Photothermal Killing of A549 Cells and Autophagy Induction by Bismuth Selenide Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Bi2Se3 Particles
2.2. Photothermal Conversion Performance of Bi2Se3 Particles
2.3. CCK-8 Cell Viability Assay
2.4. Live and Dead Assay
2.5. Annexin V-FITC/PI Double Staining
2.6. Monodansylcadaverine (MDC) Staining for Autophagy Assay
2.7. Western Blotting Analysis
2.8. Statistical Analysis
3. Results and Discussions
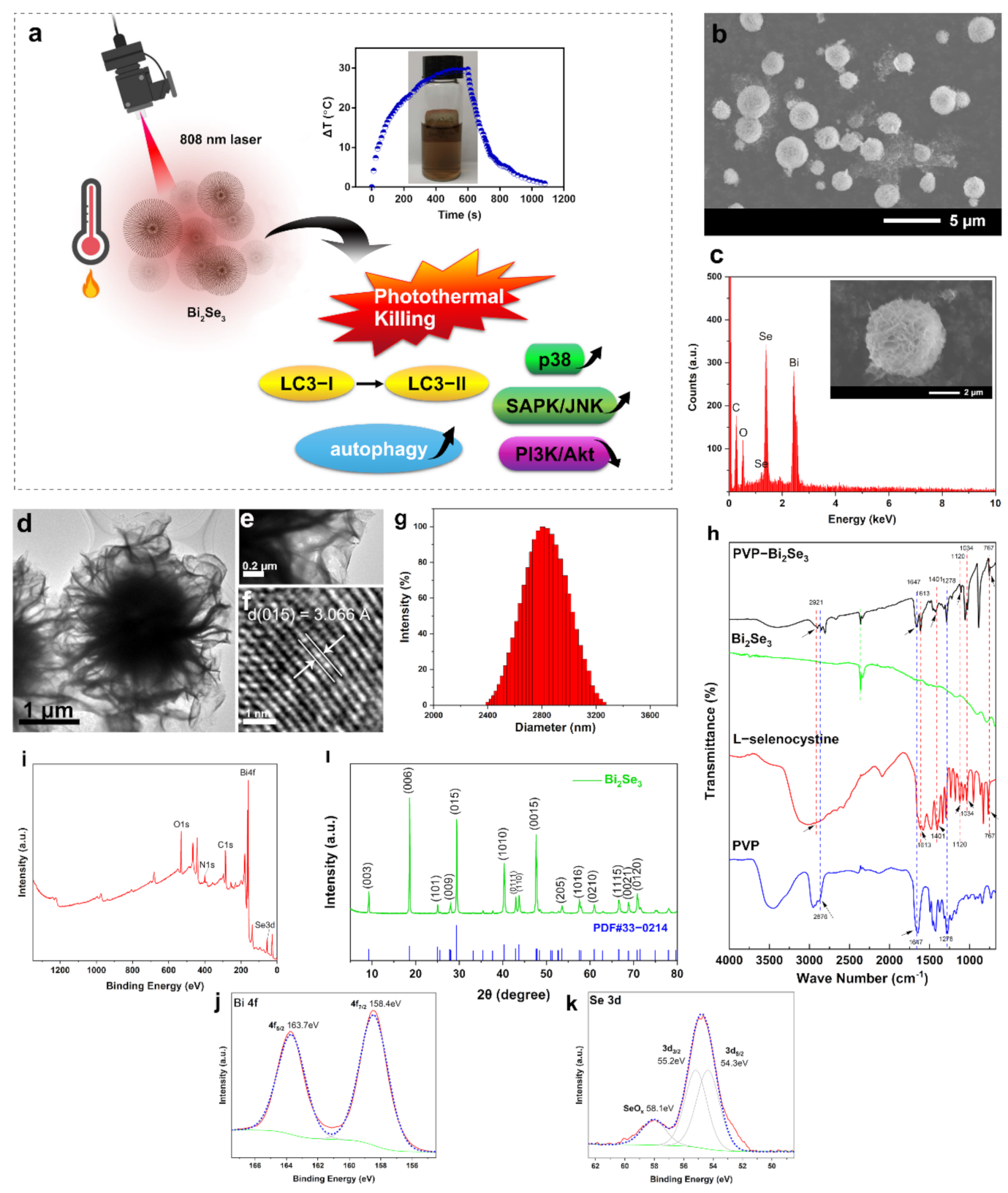
3.1. Characterization of Bi2Se3
3.2. Photothermal Profile of Bi2Se3
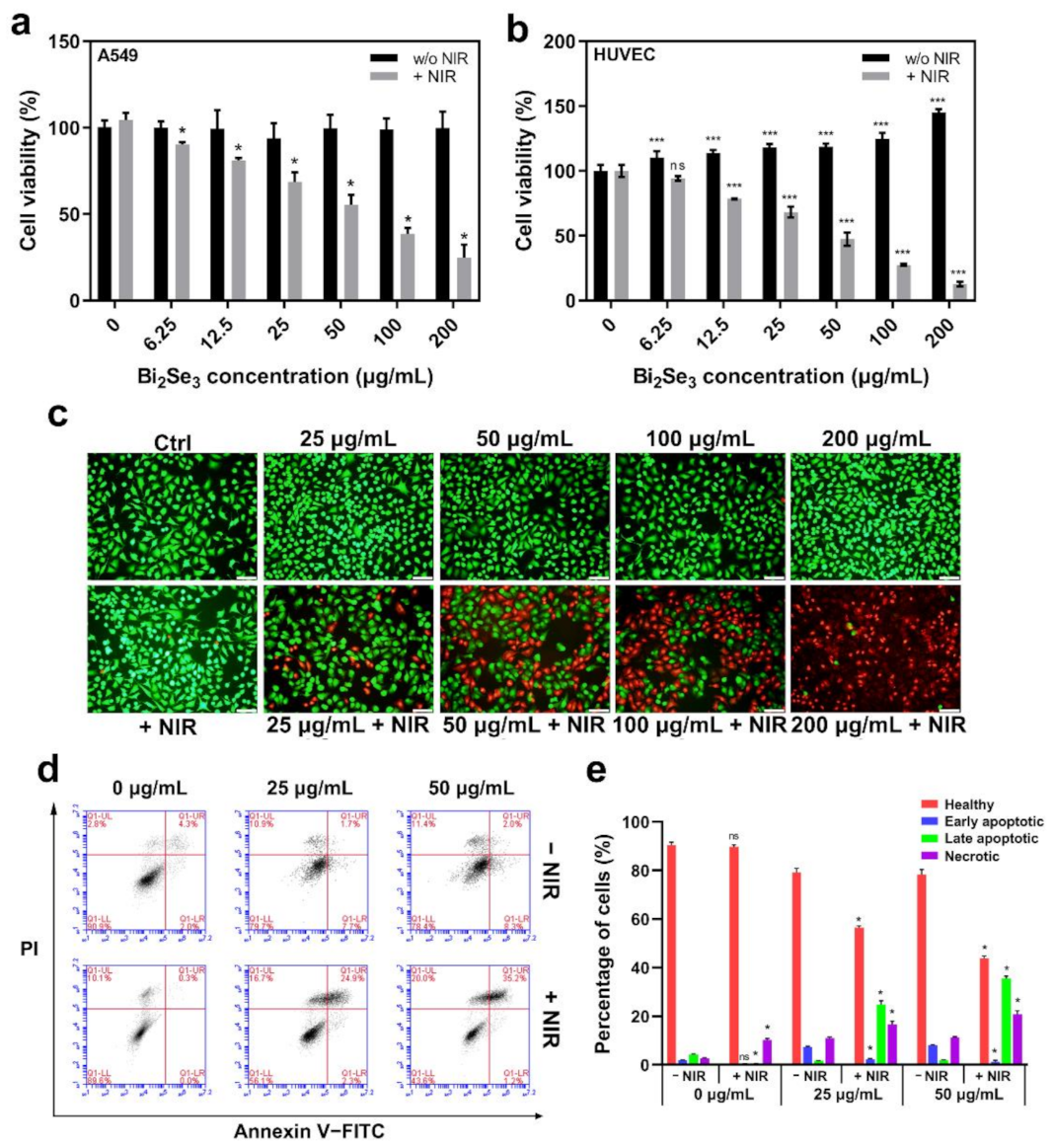
3.3. The Photothermal Effect of Bi2Se3
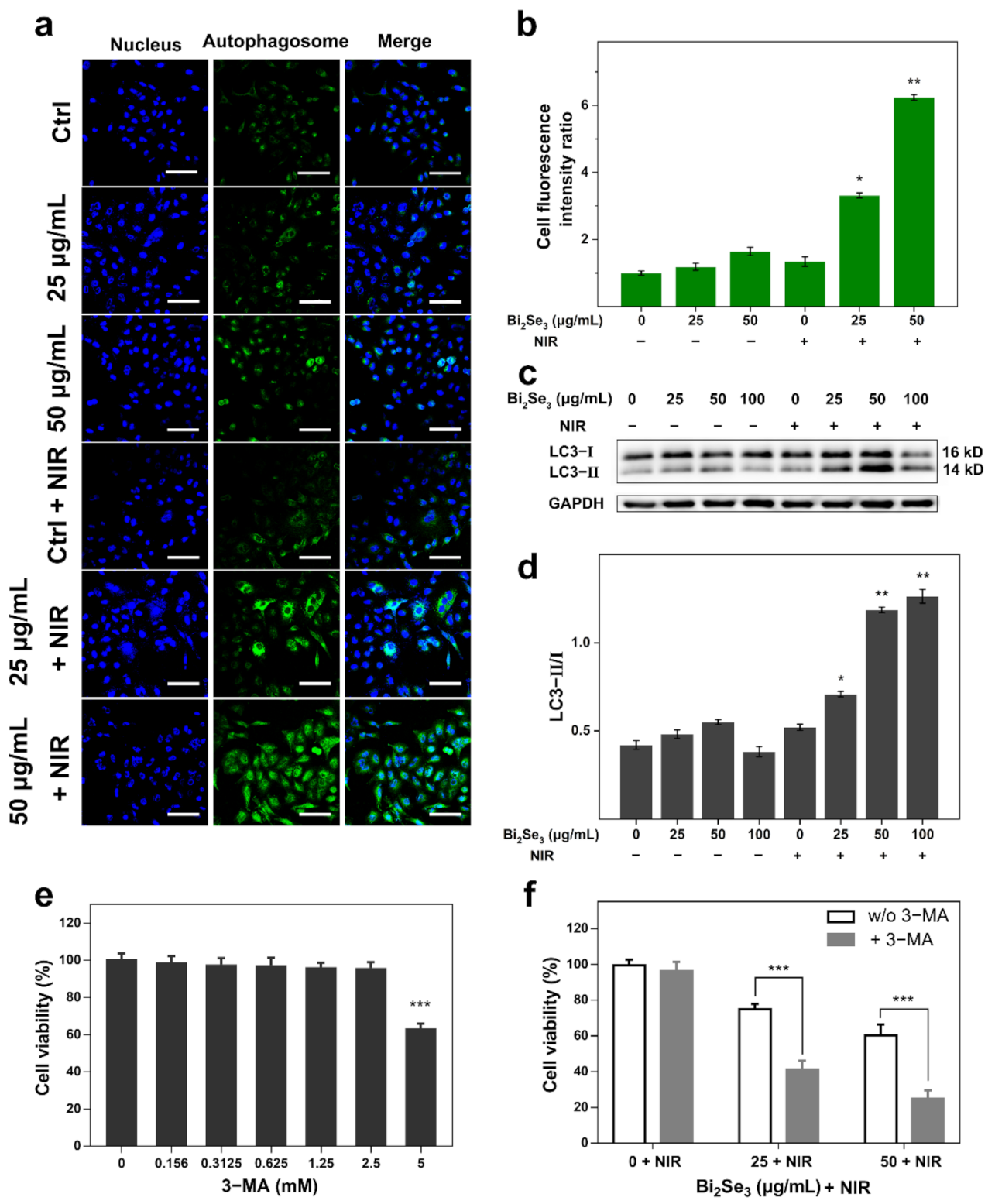
3.4. Autophagy Gets Involved in the Photothermal Killing of Bi2Se3
3.5. Activated Stress-Related p38 and SAPK/JNK Signaling Pathways Coupled with the Attenuated PI3K/Akt Signaling in the Photothermal Effect of Bi2Se3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y.; Xiao, Q.; Yu, X.-F.; Zhao, Y.; Zhang, H.; et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Lu, X.-M.; Zhu, F.; Huang, P.; Yu, Y.; Zeng, L.; Long, Z.-Y.; Wu, Y.-M. The use of a gold nanoparticle-based adjuvant to improve the therapeutic efficacy of hNgR-Fc protein immunization in spinal cord-injured rats. Biomaterials 2011, 32, 7988–7998. [Google Scholar] [CrossRef]
- Li, Z.H.; Chen, Y.J.; Yang, Y.; Yu, Y.; Zhang, Y.H.; Zhu, D.H.; Yu, X.P.; Ouyang, X.X.; Xie, Z.Y.; Zhao, Y.L.; et al. Recent Advances in Nanomaterials-Based Chemo-Photothermal Combination Therapy for Improving Cancer Treatment. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Tan, L.J.; Wu, Z.Y.; Wang, X.J.; Sun, J. Facile synthesis of CuS mesostructures with high photothermal conversion efficiency (vol 5, gp 35317, 2015). Rsc Adv. 2015, 5, 39192. [Google Scholar] [CrossRef]
