High-Temperature Oxidation Behavior of Fe–10Cr Steel under Different Atmospheres
Abstract
1. Introduction
2. Experiment
3. Results and Discussion
3.1. Oxidation Kinetics
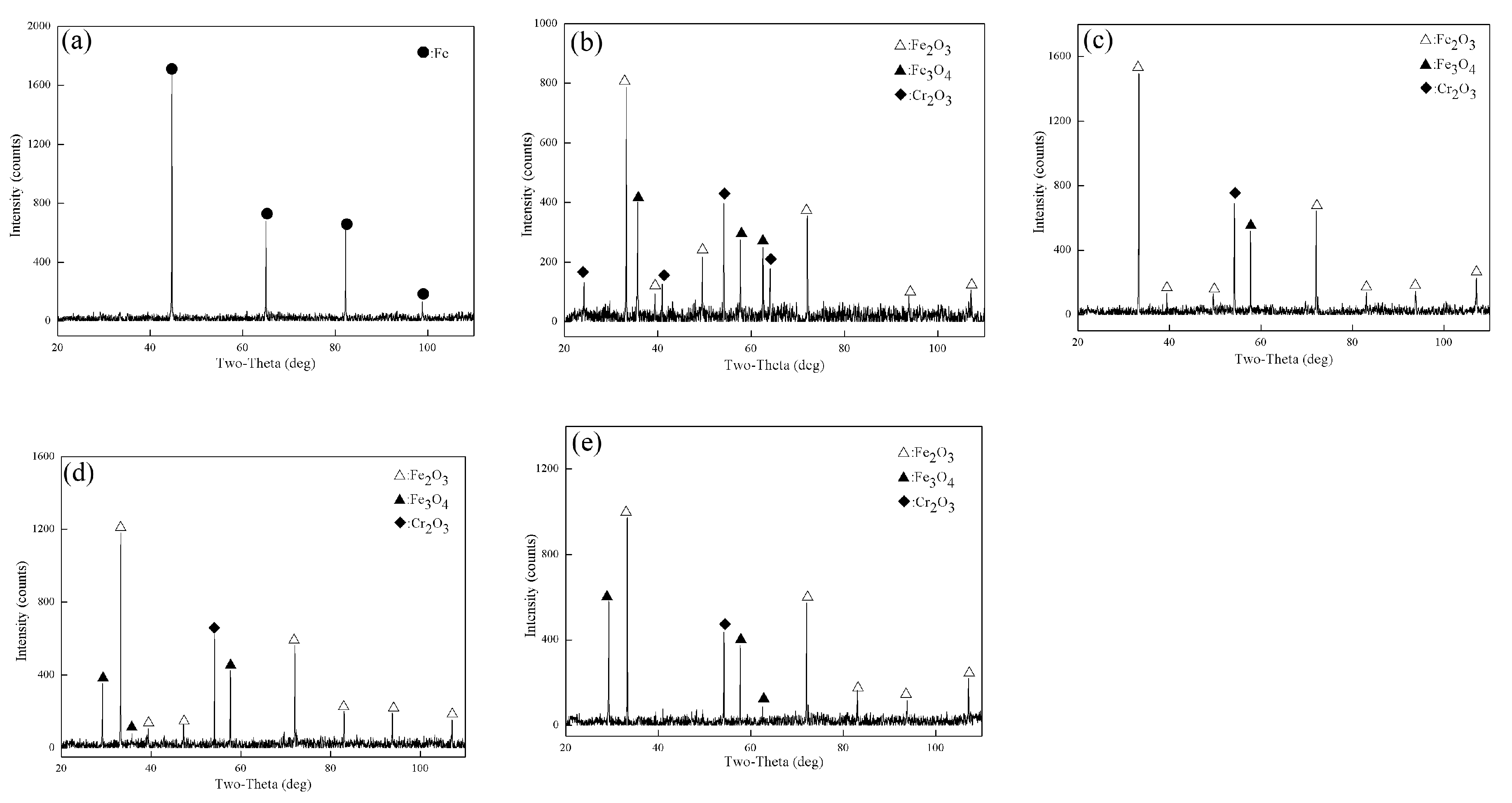
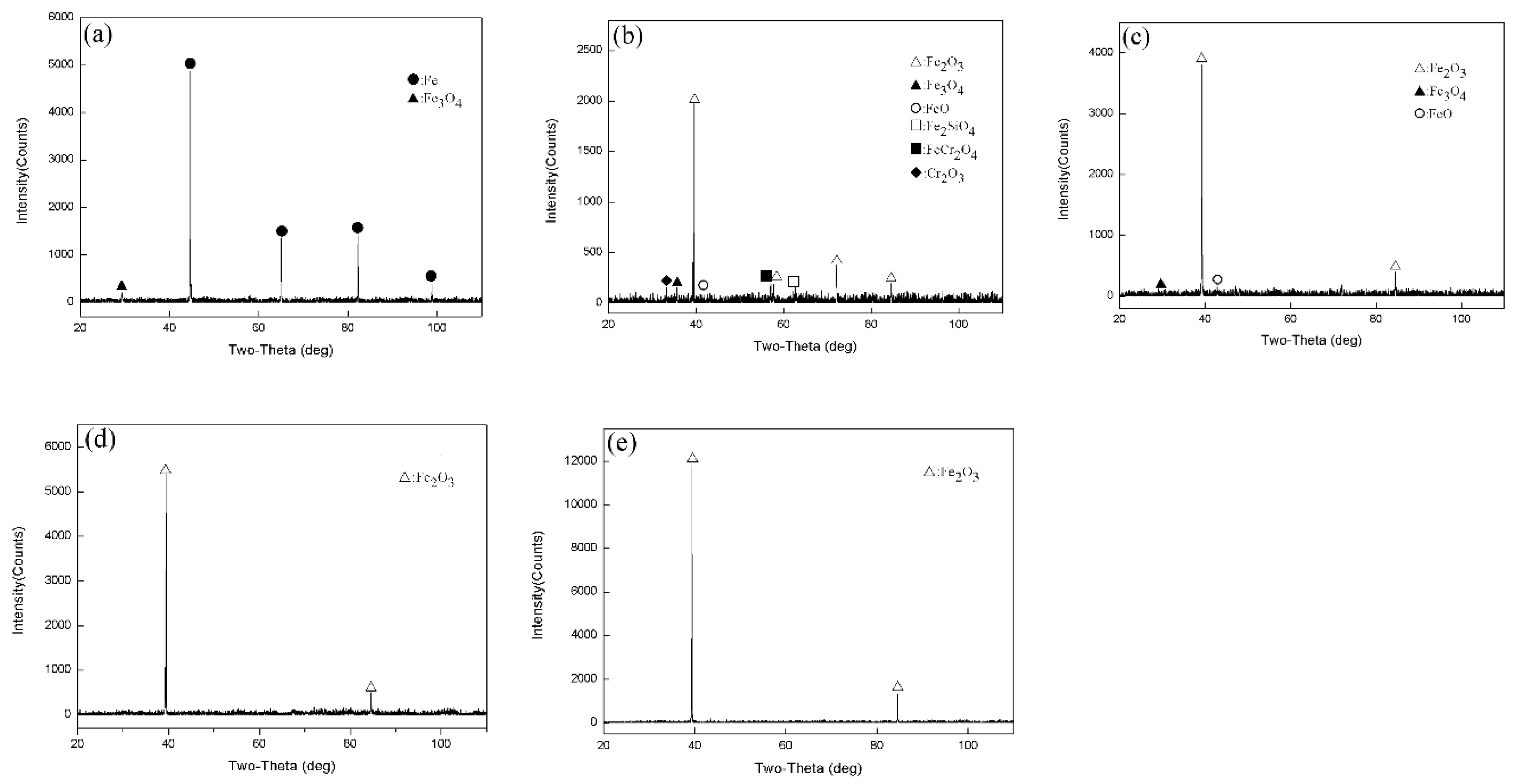
