A High-Efficiency TiO2/ZnO Nano-Film with Surface Oxygen Vacancies for Dye Degradation
Abstract
1. Introduction
2. Materials and Methods
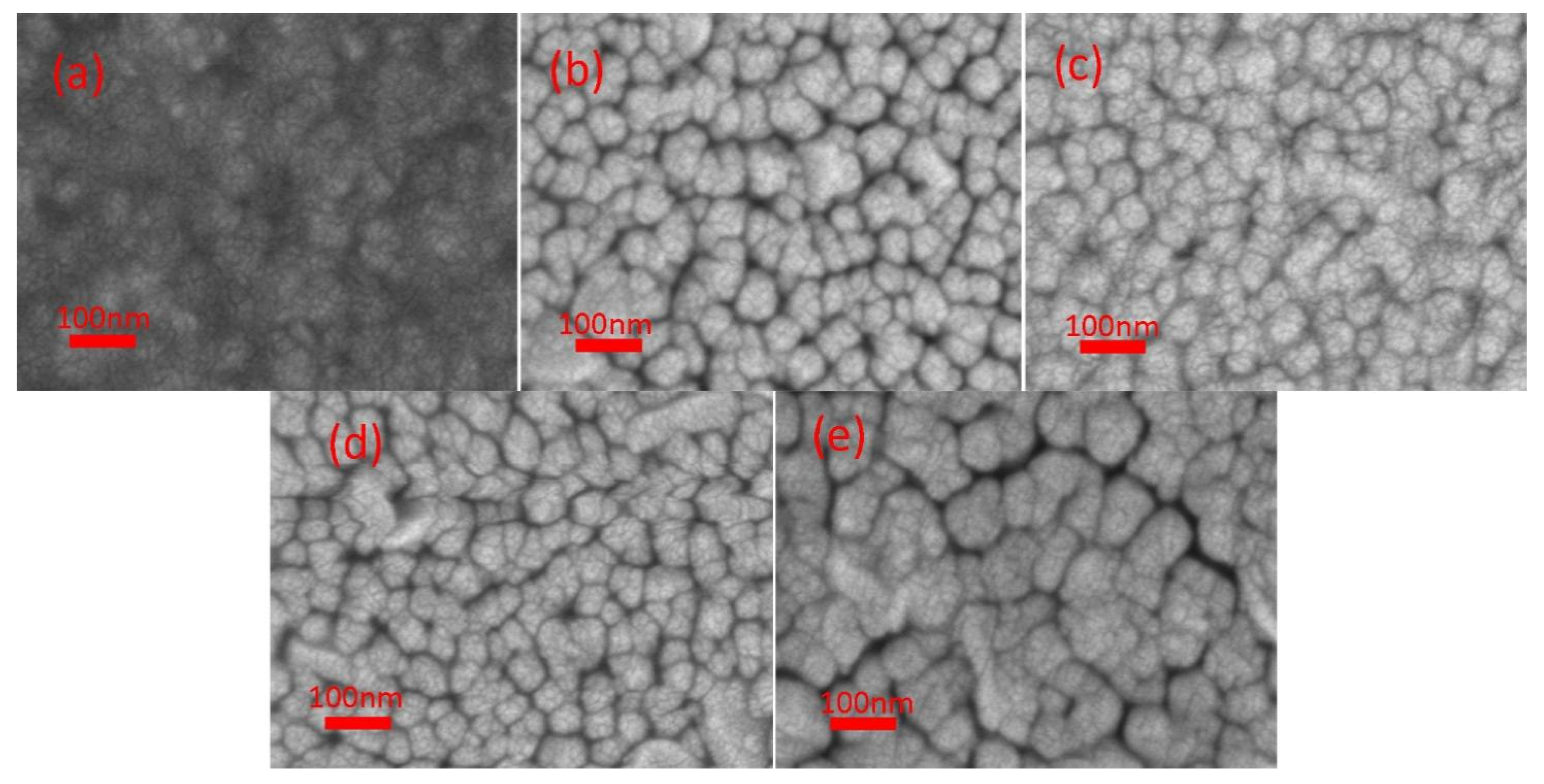
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganiyu, S.O.; Martinez-Huitle, C.A.; Rodrigo, M.A. Renewable energies driven electrochemical wastewater/soil decontamination technologies: A critical review of fundamental concepts and applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Fernandes, A.; Makos, P.; Wang, Z.; Boczkaj, G. Synergistic effect of TiO2 photocatalytic advanced oxidation processes in the treatment of refinery effluents. Chem. Eng. J. 2020, 391, 123488. [Google Scholar] [CrossRef]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Santamaria Amato, L.; Maddalena, P. Charge Carrier Processes and Optical Properties in TiO2 and TiO2-Based Heterojunction Photocatalysts: A Review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Medvids, A.; Onufrijevs, P.; Kaupuzs, J.; Eglitis, R.; Padgurskas, J.; Zunda, A.; Mimura, H.; Skadins, I.; Varnagiris, S. Anatase or rutile TiO2 nanolayer formation on Ti substrates by laser radiation: Mechanical, photocatalytic and antibacterial propertie. Opt. Laser Technol. 2021, 138, 106898. [Google Scholar] [CrossRef]
- Lettieri, S.; Gargiulo, V.; Alfe, M.; Amati, M.; Zeller, P.; Maraloiu, V.-A.; Borbone, F.; Pavone, M.; Munoz-Garcia, A.B.; Maddalena, P. Simple Ethanol Refluxing Method for Production of Blue-Colored Titanium Dioxide with Oxygen Vacancies and Visible Light-Driven Photocatalytic Propertiess. J. Phys. Chem. C 2020, 124, 3564–3576. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B Environ. 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Sturini, M.; Maraschi, F.; Cantalupi, A.; Pretali, L.; Nicolis, S.; Dondi, D.; Profumo, A.; Caratto, V.; Sanguineti, E.; Ferretti, M.; et al. TiO2 and N-TiO2 sepiolite and zeolite composites for photocatalytic removal of ofloxacin from polluted water. Materials 2020, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, J.; Zhang, J.; Niu, J.; Zhao, J.; Wei, Y.; Yao, B. Photocatalytic degradation of ciprofloxacin using Zn-doped Cu2O particles: Analysis of degradation pathways and intermediates. Chem. Eng. J. 2019, 374, 316–327. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, Y.; Xu, Y.; Li, L.; Li, C.; Liu, X.; Niu, L. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water. Chem. Eng. J. 2018, 350, 257–267. [Google Scholar] [CrossRef]
- Das, A.; Kumar, P.M.; Bhagavathiachari, M.; Nair, R.G. Hierarchical ZnO-TiO2 nanoheterojunction: A strategy driven approach to boost the photocatalytic performance through the synergy of improved surface area and interfacial charge transport. Appl. Surf. Sci. 2020, 534, 147321. [Google Scholar] [CrossRef]
