Freeze–Thaw Effect on Road Concrete Containing Blast Furnace Slag: NMR Relaxometry Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
- S 360, control mixture of 360 kg/m3 Portland cement dosage and natural aggregates;
- S 414, control mixture of 414 kg/m3 Portland cement dosage and natural aggregates;
- S 54/20, 360 kg/m3 (cement) + 54 kg/m3 (GGBS) and 20% (ACBFS)_0/4 mm + 80% (NA)_0/4;
- S 54/40, 360 kg/m3 (cement) + 54 kg/m3 (GGBS) and 40% (ACBFS)_0/4 mm + 60% (NA)_0/4;
- S 54/60, 360 kg/m3 (cement) + 54 kg/m3 (GGBS) and 60% (ACBFS)_0/4 mm + 40% (NA)_0/4.
2.3. Methods
2.3.1. Sampling, Preservation and Preparation of Samples for Testing
- ηn—the compressive strength loss after “n” freeze–thaw cycles;
- f cm water—the average compressive strength of the samples maintained in water during “n” freeze–thaw cycles;
- f cm freeze—the average compressive resistance of the samples maintained in the thermostat chamber during “n” freeze–thaw cycles.
- fc—compressive strength in N/mm2,
- F—the maximum load in N;
- Ac—cross-sectional area of the section of rupture in mm2.
2.3.2. NMR Relaxometry Approach for Determining the Pore Size Distribution
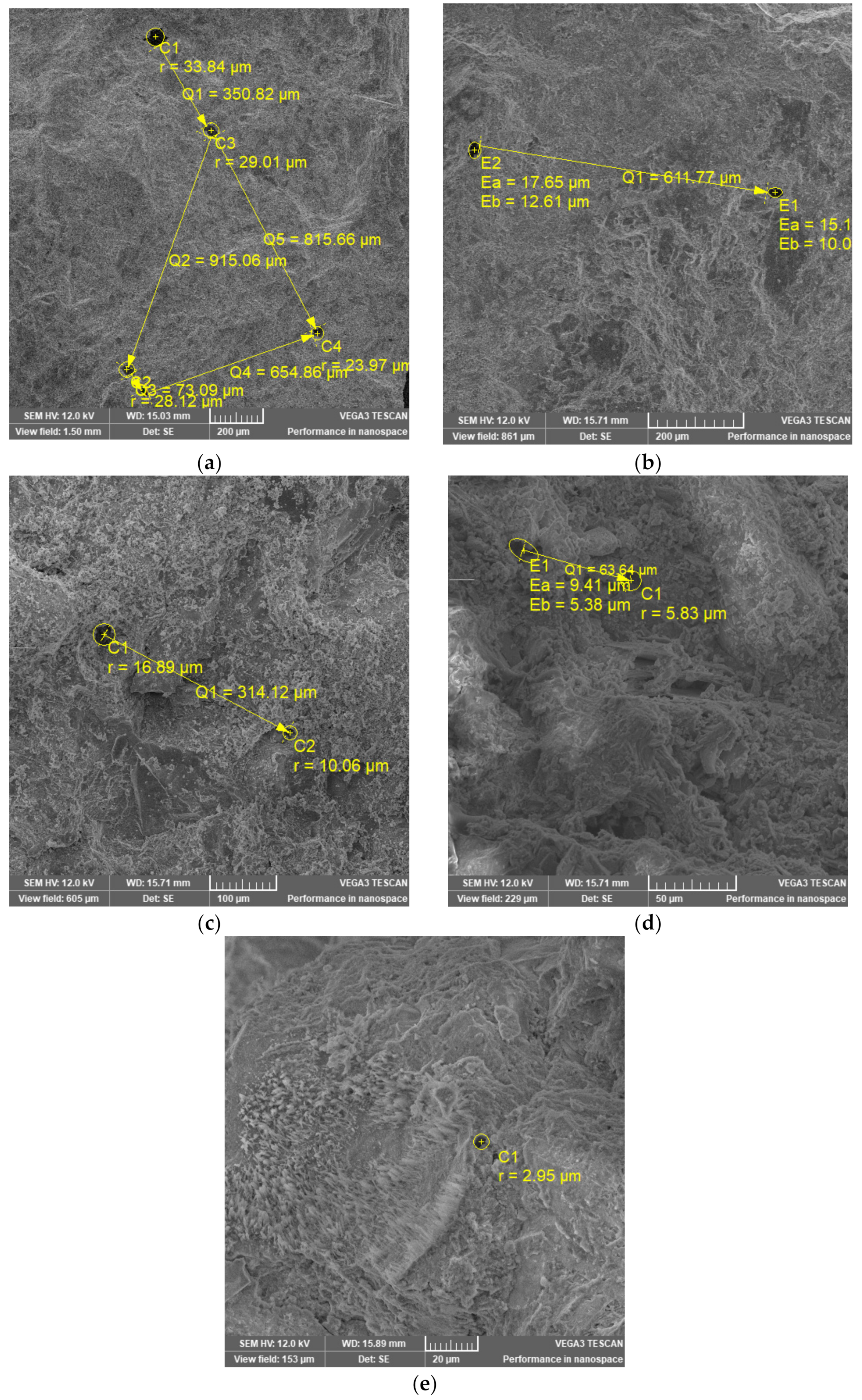
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Density after Immersion and Boiling, Water Absorption and Proportion of Permeable Pores
3. Results and Discussion
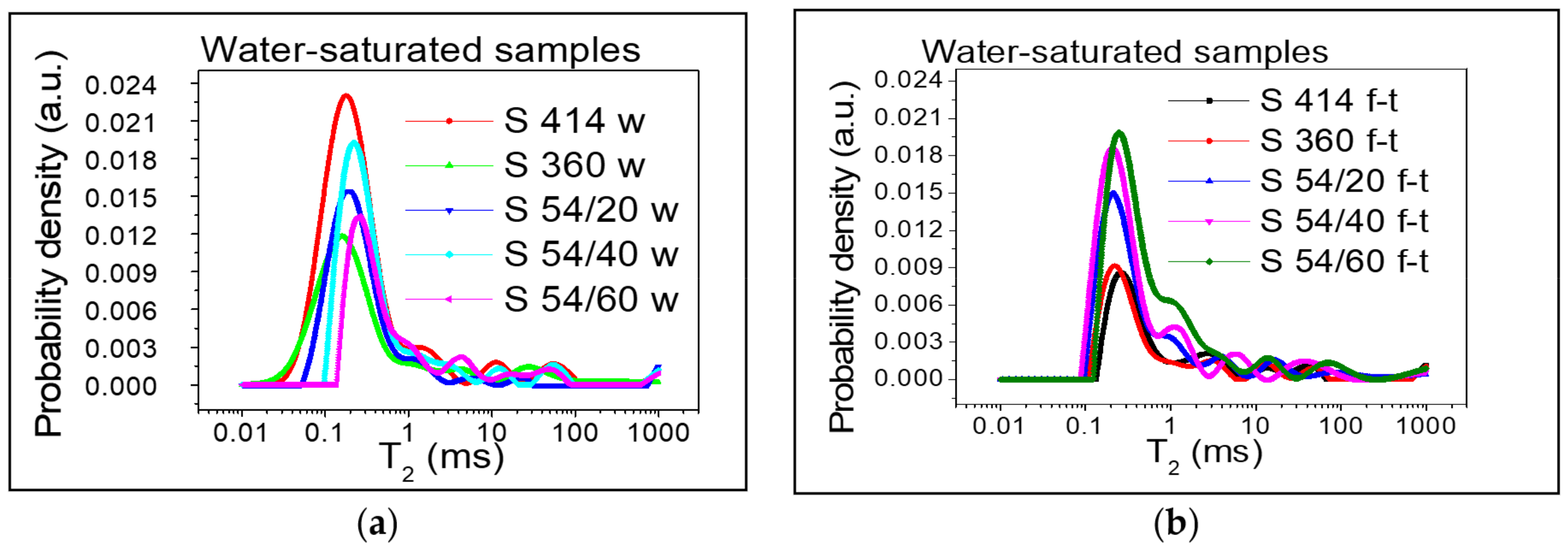
