Behavior of Ternary Mixtures of Hydrogen Bond Acceptors and Donors in Terms of Band Gap Energies
Abstract
:1. Introduction
2. Experimental Section
2.1. General Synthetic Procedure
2.2. Spectroscopic UV-VIS Analysis
2.3. Statistical Analysis
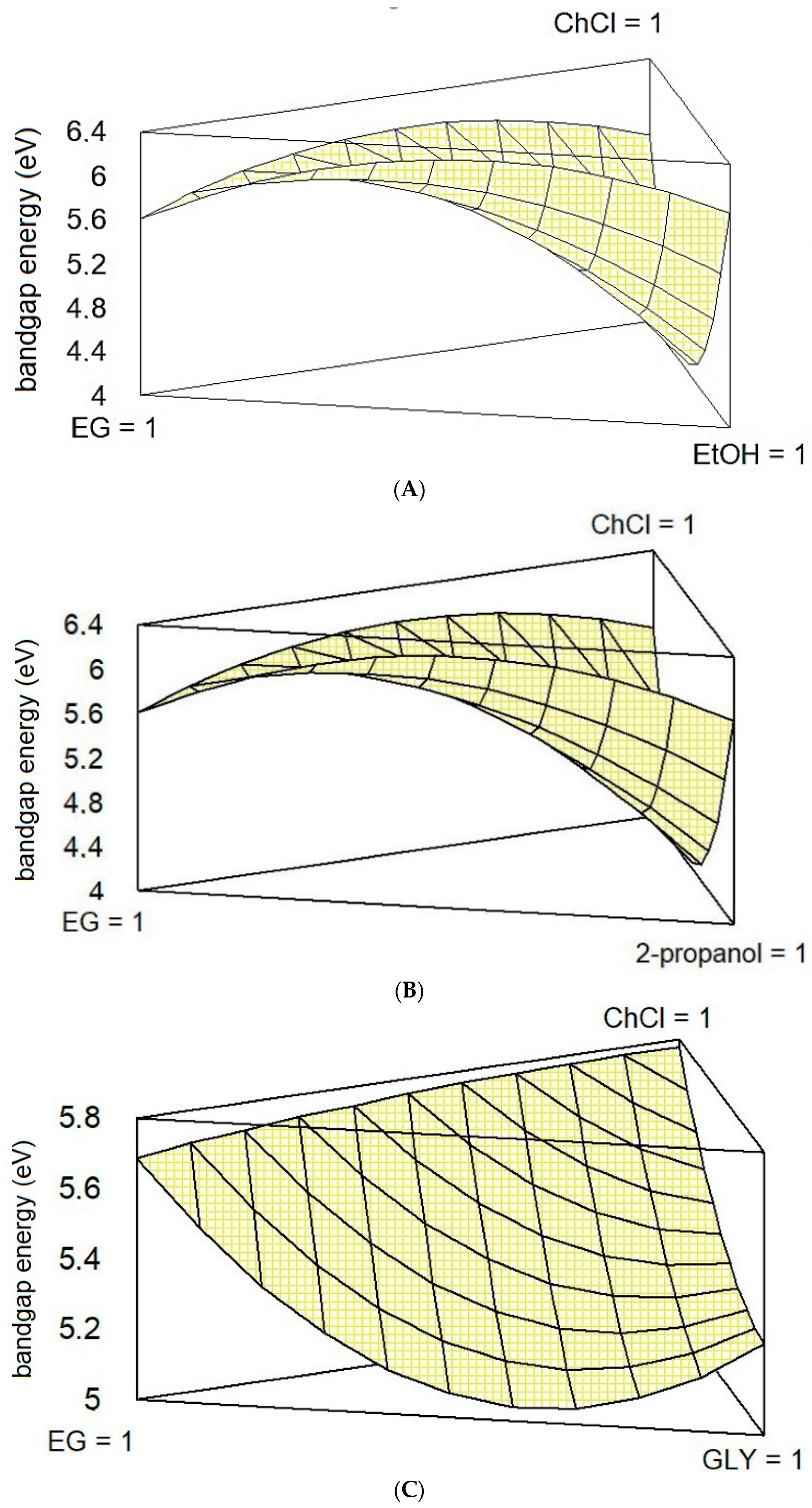
3. Results and Discussion
Multivariate Analysis of Systems A, B, and C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mannu, A.; Blangetti, M.; Baldino, S.; Prandi, C. Promising Technological and Industrial Applications of Deep Eutectic Systems. Materials 2021, 14, 2494. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for green-solvent design: From hydrophilic to hydrophobic (deep) eutectic solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F.J. Natural deep eutectic solvents: Properties, applications, and perspectives. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P.J. Insights into the nature of eutectic and deep eutectic mixtures. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef] [Green Version]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Abbott, A.P. Application of Hole Theory to the Viscosity of Ionic and Molecular Liquids. ChemPhysChem 2004, 5, 1242–1246. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of Improved Deep Eutectic Solvents Using Hole Theory. ChemPhysChem 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef]