- Jeong, K.; Deshmukh, P.R.; Park, J.; Sohn, Y.; Shin, W.G. ZnO-TiO2 Core-Shell Nanowires: A sustainable photoanode for enhanced photoelectrochemical water splitting. ACS Sustain. Chem. Eng. 2018, 6, 6518–6526. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide-From synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Huo, W.; Zhang, X.; Fang, F.; Xie, Z.; Jiang, J. Single-crystal-like black Zr-TiO2 nanotube array film: An efficient photocatalyst for fast reduction of Cr(VI). Chem. Eng. J. 2021, 403, 126331. [Google Scholar] [CrossRef]
- Kuriakose, S.; Choudhary, V.; Satpati, B.; Mohapatra, S. Facile synthesis of Ag-ZnO hybrid nanospindles for highly efficient photocatalytic degradation of methyl orange. Phys. Chem. Chem. Phys. 2014, 16, 17560–17568. [Google Scholar] [CrossRef]
- Singh, J.; Satpati, B.; Mohapatra, S. Structural, Optical and Plasmonic Properties of Ag-TiO2 hybrid plasmonic nanostructures with enhanced photocatalytic activity. Plasmonics 2017, 12, 877–888. [Google Scholar] [CrossRef]
- Vahl, A.; Veziroglu, S.; Henkel, B.; Strunskus, T.; Polonskyi, O.; Aktas, O.C.; Faupel, F. Pathways to tailor photocatalytic performance of TiO2 thin films deposited by reactive magnetron sputtering. Materials 2019, 12, 2840. [Google Scholar] [CrossRef]
- Abdallah, B.; Jazmati, A.K.; Refaai, R. Oxygen Effect on Structural and Optical Properties of ZnO thin films deposited by RF magnetron sputtering. Mater. Res. Ibero Am. J. Mater. 2017, 20, 607–612. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Rahman, K.H.; Wu, C.-C.; Chen, K.-C. A Review on the pathways of the improved structural characteristics and photocatalytic performance of Titanium Dioxide (TiO2) thin films fabricated by the magnetron-sputtering technique. Catalysts 2020, 10, 598. [Google Scholar] [CrossRef]
- Yu, H.-L.; Wu, Q.-X.; Wang, J.; Liu, L.-Q.; Zheng, B.; Zhang, C.; Shen, Y.-G.; Huang, C.-L.; Zhou, B.; Jia, J.-R. Simple fabrication of the Ag-Ag2O-TiO2 photocatalyst thin films on polyester fabrics by magnetron sputtering and its photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144075. [Google Scholar] [CrossRef]
- Yuan, X.; Liang, S.; Ke, H.; Wei, Q.; Huang, Z.; Chen, D. Photocatalytic property of polyester fabrics coated with Ag/TiO2 composite films by magnetron sputtering. Vacuum 2020, 172, 109103. [Google Scholar] [CrossRef]
- Peng, S.; Yang, Y.; Li, G.; Jiang, J.; Jin, K.; Yao, T.; Zhang, K.; Cao, X.; Wang, Y.; Xu, G. Effect of N-2 flow rate on the properties of N doped TiO2 films deposited by DC coupled RF magnetron sputtering. J. Alloys Compd. 2016, 678, 355–359. [Google Scholar] [CrossRef]
