Azadirachta indica Leaf Extract as Green Corrosion Inhibitor for Reinforced Concrete Structures: Corrosion Effectiveness against Commercial Corrosion Inhibitors and Concrete Integrity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Extract and Commercial Corrosion Inhibitors
2.2. Admixture Materials
2.2.1. Cement
2.2.2. Aggregates
2.2.3. Water
2.2.4. Additive
2.3. Concrete Admixture Design
Admixture Proportions
2.4. Fresh Concrete Characteristics
2.4.1. Concrete Sampling
2.4.2. Concrete Temperature
2.4.3. Concrete Slump
2.4.4. Concrete Unit Weight and Relative Performance
2.4.5. Air Content
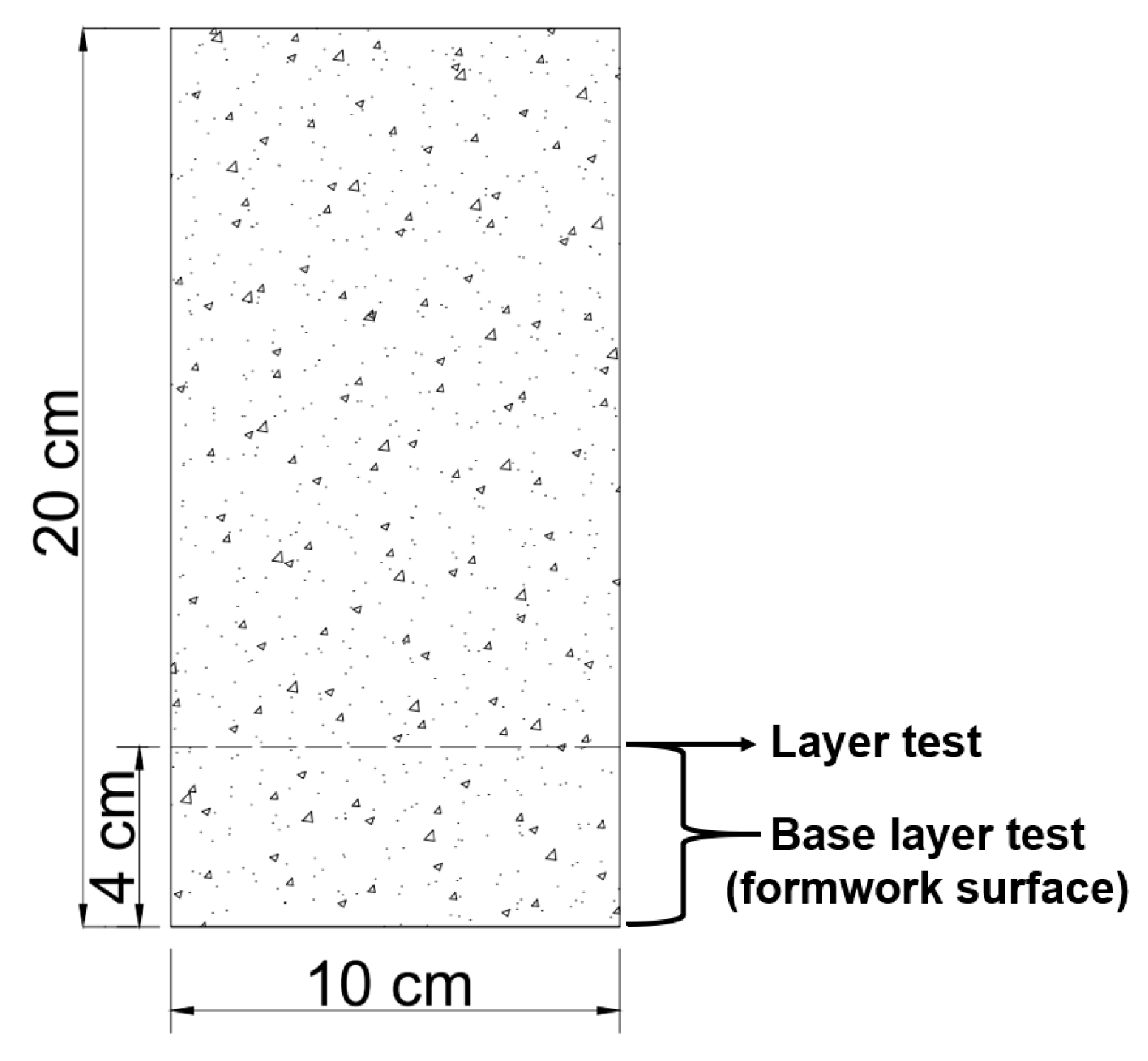
2.5. Concrete Cylinder Design
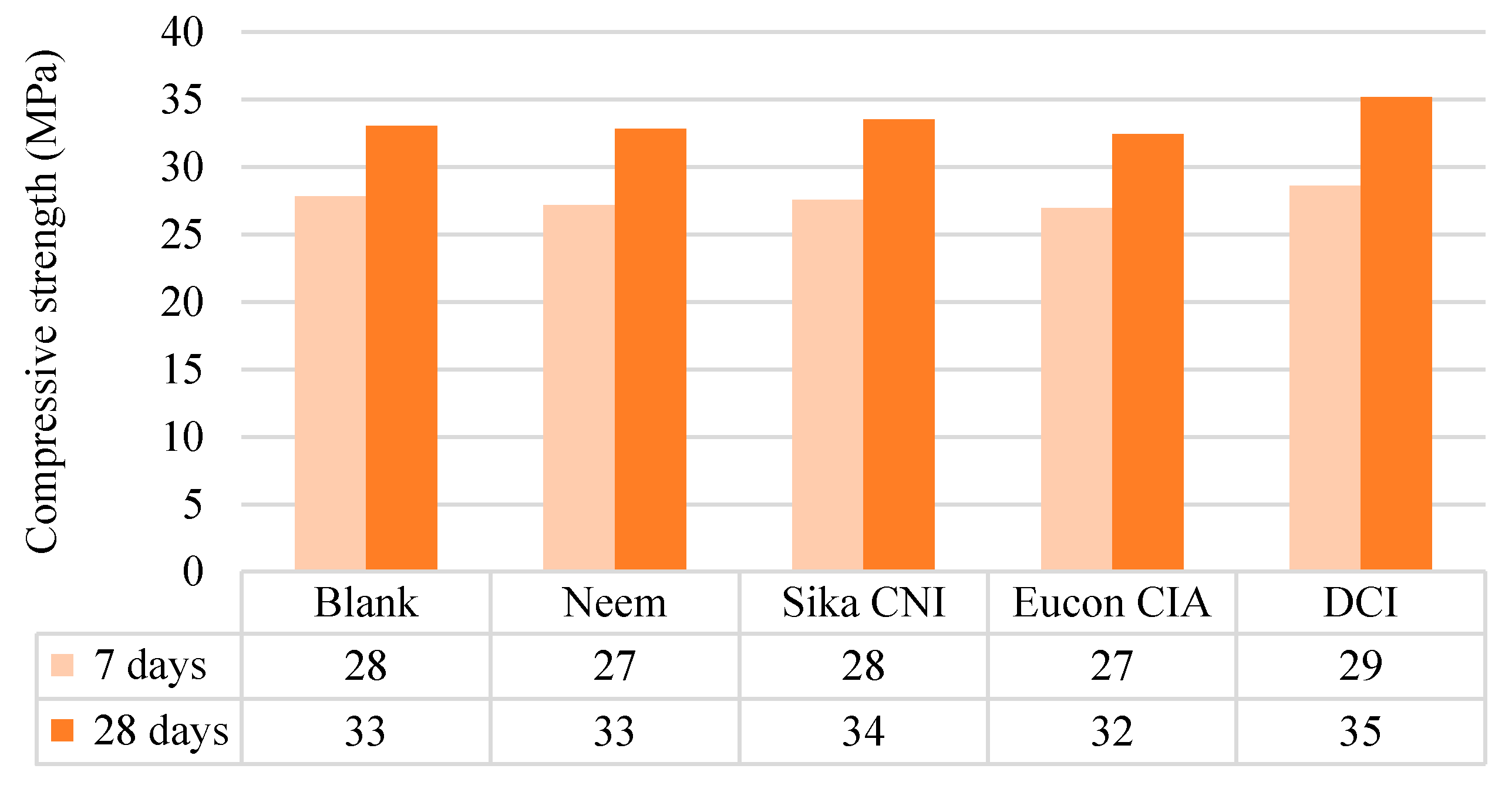
2.6. Compressive-Strength Test
2.7. Environment Conditions
2.8. Analysis Procedures for Measured Experimental Data
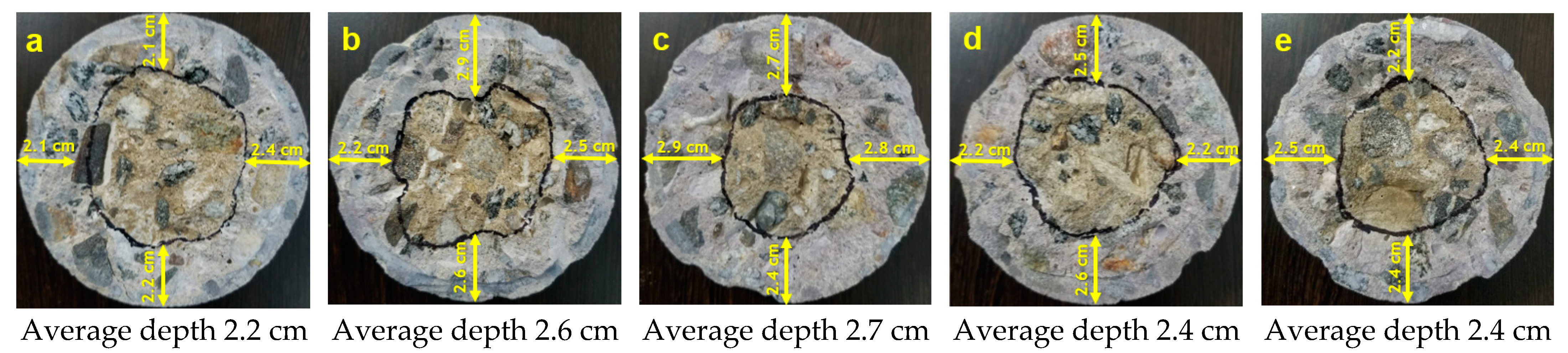
2.8.1. Chloride Ion Penetration in Concrete
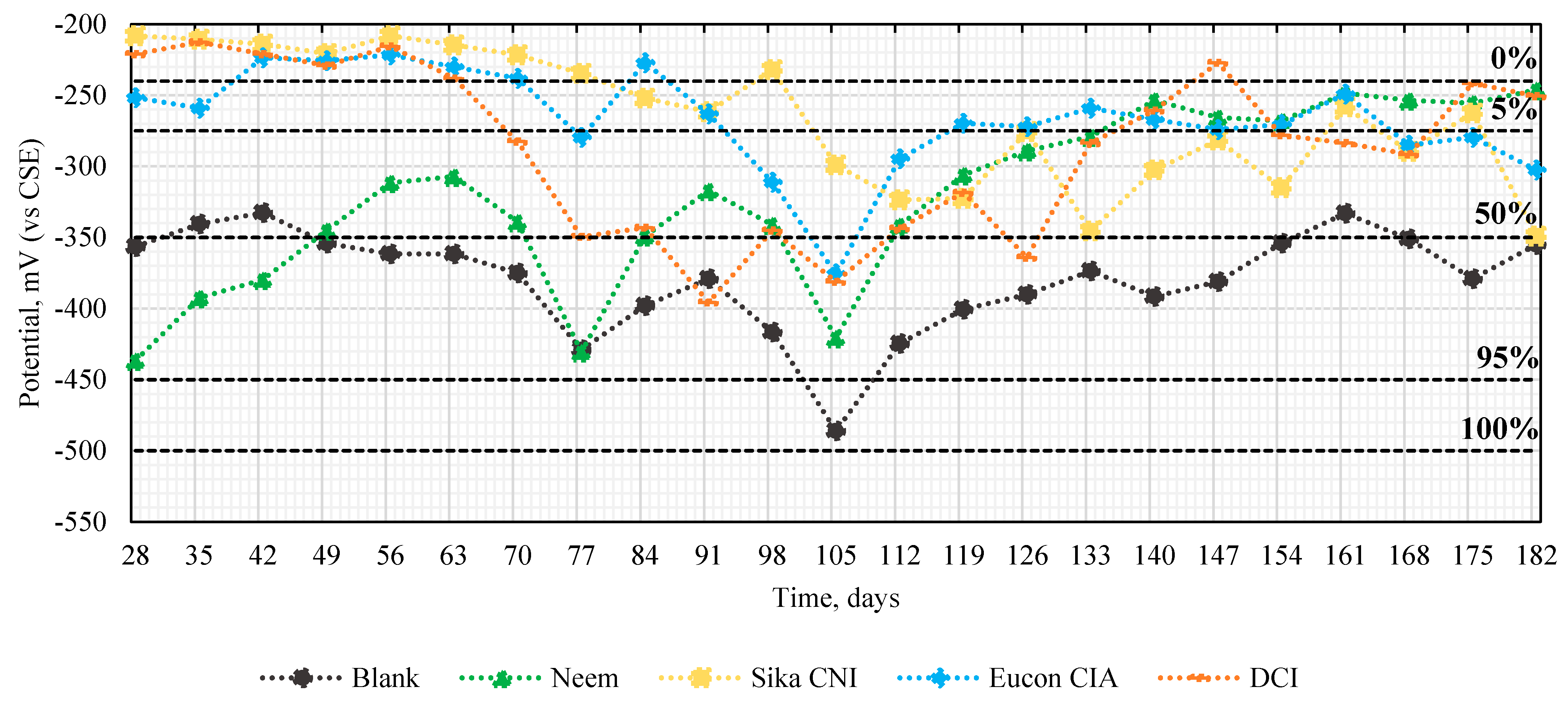
2.8.2. Half-Cell Potential
3. Results and Discussion
3.1. Concrete Integrity
3.1.1. Fresh Concrete
Concrete Temperature
Concrete Slump
Concrete Unit Weight and Relative Performance
Air Content
3.1.2. Solid Concrete
3.2. Chloride-Ion Penetration
3.3. Half-Cell Potential Record
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.S.; Sarawathy, V.; Kwon, S.J.; Karthick, S. Corrosion Inhibitors for Reinforced Concrete: A Review. In Corrosion Inhibitors, Principles and Recent Applications; IntechOpen: Rijeka, Croatia, 2017; Chapter 5. [Google Scholar]
- Bolzoni, F.; Brenna, A.; Fumagalli, G.; Goidanich, S.; Lazzari, L.; Ormellese, M.; Pedeferri, M.P. Experiences on corrosion inhibitors for reinforced concrete. Int. J. Corros. Scale Inhib. 2014, 3, 254–278. [Google Scholar] [CrossRef]
- Brenna, A.; Ormellese, M.; Pedeferri, M.P.; Bolzoni, F. Effect of binary mixtures on chloride induced corrosion of rebars in concrete. Int. J. Corros. Scale Inhib. 2018, 7, 151–164. [Google Scholar]
- Sousa, M.L.; Andreade, J.; Santana, N.; Véras, D. Corrosion inhibitors for reinforced concrete, Chapter 2. In Corrosion Inhibitors, Principles and Recent Applications; IntechOpen: Rijeka, Croatia, 2017; Chapter 2. [Google Scholar]
- Öz, G.S.; Bilgic, S. The inhibition effect of sodium tetraborate on the corrosion of steel in KOH media. Int. J. Corros. Scale Inhib. 2018, 7, 390–408. [Google Scholar]
