Mechanical and Gamma Ray Absorption Behavior of PbO-WO3-Na2O-MgO-B2O3 Glasses in the Low Energy Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mechanical Properties
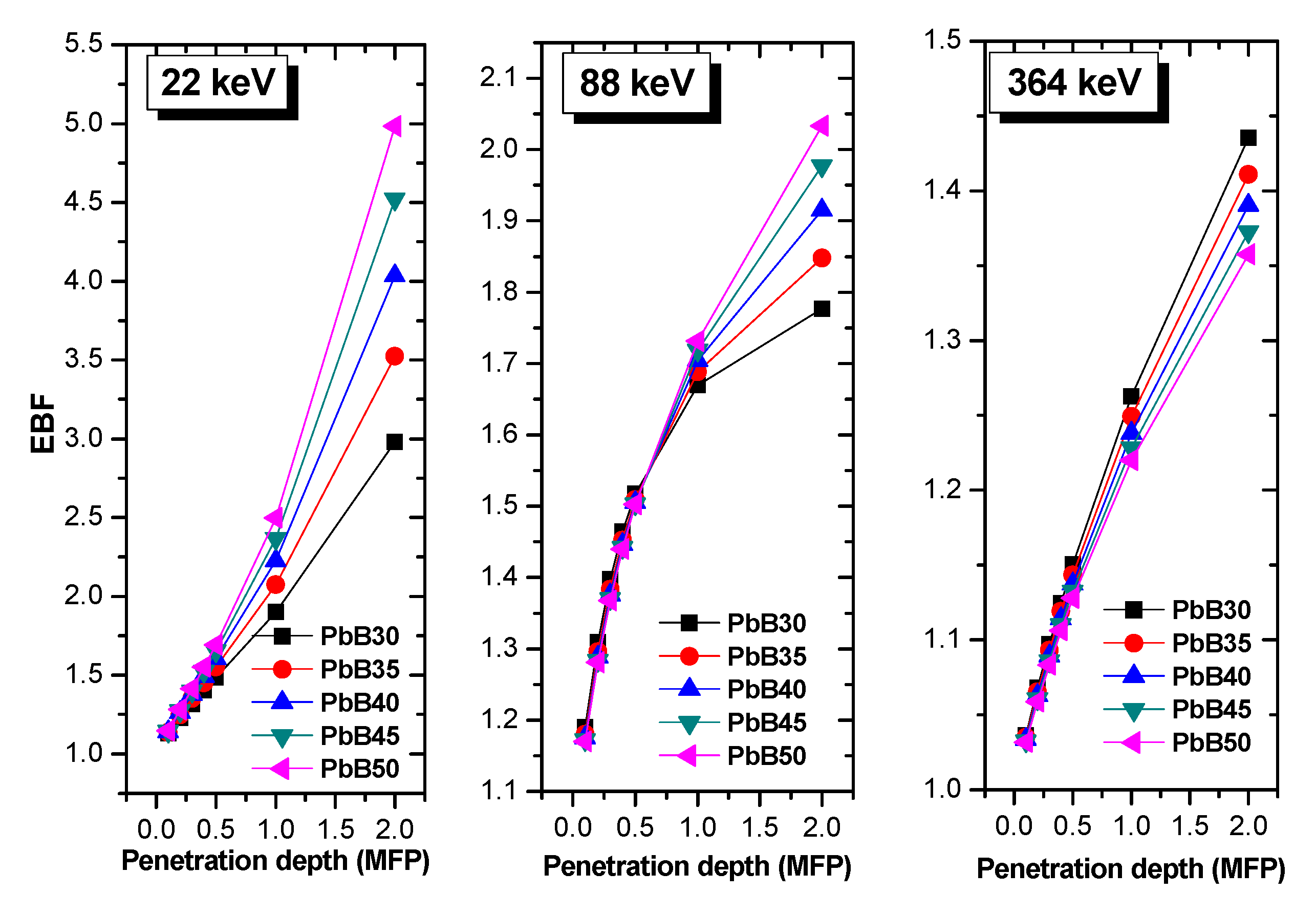
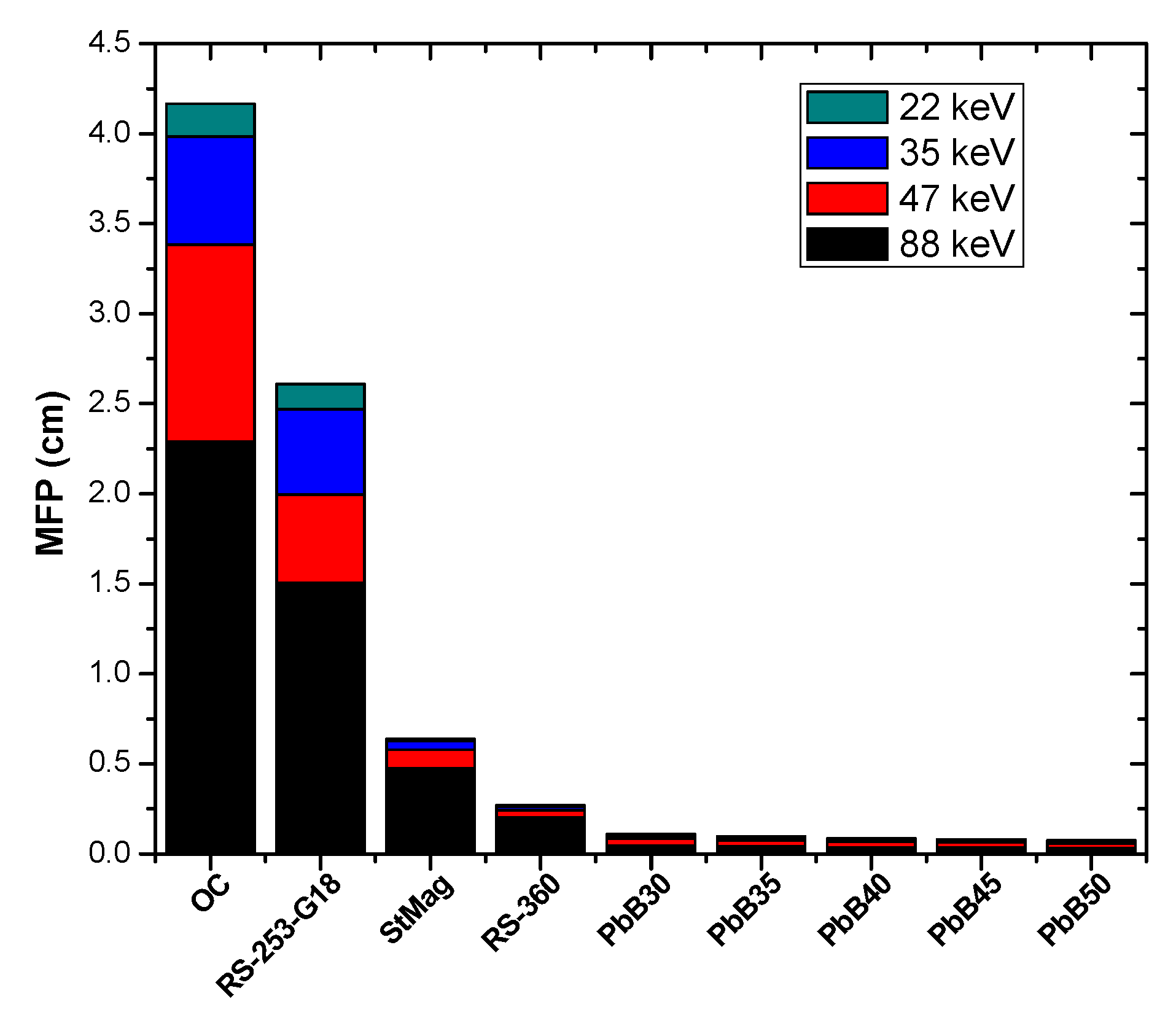
2.2. Radiation Shielding Parameters and Computation
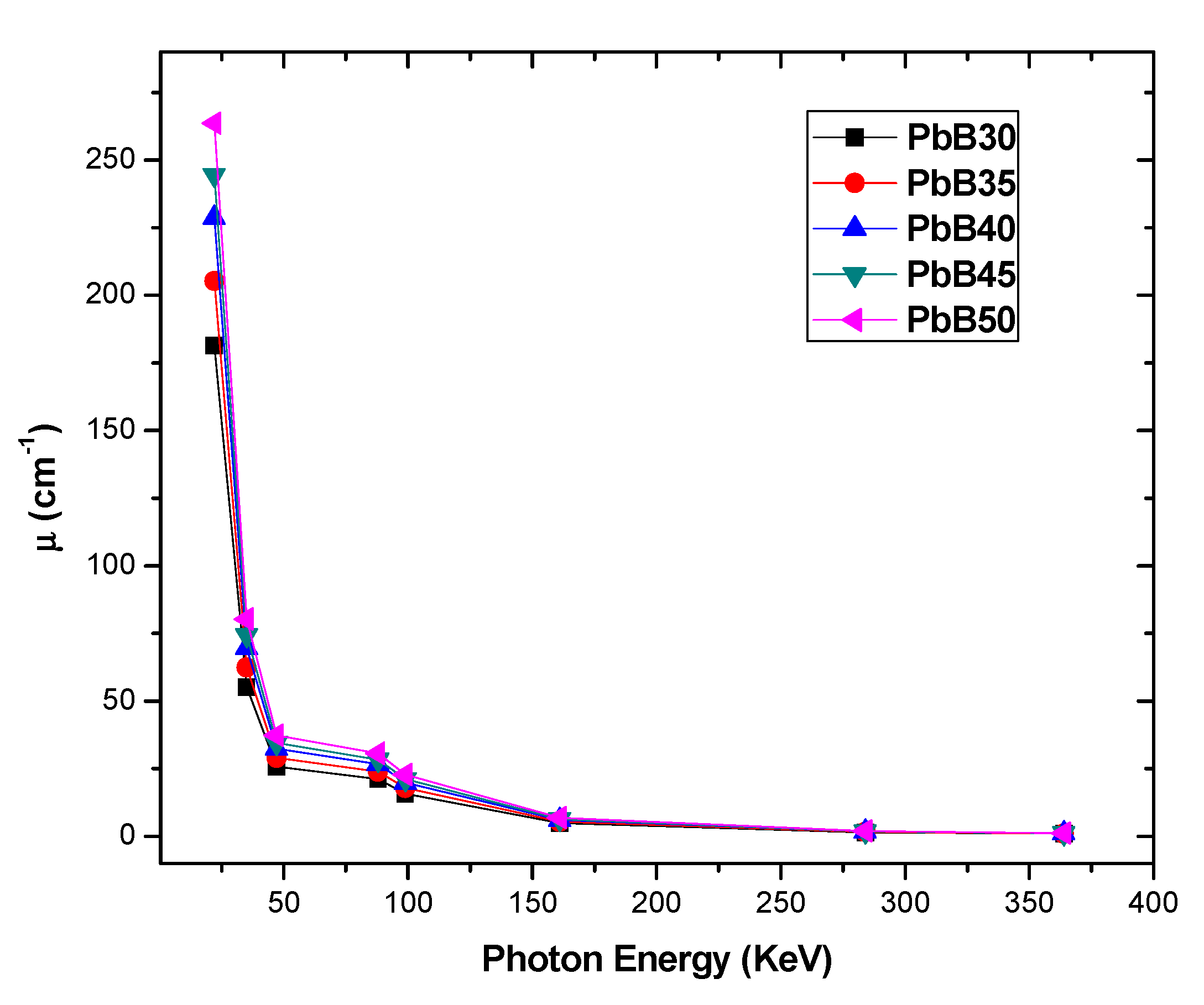
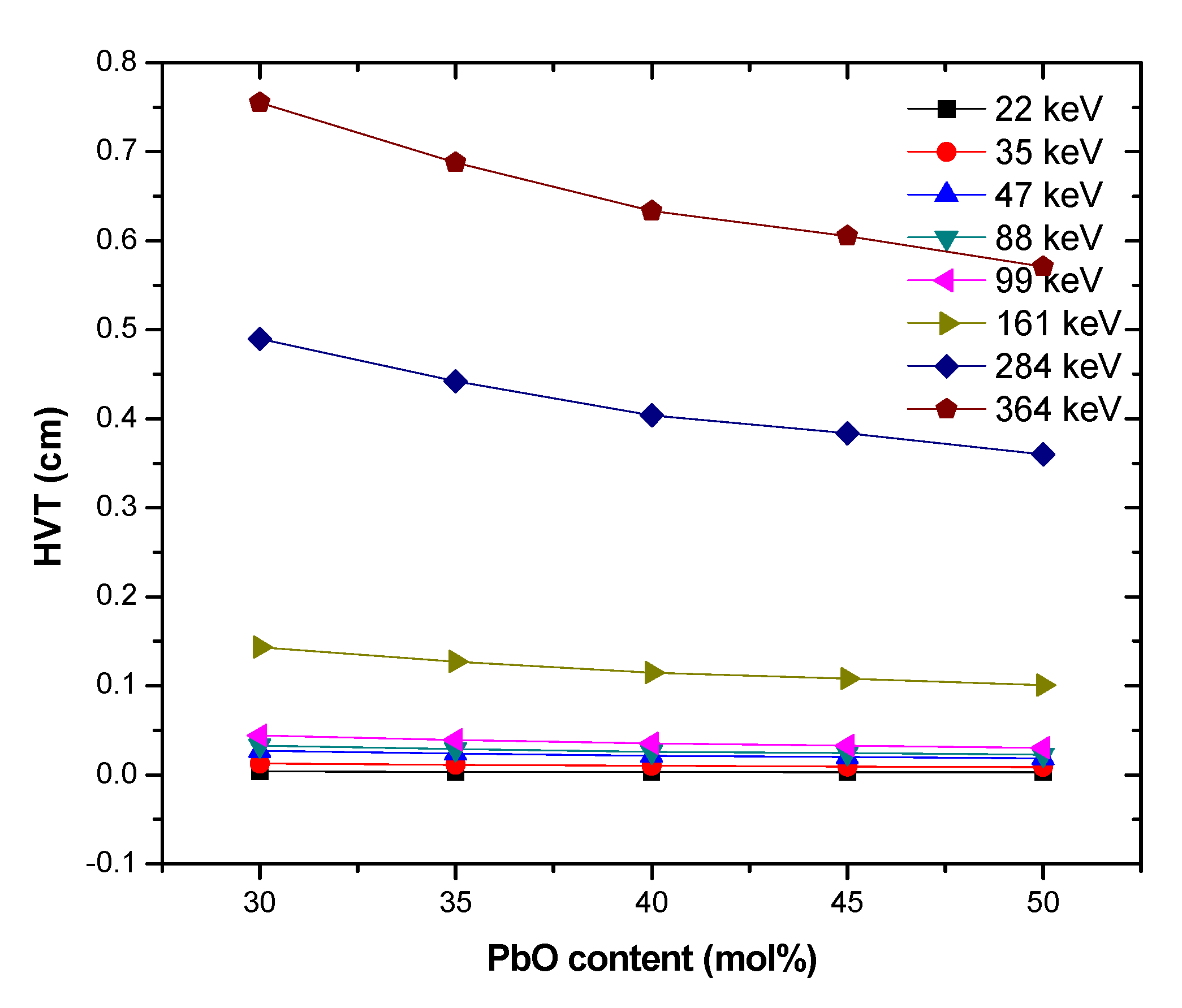
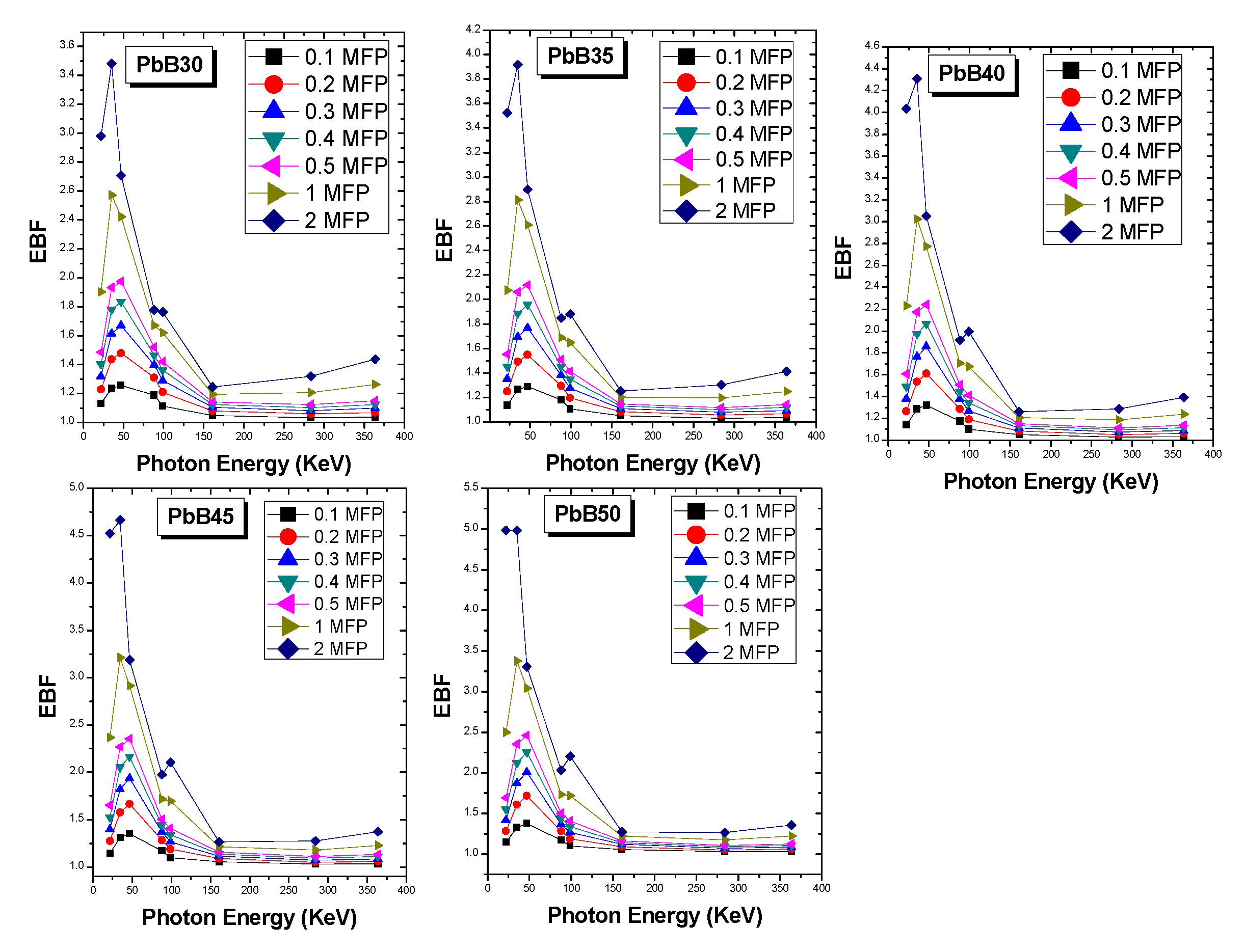
3. Results and Discussion
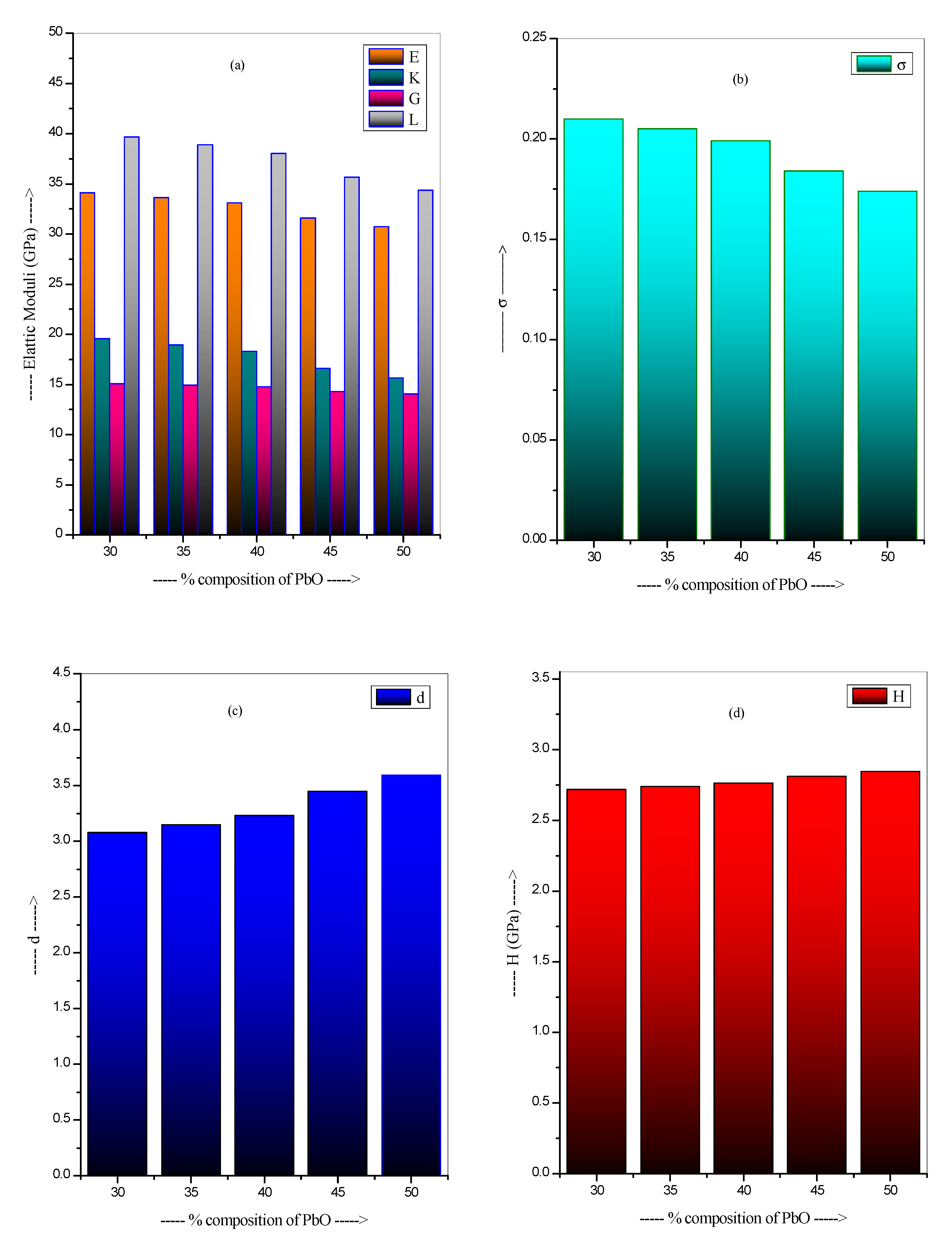
3.1. Mechanical Properties
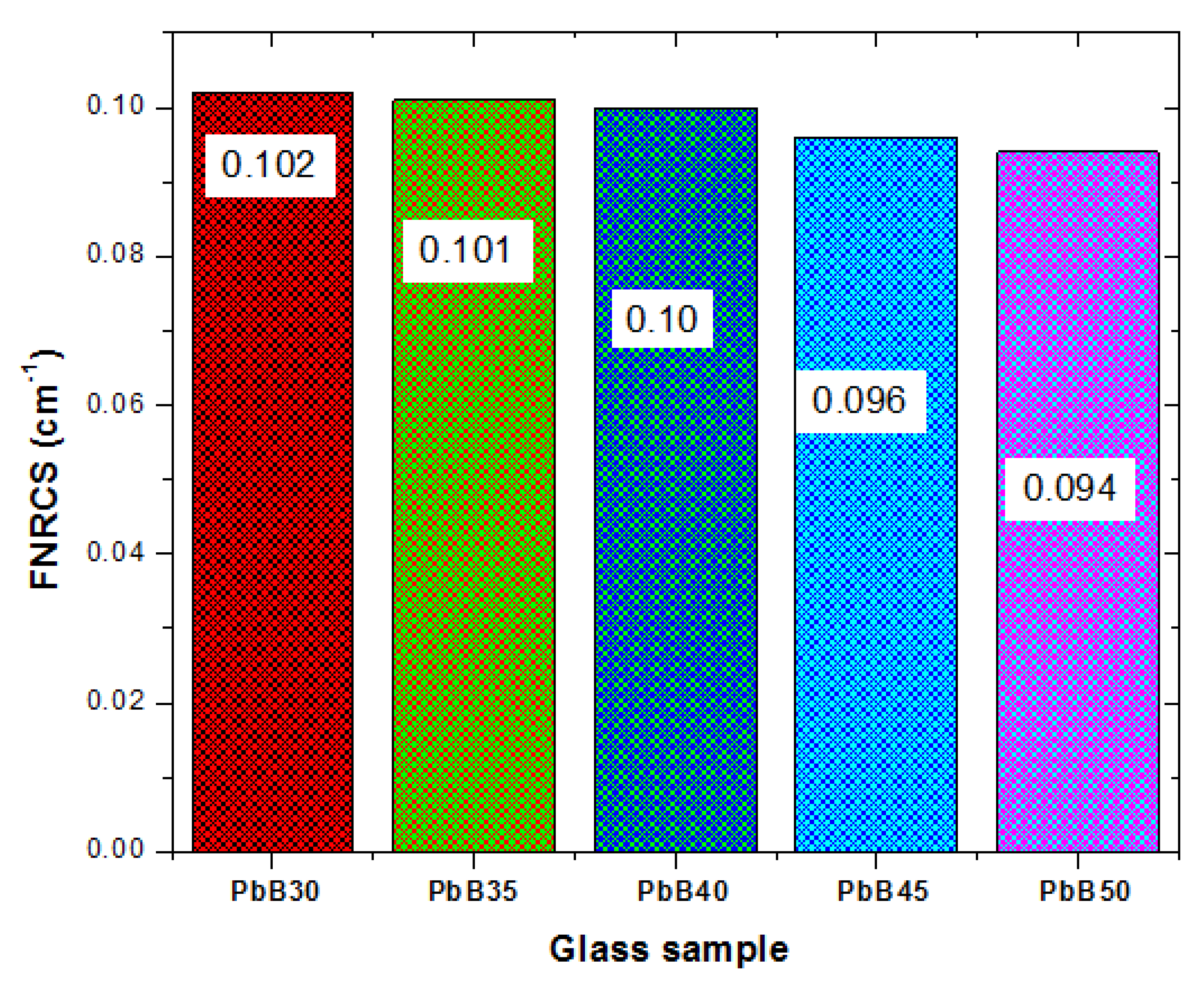
3.2. Fast Neutron Attenuation Feature
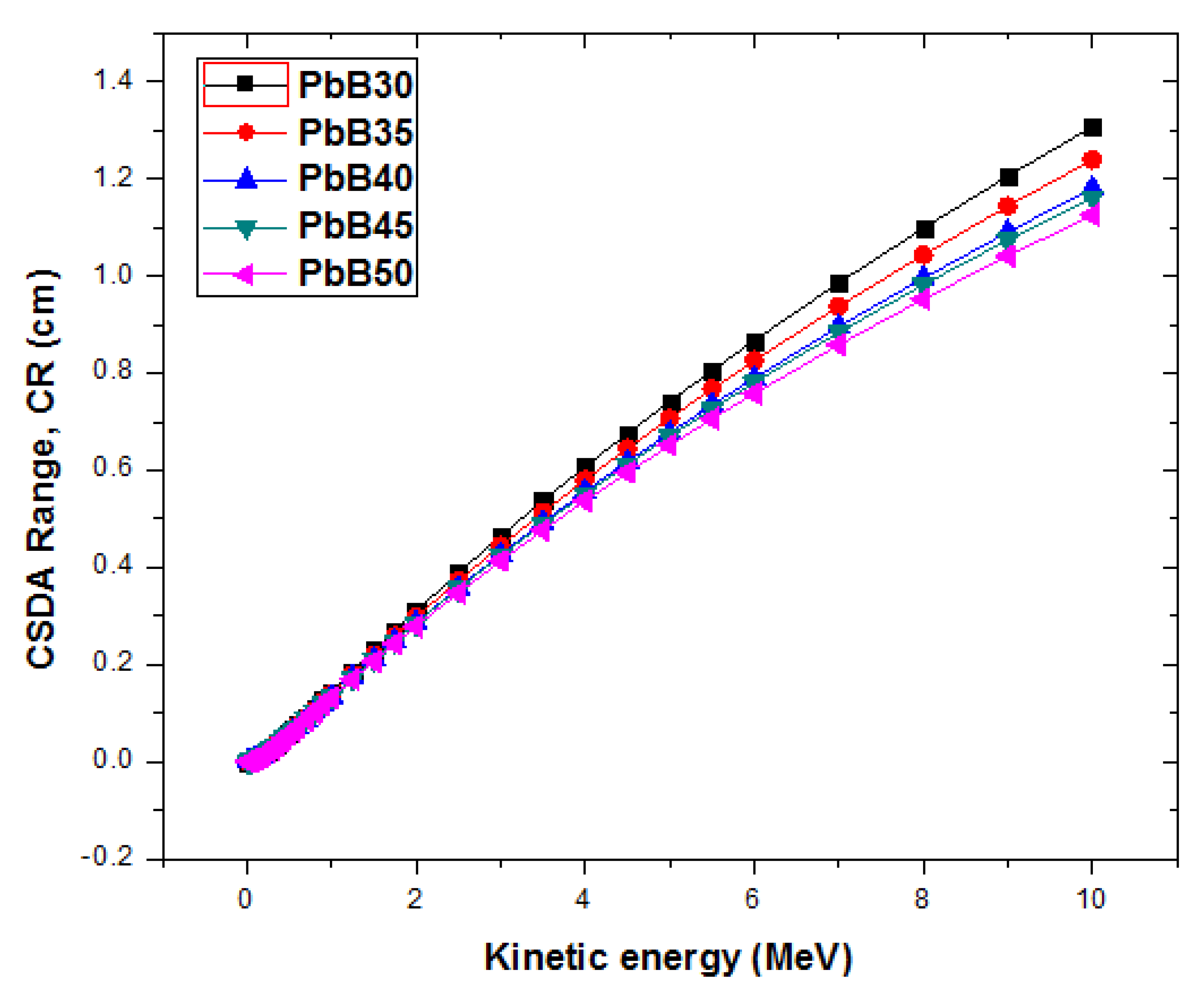
3.3. Range and Stopping Power of Electrons
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Hadeethi, Y.; Tijani, S.A. The use of lead-free transparent 50BaO-(50-x)borosilicate-xBi2O3 glass system as radiation shields in nuclear medicine. J. Alloys Compd. 2019, 803, 625–630. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Julius, F.; Jecong, M.; Frederick, C.; Hila, C.; Balderas, V.; Abdullah, M.; Alhuthali, S.; Raymund, N.; Guillermo, D.; et al. Radiation shielding characteristics of selected ceramics using the EPICS2017 library. Ceram. Int. 2021, 47, 13181–13186. [Google Scholar] [CrossRef]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Chowdhury, F.-U.