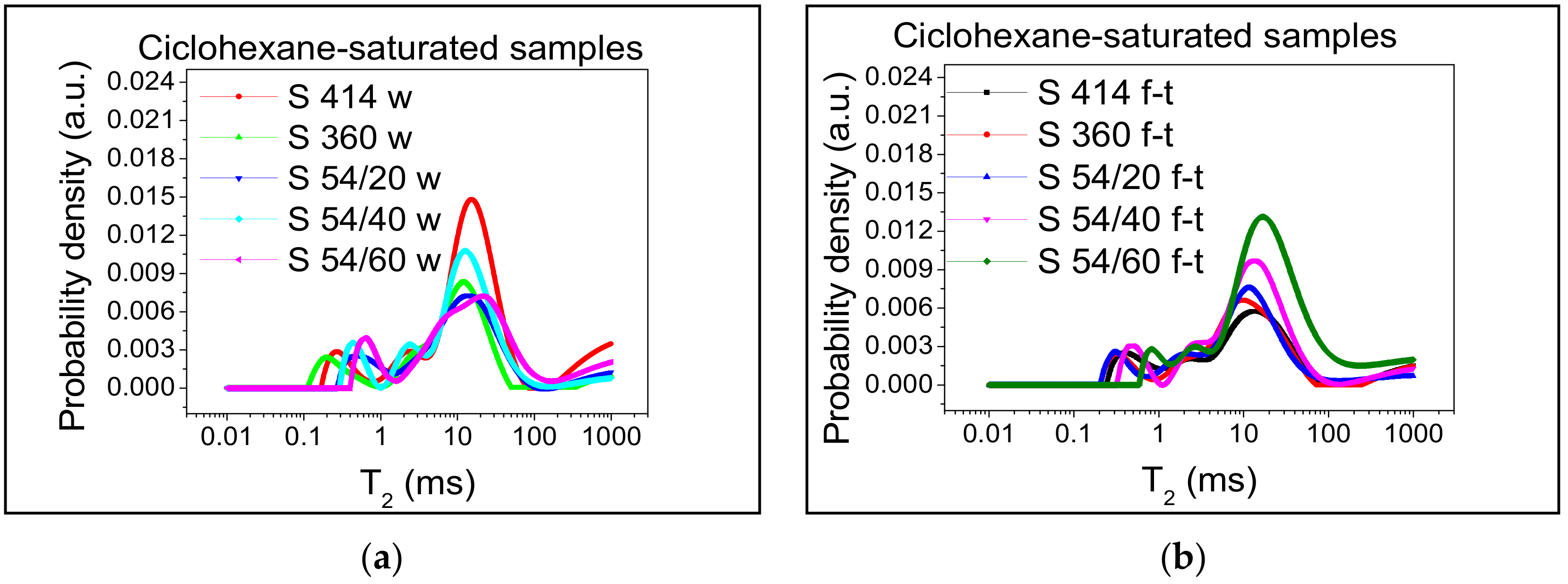
3.1. Relative Distribution of Pore Sizes
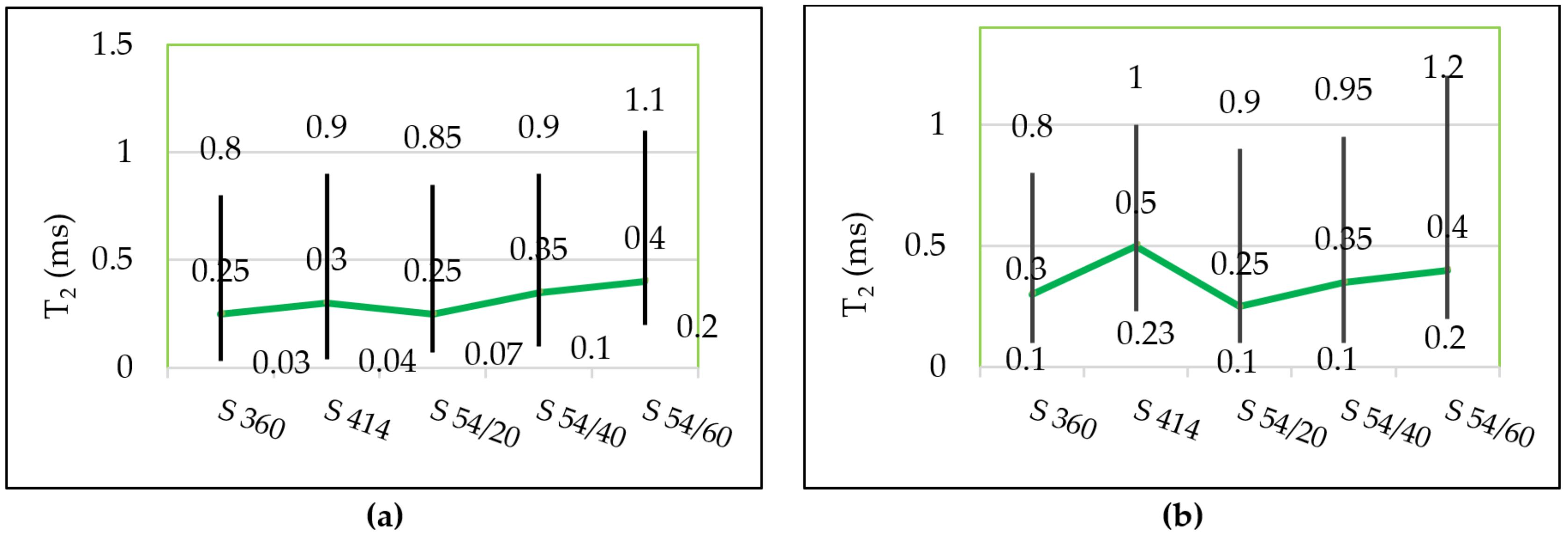
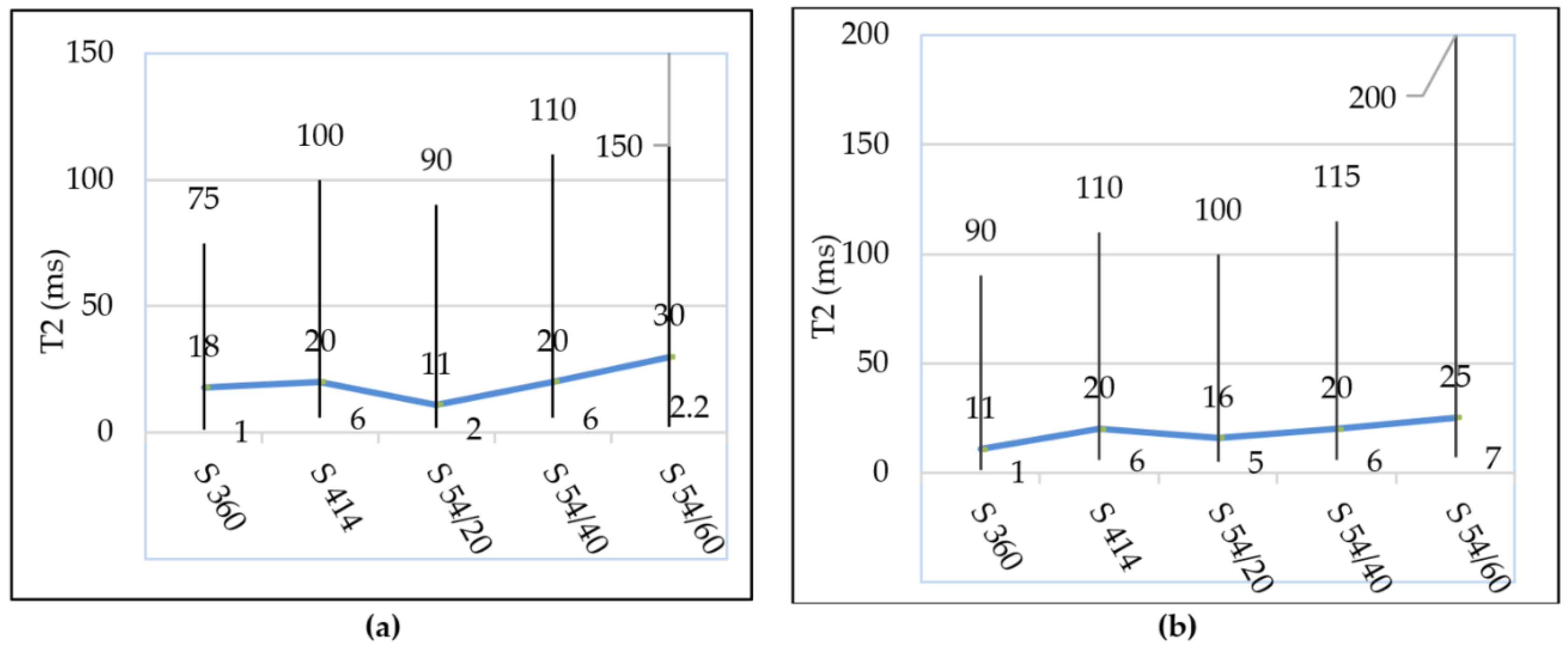
3.2. Pores Size
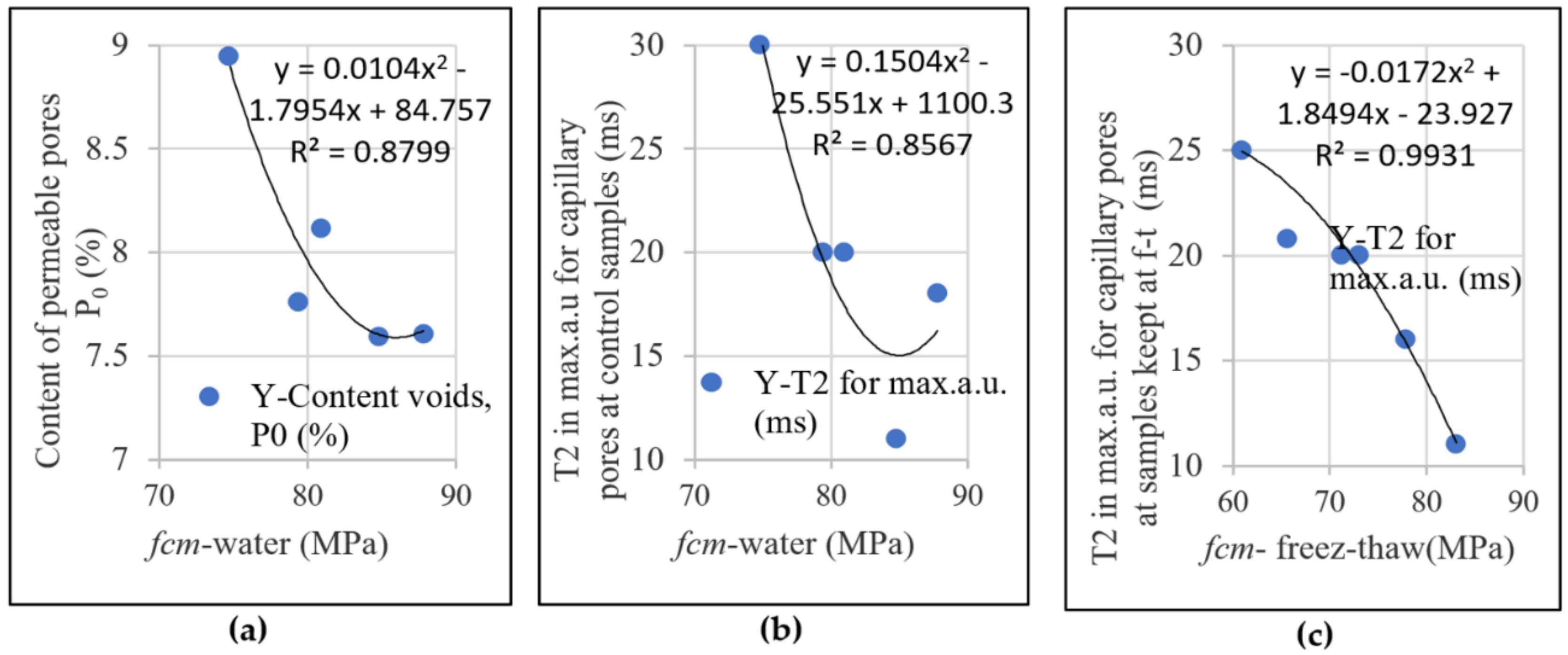
3.3. Density, Water Absorption and Permeable Pores Content of Hardened Concrete
4. Conclusions
- After the freeze–thaw cycles, the transverse relaxation time distribution interval, T2, a of the intra C-S-H pores shifted towards higher values compared to the control mixtures, which indicates the appearance of microcracks even in the (intra C-S-H) pores. In addition, the maximum probability density was close to the value indicated for the control mixtures.