- Liu, P.; Hao, J.W.; Mo, L.P.; Zhang, Z.H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Moura, L.; Moufawad, T.; Ferreira, M.; Bricout, H.; Tilloy, S.; Monflier, E.; Costa Gomes, M.F.; Landy, D.; Fourmentin, S. First evidence of cyclodextrin inclusion complexes in a deep eutectic solvent. Environ. Chem. Lett. 2017, 15, 747–753. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Colombo Dugoni, G.; Ferro, M.; Mannu, A.; Castiglione, F.; Costa Gomes, M.; Fourmentin, S.; Mele, A. Do cyclodextrins encapsulate volatiles in deep eutectic systems? ACS Sust. Chem Eng. 2019, 7, 17397–17405. [Google Scholar] [CrossRef]
- Maschita, J.; Banerjee, T.; Savasci, G.; Haase, F.; Ochsenfeld, C.; Lotsch, B.V. Ionothermal Synthesis of Imide-Linked Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2020, 59, 15750–15758. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Zhang, Y.; Meng, H.; Xu, Y.; Han, Y.; Wang, Z.; Dong, Y.; Zhang, X.J. Highly safe and ionothermal synthesis of Ti3C2 MXene with expanded interlayer spacing for enhanced lithium storage. Energy Chem. 2020, 47, 203–209. [Google Scholar] [CrossRef]
- Zhao, X.; Duan, W.; Wang, Q.; Ji, D.; Zhao, Y.; Li, G. Microwave-assisted ionothermal synthesis of Fe-LEV molecular sieve with high iron content in low-dosage of eutectic mixture. Microporous Mesoporous Mater. 2019, 275, 253–262. [Google Scholar] [CrossRef]
- Qin, H.; Hu, X.; Wang, J.; Cheng, H.; Chen, L.; Qi, Z. Overview of acidic deep eutectic solvents on synthesis, properties and applications. Green Energy Environ. 2020, 5, 8–21. [Google Scholar] [CrossRef]