3.2. XRD Test Results
3.3. Cross-Sectional Morphology of Oxide Scales
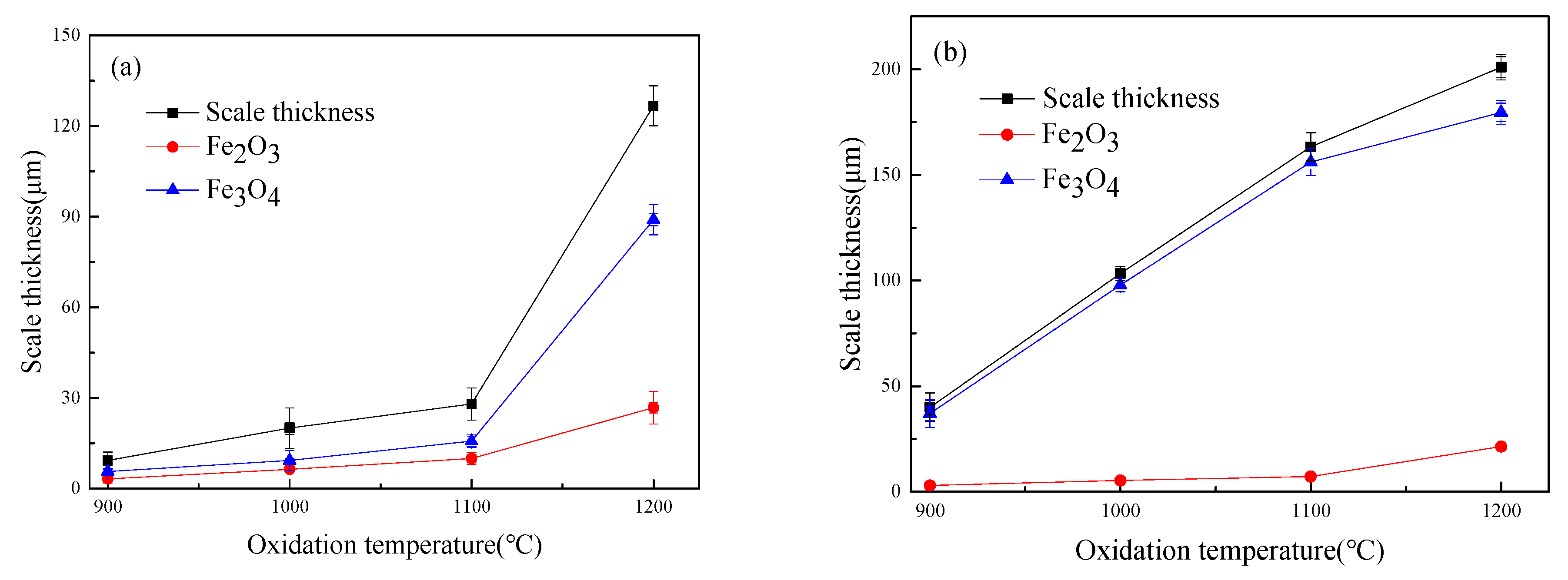
3.4. Iron Oxide Scale Thickness
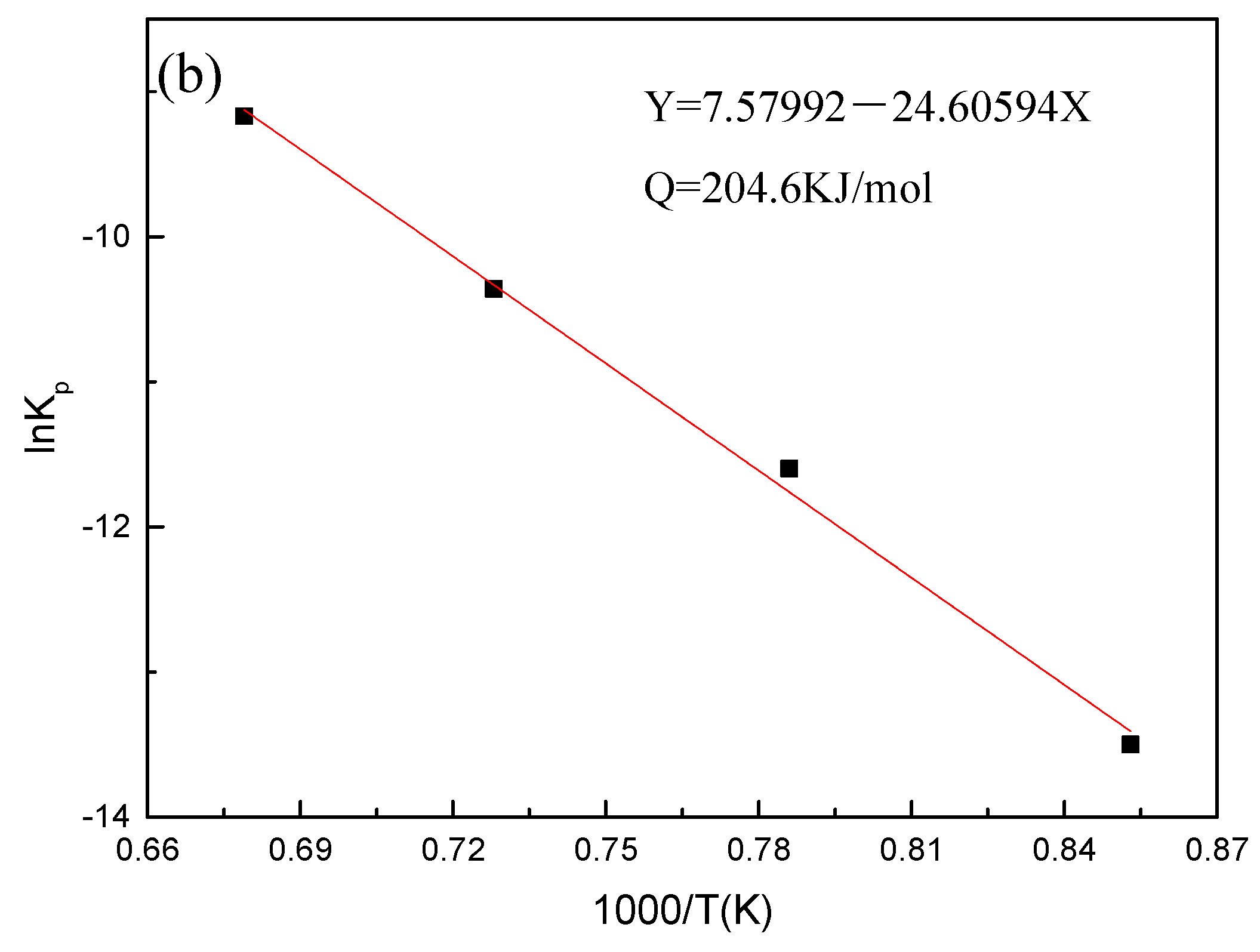
3.5. Oxidation Kinetic Model
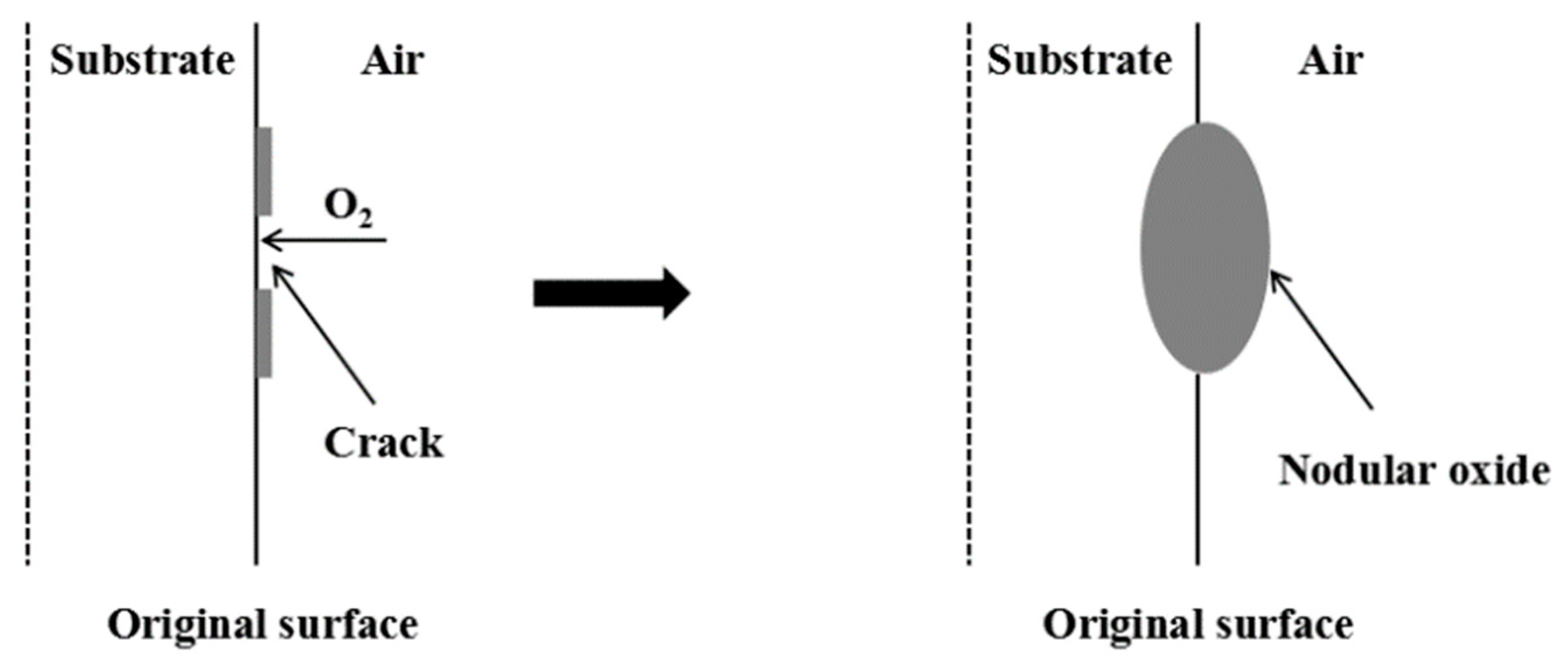
