C.D. Fabrication of nanostructured TiO2 hollow fiber photocatalytic membrane and application for wastewater treatment. Chem. Eng. J. 2014, 236. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Sui, G.; Du, L.; Zhuang, Y.; Zhang, Y.; Zou, Y. Removal of volatile organic compounds from air using supported ionic liquid membrane containing ultraviolet-visible light-driven Nd-TiO2 nanoparticles. J. Mol. Struct. 2021, 1231. [Google Scholar] [CrossRef]
- Shetty, R.; Chavan, V.B.; Kulkarni, P.S.; Kulkarni, B.D.; Kamble, S.P. Photocatalytic Degradation of Pharmaceuticals Pollutants Using N-Doped TiO2 Photocatalyst: Identification of CFX Degradation Intermediates. Indian Chem. Eng. 2017, 59. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. TiO2/PLLA Electrospun Nanofibers Membranes for Efficient Removal of Methylene Blue Using Sunlight. J. Polym. Environ. 2021, 29. [Google Scholar] [CrossRef]
- Mpelane, A.; Katwire, D.M.; Mungondori, H.H.; Nyamukamba, P.; Taziwa, R.T. Application of novel c-tio2-cfa/pan photocatalytic membranes in the removal of textile dyes in wastewater. Catalysts 2020, 10, 909. [Google Scholar] [CrossRef]
- Paredes, L.; Murgolo, S.; Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Carballa, M.; Mascolo, G. Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices. Appl. Catal. B Environ. 2019, 240. [Google Scholar] [CrossRef]







| Chemical Process | Photochemical Process |
|---|---|
| Wet air oxidation | Photo-Fenton reaction |
| Supercritical water oxidation | UV/ultrasound system |
| Ultrasound/H2O2 system | UV/O3/H2O2 system |
| Ultrasound/Sonolysis | UV/H2O2 system |
| Fenton reaction | UV/O3 system |
| Ozonation in alkaline | UV photolysis |
| O3/H2O2 system | UV/O2/TiO2 system |
| Electron—Fenton reaction | UV/H2O2/TiO2 system |
| Crystalline Forms | Anatase | Rutile | Brookite |
|---|---|---|---|
| Crystalline structure | Tetragonal | Tetragonal | Rhombohedral |
| Lattice constants (nm) | a = b = 0.3733 c = 0.9370 | a = b = 0.4584 c = 0.2953 | a = 0.5436; b = 0.9166 c = 0.5135 |
| Density (g.cm−3) | 3.83 | 4.24 | 4.17 |
| Bravais lattice | Simple, body-centered | Simple, body-centered | Simple |
| Melting point (°C) | Turning into rutile | 1870 | Turning into rutile |
| Boiling point (°C) | 2927 | - | - |
| Band gap (eV) | 3.2 | 3.0 | - |
| Refractive index (ng) | 2.5688 | 2.9467 | 2.809 |
| Standard heat capacity (cp) | 55.52 | 55.60 | - |
| Dielectric constant | 55 | 110–117 | 78 |
| TiO2 Nanotubes | Pollutant | Removal Efficiency (%) | Reference |
|---|---|---|---|
| TiO2 arrays | Acid orange 7 | 77 | [91] |
| TiO2 –Au/Ag | Acid orange 7 | 85 | [91] |
| TiO2 | Acid orange 7 | 99 | [91] |
| TiO2 | Oil | 99 | [92] |
| TiO2/CdS | Rhodamine B | 60 | [75] |
| Ag–TiO2/HPA/Al2O3 | Humic acid | 88 | [47] |
| TiO2 | Humic acid | 98 | [47] |
| Si-doped TiO2 | Reactive Red ED-28 | 40 | [83] |
| TiO2 | Methylene blue | 68 | [83] |
| Silica/TiO2 | Direct black 168 | 85 | [83] |
| Polymer Membrane | Types of TiO2 | Deposition Time (min) | Reference |
|---|---|---|---|
| Polyethersulfone (PES) Ultrafiltration membrane | Degussa 25 | 15–30 60 | [112] |
| Poly (vinylidene fluoride) Plasma-grafted poly acrylic acid (PAA) | TiO2 suspension | 30 | [113] |
| Polyamide thin film supported on polysulfide | TiO2 colloidal solution | 60 | [107] |
| Polyether sulfone (PES) Polyimide blend membrane (PI) | Degussa 25 | 15 | [109] |
| Type of Membrane | Type of TiO2 Precursor | Calcination Conditions | Reference |
|---|---|---|---|
| Al2O3 | Tetra-n-butyl titanate | 400 °C for 6 h by 100 °C/h | [18] |
| Al2O3 | Tetraethyl orthosilicate | 400 °C for 2 h by 100 °C/h | |
| ZrO2 | Tetrabutyl orthotitanate | 530 °C for 1 h by 100 °C/h | [20] |
| Al2O3 | Tetra-n-butyl titanate | 500 °C for 2 h by 2 °C/min | [115] |
| Al2O3-ZrO2 | Tetraisopropoxide titanium | 510 °C for 2 h by 5 °C/min | |
| Al2O3 | Titanium tetraisopropoxide | 500 °C for 15 min by 3 °C/min | [20] |
| Activated carbon filter | Tetra-n-butyl titanate | 200 °C for 15h | [18] |
| Al2O3 | Tetraisopropyl orthotitatnate | 450 °C by 10 °C/h | [20] |
| Al2O3 | Commercial TiO2 | 450 °C by 0.5/min | [115] |
| Membrane Type | Polymeric Membrane | Ceramic Membrane | |