- Brenna, A.; Bolzoni, F.; Pedeferri, M.P.; Ormellese, M. Corrosion inhibitors for reinforced concrete structures: A study of binary mixtures. Int. J. Corros. Scale Inhib. 2017, 6, 59–69. [Google Scholar]
- Abdulrahman, A.S.; Mohammad, I. Green Plant Extract as a passivation-promoting Inhibitor for Reinforced Concrete. Int. J. Eng. Sci. Technol. 2011, 3, 6484–6490. [Google Scholar]
- Odewunmi, N.A.; Umoren, S.A.; Gasem, Z.M. Watermelon waste products as green corrosion inhibitors for mild steel in HCl solution. J. Environ. Chem. Eng. 2015, 3, 286–296. [Google Scholar] [CrossRef]
- Salawu, A.A.; Mohammand, I.; Muhd, A.M.; Zaiton, A.M.; CheSobry, A.; Jahangir, M. Green Bambusa Arundinacea leaves extract as a sustainable corrosion inhibitor in steel reinforced concrete. J. Clean. Prod. 2014, 67, 139–146. [Google Scholar]
- Peter, A.; Sharma, S.K. Use of Azadirachta indica (AZI) as green corrosion inhibitor against mil steel in acidic medium: Anti-corrosive efficacy and adsorptive behaviour. Int. J. Corros. Scale Inhib. 2017, 6, 112–131. [Google Scholar]
- Kavya, K.; Keerthana, S.; Pradeep, T. Effecto of Green Corrosion Inhibitors on the Properties of Mortar and Concrete. Smart Technol. Sustain. Dev. 2020, 78, 395–405. [Google Scholar]
- Rajendran, S.; Srinivasan, R.; Dorothy, R.; Umasankareswari, T.; Al-Hashem, A. Green solution to corrosion problem—At a glance. Int. J. Corros. Scale Inhib. 2019, 8, 437–479. [Google Scholar]
- Cheng, N.; Valdez, B.; Moe, P.; Salvador, J.S. Optimization and characterization of commercial water-based volatile corrosion inhibitor. Int. J. Corros. Scale Inhib. 2019, 8, 366–386. [Google Scholar]
- Okafor, P.C.; Ebenso, E.E.; Ekpe, U.J. Azadirachta indica Extracts as Corrosion Inhibitor for Mild Steel in Acid Medium. Int. J. Electrochem. Sci. 2010, 5, 978–993. [Google Scholar]
- Oguzie, E.E. Adsorption and corrosion inhibitive properties of Azadirachta indica in acid solutions. Pigment Resin Technol. 2006, 35, 334–340. [Google Scholar] [CrossRef]
- Eddy, N.O.; Mamza, P.A.P. Inhibitive and Adsortion Properties of Ethanol Extract of Seeds and Leaves of Azadirachta indica on the Corrosion of Mild Stee in H2SO4. Port. Electrochim. Acta 2009, 27, 443–456. [Google Scholar] [CrossRef]