-Z.; Rashid, A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Results Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Akyildirim, H.; Kavaz, E.; El-Agawany, F.; Yousef, E.S.S.; Rammah, Y. Radiation shielding features of zirconolite silicate glasses using XCOM and FLUKA simulation code. J. Non-Crystalline Solids 2020, 545, 120245. [Google Scholar] [CrossRef]
- Aljawhara, H.; Almuqrin, M.; Sayyed, I. Radiation shielding characterizations and investigation of TeO2–WO3–Bi2O3 and TeO2–WO3–PbO glasses. Appl. Phys. A 2021, 127, 190. [Google Scholar]
- Yasaka, P.; Pattanaboonmee, N.; Kim, H.; Limkitjaroenporn, P.; Kaewkhao, J. Gamma radiation shielding and optical properties measurements of zinc bismuth borate glasses. Ann. Nucl. Energy 2014, 68, 4–9. [Google Scholar] [CrossRef]
- Yasmin, S.; Rozaila, Z.S.; Khandaker, M.U.; Barua, B.S.; Chowdhury, F.-U.-Z.; Rashid, A.; A Bradley, D. The radiation shielding offered by the commercial glass installed in Bangladeshi dwellings. Radiat. Eff. Defects Solids 2018, 173, 657–672. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Almuqrin, A.H.; Kurtulus, R.; Javier-Hila, A.M.V.; Kaky, K.; Kavas, T. X-ray shielding characteristics of P2O5–Nb2O5 glass doped with Bi2O3 by using EPICS2017 and Phy-X/PSD. Appl. Phys. A 2021, 127, 1–8. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Olarinoye, O.I.; Elsafi, M. Assessment of gamma-radiation attenuation characteristics of Bi2O3–B2O3–SiO2–Na2O glasses using Geant4 simulation code. Eur. Phys. J. Plus 2021, 136, 535. [Google Scholar] [CrossRef]