- Nejrotti, S.; Mannu, A.; Blangetti, M.; Baldino, S.; Fin, A.; Prandi, C. Optimization of Nazarov Cyclization of 2, 4-Dimethyl-1, 5-diphenylpenta-1, 4-dien-3-one in Deep Eutectic Solvents by a Design of Experiments Approach. Molecules 2020, 25, 5726. [Google Scholar] [CrossRef]
- Sanyal, U.; Yuk, S.F.; Koh, K.; Lee, M.-S.; Stoerzinger, K.; Zhang, D.; Meyer, L.C.; Lopez-Ruiz, J.A.; Karkamkar, A.; Holladay, J.D.; et al. Hydrogen bonding enhances the electrochemical hydrogenation of benzaldehyde in the aqueous phase. Angew. Chem. Int. Ed. 2021, 60, 290–296. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Afshari, R.; Ramón, D.J.; Varma, R.S. Deep eutectic solvents: Cutting-edge applications in cross-coupling reactions. Green Chem. 2020, 22, 3668–3692. [Google Scholar] [CrossRef]
- Cavallo, M.; Arnodo, D.; Mannu, A.; Blangetti, M.; Prandi, C.; Baratta, W.; Baldino, S. Deep eutectic solvents as H2-sources for Ru (II)-catalyzed transfer hydrogenation of carbonyl compounds under mild conditions. Tetrahedron 2021, 83, 131997. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Šima, J. Use of deep eutectic solvents in polymer chemistry—A review. Molecules 2019, 24, 3978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannu, A.; Ferro, M.; Colombo Dugoni, G.; Di Pietro, M.E.; Garroni, S.; Mele, A.J. From deep eutectic solvents to deep band gap systems. Mol. Liq. 2020, 301, 112441. [Google Scholar] [CrossRef]