- Lisha, C.; Rajalingam, M.; Sunilaa, G. Corrosion resistance of reinforced concrete with green corrosion inhibitors. Int. J. Eng. Sci. Invent. Res. Dev. 2017, 3, 687–691. [Google Scholar]
- Herbert, J.; Ganesh, G. Effect of green corrosion inhibitors on the corrosion behaviour of reinforced concrete. Int. Res. J. Eng. Technol. 2019, 6, 1656–1661. [Google Scholar]
- ASTM C494. Standard Specification for Chemical Admixtures for Concrete; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Sika CNI, Product Date Sheet, Version 01.01. 2018. Available online: https://gcc.sika.com/content/dam/dms/gcc/w/sika_-cni_k_.pdf (accessed on 2 June 2020).
- Eucon CIA, Product Date Sheet, Rev.03.20. Available online: https://www.euclidchemical.com/fileshare/ProductFiles/tds/Eucon_CIA.pdf (accessed on 2 June 2020).
- DCI, Product Date Sheet, Last Updated. 2018. Available online: https://gcpat.com/en/solutions/products/dci-corrosion-inhibitor/dci (accessed on 2 June 2020).
- Norma NMX-C-414-ONNCCE-2017. Industria de la Construcción—Cementos Hidráulicos—Especificaciones y Métodos de Ensayo. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5510053&fecha=04/01/2018 (accessed on 15 June 2020).
- ASTM C150. Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM C33. Standard Specification for Concrete Aggregates; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM C1602. Standard Specification for Mixing Water Used in the Production of Hydraulic Cement Concrete; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Sikament 792, 92MX, Product Date Sheet, Last Updated. 2018. Available online: http://sikastore.com.mx/wp-content/uploads/pdf/ADITIVOS/Sikament%2092MX.pdf (accessed on 15 June 2021).
- Kosmatka, S.H.; Kerkhoff, B.; Panarese, W.C. Desig and Control of Concrete Mixtures, 14th ed.; Portland Cement Association: Skokie, IL, USA, 2002; pp. 1–370. [Google Scholar]
- ACI Certificacion CP-1, Technician Workbook of Concrete Field Testing Technician—Grade I. 2019. Available online: https://www.concrete.org/store/productdetail.aspx?ItemID=CP139TH&Format=HARD_COPY&Language=English&Units=US_Units (accessed on 15 June 2021).
