Calcium Carbonate–Carboxymethyl Chitosan Hybrid Materials
Abstract
1. Introduction
2. Experimental
2.1. Materials
- Chitosan was purchased from Vanson, Inc. Company. The main characteristics of chitosan are acetylation degree—20.8%, molecular weight—415.000 g/mol, cationic charge—4500 μeq/g.
- CaCl2 (96%) and Na2CO3 (99.8%) were used as chemicals for precipitated calcium carbonate—PCC.
- Softwood bleached kraft pulp refined at 30 °SR (Schopper-Riegler degree).
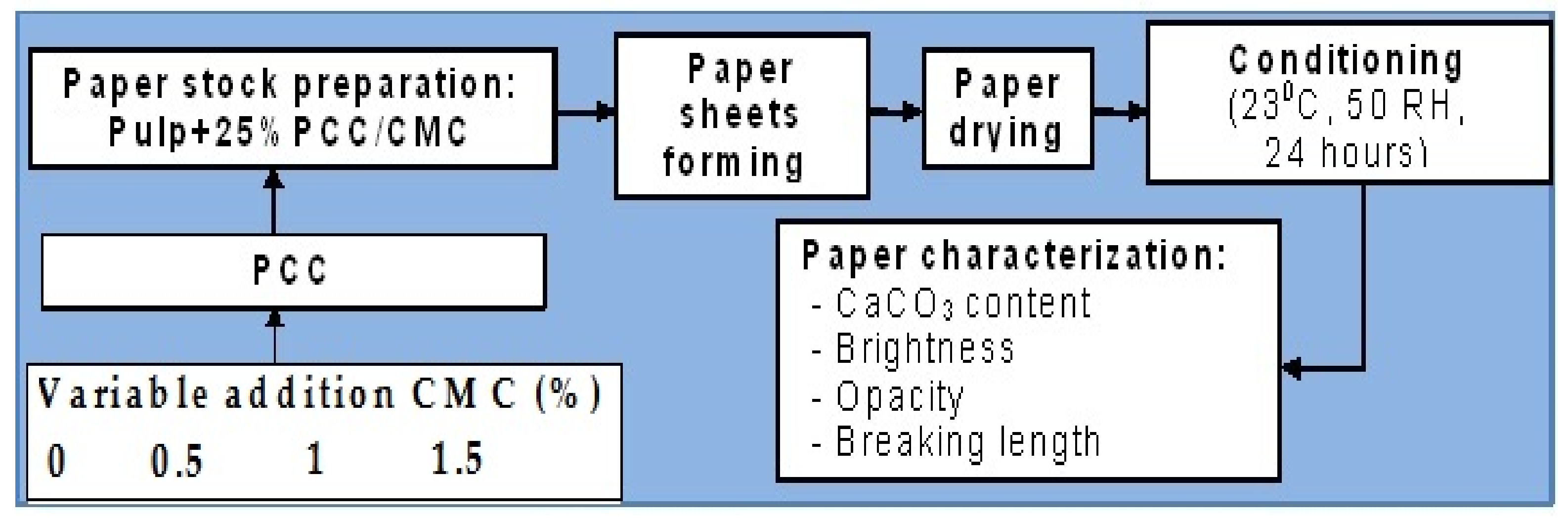
2.2. Experimental Procedure
2.3. Methods
3. Results and Discussion
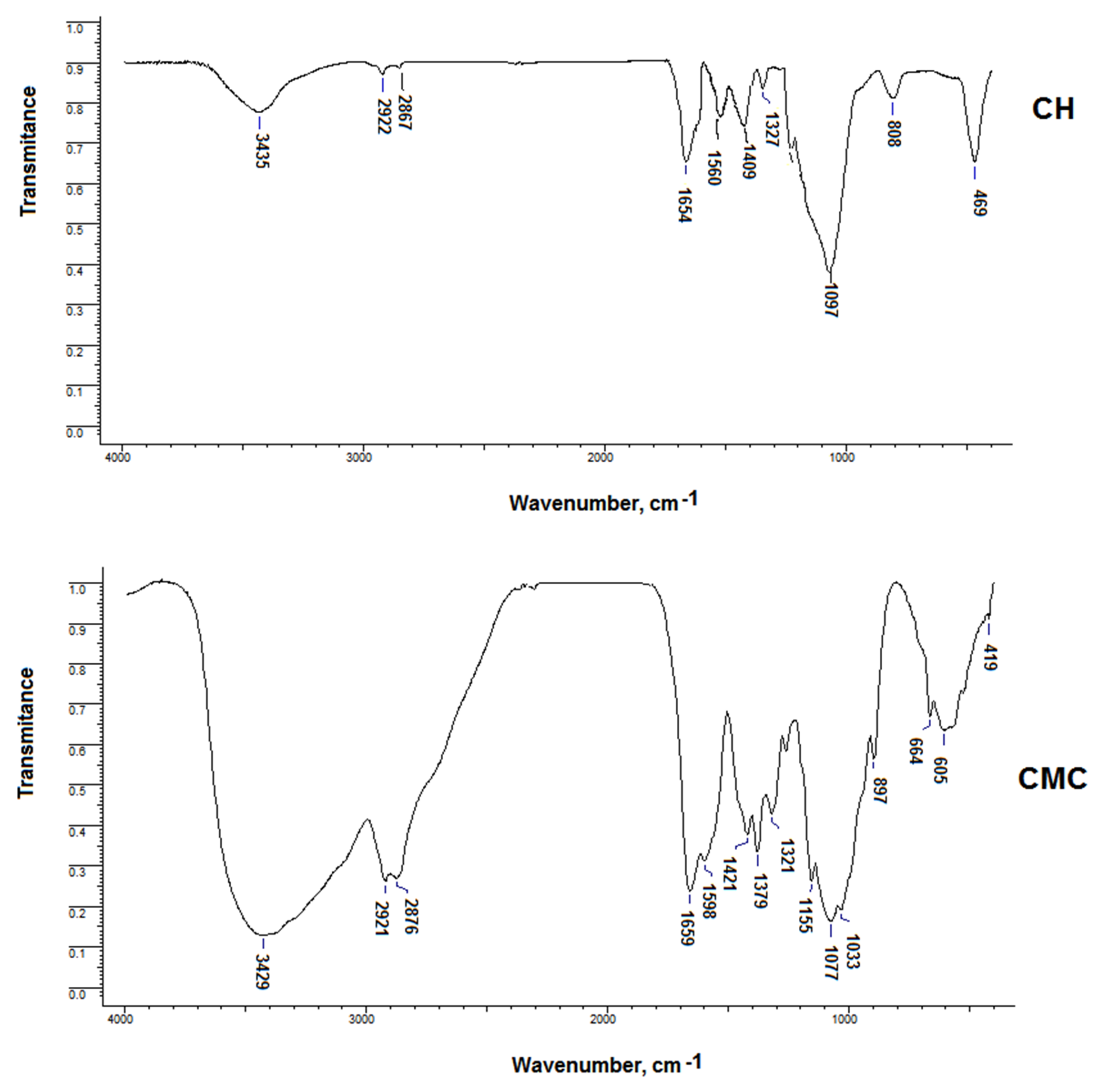
3.1. CMC Modification
3.2. Surface Morphology
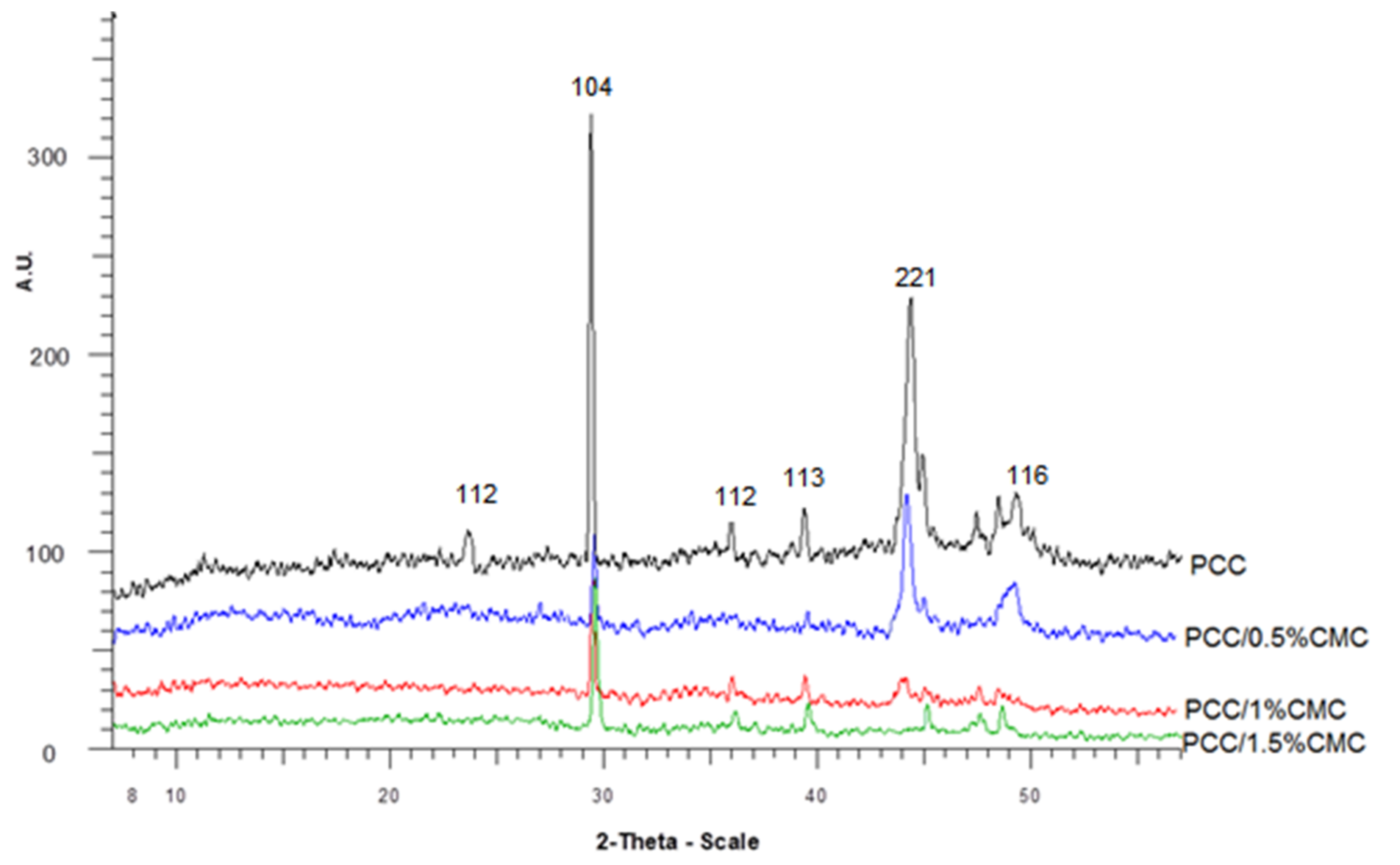
3.3. X-ray Analysis
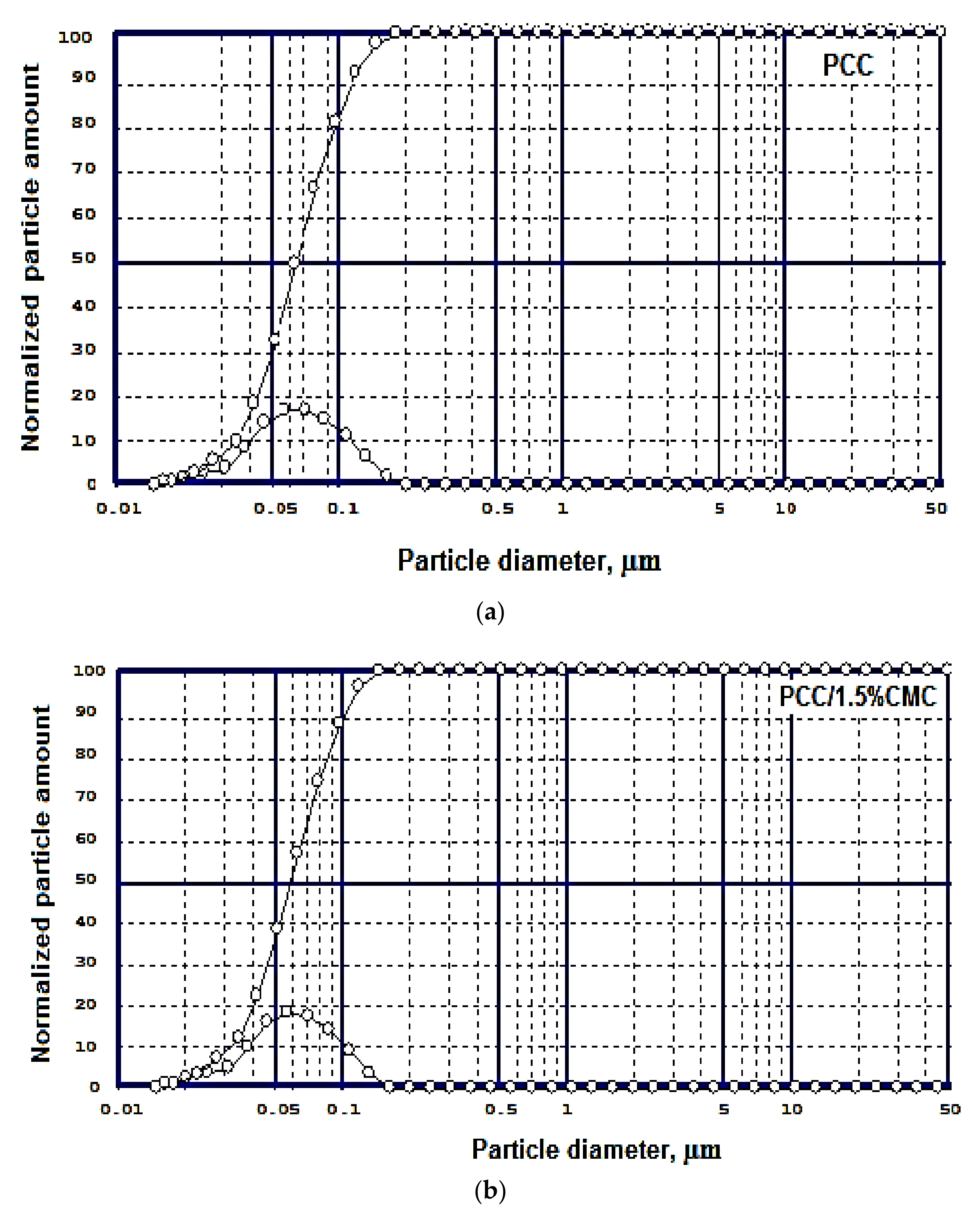
Particle Size of Modified and Unmodified PCC
3.4. Properties of Filled Paper Sheets
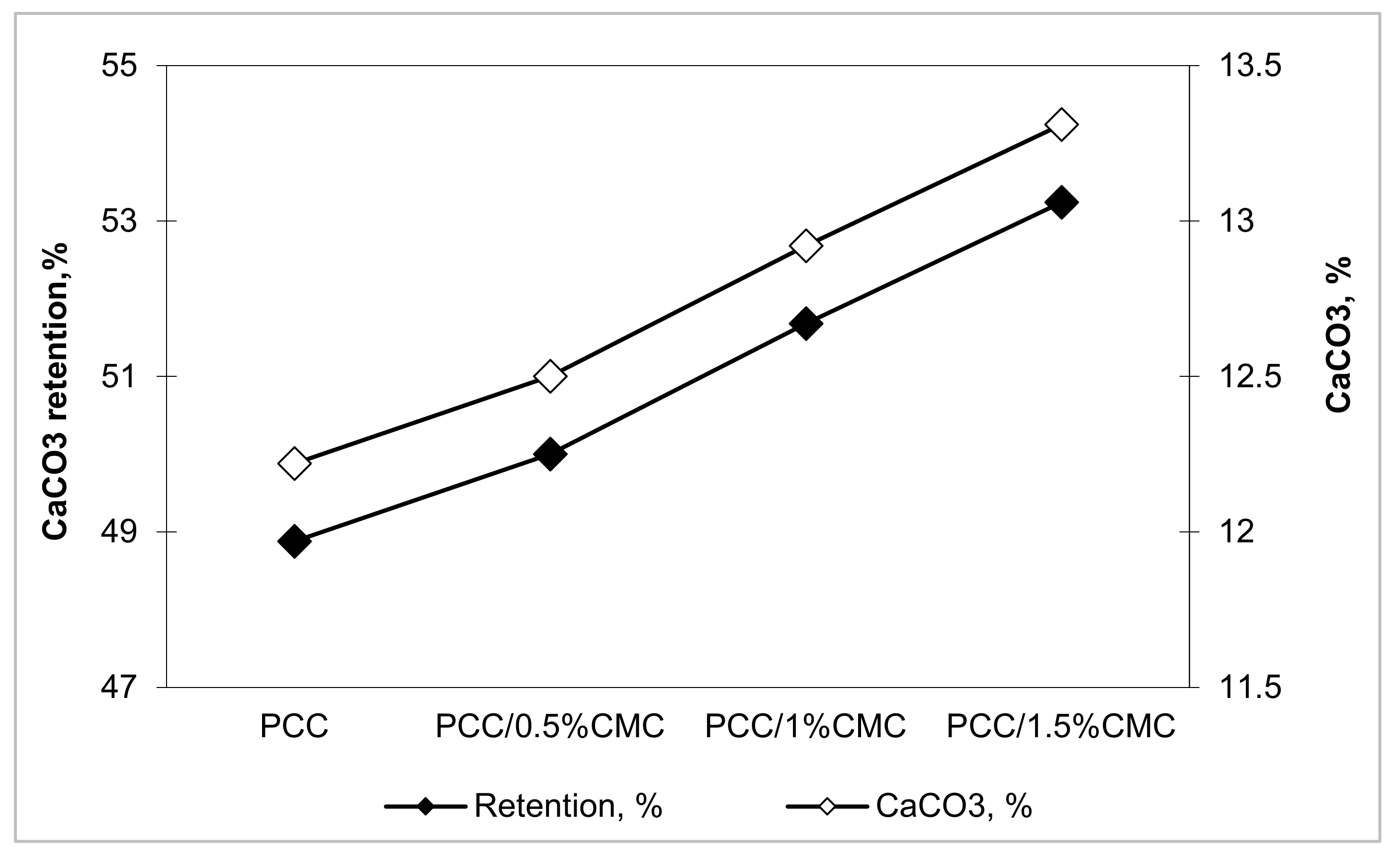
3.4.1. Retention
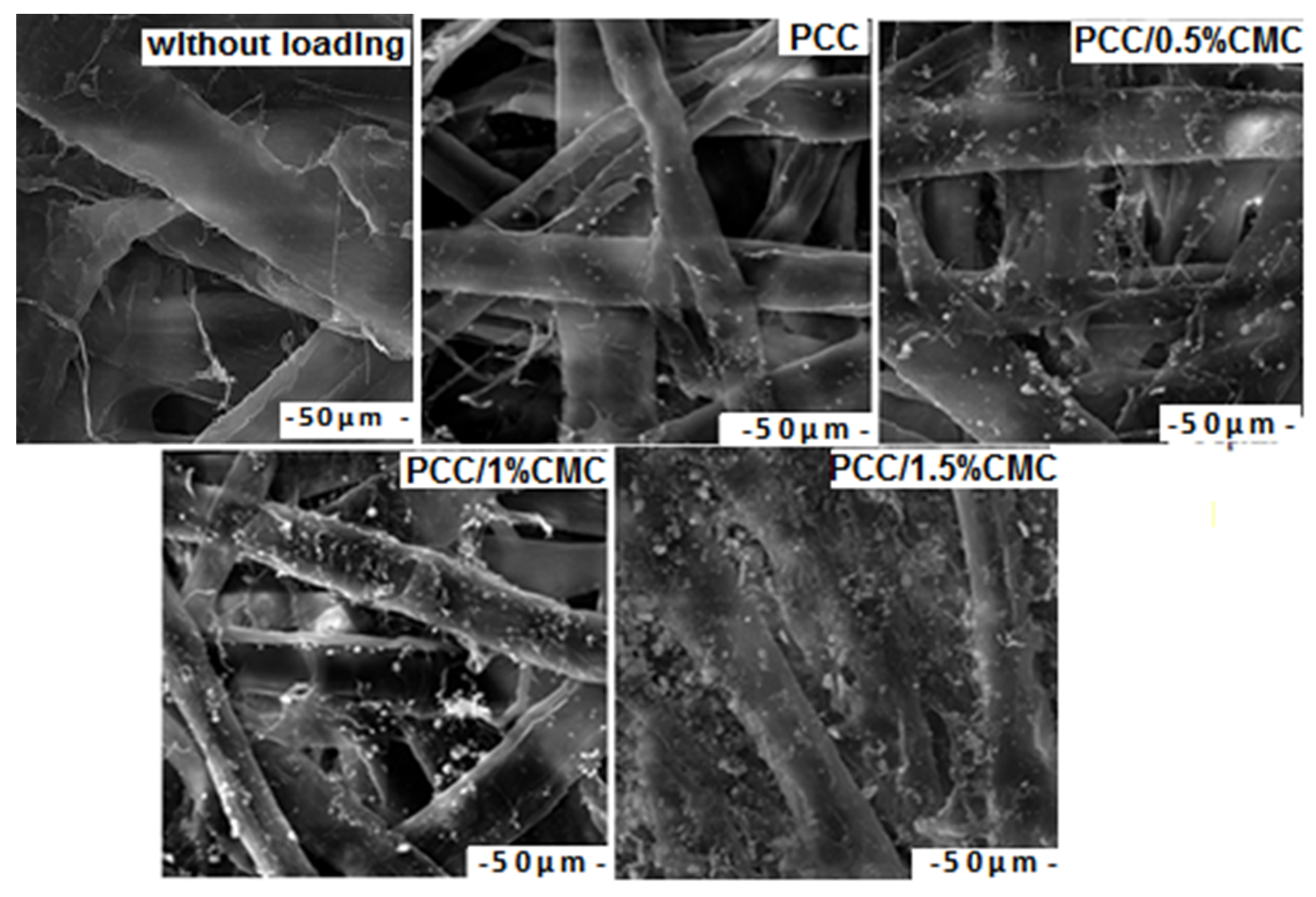