3.6. Spheroidization of Fe–10Cr Steel
3.7. Oxidation Mechanism of Fe–10Cr Steel in Air
3.8. Oxidation Mechanism of Fe–10Cr Steel under Water Vapor Conditions
4. Conclusions
- (1)
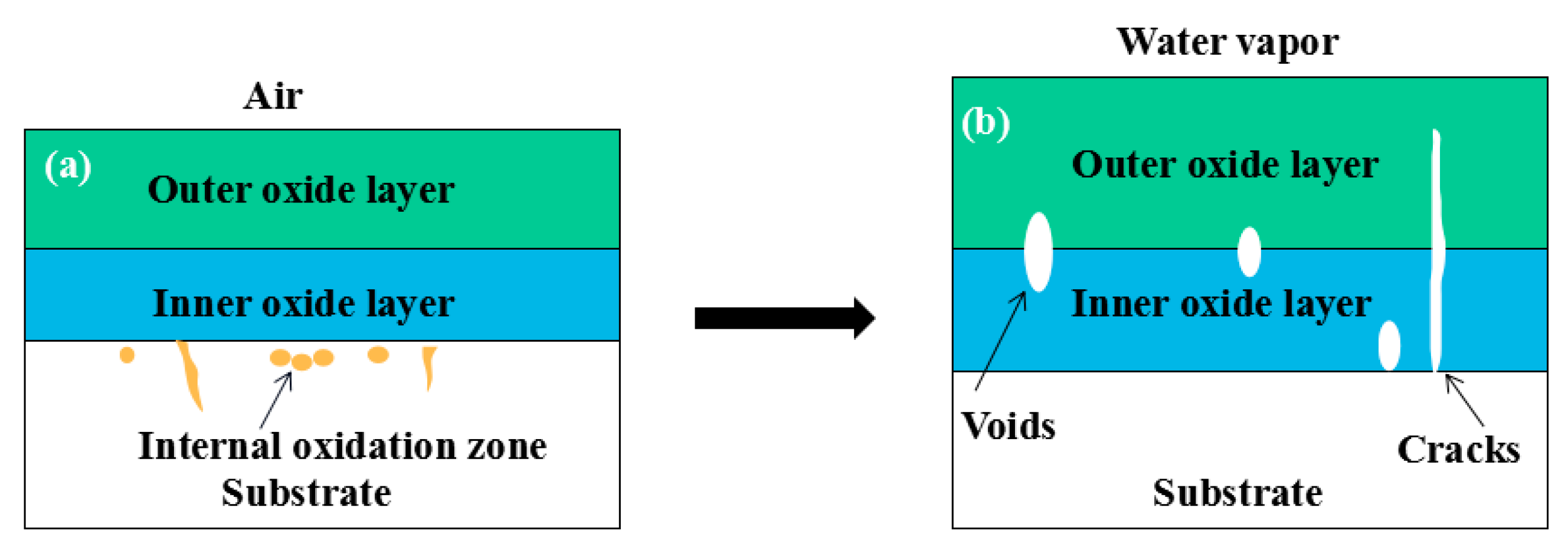
- After being oxidized in air for 1 h, Fe–10Cr steel scales include three parts: an outer oxide layer, an inner oxide layer, and an IOZ. The outer oxide layer is Fe2O3 and Fe3O4, the inner oxide layer is a mixture of Fe3O4 and Cr2O3, and the IOZ is dotted or striped Cr2O3. In water vapor atmosphere, the oxide scales are composed of two parts: an outer oxide layer and an inner oxide layer. The outer oxide layer is iron oxide, and the inner oxide layer is a product with chromium oxide and spinel structures.