- ASTM C172. Standard Practice for Sampling Freshly Mixed Concrete; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM C1064. Standard Test Method for Temperature of Freshly Mixed Hydraulic-Cement Concrete; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM C143. Standard Test Method for Slump of Hydraulic Cement Concrete; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM C138. Standard Test Method for Density (Unit Weight) Yield, and Air Content (Gravimetric) of Concrete; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM C231. Standard Test Method for Air Content of Freshly Mixed Concrete by the Pressure Method; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM C31. Standard Practice for Making and Curing Concrete Test Specimens in the Field; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- ASTM C39. Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- Italian Standard 79-28. Determination of the Chloride Ion Penetration; UNI: Rome, Italy, 1978. [Google Scholar]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Real, L.V.; Oliveira, D.R.B.; Soares, T.; Medeiros, M.H.F. AgNO3 spray method for measurement of chloride penetration: The state of art. ALCONPAT J. 2015, 5, 141–151. [Google Scholar]
- ASTM C876. Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Villagrán, Y.A.; Di-Maio, A.A.; Traversa, L.P. Deficiencias Constructivas como Causa de Corrosión en Estructura de Hormigón Armado Ubicada en Ambiente Marino. In Proceedings of the VI Congreso Internacional Sobre Patología y Recuperación de Estructuras, Córdoba, Argentina, 2–4 June 2010. [Google Scholar]
- Valle, A.; Pérez, T.; Maerínez, M. El fenómeno de la corrosión en estructuras de concreto reforzado. Inst. Mex. Transp. 2001, 182, 1–73. [Google Scholar]
- Stratful, R.F. Half-Cell Potentials and the Corrosion of Steel in Concrete. Am. Highw. Res. Rec. 1973, 433, 12–21. [Google Scholar]
- IMCYC. ACI PRC-305S-10 Guide to Hot-Weather Concreting/306S-10 Guide to Cold Weather Concreting; American Concrete Institute: Farmington Hills, MI, USA, 2010; pp. 1–102. [Google Scholar]
- Technical Consultant Team. Technical Manual-Cement & Concrete, 1st ed.; Holcim (Vietnam) Ltd.: Hon Chong, Vietnam, 2013; pp. 1–104. [Google Scholar]
- Soudki, K.A.; El-Salakawy, E.F.; Elkum, N.B. Full Factorial Optimization of Concrete Mix Design for Hot Climates. J. Mater. Civ. Eng. 2001, 13, 427–433. [Google Scholar] [CrossRef]