- From the diagrams of the distribution of inter C-S-H gel pores and of the capillary pores of the specimens tested at freeze–thaw, displacements are observed for higher values of T2 in all compositions with blast furnace slag compared to the control samples, which indicates an increase in the relative size of the pores.
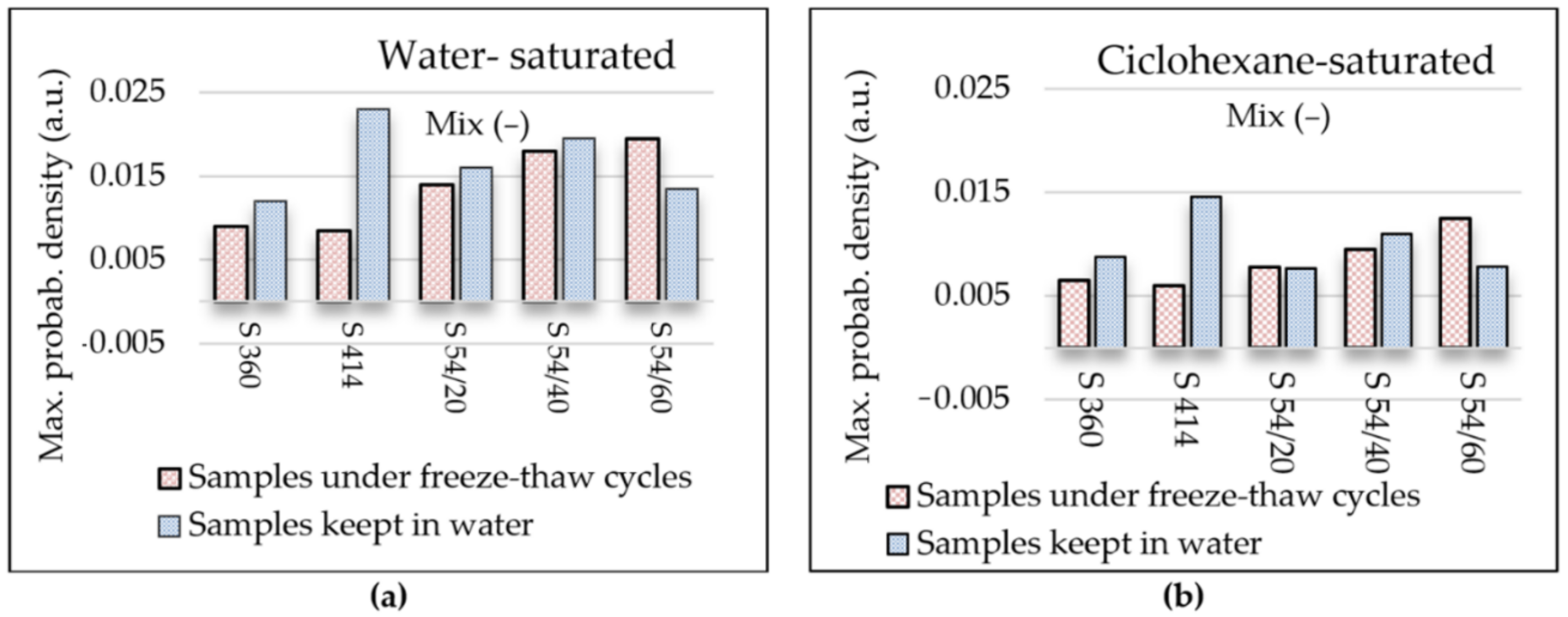
- Furthermore, the maximum probability density of samples maintained at freeze–thaw cycles, measured on the y-axis, increased with the increase in the substitution level of crushed aggregates, indicating the increase in the density of the pore distribution in concrete with the highest increase registered in the S 54/60 mixture.