- Mannu, A.; Di Pietro, M.E.; Mele, A. Band-Gap energies of choline chloride and triphenylmethylphosphoniumbromide-based systems. Molecules 2020, 25, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannu, A.; Cardano, F.; Fin, A.; Baldino, S.; Prandi, C.J. Choline chloride-based ternary deep band gap systems. Mol. Liq. 2021, 330, 115717. [Google Scholar] [CrossRef]
- Lambrakis, D.P. Experiments with mixtures: A generalization of the simplex-lattice design. J. R. Stat. Soc. Ser. B Methodol. 1968, 30, 123–136. [Google Scholar] [CrossRef]
- Fang, K.-T.; Liu, M.-Q.; Qin, H.; Zhou, Y.-D. Theory and Application of Uniform Experimental Designs; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-981-13-2041-5. [Google Scholar]
| HBA (χ) 1 | HBD (χ) | HBD (χ) | Name | BGE (eV) |
|---|---|---|---|---|
| ChCl (1) | EG (0) | EtOH (0) | A1 | 5.75 |
| ChCl (0) | EG (1) | EtOH (0) | A2 | 5.66 |
| ChCl (0) | EG (0) | EtOH (1) | A3 | 5.88 |
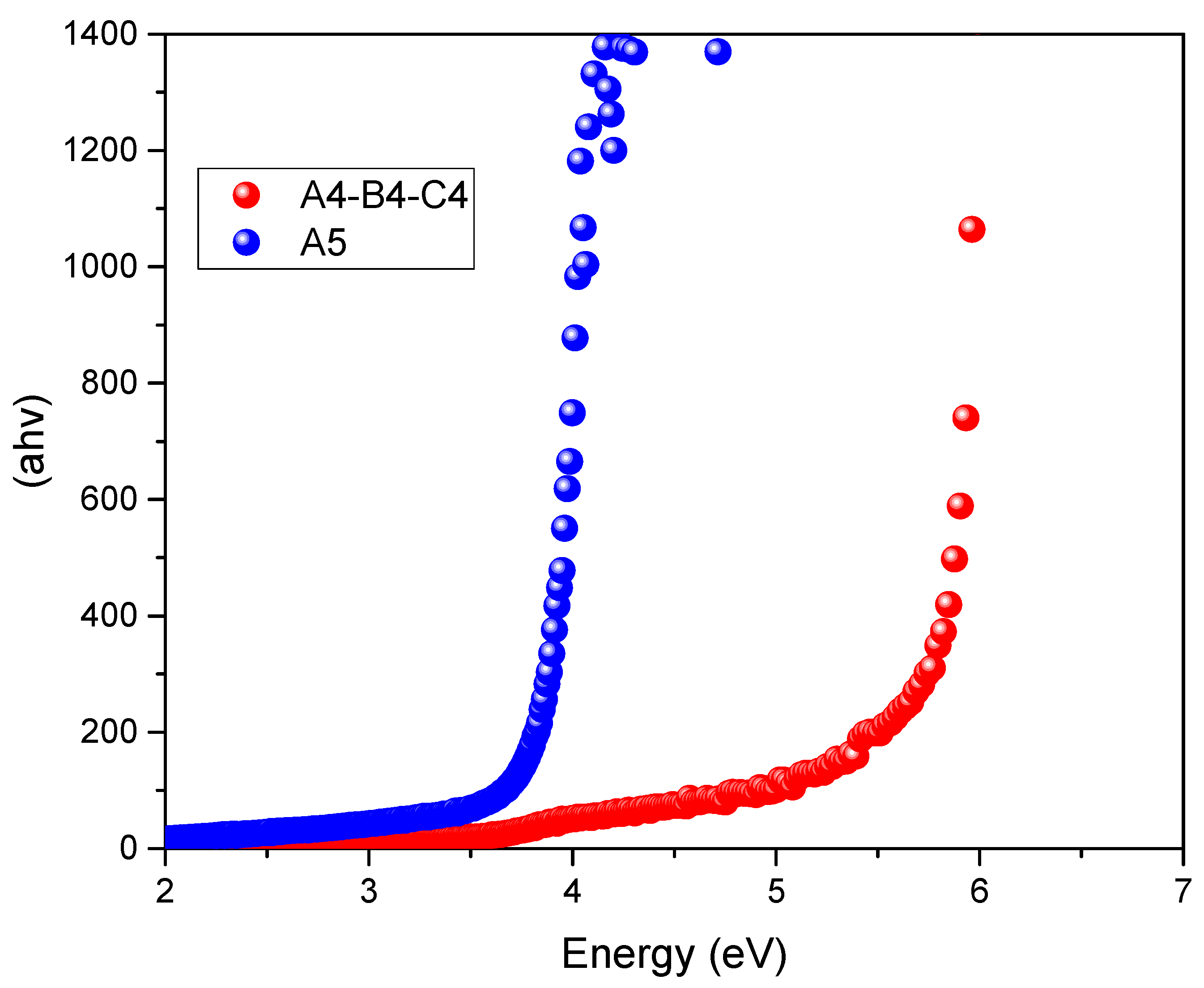
| ChCl (0.5) | EG (0.5) | EtOH (0) | A4 | 5.86 |
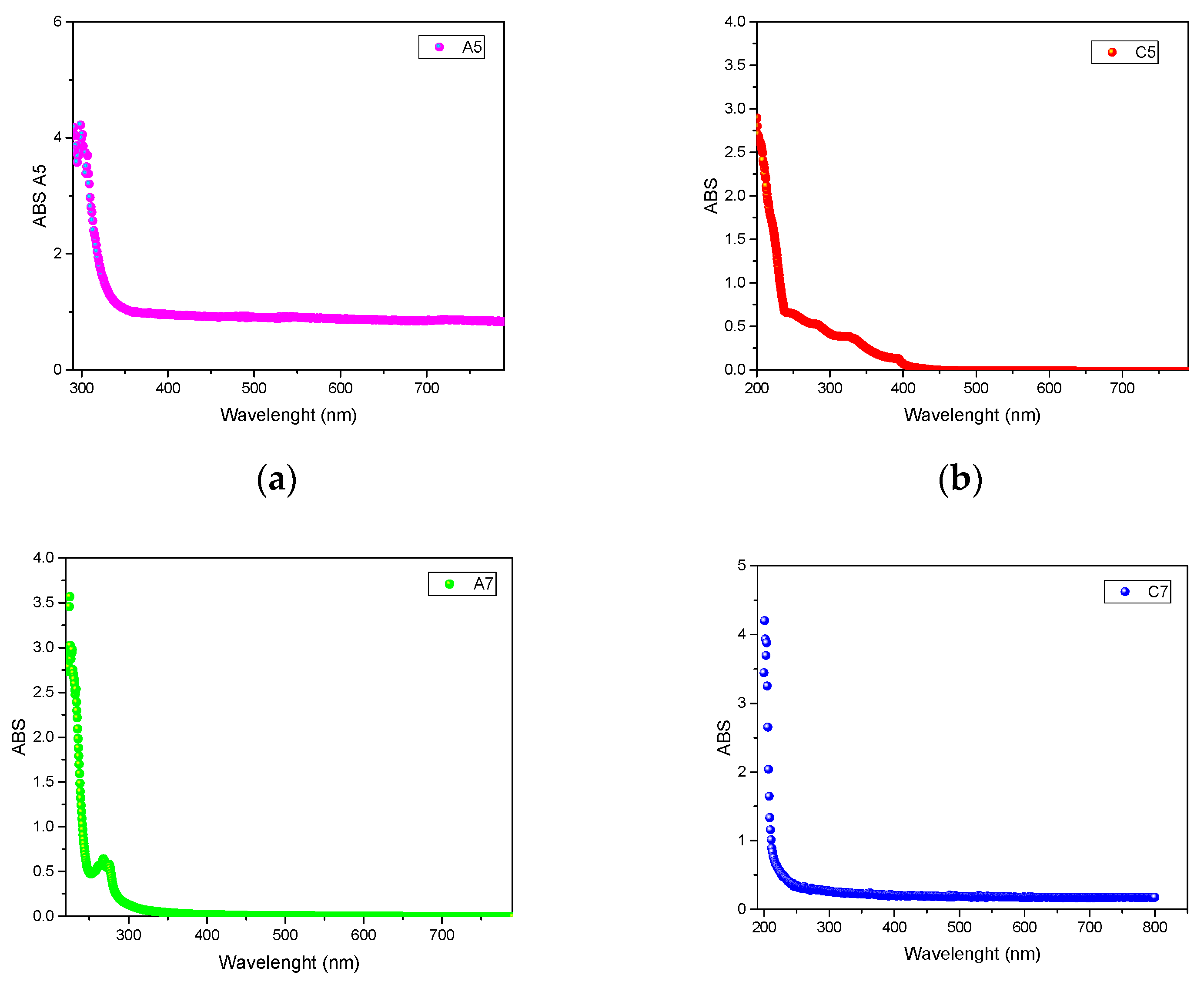
| ChCl (0.5) | EG (0) | EtOH (0.5) | A5 | 3.85 |
| ChCl (0) | EG (0.5) | EtOH (0.5) | A6 | 6.05 |
| ChCl (0.33) | EG (0.33) | EtOH (0.33) | A7 | 5.87 |
| ChCl (1) | EG (0) | 2-propanol (0) | B1 | 5.75 |
| ChCl (0) | EG (1) | 2-propanol (0) | B2 | 5.66 |
| ChCl (0) | EG (0) | 2-propanol (1) | B3 | 5.88 |
| ChCl (0.5) | EG (0.5) | 2-propanol (0) | B4 | 5.87 |
| ChCl (0.5) | EG (0) | 2-propanol (0.5) | B5 | 3.85 |
| ChCl (0) | EG (0.5) | 2-propanol (0.5) | B6 | 6.05 |
| ChCl (0.33) | EG (0.33) | 2-propanol (0.33) | B7 | 5.84 |
| ChCl (1) | EG (0) | GLY (0) | C1 | 5.75 |
| ChCl (0) | EG (1) | GLY (0) | C2 | 5.66 |