3.4.2. SEM Images of Paper Samples
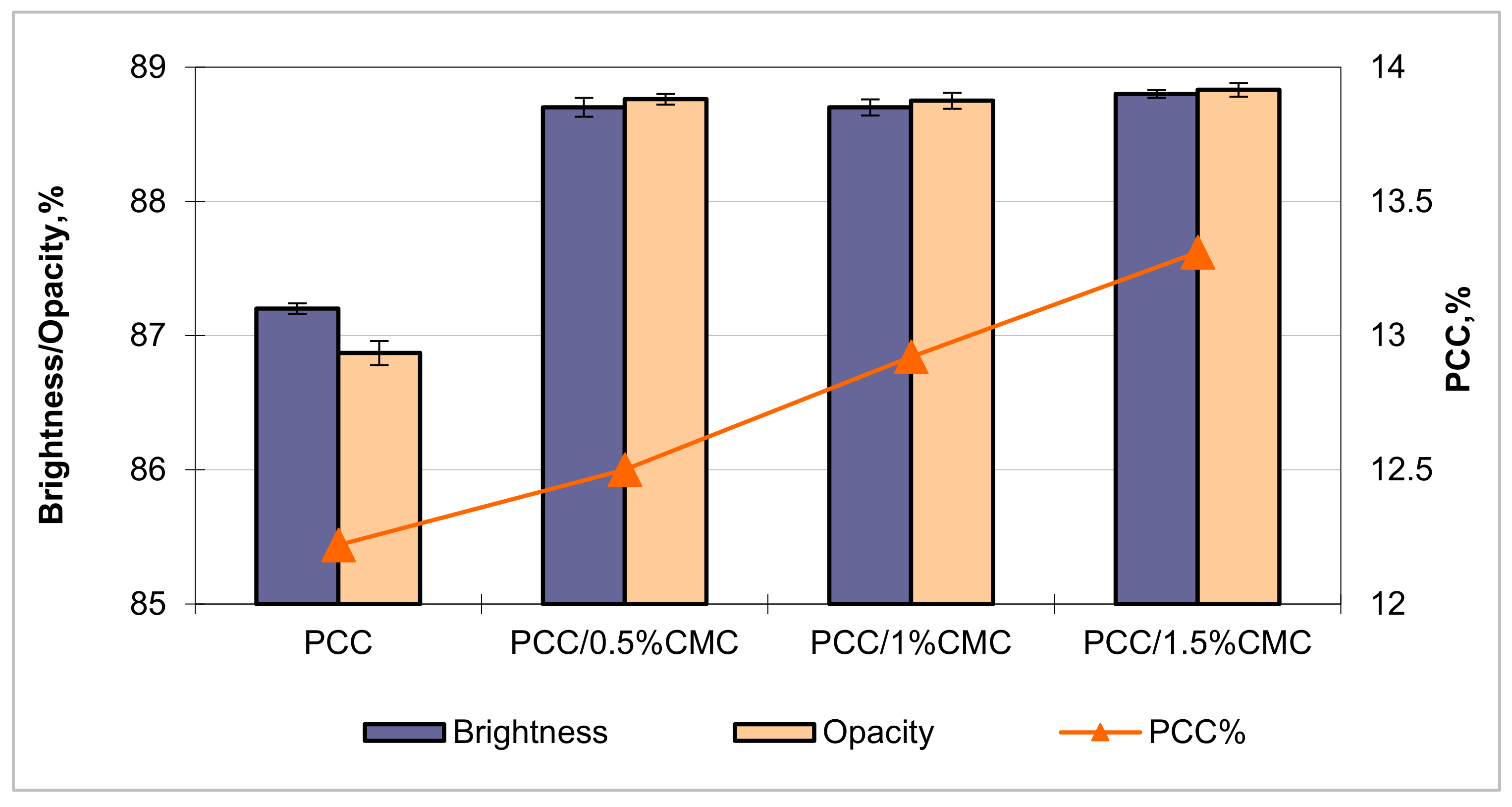
3.4.3. Optical Properties
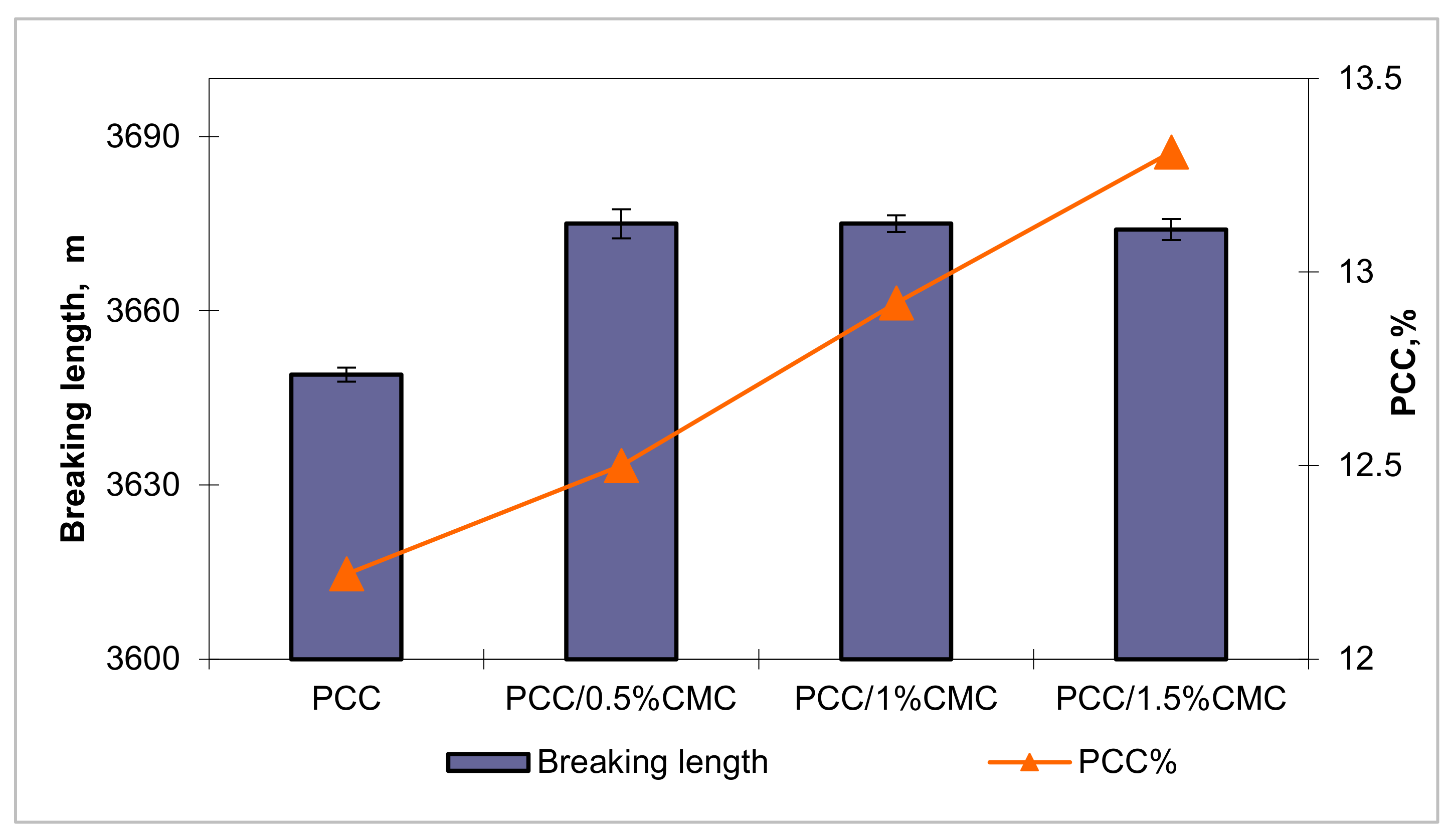
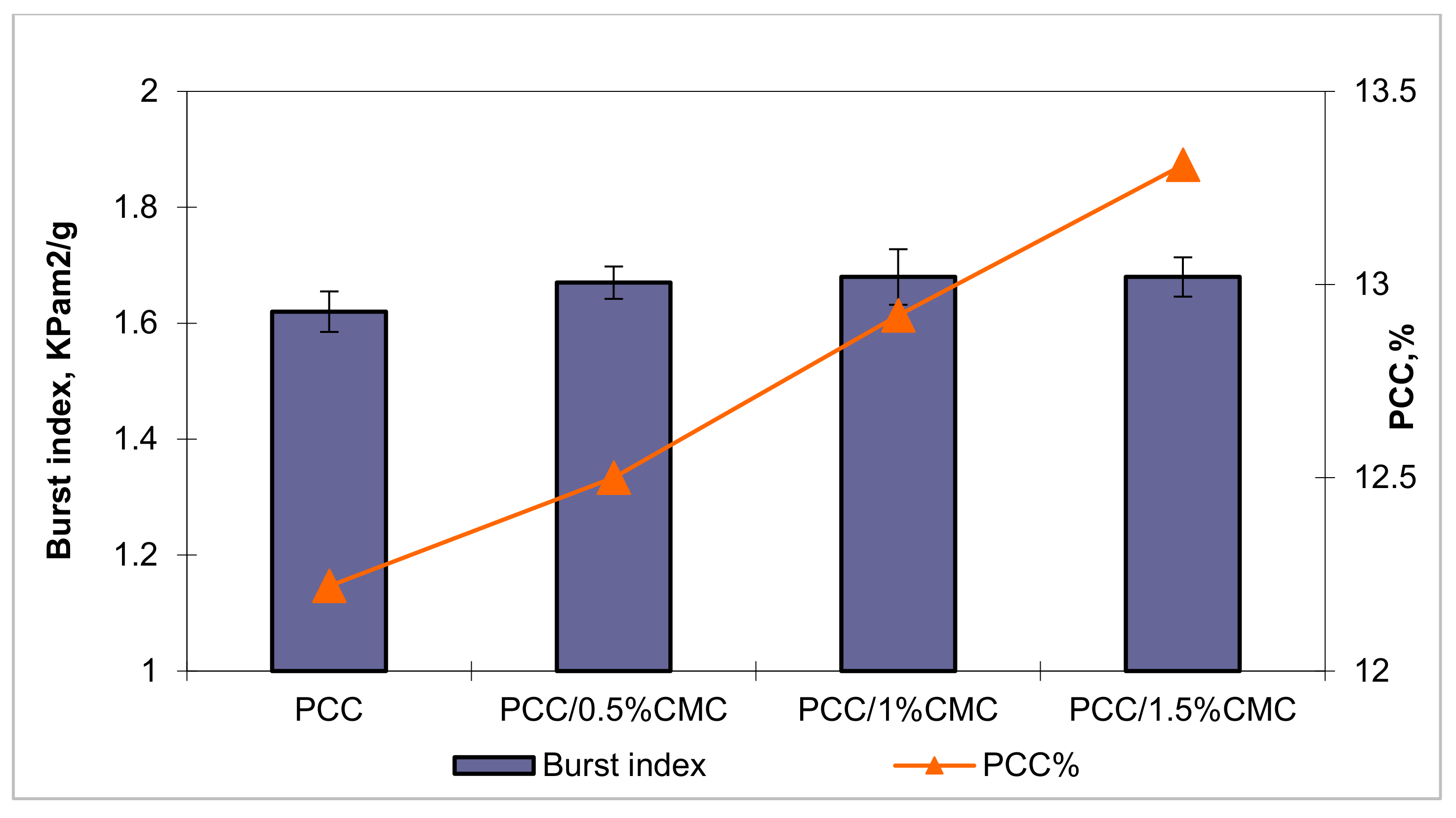
3.4.4. Mechanical Strength Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Wang, R.; Liu, Z.; Zhang, H. Preparation and characterization of calcium carbonate microspheres and their potential application as drug carriers. Mol. Med. Rep. 2018, 17, 8403–8408. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.S.; Zong, J.Y.; Zhao, D.; Li, F.; Zhuo, R.X.; Cheng, S.X. Calcium Carbonate/Carboxymethyl Chitosan Hybrid Microspheres and Nanospheres for Drug Delivery. J. Phys. Chem. C 2010, 114, 18940–18945. [Google Scholar] [CrossRef]
- Samyn, P.; Barhoum, A.; Öhlund, T.; Dufresne, A. Review: Nanoparticles and nanostructured materials in papermaking. J. Mater. Sci. 2018, 53, 146–184. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Gill, R.A. Fillers for Papermaking: A Review of their properties, usage practices, and their mechanistic role. BioResources 2016, 11, 2886–2963. [Google Scholar] [CrossRef]
- Gamelas, J.A.F.; Lourenco, A.F.; Xavier, M.; Ferreira, P.J. Modification of precipitated calcium carbonate with cellulose esters and use as filler in papermaking. Chem. Eng. Res. Des. 2014, 92, 2425–2430. [Google Scholar] [CrossRef]
- Liang, P.; Zhao, Y.; Shen, Q.; Wang, D.; Xu, D. The effect of carboxymethyl chitosan on the precipitation of calcium carbonate. J. Cryst. Growth 2004, 261, 571–576. [Google Scholar] [CrossRef]
- Fortuna, M.E.; Lobiuc, A.; Cosovanu, M.L.; Harja, M. Effects of in-situ filler loading vs. conventional filler and the use of retention-related additives on properties of paper. Materials 2020, 13, 5066. [Google Scholar] [CrossRef] [PubMed]
- Kalliola, S.; Repo, E.; Srivastava, V.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. The pH sensitive properties of carboxymethyl chitosan nanoparticles cross-linked with calcium ions. Colloid Surface B. 2017, 153, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Mousavipazhouh, H.; Azadfallah, M.; Jouybari, I.R. Encapsulation of precipitated calcium carbonate fillers using carboxymethyl cellulose /polyaluminium chloride: Preparation and its influence on mechanical and optical properties of paper. Maderas-Cienc. Tecnol. 2018, 20, 703–714. [Google Scholar] [CrossRef]
- Lourenço, A.F.; Gamelas, J.A.F.; Ferreira, P.J. Precipitated calcium carbonate modified by the layer-by-layer deposition method—Its potential as papermaking filler. Chem. Eng. Res. Des. 2015, 104, 807–813. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N.; Tiwari, A. Carboxymethyl chitosan and its applications. Adv. Mat. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Z.; Ragauskas, A.; Deng, Y. Improvement of paper properties using starch-modified precipitated calcium carbonate filler. Tappi J. 2005, 4, 3–7. [Google Scholar]