- Chen, J.Q.; Ning, C.Y.; Zhou, Z.N.; Yu, P.; Zhu, Y.; Tan, G.X.; Mao, C.B. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Shibu, E.S.; Hamada, M.; Murase, N.; Biju, V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 53–72. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Cheng, X.J.; Yong, Y.; Dai, Y.H.; Song, X.; Yang, G.; Pan, Y.; Ge, C.C. Enhanced Radiotherapy using Bismuth Sulfide Nanoagents Combined with Photo-thermal Treatment. Theranostics 2017, 7, 4087–4098. [Google Scholar] [CrossRef]
- Wang, L.P.; Long, N.J.; Li, L.H.; Lu, Y.; Li, M.; Cao, J.K.; Zhang, Y.; Zhang, Q.Y.; Xu, S.H.; Yang, Z.M.; et al. Multi-functional bismuth-doped bioglasses: Combining bioactivity and photothermal response for bone tumor treatment and tissue repair. Light. Sci. Appl. 2019, 8, 54. [Google Scholar] [CrossRef]
- Shahbazi, M.-A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The versatile biomedical applications of bismuth-based nanoparticles and composites: Therapeutic, diagnostic, biosensing, and regenerative properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Liu, T.; Zeng, L.; Jiang, W.; Fu, Y.; Zheng, W.; Chen, T. Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 947–958. [Google Scholar] [CrossRef]
- Lee, S.-C.; Lee, N.-H.; Patel, K.D.; Jang, T.-S.; Knowles, J.C.; Kim, H.-W.; Lee, H.-H.; Lee, J.-H. The Effect of Selenium Nanoparticles on the Osteogenic Differentiation of MC3T3-E1 Cells. Nanomaterials 2021, 11, 557. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Min, Y.; Park, G.; Shen, X.; Song, S.-S.; Sun, Y.-M.; Wang, H.; Long, W.; Xie, J.; et al. Metabolizable Bi2Se3 Nanoplates: Biodistribution, Toxicity, and Uses for Cancer Radiation Therapy and Imaging. Adv. Funct. Mater. 2014, 24. [Google Scholar] [CrossRef]
- Li, J.; Jiang, F.; Yang, B.; Song, X.R.; Liu, Y.; Yang, H.H.; Cao, D.R.; Shi, W.R.; Chen, G.N. Topological insulator bismuth selenide as a theranostic platform for simultaneous cancer imaging and therapy. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Xie, H.H.; Li, Z.B.; Sun, Z.B.; Shao, J.D.; Yu, X.F.; Guo, Z.N.; Wang, J.H.; Xiao, Q.L.; Wang, H.Y.; Wang, Q.Q.; et al. Metabolizable Ultrathin Bi2Se3 Nanosheets in Imaging-Guided Photothermal Therapy. Small 2016, 12, 4136–4145. [Google Scholar] [CrossRef]