- For the S 54/40 and S 54/60 mixtures tested on freeze–thaw, the distribution range of the capillary pores was shifted to higher values compared to the control mixtures, which showed an increase in pore size due to the increase in the amount of water in the capillary pore mixtures.
- However, for the mixture S 54/20, the distribution range and the maximum intensity of the capillary pores were close to the control mixture S 360 and lower than the control mixture S 414, which indicates that this mixture contains the optimal dosages of slag built-in furnace.
- The results obtained for the content of permeable pores and the mechanical strengths obtained through standard methods and of capillary pore distribution obtained through NMR technique were consistent.
- The analysis of SEM images for slag mixtures shows that the pore density for S 54/20 mixture is the lowest, which confirms the highest compressive strength.
- Using the CPMG technique, the distribution and relative size of gel pores and capillaries on concrete samples tested on freeze–thaw were revealed, which permits the additional extraction of gel pore information compared to the standard method in which only the content of permeable pores is extracted.
- The CPMG technique can reflect the effect of freeze–thaw cycles on the total porosity of the concrete internal structure; however, the results must be correlated with standardized methods.
- An important advantage of this technique is that it allows the progressive and repeated evaluation of the distribution of gel pores and capillaries throughout the freeze–thaw test.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kocáb, D.; Kucharczyková, B.; Daněk, P.; Vymazal, T.; Hanuš, P.; Halamová, R. Destructive and non-destructive assessment of the frost resistance of concrete with different aggregate. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Moscow, Russia, 25–27 April 2018; Volume 379, p. 012022. [Google Scholar]
- Aïtcin, P.C. High-Performance Concrete; Stern B 624; E. & F.N. Spon: New York, NY, USA, 1998. [Google Scholar]
- Collepardi, M. The New Concrete; Grafiche Tintoretto: Lancenigo, Italy, 2006. [Google Scholar]
- Neville, A.M. Properties of Concrete, 5th ed.; Longman: Harlow, UK, 2011. [Google Scholar]
- Pop, A.; Badea, C.; Ardelean, I. The Effects of Different Superplasticizers and Water-to-Cement Ratios on the Hydration of Gray Cement Using T2-NMR. Appl. Magn. Reson. 2013, 44, 1223–1234. [Google Scholar] [CrossRef]
- Taylor, P.; Van Dam, T.; Sutter, L.; Fick, G. Integrated Materials and Construction Practices for Concrete Pavement: A State-of-the-Practice Manual, 2nd ed.; Part of InTrans Project 13-482; National Concrete Pavement Technology Center at Iowa State University: Ames, IA, USA, 2019. [Google Scholar]
- Verbeck, G.J. Hardened Concrete—Pore Structure; ASTM: West Conshohocken, PA, USA, 1955; pp. 136–142. [Google Scholar]
- Bede, A.; Scurtu, A.; Ardelean, I. NMR relaxation of molecules confined inside the cement paste pores under partially saturated conditions. Cem. Concr. Res. 2016, 89, 56–62. [Google Scholar] [CrossRef]
- Neville, A.M. Properties of Concrete; Bucharest Technical Publishing House: Bucharest, Romania, 1979. [Google Scholar]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials: Testing, Interpretation and Requirements; Taylor & Francis: London, UK; New York, NY, USA, 2006; p. 432. [Google Scholar]
- Polish Committee for Standardization. Standard for Admixtures for Concrete, Mortar and Grout—Test Methods—Part 11: Determination of Air Void Characteristics in Hardened Concrete; PN-EN 480-11; Polish Committee for Standardization: Warsaw, Poland, 1998. [Google Scholar]
- Wawrzeńczyk, J.; Kozak, W. A method of analyzing the porous microstructure in air-entrained concrete on the basis on 2D image analysis. Procedia Eng. 2015, 108, 102–107. [Google Scholar] [CrossRef]
- Shpak, A.; Jacobsen, S. Requirements and Recommendations for Frost Durable Concrete: Durable Advanced Concrete Structures (DaCS); Norwegian University of Science and Technology: Trondheim, Norway, 2019; ISBN 978-82-7482-116-3. [Google Scholar]
- Yuan, J.; Liu, Y.; Li, H.; Yang, C. Experimental Investigation of the Variation of Concrete Pores under the Action of Freeze-thaw Cycles. Procedia Eng. 2016, 161, 583–588. [Google Scholar] [CrossRef]
- Tracz, T.; Zdeb, T. Effect of hydration and carbonation progress on the porosity and permeability of cement pastes. Materials 2019, 12, 192. [Google Scholar] [CrossRef]
- Rao, S.K.; Sravana, P.; Rao, T.C. Abrasion resistance and mechanical properties of Roller Compacted Concrete with GGBS. Constr. Build. Mater. 2016, 114, 925–933. [Google Scholar] [CrossRef]
- Aghaeipour, A.; Madhkhan, M. Effect of ground granulated blast furnace slag (GGBFS) on RCCP durability. Constr. Build. Mater. 2017, 141, 533–541. [Google Scholar] [CrossRef]
- Limbachiya, V.; Ganjian, E.; Claisse, P. Strength, durability and leaching properties of concrete paving blocks incorporating GGBS and SF. Constr. Build. Mater. 2016, 113, 273–279. [Google Scholar] [CrossRef]
- Zhang, C.; Han, S.; Hua, Y. Flexural performance of reinforced self-consolidating concrete beams containing hybrid fibers. Constr. Build. Mater. 2018, 174, 11–23. [Google Scholar] [CrossRef]
- Li, L.; Cao, M. Influence of calcium carbonate whisker and polyvinyl alcohol- steel hybrid fiber on ultrasonic velocity and resonant frequency of cementitious composites. Constr. Build. Mater. 2018, 188, 737–746. [Google Scholar] [CrossRef]
- Lam, M.N.-T.; Jaritngam, S.; Le, D.-H. Roller-compacted concrete pavement made of Electric Arc Furnace slag aggregate: Mix design and mechanical properties. Constr. Build. Mater. 2017, 154, 482–495. [Google Scholar] [CrossRef]