- ASTM C94. Standard Specification for Ready-Mixed Concrete; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar]
- ACI Committee 211. 1-91. Standard Practice for Selecting Proportions for Normal, Heavyweight and Mass Concrete; American Concrete Institute: Farmington Hills, MI, USA, 2009; pp. 1–38. [Google Scholar]
- ACI Committee 318. Building Code Requirements for Structural Concrete, ACI 318R-02; American Concrete Institute: Farmington Hills, MI, USA, 2019; pp. 1–624. [Google Scholar]
- Castro, P.; Castillo, R.; Carpio, J.; Genescá, J.; Helene, P.; López, W.; Pazini, E.; Sanjuán, M.; Veleva, L. Corrosión en Estructuras de Concreto Armado-Teoría, Inspección, Diagnostico, Vida Útil y Reparación; Instituto Mexicano del Cemento y del Concreto: Ciudad de México, Mexico, 1998; pp. 1–125. [Google Scholar]
- Sanjuan, M.; Castro, P. Acción de Los Agentes Químicos y Físicos Sobre El Concreto; Instituto Mexicano del Cemento y del Concreto: Ciudad de México, Mexico, 2001; pp. 1–46. [Google Scholar]
- Nmai, C.K.; Farrington, S.A.; Bobrowski, G.S. Organic-Based Corrosion-Inhibiting Admixture for Reinforced Concrete. Concr. Int. 1992, 14, 45–51. [Google Scholar]
- Ugi, B.U.; Bassey, V.M.; Obeten, M.E.; Adalikwu, S.A.; Namdi, D.O. Secondary plant metabolites of natural product origin-Strongylodon macrobotrys as pitting corrosion inhibitors of steel around heavy salt desposits in Gabu, Nigeria. J. Mater. Sci. Chem. Eng. 2020, 8, 38–60. [Google Scholar]
- Li, H.; Zhang, W.; Wu, Y. Anti-Corrosive Properties of Alkaloids on Metals. In Alkaloids—Their Importance in Nature and Human Life; IntechOpen: Rijeka, Croatia, 2018; Chapter 5; pp. 1–15. [Google Scholar]
- Miralrio, A.; Espinoza, A. Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review. Processes 2020, 8, 942. [Google Scholar] [CrossRef]
- Priyanka, S.; Dixit, S.; Sahoo, S. Phytochemical and Biochemical Characterizations from Leaf Extracts from Azadirachta indica: An Important Medicinal Plant. Biochem. Anal. Biochem. 2017, 6, 1000323. [Google Scholar] [CrossRef]
- Sharma, S.K.; Peter, A.; Obot, I.B. Potential of Azadirachta indica as a green corrosion inhibitor against mild steel, aluminium and tin: A review. J. Anal. Sci. Technol. 2015, 6, 26. [Google Scholar] [CrossRef]