- Oto, B.; Kavaz, E.; Durak, H.; Aras, A.; Madak, Z. Effect of addition of molybdenum on photon and fast neutron radiation shielding properties in ceramics. Ceram. Int. 2019, 45, 23681–23689. [Google Scholar] [CrossRef]
- Yasmin, S.; Khandaker, M.U.; Barua, B.S.; Mustafa, N.; Chowdhury, F.-U.-Z.; Rashid, M.A.; Bradley, D.A. Ionizing radiation shielding effectiveness of decorative building materials (porcelain and ceramic tiles) used in Bangladeshi dwellings. Indoor Built Environ. 2019, 28, 825–836. [Google Scholar] [CrossRef]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Sayyed, M.; Mahmoud, K.; Tashlykov, O.; Khandaker, M.U.; Faruque, M. Enhancement of the Shielding Capability of Soda–Lime Glasses with Sb2O3 Dopant: A Potential Material for Radiation Safety in Nuclear Installations. Appl. Sci. 2020, 11, 326. [Google Scholar] [CrossRef]
- Ali, A.M.; Sayyed, M.I.; Rashad, M.; Kumar, A.; Kaur, R.; Aşkın, A.; Algarni, H. Gamma ray shielding behavior of Li2O doped PbO–MoO3–B2O3 glass system. Appl. Phys. A 2019, 125, 671. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Kumar, A.; Tekin, H.; Issa, S.A.; Al-Buriahi, M.; Lee, D.-E.; Yoon, J.; Park, T. Binary B2O3–Bi2O3 glasses: Scrutinization of directly and indirectly ionizing radiations shielding abilities. J. Mater. Res. Technol. 2020, 9, 14549–14567. [Google Scholar] [CrossRef]