- Zhao, H.J.; Li, L.; Zhang, J.L.; Zheng, C.X.; Ding, K.L.; Xiao, H.F.; Wang, L.; Zhang, Z.Z. C-C Chemokine Ligand 2 (CCL2) Recruits Macrophage-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles To Facilitate Photothermal Sensitivity and Inhibit Lung Metastasis of Breast Cancer. ACS Appl. Mater. Interfaces 2018, 10, 31124–31135. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J.H. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Bravo-San Pedro, J.M.; Amaravadi, R.K.; Baehrecke, E.H.; Cecconi, F.; Codogno, P.; Debnath, J.; Gewirtz, D.A.; Karantza, V.; et al. Autophagy in malignant transformation and cancer progression. Embo J. 2015, 34, 856–880. [Google Scholar] [CrossRef]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Levine, B.; Green, D.R.; Kroemer, G. Pharmacological modulation of autophagy: Therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 2017, 16, 487–511. [Google Scholar] [CrossRef]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Hale, B.J.; Hager, C.L.; Seibert, J.T.; Selsby, J.T.; Baumgard, L.H.; Keating, A.F.; Ross, J.W. Heat stress induces autophagy in pig ovaries during follicular development. Biol. Reprod. 2017, 97, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.J.; Yan, Y.; Hu, K.W.; Zou, Y.; Li, Y.W.; Ma, R.; Zhang, Q.; Cheng, Y.Y. Autophagy inhibition enabled efficient photothermal therapy at a mild temperature. Biomaterials 2017, 141, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.J.; Yan, Y.; Wang, L.; Zhang, Q.; Cheng, Y.Y. Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy. Biomaterials 2019, 203, 63–72. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Li, Y.; Jiang, W.Q.; Zhou, L.F. MAPK/JNK signalling: A potential autophagy regulation pathway. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef]