- (2)
- At 800 °C, the oxidation kinetics of Fe–10Cr steel under the two atmospheres follows a linear law. The oxidation kinetics curve at 900–1200 °C follows a linear law in the preliminary stage and a parabolic law in the middle and late stages. The oxidation activation energy in air and water vapor is 311.4 kJ/mol and 204.6 kJ/mol, respectively. Fe–10Cr steel is more easily oxidized in water vapor, and oxidation is greater.
- (3)
- Under the action of growth stress, at 800 °C, Fe–10Cr steel will be spheroidized under the two atmospheres.
- (4)
- When oxidized in dry air at 1200 °C, the inner oxide layer of Fe–10Cr steel will be partially damaged, which will result in a significant increase in the thickness of the iron oxide scales and a decrease in the thickness of the Fe2O3 layer.
- (5)
- When oxidized in a mixed atmosphere containing 50% water vapor, a large number of voids and microcracks are generated in the iron oxide layer of Fe–10Cr steel; due to the existence of voids and microcracks, the oxidation rate of Fe–10Cr steel is accelerated, and the thickness of Fe2O3 decreases. Under the combined action of volatilization and internal and external oxidation, the IOZ of Fe–10Cr steel decreases or even disappears.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.F.; Cao, G.M.; He, Y.Q.; Liu, Z.Y.; Wang, G.D. Effect of chromium and water vapor of low carbon steel on oxidation behavior at 1050 °C. Steel Res. Int. 2016, 87, 1–9. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yuen, W.Y.D. A study of the structure of hot-rolled steel strip by simulated coiling and cooling. Oxid. Met. 2000, 53, 539–560. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yeun, W.Y.D. Review of the high-temperature oxidation of iron and carbon steels in air or oxygen. Oxid. Met. 2003, 59, 433–468. [Google Scholar] [CrossRef]
- Liu, X.F.; Sun, B.; Wang, J.M.; Cao, G.Z. Research progress of high temperature iron oxide scale of steel and iron material during hot rolling. Hot Work. Technol. 2018, 47, 10–14+19. [Google Scholar]
- Ding, M.-L.; Ding, B.; Guan, J.-H. Effect of heating temperature on scale pf W470 high-silicon steel continuous casting billet. Heat Treat. Met. 2014, 39, 45–48. [Google Scholar]
- Tanei, H.; Kondo, Y. Strain development in oxide scale during phase transformation of FeO. ISIJ Int. 2017, 57, 506–510. [Google Scholar] [CrossRef]
- He, Y.; Liu, H.Y.; Sun, B.; Liu, X.J.; Cao, G.M.; Liu, Z.Y. Structure development of oxide on low carbon steel strip under continue conditions. Trans. Met. Heat Treat. 2015, 36, 178–182. [Google Scholar]
- Shizukawa, Y.; Hayashi, S.; Yoneda, S.; Kondo, Y.; Tanei, H.; Ukai, S. Mechanism of magnetite seam formation and its role for FeO scale transformation. Oxid. Met. 2016, 86, 315–356. [Google Scholar] [CrossRef]