- Al-Buriahi, M.; Alajerami, Y.; Abouhaswa, A.; Alalawi, A.; Nutaro, T.; Tonguc, B. Effect of chromium oxide on the physical, optical, and radiation shielding properties of lead sodium borate glasses. J. Non-Crystalline Solids 2020, 544, 120171. [Google Scholar] [CrossRef]
- Cheewasukhanont, W.; Limkitjaroenporn, P.; Kothan, S.; Kedkaew, C.; Kaewkhao, J. The effect of particle size on radiation shielding properties for bismuth borosilicate glass. Radiat. Phys. Chem. 2020, 172, 108791. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Khattari, Z.Y.; Kumarm, A.; Al-Jundi, J.; Dong, M.G.; AlZaatreh, M.Y. Radiation shielding parameters of BaO–Nb2O5–P2O5 glass system using MCNP5 code and XCOM software. Mater. Res. Express 2018, 5, 115203. [Google Scholar] [CrossRef]
- Kaewjaeng, S.; Kothan, S.; Chaiphaksa, W.; Chanthima, N.; Rajaramakrishna, R.; Kim, H.J.; Kaewkhao, J. High transparency La2O3-CaO-B2O3-SiO2 glass for diagnosis x-rays shielding material application. Radiat. Phys. Chem. 2019, 160, 41–47. [Google Scholar] [CrossRef]
- Tijani, S.; Kamal, S.M.; Al-Hadeethi, Y.; Arib, M.; Hussein, M.; Wageh, S.; Dim, L. Radiation shielding properties of transparent erbium zinc tellurite glass system determined at medical diagnostic energies. J. Alloys Compd. 2018, 741, 293–299. [Google Scholar] [CrossRef]