- Lam, M.N.-T.; Le, D.-H.; Jaritngam, S. Compressive strength and durability properties of roller-compacted concrete pavement containing electric arc furnace slag aggregate and fly ash. Constr. Build. Mater. 2018, 191, 912–922. [Google Scholar] [CrossRef]
- Lei, D.; Guo, L.; Chen, B.; Curosu, I.; Mechtcherine, V. The connection between microscopic and macroscopic properties of ultra-high strength and ultra-high ductility cementitious composites (UHS-UHDCC). Compos. B Eng. 2019, 164, 144–157. [Google Scholar] [CrossRef]
- Nedunuri, S.S.S.A.; Sertse, S.G.; Muhammad, S. Microstructural study of Portland cement partially replaced with fly ash, ground granulated blast furnace slag and silica fume as determined by pozzolanic activity. Constr. Build. Mater. 2020, 238, 117561. [Google Scholar] [CrossRef]
- Majhi, R.K.; Nayak, A.N. Bond, durability and microstructural characteristics of ground granulated blast furnace slag based recycled aggregate concrete. Constr. Build. Mater. 2019, 212, 578–595. [Google Scholar] [CrossRef]
- De Castro Pessoa, J.R.; Dominguez, J.S.; De Carvalho, G.; De Assis, J.T. Obtaining Porosity of Concrete Using X-ray Microtomography or Digital Scanner. J. Chem. Chem. Eng. 2014, 8, 371–377. [Google Scholar] [CrossRef][Green Version]
- Diamond, S. Mercury porosimetry. Cem. Concr. Res. 2000, 30, 1517–1525. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Cao, M.; Tang, Y.; Zhang, Z. Nanoindentation and Porosity Fractal Dimension of Calcium Carbonate Whisker Reinforced Cement Paste After Elevated Temperatures (up to 900 °C). Fractals 2021, 29, 2140001. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, J.; Huang, B. Fractal analysis of effect of air void on freeze–thaw resistance of concrete. Constr. Build. Mater. 2013, 47, 126–130. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, T.; Wang, F. Pore structure and strength of waste fiber recycled concrete. J. Eng. Fibers Fabr. 2019, 14, 1558925019874701. [Google Scholar] [CrossRef]
- Kowalczyk, R.M.; Gajewicz, A.M.; McDonald, P.J. The mechanism of water\isopropanol exchange in cement pastes evidenced by NMR relaxometry. RSC Adv. 2014, 4, 20709–20715. [Google Scholar] [CrossRef]
- Vicente, M.A.; Mínguez, J.; González, D.C. Variation of the Pore Morphology during the Early Age in Plain and Fiber-Reinforced High-Performance Concrete under Moisture-Saturated Curing. Materials 2019, 12, 975. [Google Scholar] [CrossRef]
- Vicente, M.A.; Mínguez, J.; González, D.C. Recent advances in the use of computed tomography in concrete technology and other engineering fields. Micron 2019, 118, 22–34. [Google Scholar] [CrossRef]
- European Committee for Standardization. Testing the Freeze-Thaw Resistance of Concrete—Internal Structural Damage; CEN/TR 15177; Committee for Standardizațion: Brussels, Belgium, 2006. [Google Scholar]
- Badea, C.; Pop, A.; Mattea, C.; Stapf, S.; Ardelean, I. The Effect of Curing Temperature on Early Hydration of Gray Cement Via Fast Field Cycling-NMR Relaxometry. Appl. Magn. Reson. 2014, 45, 1299–1309. [Google Scholar] [CrossRef]
- McDonald, P.J.; Korb, J.P.; Mitchell, J.; Monteilhet, L. Surface relaxation and chemical exchange in hydrating cement pastes: A two-dimensional NMR relaxation study. Phys. Rev. E 2005, 72, 011409. [Google Scholar] [CrossRef]
- Holly, R.; Reardon, E.J.; Hansson, C.M.; Peemoeller, H. Proton Spin-Spin Relaxation Study of the Effect of Temperature on White Cement Hydration. J. Am. Ceram. Soc. 2007, 90, 570–577. [Google Scholar] [CrossRef]
- Muller, A.C.A.; Scrivener, K.L.; Gajewicz, A.M.; McDonald, P.J. Use of bench-top NMR to measure the density, composition and desorption isotherm of CSH in cement paste. Microporous Mesoporous Mater. 2013, 178, 99–103. [Google Scholar] [CrossRef]
- Faure, P.F.; Caré, S.; Magat, J.; Chaussadent, T. Drying effect on cement paste porosity at early age observed by NMR methods. Constr. Build. Mater. 2012, 29, 496–503. [Google Scholar] [CrossRef]
- Pop, A.; Bede, A.; Dudescu, M.C.; Popa, F.; Ardelean, I. Monitoring the Influence of Aminosilane on Cement Hydration Via Low-field NMR Relaxometry. Appl. Magn. Reson. 2015, 47, 191–199. [Google Scholar] [CrossRef]
- Burduhos Nergis, D.D.; Vizureanu, P.; Ardelean, I.; Sandu, A.V.; Corbu, O.C.; Matei, E. Revealing the influence of microparticles on geopolymers’ synthesis and porosity. Materials 2020, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Nicula, L.M.; Corbu, O.; Iliescu, M. Methods for assessing the frost-thaw resistance of road concrete used in our country and at European level. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 877, p. 012025. [Google Scholar]
- Romanian Standards Association (ASRO). Standard Cement—Part 1: Composition, Specification, and Conformity Criteria Common Cements; Romanian Standard EN 197-1; Romanian Standards Association (ASRO): Bucharest, Romania, 2011. [Google Scholar]
- Romanian Standards Association (ASRO). Standard Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout Part 1: Definitions, Specifications and Conformity Criteria; Romanian Standard EN 15167/1; Romanian Standards Association (ASRO): Bucharest, Romania, 2007. [Google Scholar]
- Romanian Standards Association (ASRO). The Norm for the Execution of Cement Concrete Road Pavements in A Fixed and Sliding Formwork System; Romanian Norms NE 014; Romanian Standards Association (ASRO): Bucharest, Romania, 2002. [Google Scholar]
- Romanian Standards Association (ASRO). Aggregates for Concrete; Romanian Standard EN 12620; EN 12620+A1; Romanian Standards Association (ASRO): Bucharest, Romania, 2008. [Google Scholar]
- Smith, K.D.; Morian, D.A.; Van Dam, T.J. Use of Air-Cooled Blast Furnace Slag as Coarse Aggregate in Concrete Pavements—A Guide to Best Practice; Quality Engineering Solutions; Federal Highway Administration: Washington, DC, USA, 2012.