- Vinutha, M.R.; Venkatesha, T.V. Review on mechanistic action of inhibitors on steel corrosion in acidic media. Port. Electrochem. Acta 2016, 34, 157–184. [Google Scholar] [CrossRef]
- Valek, L.; Martinez, S. Copper corrosion inhibition by Azadirachta indica leaves extract in 0.5 M sulphuric acid. Mater. Lett. 2007, 61, 148–151. [Google Scholar] [CrossRef]
- Silveira, R.; Cassel, E.; Schermann, D. Black wattle tannin as steel corrosion inhibitor. ISRN Corros. 2012, 1–9. [Google Scholar] [CrossRef]
- Oh, B.H.; Jang, S.Y.; Shin, S. Experimental investigation of the threshold chloride concentration for corrosion initiation in reinforced concrete structures. Mag. Concr. Res. 2003, 55, 117–124. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M.; Solomon, M.M.; Udoh, A.P. Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab. J. Chem. 2016, 9, 209–224. [Google Scholar] [CrossRef]




| Chemical Composition | Standard Requirement | Result | Unit | |||
|---|---|---|---|---|---|---|
| ASTM C 150/C150M-16 | NMX-C-414-ONNCCE-2017 | |||||
| Minimum | Maximum | Minimum | Maximum | |||
| SiO2 | - | - | - | - | 24.72 | % |
| Al2O3 | - | - | - | - | 5.09 | % |
| Fe2O3 | - | - | - | - | 2.90 | % |
| CaO | - | - | - | - | 55.87 | % |
| MgO | - | 6.0 | - | - | 1.33 | % |
| SO3 | - | 3.0/3.5 (A) | - | 4.0 (B) | 3.40 | % |
| K2O | - | - | - | - | 0.73 | % |
| Na2O | - | - | - | - | 0.81 | % |
| Loss on ignition | - | 3.0/3.5 (A) | - | - | 4.70 | % |
| Insoluble residue | - | - | - | - | 0.40 | % |
| Equivalent alkalis | - | - | - | - | 1.28 | % |
| Physical Composition | Standard Requirement | Result | Unit | ||||
|---|---|---|---|---|---|---|---|
| ASTM C 150/C150M-16 | NMX-C-414-ONNCCE-2017 | ||||||
| Minimum | Maximum | Minimum | Maximum | ||||
| Air content | - | 12 | - | - | 1.6 | Volume, % | |
| Fineness, specific surface | 260 | - | - | - | 490 | m2/kg | |
| Autoclave expansion | - | 0.80 | −0.20 | 0.80 | 0.03 | % | |
| Compressive strength | 3 days | 12 | - | - | - | 31.5 | MPa (N/mm2) |
| 7 days | 19 | - | - | - | 37.5 | MPa (N/mm2) | |
| 28 days | 28 | - | 40.0 | - | 47.4 | MPa (N/mm2) | |
| Time of setting, Vicat test | Initial | 45 | - | 45 | - | 115 | Minutes |
| Final | - | 375 | - | 600 | 163 | Minutes | |