| ChCl (0) | EG (0) | GLY (1) | C3 | 5.23 |
| ChCl (0.5) | EG (0.5) | GLY (0) | C4 | 5.86 |
| ChCl (0.5) | EG (0) | GLY (0.5) | C5 | 5.51 |
| ChCl (0) | EG (0.5) | GLY (0.5) | C6 | 5.17 |
| ChCl (0.33) | EG (0.33) | GLY (0.33) | C7 | 5.12 |
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| Quadratic model | 3.1813 | 5 | 0.63626 | 1.48 | 0.5435 |
| Total error | 0.430699 | 1 | 0.430699 | ||
| Total (corr.) | 3.612 | 6 |
| System | Statistical Model | R-Squared (%) |
|---|---|---|
| A | quadratic | 88.0759 |
| B | quadratic | 89.2872 |
| C | quadratic | 83.3460 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannu, A.; Cardano, F.; Baldino, S.; Fin, A. Behavior of Ternary Mixtures of Hydrogen Bond Acceptors and Donors in Terms of Band Gap Energies. Materials 2021, 14, 3418. https://doi.org/10.3390/ma14123418
Mannu A, Cardano F, Baldino S, Fin A. Behavior of Ternary Mixtures of Hydrogen Bond Acceptors and Donors in Terms of Band Gap Energies. Materials. 2021; 14(12):3418. https://doi.org/10.3390/ma14123418
Chicago/Turabian StyleMannu, Alberto, Francesca Cardano, Salvatore Baldino, and Andrea Fin. 2021. "Behavior of Ternary Mixtures of Hydrogen Bond Acceptors and Donors in Terms of Band Gap Energies" Materials 14, no. 12: 3418. https://doi.org/10.3390/ma14123418
APA StyleMannu, A., Cardano, F., Baldino, S., & Fin, A. (2021). Behavior of Ternary Mixtures of Hydrogen Bond Acceptors and Donors in Terms of Band Gap Energies. Materials, 14(12), 3418. https://doi.org/10.3390/ma14123418