- Salama, A. Recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int. J. Biol. Macromol. 2021, 178, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, C.; Song, Z.; Qian, X. Modification of precipitated calcium carbonate filler for papermaking with adsorption of cationically derivatized chitosan and carboxymethyl chitosan. BioResources 2014, 9, 5917–5927. [Google Scholar] [CrossRef][Green Version]
- François, R.; Bertrand, S.; Pierre-Marie, B.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2008, 45, 1337–1348. [Google Scholar]
- Iftime, M.M.; Ailiesei, G.L.; Ungureanu, E.; Marin, L. Designing chitosan based eco-friendly multifunctional soil conditioner systems with urea controlled release and water retention. Carbohydr. Polym. 2019, 223, 115140. [Google Scholar] [CrossRef]
- Guo, J.; Liu, J.; Qie, H.; Zhao, F.; Niu, C.H. Efficient synthesis strategy of folate-modified carboxymethyl chitosan/CaCO3 hybrid nanospheres and their drug-carrying and sustained release properties. J. Biomater. Sci. Polym. Ed. 2021, 32, 799–812. [Google Scholar] [CrossRef]
- Ali, Z.M.; Laghari, A.J.; Ansari, A.K.; Khuhawar, M.Y. Synthesis and characterization of carboxymethyl chitosan and its effect on turbidity removal of river water. J. Appl. Chem. 2013, 5, 72–79. [Google Scholar]
- Chen, X.; Park, H. Chemical characteristics of O-carboxymethyl chitosansrelated to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- El-Sherbiny, S.; El-Sheikh, S.M.; Barhoum, A. Preparation and modification of nano calcium carbonate filler from waste marble dust and commercial limestone for papermaking wet end application. Powder Technol. 2015, 279, 290–300. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K.D.J. Antibacterial action of chitosan and carboxymethylated chitosan. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Aiping, Z.; Jianhong, L.; Wenhui, Y. Effective loading and controlled release of camptothecin by O-carboxymethylchitosan aggregates. Carbohydr. Polym. 2006, 63, 89–96. [Google Scholar] [CrossRef]
- Nada, A.M.A.; El-Sakhawy, M.; Kamel, S.; Eid, M.A.M.; Adel, A.M. Mechanical and electrical properties of paper sheets treated with chitosan and its derivatives. Carbohydr. Polym. 2006, 63, 113–121. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, X.; Song, H. Morphological investigation of calcium carbonate during ammonification-carbonization process of low concentration calcium solution. J. Nanomater. 2014, 2014, 503696. [Google Scholar] [CrossRef]
- Widyastuti, S.; Kusuma, I.A. Synthesis and characterization of CaCO3 (calcite) nano particles from cockle shells (Anadara granosa Linn) by precipitation method, Green Process, Material, and Energy: A Sustainable Solution for Climate Change. In Proceedings of the 3rd International Conference on Engineering, Technology, and Industrial Application, Surakarta, Indonesia, 7–8 December 2016. [Google Scholar]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortună, M.E.; Ungureanu, E.; Jitareanu, C.D. Calcium Carbonate–Carboxymethyl Chitosan Hybrid Materials. Materials 2021, 14, 3336. https://doi.org/10.3390/ma14123336
Fortună ME, Ungureanu E, Jitareanu CD. Calcium Carbonate–Carboxymethyl Chitosan Hybrid Materials. Materials. 2021; 14(12):3336. https://doi.org/10.3390/ma14123336
Chicago/Turabian StyleFortună, Maria E., Elena Ungureanu, and Carmen D. Jitareanu. 2021. "Calcium Carbonate–Carboxymethyl Chitosan Hybrid Materials" Materials 14, no. 12: 3336. https://doi.org/10.3390/ma14123336
APA StyleFortună, M. E., Ungureanu, E., & Jitareanu, C. D. (2021). Calcium Carbonate–Carboxymethyl Chitosan Hybrid Materials. Materials, 14(12), 3336. https://doi.org/10.3390/ma14123336