- Hao, M.; Sun, B.; Wang, H. High temperature oxidation kinetics of Fe-1Cr steel. Hot Work. Technol. 2016, 45, 46–48+52. [Google Scholar]
- Han, M.-F.; Yang, Z.B.; Zhao, X.L.; Wang, H. Research of Fe-Cr alloys properties at high temperature. Mater. Sci. Technol. 2007, 15, 534–536. [Google Scholar]
- Liang, Y.; Long, Y.; Fu, G. Study on high Temperature Oxidation of Fe-Cr Alloys. Liaoning Chem. Ind. 2004, 33, 263–266. [Google Scholar]
- Saunders, S.R.J.; Monteiro, M.; Rizzo, F. The oxidation behaviour of metals and alloys at high temperatures in atmospheres containing water vapour:A review. Prog. Mater. Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Yang, C.W.; Kim, J.H.; Triambulo, R.E.; Kang, Y.H.; Lee, J.S.; Park, J.W. The mechanical property of the oxide scale on Fe-Cr alloy steels. J. Alloys Compd. 2013, 549, 6–10. [Google Scholar] [CrossRef]
- Cao, G.; Liu, X.; Xue, J.; Xu, R.; Li, C.; Liu, Z. Picking mechanisms of hot-rolled steel strip. J. Iron Steel Res. 2012, 24, 36–41. [Google Scholar]
- Cheng, X.W.; Jiang, Z.Y.; Wei, D.B.; Hao, L.; Zhao, J.W.; Peng, J.G.; Jiang, L.Z. Effect of water vapor on oxidation of ferritic stainless steel 21Cr-0. 6Mo-Nb-Ti in simulated reheating environment. Adv. Mater. Res. 2013, 609–693, 280–289. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z. State of the art development on technology for new generation to controlling oxide scale of hot rolled plate and strip. Steel Roll. 2020, 37, 1–6+23. [Google Scholar]
- Li, Z. Evolution Mechanism of Oxide Scale of Hot Rolled Steel and Development of Acid-Free Picking Technology; Northeast University: Shenyang, China, 2018. [Google Scholar]
- Lu, K.Y.; Yu, J. Technology reformation of preventing spalling of oxide film on roll surface to improve the surface quality of strip. Stell Roll. 2020, 37, 62–64+85. [Google Scholar]
- Li, M.S. High Temperature Corrosion of Metals; Metallurgical Industry Press: Beijing, China, 2001; pp. 247–248. [Google Scholar]
- Kofstad, P. Prediction the numerical model of mental non isothermal oxidation kinetics. Acta Chem. Scand. 1958, 12, 701. [Google Scholar] [CrossRef][Green Version]
- Neviobalo, S.; Yakuphanoglu, F. The effects of Cr on isothermal oxidation behavior of Fe-30Mn-6Si alloy. Thermochim. Acta 2013, 560, 43–46. [Google Scholar]
- Wood, G.C. High-temperature oxidation of alloys. Oxid. Met. 1970, 2, 11–57. [Google Scholar] [CrossRef]
- Adachi, T.; Meier, G. Oxidation of iron-silicon alloys. Oxid. Met. 1987, 27, 347–366. [Google Scholar] [CrossRef]
- Fukumoto, M.; Maeda, S.; Hayashi, S.; Narita, T. Effect of water vapor on the oxidation behaviour of Fe-1. 5Si in air at 1073 and 1273K. Oxid. Met. 2001, 55, 401–422. [Google Scholar] [CrossRef]
- Li, T.F. High Temperature Oxidation and Hot Corrosion of Metals; Chemical Industry Press: Beijing, China, 2003; pp. 202–203. [Google Scholar]