- Zhang, L.; Tse, M.S.; Tan, O.K.; Wang, Y.X.; Han, M. Facile fabrication and characterization of multi-type carbon-doped TiO2 for visible light-activated photocatalytic mineralization of gaseous toluene. J. Mater. Chem. A 2013, 1, 4497–4507. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhang, Y.Y.; Zhang, J.; Shi, Y.; Li, Z.; Feng, Z.C.; Li, C. In situ loading of CuS nanoflowers on rutile TiO2 surface and their improved photocatalytic performance. Appl. Surf. Sci. 2016, 370, 312–319. [Google Scholar] [CrossRef]
- Singh, J.; Khan, S.A.; Shah, J.; Kotnala, R.K.; Mohapatra, S. Nanostructured TiO2 thin films prepared by RF magnetron sputtering for photocatalytic applications. Appl. Surf. Sci. 2017, 422, 953–961. [Google Scholar] [CrossRef]
- Barthomeuf, M.; Castel, X.; Le Gendre, L.; Louis, J.; Denis, M.; Pissavin, C. Effect of titanium dioxide film thickness on photocatalytic and bactericidal activities against listeria monocytogenes. Photochem. Photobiol. 2019, 95, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Kaltzoglou, A.; Athanasekou, C.; Katsaros, F.; Devlin, E.; Kontos, A.G.; Ioannidis, N.; Perraki, M.; Tsakiridis, P.; Sygellou, L.; et al. Magnetically separable TiO2/CoFe2O4/Ag nanocomposites for the photocatalytic reduction of hexavalent chromium pollutant under UV and artificial solar light. Chem. Eng. J. 2020, 381, 122730. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Shi, Y.; Cui, J.; Shu, X.; Wang, Y.; Qin, Y.; Liu, J.; Tan, H.H.; Wu, Y. Fabrication of WO3/TiO2 core-shell nanowire arrays: Structure design and high electrochromic performance. Electrochim. Acta 2020, 330, 135189. [Google Scholar] [CrossRef]
- Huang, C.; Bian, J.; Guo, Y.; Huang, M.; Zhang, R.-Q. Thermal vacuum de-oxygenation and post oxidation of TiO2 nanorod arrays for enhanced photoelectrochemical properties. J. Mater. Chem. A 2019, 7, 5434–5441. [Google Scholar] [CrossRef]
- Yang, Y.; Kao, L.C.; Liu, Y.; Sun, K.; Yu, H.; Guo, J.; Liou, S.Y.H.; Hoffmann, M.R. Cobalt-doped black TiO2 nanotube array as a stable anode for oxygen evolution and electrochemical wastewater treatment. ACS Catal. 2018, 8, 4278–4287. [Google Scholar] [CrossRef]
- Bakos, L.P.; Justh, N.; Moura da Silva Bezerra da Costa, U.C.; László, K.; Lábár, J.L.; Igricz, T.; Varga-Josepovits, K.; Pasierb, P.; Färm, E.; Ritala, M.; et al. Photocatalytic and gas sensitive multiwalled carbon Nanotube/TiO2-ZnO and ZnO-TiO2 composites prepared by atomic layer deposition. Nanomaterials 2020, 10, 252. [Google Scholar] [CrossRef]
- Perez-Gonzalez, M.; Tomas, S.A.; Santoyo-Salazar, J.; Gallardo-Hernandez, S.; Tellez-Cruz, M.M.; Solorza-Feria, O. Sol-gel synthesis of Ag-loaded TiO2-ZnO thin films with enhanced photocatalytic activity. J. Alloys Compd. 2019, 779, 908–917. [Google Scholar] [CrossRef]