- Zhong, W.; Zhu, H.; Sheng, F.; Tian, Y.; Zhou, J.; Chen, Y.; Li, S.; Lin, J. Activation of the MAPK11/12/13/14 (p38 MAPK) pathway regulates the transcription of autophagy genes in response to oxidative stress induced by a novel copper complex in HeLa cells. Autophagy 2014, 10, 1285–1300. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Hamdi, M.; Kool, J.; Cornelissen-Steijger, P.; Carlotti, F.; Popeijus, H.E.; van der Burgt, C.; Janssen, J.M.; Yasui, A.; Hoeben, R.C.; Terleth, C.; et al. DNA damage in transcribed genes induces apoptosis via the JNK pathway and the JNK-phosphatase MKP-1. Oncogene 2005, 24, 7135–7144. [Google Scholar] [CrossRef]
- Hammouda, M.B.; Ford, A.E.; Liu, Y.; Zhang, J.Y. The JNK Signaling Pathway in Inflammatory Skin Disorders and Cancer. Cells 2020, 9, 857. [Google Scholar] [CrossRef]
- Bubici, C.; Papa, S. JNK signalling in cancer: In need of new, smarter therapeutic targets. Br. J. Pharmacol. 2014, 171, 24–37. [Google Scholar] [CrossRef]
- Saeki, K.; Kobayashi, N.; Inazawa, Y.; Zhang, H.; Nishitoh, H.; Ichijo, H.; Saeki, K.; Isemura, M.; Yuo, A. Oxidation-triggered c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein (MAP) kinase pathways for apoptosis in human leukaemic cells stimulated by epigallocatechin-3-gallate (EGCG): A distinct pathway from those of chemically induced and recep. Biochem. J. 2002, 368, 705–720. [Google Scholar] [CrossRef]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- Varghese, J.; Chattopadhaya, S.; Sarin, A. Inhibition of p38 kinase reveals a TNF-alpha-mediated, caspase-dependent, apoptotic death pathway in a human myelomonocyte cell line. J. Immunol. 2001, 166, 6570–6577. [Google Scholar] [CrossRef]
- Basmaciyan, L.; Azas, N.; Casanova, M. Calcein+/PI- as an early apoptotic feature in Leishmania. PLoS ONE 2017, 12, e0187756. [Google Scholar] [CrossRef]
- Loría-Bastarrachea, M.I.; Herrera-Kao, W.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M.; Vázquez-Torres, H.; Ávila-Ortega, A. A TG/FTIR study on the thermal degradation of poly(vinyl pyrrolidone). J. Therm. Anal. Calorim. 2011, 104, 737–742. [Google Scholar] [CrossRef]