| False set, final penetration | 50 | - | - | - | 95 | Minutes | |
| Expansion in submerged bars to 14 days | - | - | - | 0.020 | −0.001 | % | |
| Specific gravity | - | - | - | - | 3.02 | Kg/cm3 | |
| Physical Properties. | Units | Sand | Coarse (GT) | Coarse (GS) |
|---|---|---|---|---|
| Specific gravity | kg/m3 | 2.60 | 2.65 | 2.67 |
| Absorption | % | 1.42 | 0.78 | 1.0 |
| Moisture | % | 0.40 | 0.11 | 0.2 |
| Loose bulk density | kg/m3 | 1561 | 1398 | 1422 |
| Bulk density by rodding | kg/m3 | 1701 | 1532 | 1549 |
| Loss by washing | % | 0.75 | 0.56 | 0.30 |
| Coarse or sand contamination | % | 3.05 | 0.24 | 2.3 |
| Fineness Modulus | Adim | 2.4 | 6.0 | 6.0 |
| Sieve | Sand | Coarse (GS) | Coarse (GT) | |||
|---|---|---|---|---|---|---|
| No. | Mass Detained | Percent Passing | Mass Detained | Percent Passing | Mass Detained | Percent Passing |
| 2” | 0.0 | 100.0 | 0 | 100.0 | 0.0 | 100.0 |
| 1 1/2” | 0.0 | 100.0 | 0 | 100.0 | 0.0 | 100.0 |
| 1” | 0.0 | 100.0 | 0 | 100.0 | 639 | 92.0 |
| 3/4” | 0.0 | 100.0 | 0 | 100.0 | 3772 | 44.8 |
| 1/2” | 0.0 | 100.0 | 110 | 96.3 | 2793 | 9.9 |
| 3/8” | 0.0 | 100.0 | 922 | 65.6 | 663 | 1.6 |
| No. 4 | 60.5 | 96.9 | 1897 | 2.3 | 112 | 0.2 |
| No. 8 | 82.5 | 92.8 | 64 | 0.2 | 4 | 0.2 |
| No. 16 | 109.0 | 87.3 | 0 | 0.2 | 0 | 0.2 |
| No. 30 | 334.0 | 70.5 | 0 | 0.2 | 0 | 0.2 |
| No. 50 | 1127.5 | 13.6 | 0 | 0.2 | 0 | 0.2 |
| No. 100 | 198.0 | 3.6 | 0 | 0.2 | 0 | 0.2 |
| No. 200 | 64.0 | 0.4 | 0 | 0.2 | 0 | 0.2 |
| Ch. | 8.0 | 0.0 | 5 | 0.0 | 16 | 0.0 |
| Total | 1984 | - | 2996 | - | 7998 | - |
| Mix | Composition |
|---|---|
| 1 | Blank |
| 2 | Neem |
| 3 | Sika CNI |
| 4 | Eucon CIA |
| 5 | DCI |
| Materials | Characteristic | Unit | Original Design Per m3 | Mixing Quantities for 35 L | ||||
|---|---|---|---|---|---|---|---|---|
| Mix 1 | Mix 2 | Mix 3 | Mix 4 | Mix 5 | ||||
| Cement | CPC40 | (kg) | 277 | 277 | 277 | 277 | 277 | 9.70 |
| Coarse 1 | GS | (kg) | 385 | 385 | 385 | 385 | 385 | 13.48 |
| Coarse 2 | GT | (kg) | 890 | 890 | 890 | 890 | 890 | 31.15 |
| Sand 1 | AN | (kg) | 643 | 643 | 643 | 643 | 643 | 22.51 |
| Water | Water | (L) | 180 | 180 | 180 | 180 | 180 | 6.30 |
| Additive 1 | Sikament 792 | (L) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 0.053 |
| Additive 2 | Neem | (L) | - | 10 | - | - | - | 0.350 |