- Romanian Standards Association (ASRO). Concrete Additives; Romanian Standard EN 934-2; Romanian Standards Association (ASRO): Bucharest, Romania, 2010. [Google Scholar]
- Romanian Standards Association (ASRO). Standard for Mixing Water for Concrete; Romanian Standard EN 1008; Romanian Standards Association (ASRO): Bucharest, Romania, 2003. [Google Scholar]
- Romanian Standards Association (ASRO). Standard for Concrete-Part 1: Specification, Performance, Production and Conformity; Romanian Standard EN 206-1; EN 206+A1; Romanian Standards Association (ASRO): Bucharest, Romania, 2017. [Google Scholar]
- Georgescu, D.P. Design Guide for Concrete Durability in Accordance with the National Annex for the Application of SR EN 206-1, Durability Classes; Romanian Standards Association (ASRO): Bucharest, Romania, 2001; Everest; ISBN 978-973-0-04914-5. [Google Scholar]
- Corbu, O.; Ioani, A.; Al Bakri, A.M.; Meita, V.; Szilagyi, H.; Sandu, A.V. The Pozzoolanic Activity Level of Powder Waste Glass in Comparisons with other Powders. Key Eng. Mater. 2015, 660, 237–243. [Google Scholar] [CrossRef]
- Nicula, L.M.; Corbu, O.; Iliescu, M. The Influence of Blast Furnace Slag on Abrasion Resistance for Road Concretes. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 877, p. 012040. [Google Scholar]
- American Society for Testing and Materials. C642 Standard test method for Density, Absorption and Voids in Hardened Concrete. In American Society of Testing Materials; American Society for Testing and Materials: Philadelphia, PA, USA, 2013. [Google Scholar]
- Romanian Standards Association (ASRO). Tests on Concrete: Determination of the Freeze-Tawing Resistance by Measuring the Variations of the Resistance Strength and/or of the Dynamic Relative Elastics Modulus; Romanian Standard SR 3518; Romanian Standards Association (ASRO): Bucharest, Romania, 2009. [Google Scholar]
- Romanian Standards Association (ASRO). Testing Hardened Concrete—Compessive Strenght of Test Specimens; SR EN 12390-3; Romanian Standards Association (ASRO): Bucharest, Romania, 2002. [Google Scholar]
- Meiboom, S.; Gill, D. Modified spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Venkataramanan, L.; Song, Y.-Q.; Hurlimann, M.D. Solving Fredholm integrals of the first kind with tensor product structure in 2 and 2.5 dimensions. IEEE Trans. Signal Process. 2002, 50, 1017–1026. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard test method for resistance of concrete to rapid freezing and thawing. In Annual Book of ASTM Standards; ASTM-C666/C666M-03; American Society for Testing and Materials: Philadelphia, PA, USA, 2008. [Google Scholar]
- Magureanu, C.; Sosa, I.; Negrutiu, C.; Heghes, B. Physical and mechanical properties of ultra high strength fiber reinforced cementitious composites. In Fracture Mechanics of Concrete and Concrete Structures—High Performance, Fiber Reinforced Concrete, Special Loadings and Structural Application; Korea Concrete Institute: Seoul, Korea, 2010; ISBN 978-89-5708-182-2. [Google Scholar]
- Mardani-Aghabaglou, A.; Andiç-Çakir, Ö.; Ramyar, K. Freeze thaw resistance and transport properties of high-volume fly ash roller compacted concrete designed by maximum density method. Cem. Concr. Compos. 2013, 37, 259–266. [Google Scholar] [CrossRef]
- Ethington, E.F. Interfacial Contact Angle Measurements of Water, Mercury, and 20 Organic Liquids on Quartz, Calcite, Biotite, and Ca-Montmorillonite Substrates; Open-File Report, No. 90–409; US Geological Survey: Golden, CO, USA, 1990.