- Xu, X.H.; Zhang, T.; Wang, L.; Zhang, H.H.; Men, D.D.; Xiang, J.H.; An, J.S. Oxidation behaviour of Fe-Cr alloys with high Cr content at 800 °C. Chin. J. Nonferrous Met. 2020, 30, 580–586. [Google Scholar]
- Cheng, X.; Jiang, Z.; Wei, D.; Zhao, J.; Monaghan, B.J.; Longbottom, R.J.; Jiang, L. High Temperature Oxidation Behaviour of Ferritic Stainless Steel SUS 430 in Humid Air. Met. Mater. Int 2015, 21, 251–259. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, J.; Zhao, G.; Guan, Y.; Li, M. Oxidation characteristic of ferritic-martenstitic steel T91in water-vapor atmosphere. Chin. J. Mater. Res. 2008, 22, 599–605. [Google Scholar]



| Sample | C | Si | Mn | P | S | Cr | Fe |
|---|---|---|---|---|---|---|---|
| Fe–10Cr steel | 0.094 | 0.23 | 0.51 | 0.07 | 0.01 | 9.88 | Bal |
| Sample | Oxidation Time (h) | Temperature (℃) | Atmosphere |
|---|---|---|---|
| 1 | 1 | 800, 900, 1000, 1100, 1200 | Air |
| 2 | 1 | 800, 900, 1000, 1100, 1200 | Air + 50% water vapor |
| Oxidation Temperature | Air | Air + 50% Water Vapor |
|---|---|---|
| 800 °C |  | 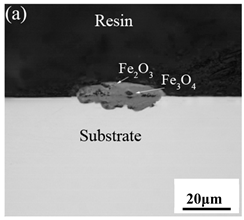 |
| 900 °C | 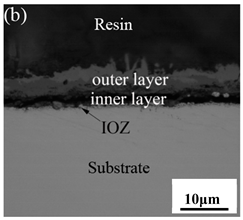 | 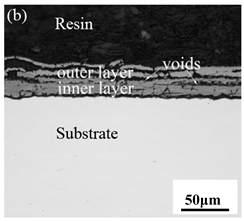 |
| 1000 °C | 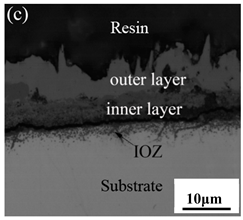 |  |
| 1100 °C | 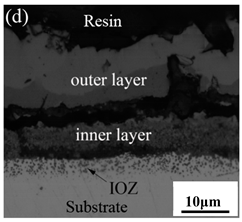 |  |
| 1200 °C |  |  |
| Temperature/°C | 800 | 900 | 1000 | 1100 | 1200 | |
|---|---|---|---|---|---|---|
| K1/(mg/mm4·s) | Air | 3.040 × 10−8 (0–3600 s) | 3.508 × 10−6 (0–1500 s) | 8.595 × 10−6 (0–1100 s) | 9.918 × 10−6 (0–800 s) | 3.840 × 10−5 (0–500 s) |
| Air + 50% Water vapor | 4.906 × 10−7 (0–3600 s) | 2.454 × 10−5 (0–800 s) | 5.427 × 10−5 (0–550 s) | 1.257 × 10−4 (0–400 s) | 4.670 × 10−4 (0–200 s) | |
| Temperature/°C | 800 | 900 | 1000 | 1100 | 1200 | |
|---|---|---|---|---|---|---|
| Kp/(mg/mm4·s) | Air | - | 2.800 × 10−8 | 1.664 × 10−7 | 1.196 × 10−6 | 2.241 × 10−5 |
| Air + 50% Water vapor | - | 1.374 × 10−6 | 9.122 × 10−6 | 3.182 × 10−5 | 1.036 × 10−4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.; Sun, B.; Du, C.; Gao, W.; Cao, G. High-Temperature Oxidation Behavior of Fe–10Cr Steel under Different Atmospheres. Materials 2021, 14, 3453. https://doi.org/10.3390/ma14133453
Cheng L, Sun B, Du C, Gao W, Cao G. High-Temperature Oxidation Behavior of Fe–10Cr Steel under Different Atmospheres. Materials. 2021; 14(13):3453. https://doi.org/10.3390/ma14133453
Chicago/Turabian StyleCheng, Lei, Bin Sun, Chongyang Du, Wei Gao, and Guangming Cao. 2021. "High-Temperature Oxidation Behavior of Fe–10Cr Steel under Different Atmospheres" Materials 14, no. 13: 3453. https://doi.org/10.3390/ma14133453
APA StyleCheng, L., Sun, B., Du, C., Gao, W., & Cao, G. (2021). High-Temperature Oxidation Behavior of Fe–10Cr Steel under Different Atmospheres. Materials, 14(13), 3453. https://doi.org/10.3390/ma14133453