- Borodko, Y.; Habas, S.E.; Koebel, M.; Yang, P.; Frei, H.; Somorjai, G.A. Probing the interaction of poly (vinylpyrrolidone) with platinum nanocrystals by \mbox{UV}-\mbox{R}aman and \mbox{FTIR}. J. Phys. Chem. B 2006, 110, 23052–23059. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, P.; Chen, W.; Dong, J. Studies of UV crosslinked poly(N-vinylpyrrolidone) hydrogels by FTIR, Raman and solid-state NMR spectroscopies. Polymer 2010, 51, 3054–3063. [Google Scholar] [CrossRef]
- Han, G.; Chen, Z.-G.; Yang, L.; Wang, L.; Drennan, J.; Zou, J. In-Doped Bi2Se3 Hierarchical Nanostructures as Anode Materials for Li-Ion Batteries. J. Mater. Chem. A 2014, 2, 7109–7116. [Google Scholar] [CrossRef]
- Du, J.; Gu, Z.; Yan, L.; Yong, Y.; Yi, X.; Zhang, X.; Liu, J.; Wu, R.; Ge, C.; Chen, C.; et al. Poly(Vinylpyrollidone)- and Selenocysteine-Modified Bi2 Se3 Nanoparticles Enhance Radiotherapy Efficacy in Tumors and Promote Radioprotection in Normal Tissues. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Kong, D.; Randel, J.C.; Peng, H.; Cha, J.J.; Meister, S.; Lai, K.; Chen, Y.; Shen, Z.-X.; Manoharan, H.C.; Cui, Y. Topological Insulator Nanowires and Nanoribbons. Nano Lett. 2010, 10, 329–333. [Google Scholar] [CrossRef]
- Kong, D.; Cha, J.; Lai, K.; Peng, H.; Analytis, J.; Meister, S.; Chen, Y.; Zhang, H.-J.; Fisher, I.; Shen, Z.-X.; et al. Rapid Surface Oxidation as a Source of Surface Degradation Factor for Bi2Se3. ACS Nano 2011, 5, 4698–4703. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Lou, Y.; Qiu, X.; Zhu, J.; Burda, C. Plasmonic Cu2−xS Nanocrystals: Optical and Structural Properties of Copper-Deficient Copper(I) Sulfides. J. Am. Chem. Soc. 2009, 131, 4253–4261. [Google Scholar] [CrossRef]