- Badr, A.; Ashour, A.F.; Platten, A.K. Statistical variations in impact resistance of polypropylene fibre-reinforced concrete. Int. J. Impact Eng. 2006, 32, 1907–1920. [Google Scholar] [CrossRef]
- Ionescu, I.; Ispas, T. Properties and Technology of Concrete; Bucharest Technical Publishing House: Bucharest, Romania, 1997. [Google Scholar]





| Oxides | SiO2 | Al2O3 | MnO | MgO | CaO | Fe2O3 | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| CEM I 42.5R | 18.57 | 3.09 | 3.72 | 0.70 | 63.93 | 4.84 | 0.12 | 0.74 |
| GGBS | 36.44 | 11.60 | 0.55 | 5.8 | 41.81 | 0.78 | 0.345 | 0.428 |
| Technical Characteristics | Obtained Values ACBSF_0/4 mm | Obtained Values NA_0/4 mm | Limits SR EN 12620 |
|---|---|---|---|
| Granularity | GF 85 | GF 85 | GF 85 |
| Coefficient of water absorption | WA242 | WA242 | - |
| Content of fine particles <0.063 mm, % | f3.5 | f3 | (3 ÷ 22) |
| Sulphate soluble in acid, % | AS 0.52 | - | ≤1.0 |
| Total sulphate, % | 0.96 | - | ≤2.0 |
| Disintegration of iron from blast furnace slag | Does not present cracks and disintegrate | - | Visual aspect |
| Disintegration of dicalcium silicate from blast furnace slag | Presents a uniform violet color, with shining stains in small quantities uniformly distributed | - | Visual aspect |
| Quantities (kg/m3) | Mixtures (kg/m3) | ||||
|---|---|---|---|---|---|
| S 360 | S 414 | S 54/20 | S 54/40 | S 54/60 | |
| Cement (C) | 360 | 414 | 360 | 360 | 360 |
| Blast furnace slag powder (GGBS) | - | - | 54 | 54 | 54 |
| Total binder (L) | 360 | 414 | 414 | 414 | 414 |
| Water (W) | 156.60 | 169.74 | 167.67 | 178.02 | 178.02 |
| W/L, (water/binder) | 0.44 | 0.41 | 0.41 | 0.43 | 0.43 |
| Natural sand (NA 0/4 mm) | 596 | 586 | 477 | 347 | 232 |
| Blast furnace slag aggregate (ACBFS 0/4 mm) | - | - | 119 | 232 | 347 |
| Coarse aggregate (CA 4/25 mm) | 1268 | 1245 | 1267 | 1231 | 1231 |
| Total aggregate | 1864 | 1831 | 1863 | 1810 | 1810 |
| Superplasticizer additive | 3.60 | 4.14 | 4.14 | 4.97 | 4.97 |
| Air training additive | 1.08 | 2.07 | 2.07 | 2.07 | 2.07 |
| Slump (mm) | 14 | 15 | 13 | 16 | 15 |
| Fresh state density | 2380 | 2415 | 2444 | 2402 | 2402 |
| Name of Tests (Test Method) | Number of Samples (Pieces) | Dimension (mm) | Trial Age (Days) |
|---|---|---|---|
| Dry density, density after immersion and boiling and content of permeable pores (ASTM C642: 2006) | 3 | 71 mm | 150 |
| Control samples kept in water (w) (SR 3518: 2009) | 3 | 150 mm | 150 |
| Tests tested at 300 cycles (f-t) (SR 3518: 2009) | 3 | 150 mm | 150 |
| Control samples kept in water (w) (NMR relaxometry and SEM) | 1 | 150 mm | 150 |
| Tests tested at 300 cycles (f-t) (NMR relaxometry) | 1 | 150 mm | 150 |
| Mixture | S 360 | S 414 | S 54/20 | S 54/40 | S 54/60 |
|---|---|---|---|---|---|
| SD-ρ1 (g/cm3) | 0.005 | 0.019 | 0.007 | 0.001 | 0.013 |
| CoV (%) | 0.002 | 0.008 | 0.003 | 0.000 | 0.006 |
| SD-ρ2 (g/cm3) | 0.003 | 0.041 | 0.009 | 0.019 | 0.033 |
| CoV (%) | 0.001 | 0.016 | 0.004 | 0.008 | 0.013 |
| SD-P0 (g/cm3) | 0.195 | 1.425 | 0.763 | 0.730 | 1.717 |
| CoV (%) | 0.026 | 0.176 | 0.100 | 0.094 | 0.192 |
| Mixture | S 360 | S 414 | S 54/20 | S 54/40 | S 54/60 |
|---|---|---|---|---|---|
| fcm—water (MPa) | 87.78 | 80.96 | 84.80 | 79.38 | 74.77 |
| SD (MPa) | 4.802 | 1.078 | 0.855 | 1.528 | 4.748 |
| CoV (%) | 0.055 | 1.300 | 0.010 | 0.019 | 0.064 |
| fcm—300 cycles (f-t) (MPa) | 83.06 | 71.15 | 77.82 | 73.06 | 60.95 |
| SD (MPa) | 0.492 | 2.630 | 0.147 | 4.040 | 5.525 |
| CoV (%) | 0.006 | 0.037 | 0.002 | 0.055 | 0.091 |
| Reduction in compressive strength (η300) | 5.38 | 12.10 | 8.32 | 7.96 | 18.47 |
| SD (MPa) | 4.633 | 2.078 | 1.089 | 3.325 | 2.220 |
| CoV (%) | 0.862 | 0.171 | 0.132 | 0.415 | 0.120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicula, L.M.; Corbu, O.; Ardelean, I.; Sandu, A.V.; Iliescu, M.; Simedru, D. Freeze–Thaw Effect on Road Concrete Containing Blast Furnace Slag: NMR Relaxometry Investigations. Materials 2021, 14, 3288. https://doi.org/10.3390/ma14123288
Nicula LM, Corbu O, Ardelean I, Sandu AV, Iliescu M, Simedru D. Freeze–Thaw Effect on Road Concrete Containing Blast Furnace Slag: NMR Relaxometry Investigations. Materials. 2021; 14(12):3288. https://doi.org/10.3390/ma14123288
Chicago/Turabian StyleNicula, Liliana Maria, Ofelia Corbu, Ioan Ardelean, Andrei Victor Sandu, Mihai Iliescu, and Dorina Simedru. 2021. "Freeze–Thaw Effect on Road Concrete Containing Blast Furnace Slag: NMR Relaxometry Investigations" Materials 14, no. 12: 3288. https://doi.org/10.3390/ma14123288
APA StyleNicula, L. M., Corbu, O., Ardelean, I., Sandu, A. V., Iliescu, M., & Simedru, D. (2021). Freeze–Thaw Effect on Road Concrete Containing Blast Furnace Slag: NMR Relaxometry Investigations. Materials, 14(12), 3288. https://doi.org/10.3390/ma14123288