- Tijani, S.; Al-Hadeethi, Y. The influence of TeO2 and Bi2O3 on the shielding ability of lead-free transparent bismuth tellurite glass at low gamma energy range. Ceram. Int. 2019, 45, 23572–23577. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Kaky, K.M.; Baki, S.; Lira, A.; Caldiño, U.; Kityk, I.; Mahdi, M. Optical absorption, luminescence, and energy transfer processes studies for Dy 3+ /Tb 3+ -codoped borate glasses for solid-state lighting applications. Opt. Mater. 2017, 72, 380–391. [Google Scholar] [CrossRef]
- Kaky, K.M.; Hassib, M.D.; Taki, M.M.; Baki, S. Optical properties of zinc boro-tellurite alumina glasses. Mater. Today Proc. 2020, 29, 39–42. [Google Scholar] [CrossRef]
- Laariedh, F.; Sayyed, M.I.; Kumar, A.; Tekin, H.; Kaur, R.O.; Badeche, T.-B. Studies on the structural, optical and radiation shielding properties of (50–x) PbO–10 WO3–10 Na2O–10 MgO–(20 + x) B2O3 glasses. J. Non-Crystalline Solids 2019, 513, 159–166. [Google Scholar] [CrossRef]
- Hager, I.Z.; El-Mallawany, R. Preparation and structural studies in the (70 − x)TeO2–20WO3–10Li2O–xLn2O3 glasses. J. Mater. Sci. 2010, 45, 897–905. [Google Scholar] [CrossRef]
- Aloraini, D.A.; Sayyed, M.I.; Kumar, A.; Kurtulus, R.; Aljawhara, H.; Almuqrin, T.K. Synthesis, structural investigation, mechanical calculations and photon shielding properties of CaO–K2O–Na2O–P2O5 glass system. Opt. Mater. 2021, 117, 111178. [Google Scholar] [CrossRef]