- Perez-Gonzalez, M.; Tomas, S.A.; Santoyo-Salazar, J.; Morales-Luna, M. Enhanced photocatalytic activity of TiO2-ZnO thin films deposited by dc reactive magnetron sputtering. Ceram. Int. 2017, 43, 8831–8838. [Google Scholar] [CrossRef]
- Correia, F.C.; Calheiros, M.; Marques, J.; Ribeiro, J.M.; Tavares, C.J. Synthesis of Bi2O3/TiO2 nanostructured films for photocatalytic applications. Ceram. Int. 2018, 44, 22638–22644. [Google Scholar] [CrossRef]
- Ben Jemaa, I.; Chaabouni, F.; Ranguis, A. Cr doping effect on the structural, optoelectrical and photocatalytic properties of RF sputtered TiO2 thin films from a powder target. J. Alloys Compd. 2020, 825, 153988. [Google Scholar] [CrossRef]
- Sabri, M.; Habibi-Yangjeh, A.; Chand, H.; Krishnan, V. Activation of persulfate by novel TiO2/FeOCl photocatalyst under visible light: Facile synthesis and high photocatalytic performance. Sep. Purif. Technol. 2020, 250, 117268. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Mueller, M.M.; Kleebe, H.-J.; Ziegler, J.; Jaegermann, W. Nanostructured SnO2-ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef] [PubMed]










| Samples | Ti (at%) | Zn (at%) | O (at%) |
|---|---|---|---|
| ZT3 | 24.52 | 5.59 | 68.89 |
| ZT5 | 22.87 | 4.63 | 72.49 |
| Samples | Dye Concentration | Removal | kapp (10−3 min−1) |
|---|---|---|---|
| (This work) | RhB (5 mg/L) in 50 mL | 90.8% in 2 h | 21.9 |
| CNT-ZnO Composition [34] | Methyl orange | 41.5% in 4 h | 2.6 |
| Ag-loaded TiO2-ZnO film [35] | Methyl blue | 80% in 2 h | 12.6 |
| Ag-Ag2O-TiO2 film [23] | RhB (5 mg/L) in 50 mL | 68.7% in 6 h | - |
| Ag/TiO2 film [24] | RhB (5 mg/L) | 72.1% in 6 h | 3.2 |
| TiO2-ZnO film [36] | Methyl blue (10 ppm) | 44% in 2 h | - |
| Bi2O3/TiO2 film [37] | Methyl blue (10–5 M) | 40% in 6 h | 1.32 |
| Cr doped TiO2 film [38] | Methyl blue (3 mg/L) | 75% in 2 h | 11.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Hao, B.; Song, W.; Guo, J.; Li, M.; Zhang, L. A High-Efficiency TiO2/ZnO Nano-Film with Surface Oxygen Vacancies for Dye Degradation. Materials 2021, 14, 3299. https://doi.org/10.3390/ma14123299
Ma H, Hao B, Song W, Guo J, Li M, Zhang L. A High-Efficiency TiO2/ZnO Nano-Film with Surface Oxygen Vacancies for Dye Degradation. Materials. 2021; 14(12):3299. https://doi.org/10.3390/ma14123299
Chicago/Turabian StyleMa, Huizhong, Baofei Hao, Wentao Song, Jinpeng Guo, Mingyuan Li, and Lan Zhang. 2021. "A High-Efficiency TiO2/ZnO Nano-Film with Surface Oxygen Vacancies for Dye Degradation" Materials 14, no. 12: 3299. https://doi.org/10.3390/ma14123299
APA StyleMa, H., Hao, B., Song, W., Guo, J., Li, M., & Zhang, L. (2021). A High-Efficiency TiO2/ZnO Nano-Film with Surface Oxygen Vacancies for Dye Degradation. Materials, 14(12), 3299. https://doi.org/10.3390/ma14123299