- Tian, Q.; Jiang, F.; Zou, R.; Liu, Q.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic Cu9S5 Nanocrystals: A Photothermal Agent with a 25.7% Heat Conversion Efficiency for Photothermal Ablation of Cancer Cells in Vivo. ACS Nano 2011, 5, 9761–9771. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.F. Structural Inorganic Chemistry, 5th ed.; Clarendon Press: Oxford, UK, 1990. [Google Scholar]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z.; et al. High-Throughput Synthesis of Single-Layer MoS2 Nanosheets as a Near-Infrared Photothermal-Triggered Drug Delivery for Effective Cancer Therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, C.L.; Colombo, M.I. Assays to assess autophagy induction and fusion of autophagic vacuoles with a degradative compartment, using monodansylcadaverine (MDC) and DQ-BSA. Methods Enzymol. 2009, 452, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Gomes-da-Silva, L.C.; Jimenez, A.J.; Sauvat, A.; Xie, W.; Souquere, S.; Divoux, S.; Storch, M.; Sveinbjornsson, B.; Rekdal, O.; Arnaut, L.G.; et al. Recruitment of LC3 to damaged Golgi apparatus. Cell Death Differ. 2019, 26, 1467–1484. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.L.; Wei, Y.Q.; Peng, Y.; Wei, X.W. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Y.; Li, J.; Chen, L.; Wang, M.; Dong, X.; Yan, L.; Zhang, A.; Zhao, F. Photothermal Killing of A549 Cells and Autophagy Induction by Bismuth Selenide Particles. Materials 2021, 14, 3373. https://doi.org/10.3390/ma14123373
You Y, Li J, Chen L, Wang M, Dong X, Yan L, Zhang A, Zhao F. Photothermal Killing of A549 Cells and Autophagy Induction by Bismuth Selenide Particles. Materials. 2021; 14(12):3373. https://doi.org/10.3390/ma14123373
Chicago/Turabian StyleYou, Yue, Jinxia Li, Linlin Chen, Mei Wang, Xinghua Dong, Liang Yan, Aiping Zhang, and Feng Zhao. 2021. "Photothermal Killing of A549 Cells and Autophagy Induction by Bismuth Selenide Particles" Materials 14, no. 12: 3373. https://doi.org/10.3390/ma14123373
APA StyleYou, Y., Li, J., Chen, L., Wang, M., Dong, X., Yan, L., Zhang, A., & Zhao, F. (2021). Photothermal Killing of A549 Cells and Autophagy Induction by Bismuth Selenide Particles. Materials, 14(12), 3373. https://doi.org/10.3390/ma14123373