| Additive 3 | Sika CNI | (L) | - | - | 10 | - | - | 0.350 |
| Additive 4 | Eucon CIA | (L) | - | - | - | 10 | - | 0.350 |
| Additive 5 | DCI | (L) | - | - | - | - | 10 | 0.350 |
| mV vs. CSE | Corrosion Probability |
|---|---|
| −240 | 0% |
| −275 | 5% |
| −350 | 50% |
| −450 | 95% |
| −500 | 100% |
| Mix | Temperature (°C) | Slump (cm) | Density (Unit Weight) Concrete (kg/m3) | Relative Yield | Air Content (%) |
|---|---|---|---|---|---|
| 1 | 29 | 6 | 2379 | 0.99 | 1.4 |
| 2 | 30 | 11 | 2374 | 1.00 | 1.2 |
| 3 | 30 | 11 | 2383 | 1.00 | 1.1 |
| 4 | 30 | 11 | 2380 | 1.00 | 0.9 |
| 5 | 30 | 12 | 2376 | 1.00 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdez-Salas, B.; Vazquez-Delgado, R.; Salvador-Carlos, J.; Beltran-Partida, E.; Salinas-Martinez, R.; Cheng, N.; Curiel-Alvarez, M. Azadirachta indica Leaf Extract as Green Corrosion Inhibitor for Reinforced Concrete Structures: Corrosion Effectiveness against Commercial Corrosion Inhibitors and Concrete Integrity. Materials 2021, 14, 3326. https://doi.org/10.3390/ma14123326
Valdez-Salas B, Vazquez-Delgado R, Salvador-Carlos J, Beltran-Partida E, Salinas-Martinez R, Cheng N, Curiel-Alvarez M. Azadirachta indica Leaf Extract as Green Corrosion Inhibitor for Reinforced Concrete Structures: Corrosion Effectiveness against Commercial Corrosion Inhibitors and Concrete Integrity. Materials. 2021; 14(12):3326. https://doi.org/10.3390/ma14123326
Chicago/Turabian StyleValdez-Salas, Benjamin, Ramiro Vazquez-Delgado, Jorge Salvador-Carlos, Ernesto Beltran-Partida, Ricardo Salinas-Martinez, Nelson Cheng, and Mario Curiel-Alvarez. 2021. "Azadirachta indica Leaf Extract as Green Corrosion Inhibitor for Reinforced Concrete Structures: Corrosion Effectiveness against Commercial Corrosion Inhibitors and Concrete Integrity" Materials 14, no. 12: 3326. https://doi.org/10.3390/ma14123326
APA StyleValdez-Salas, B., Vazquez-Delgado, R., Salvador-Carlos, J., Beltran-Partida, E., Salinas-Martinez, R., Cheng, N., & Curiel-Alvarez, M. (2021). Azadirachta indica Leaf Extract as Green Corrosion Inhibitor for Reinforced Concrete Structures: Corrosion Effectiveness against Commercial Corrosion Inhibitors and Concrete Integrity. Materials, 14(12), 3326. https://doi.org/10.3390/ma14123326