- Makishima, A.; Mackenzie, J.D. Direct calculations of Young modulus of glass. J. Non-Crystalline Solids 1973, 12, 35–45. [Google Scholar] [CrossRef]
- Makishima, A.; MacKenzie, J.D. Calculation of bulk modulus, shear modulus and Poisson’s ratio of glass. J. Non-Crystalline Solids 1975, 17, 147–157. [Google Scholar] [CrossRef]
- Olarinoye, I.; Odiaga, R.; Paul, S. EXABCal: A program for calculating photon exposure and energy absorption buildup factors. Heliyon 2019, 5, e02017. [Google Scholar] [CrossRef] [Green Version]
- Harima, Y. An historical review and current status of buildup factor calculations and applications. Radiat. Phys. Chem. 1993, 41, 631–672. [Google Scholar] [CrossRef]
- Olarinoye, I.O. Variation of Effective Atomic Numbers of Some Thermoluminescence and Phantom Materials with Photon Energies. Res. J. Chem. Sci. 2011, 1, 64–69. [Google Scholar]
- Kumar, A. Gamma ray shielding properties of PbO-Li2O-B2O3 glasses. Radiat. Phys. Chem. 2017, 136, 50–53. [Google Scholar] [CrossRef]
- Singh, N.; Singh, K.J.; Singh, K.; Singh, H. Gamma-ray attenuation studies of PbO-BaO-B2O3 glass system. Radiat. Meas. 2006, 41, 84–88. [Google Scholar] [CrossRef]
- Rammah, Y.; Olarinoye, I.; El-Agawany, F.; El-Adawy, A.; Yousef, E.S. The f-factor, neutron, gamma radiation and proton shielding competences of glasses with Pb or Pb/Bi heavy elements for nuclear protection applications. Ceram. Int. 2020, 46, 27163–27174. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Sayyed, M. The influence of PbO on the radiation attenuation features of tellurite glass. Ceram. Int. 2019, 45, 24230–24235. [Google Scholar] [CrossRef]
- Bashter, I. Calculation of radiation attenuation coefficients for shielding concretes. Ann. Nucl. Energy 1997, 24, 1389–1401. [Google Scholar] [CrossRef]




| Sample | Mole Fraction | Wt. Fraction of Elements Present in the Sample | Density (g/cm3) | Molar Volume (cm3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PbO | WO3 | Na2O | MgO | B2O3 | B | O | Na | Mg | W | Pb | |||
| PbB30 | 30 | 10 | 10 | 10 | 40 | 0.067 | 0.249 | 0.036 | 0.019 | 0.143 | 0.485 | 4.549 | 28.187 |
| PbB35 | 35 | 10 | 10 | 10 | 35 | 0.056 | 0.224 | 0.034 | 0.018 | 0.135 | 0.534 | 4.81 | 28.254 |
| PbB40 | 40 | 10 | 10 | 10 | 30 | 0.045 | 0.200 | 0.032 | 0.017 | 0.128 | 0.577 | 5.061 | 28.369 |
| PbB45 | 45 | 10 | 10 | 10 | 25 | 0.036 | 0.179 | 0.030 | 0.016 | 0.122 | 0.616 | 5.149 | 29.376 |
| PbB50 | 50 | 10 | 10 | 10 | 20 | 0.027 | 0.161 | 0.029 | 0.015 | 0.116 | 0.652 | 5.326 | 29.840 |
| Sample | Mechanical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| nb (× 1022 cm−3) | E (GPa) | K (GPa) | G (GPa) | L (GPa) | σ | d | H (GPa) | ||
| PbB30 | 9.40 | 2.000 | 34.10 | 19.57 | 15.08 | 39.67 | 0.210 | 3.08 | 2.719 |