|---|---|---|---|
| Membrane morphology | Hollow fiber | Flat sheet | Tubular membrane |
| Stability | Medium | Medium | Good |
| Price | Less expensive | Less expensive | Expensive |
| Configuration mode | Internal or external | Internal or external | Internal or external |
| Module processing | Quite simple | Easy | Hard |
| Synthesis Technique Type | Advantage | Disadvantage |
|---|---|---|
| Chemical vapor deposition | Processing time is short. Suitable for high-deposition-rate uniform films. | High deposition temperature (>600 °C) is required. |
| Due to varying evaporation times, deposition of many sources of precursors is difficult. When a vacuum system is used, the price skyrockets. | ||
| Physical vapor deposition | Low-to-medium deposition temperature (<600 °C) required. Does not involve complex chemical reactions. | Expensive as vacuum systems are used. Deposition of multiple sources of precursors are difficult. |
| Sol–gel | High purity of materials | Hydrolysis rate is difficult to control. |
| Homogeneity is achievable. | ||
| Versatile means processing and control of parameters. | ||
| Large surface area materials are produced. | Longer processing time required. | |
| Chemical bonding results in strong adherence of coating to the substrate. | Calcination at higher temperatures is required. |
| Membrane Types | Modification Methods | Characterization Methods | Reference |
|---|---|---|---|
| TiO2–Polysulfone (PS) | Blending | FTIR, SEM/EDS. XRD, contact angle, zeta potential | [105] |
| TiO2–Polyethersulfone (PES) | Sol–gel | FTIR, XRD, SEM/EDS | [118] |
| TiO2/PES | Phase inversion | Contact angle, FT-IR, TGA, pore size distribution, SEM | [123] |
| TiO2/Cellulose Acetate | Dip-coating | Contact angle, viscosity, SEM, FTIR | [113] |
| ZrO2 PVDF | Blending | SEM, TEM, AFM, XPS, TGA, contact angle | [95] |
| Cellulose acetate- Polyurethane (CA-PU) | Blending | SEM, TEM, AFM, XPS, TGA, contact angle | [19] |
| Polyvinylchloride (PVC) | Sol–gel | SEM, FTIR, DLS, TGA | [105] |
| Hybrid Membranes. | Pollutants | Removal Efficiency (%) | Reference |
|---|---|---|---|
| UV-ZrO2/TiO2–Al2O3 | Methylene blue | 95 | [25] |
| Solar-Ag/TiO2 | Methylene blue | 80 | [18] |
| UV-TiO2/AL2O3 | Direct black 168 | 63 | [97] |
| UV-ZrO2/TiO2–Al2O3 | Methyl orange | 95 | [132] |
| UV-TiO2/PVDF | Reactive black 5 | 99 | [51] |
| UV-TiO2–Al2O3 | Reactive black 5 | 70 | [97] |
| UV-TiO2 nanotube | Rhodamine B | 70 | [131] |
| TiO2 nanotube | Humic acid | 28 | [112] |
| UV-TiO2 nanowire | Humic acid | 60 | [102] |
| UV-TiO2/Al2O3 | Humic acid | 97 | [112] |
| UV-TiO2 nanotube | Humic acid | 95 | [102] |
| Composition | Pollutant | Operating Condition | Efficiency (%) | Reference |
|---|---|---|---|---|
| Fe3O4 (0.075 gL−1) | E. coli | UV @ t = 60 min | 50.5 | [41] |
| Fe3O4 –TiO2 | Bisphenol (10 ppm) | UV @ t = 60 min | 92 | [106] |
| Fe3O4–ZnO | Rhodamine B (7 ppm) | UV @ t = 60 min | 99.3 | [52] |
| Fe3O4–ZnO-rGO | Methylene violet (408 ppm) | UV vis @ t = 120 min | 83.5 | [52] |
| Bi2O3–Fe3O4 | Ibuprofen (2.1 ppm) | UV vis @ t = 120 min | >95 | [39] |
| Fe3O4–CuO–ZnO–nano graphene | Methylene blue (30 ppm) | UV @ t = 120 min | 93 | [67] |
| Fe3O4–ZnO–CoWO4 | Rhodamine B (4.8 ppm) | UV vis @ t = 405 min | 98.3 | [134] |
| Fe3O4–Bi2O3 | Ciprofloxacin | UV vis @ t = 240 min | 98.3 | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetteh, E.K.; Rathilal, S.; Asante-Sackey, D.; Chollom, M.N. Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review. Materials 2021, 14, 3524. https://doi.org/10.3390/ma14133524
Tetteh EK, Rathilal S, Asante-Sackey D, Chollom MN. Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review. Materials. 2021; 14(13):3524. https://doi.org/10.3390/ma14133524
Chicago/Turabian StyleTetteh, E. Kweinor, S. Rathilal, D. Asante-Sackey, and M. Noro Chollom. 2021. "Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review" Materials 14, no. 13: 3524. https://doi.org/10.3390/ma14133524
APA StyleTetteh, E. K., Rathilal, S., Asante-Sackey, D., & Chollom, M. N. (2021). Prospects of Synthesized Magnetic TiO2-Based Membranes for Wastewater Treatment: A Review. Materials, 14(13), 3524. https://doi.org/10.3390/ma14133524