| PbB35 | 9.70 | 2.138 | 33.63 | 18.96 | 14.94 | 38.88 | 0.205 | 3.15 | 2.741 |
| PbB40 | 9.98 | 2.285 | 33.10 | 18.30 | 14.78 | 38.01 | 0.199 | 3.23 | 2.765 |
| PbB45 | 9.94 | 2.444 | 31.59 | 16.60 | 14.31 | 35.67 | 0.184 | 3.45 | 2.812 |
| PbB50 | 10.09 | 2.615 | 30.72 | 15.64 | 14.04 | 34.36 | 0.174 | 3.59 | 2.847 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuqrin, A.H.; Albarzan, B.; Olarinoye, O.I.; Kumar, A.; Alwadai, N.; Sayyed, M.I. Mechanical and Gamma Ray Absorption Behavior of PbO-WO3-Na2O-MgO-B2O3 Glasses in the Low Energy Range. Materials 2021, 14, 3466. https://doi.org/10.3390/ma14133466
Almuqrin AH, Albarzan B, Olarinoye OI, Kumar A, Alwadai N, Sayyed MI. Mechanical and Gamma Ray Absorption Behavior of PbO-WO3-Na2O-MgO-B2O3 Glasses in the Low Energy Range. Materials. 2021; 14(13):3466. https://doi.org/10.3390/ma14133466
Chicago/Turabian StyleAlmuqrin, Aljawhara H., Badriah Albarzan, O. I. Olarinoye, Ashok Kumar, Norah Alwadai, and M. I. Sayyed. 2021. "Mechanical and Gamma Ray Absorption Behavior of PbO-WO3-Na2O-MgO-B2O3 Glasses in the Low Energy Range" Materials 14, no. 13: 3466. https://doi.org/10.3390/ma14133466
APA StyleAlmuqrin, A. H., Albarzan, B., Olarinoye, O. I., Kumar, A., Alwadai, N., & Sayyed, M. I. (2021). Mechanical and Gamma Ray Absorption Behavior of PbO-WO3-Na2O-MgO-B2O3 Glasses in the Low Energy Range. Materials, 14(13), 3466. https://doi.org/10.3390/ma14133466








