Dry Sliding Behavior of an Aluminum Alloy after Innovative Hard Anodizing Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Microstructure and Mechanical Properties
2.2. Wear Tests
3. Results
3.1. Microstructure and Mechanical Properties
3.2. Wear Tests
4. Discussion
5. Conclusions
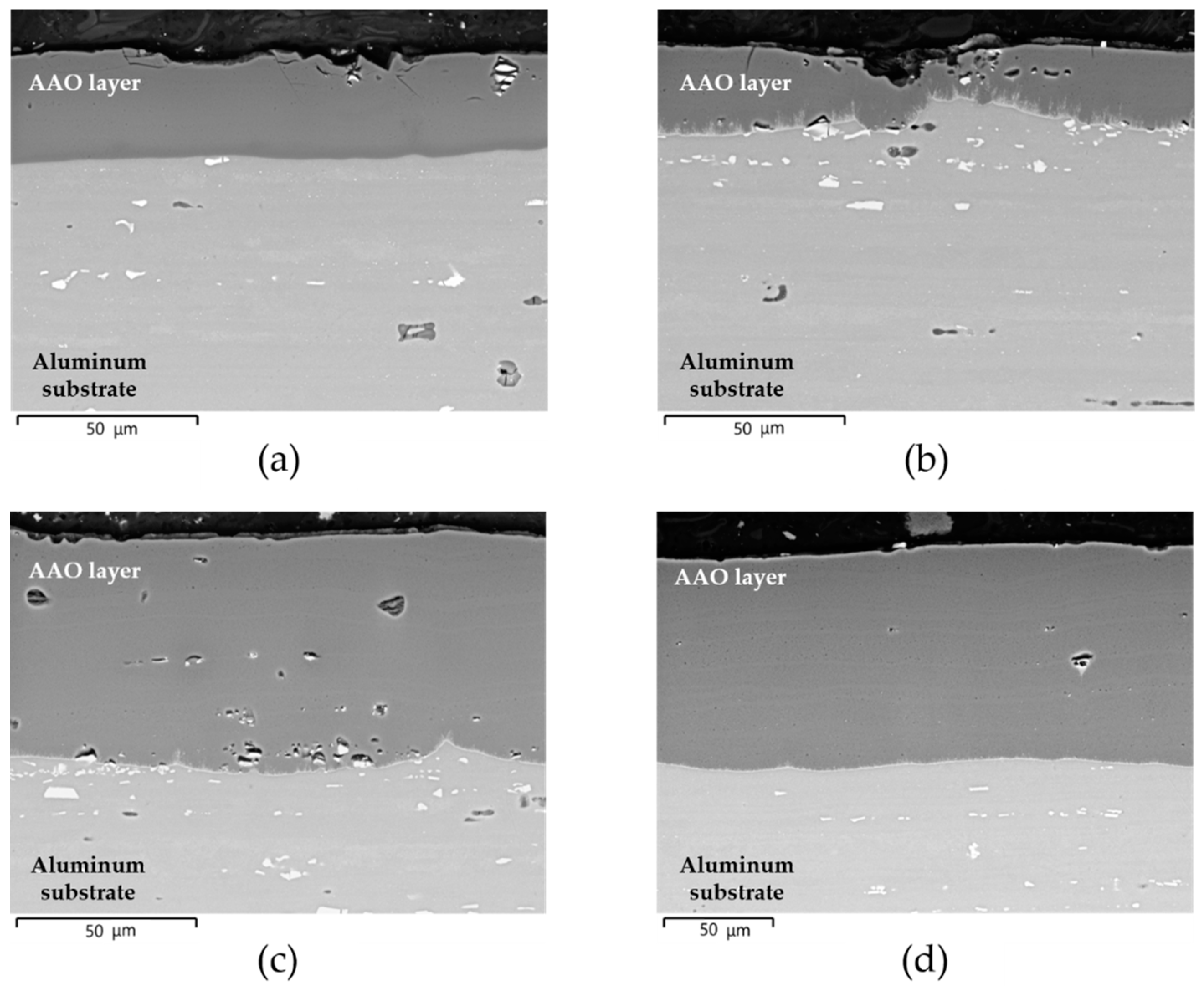
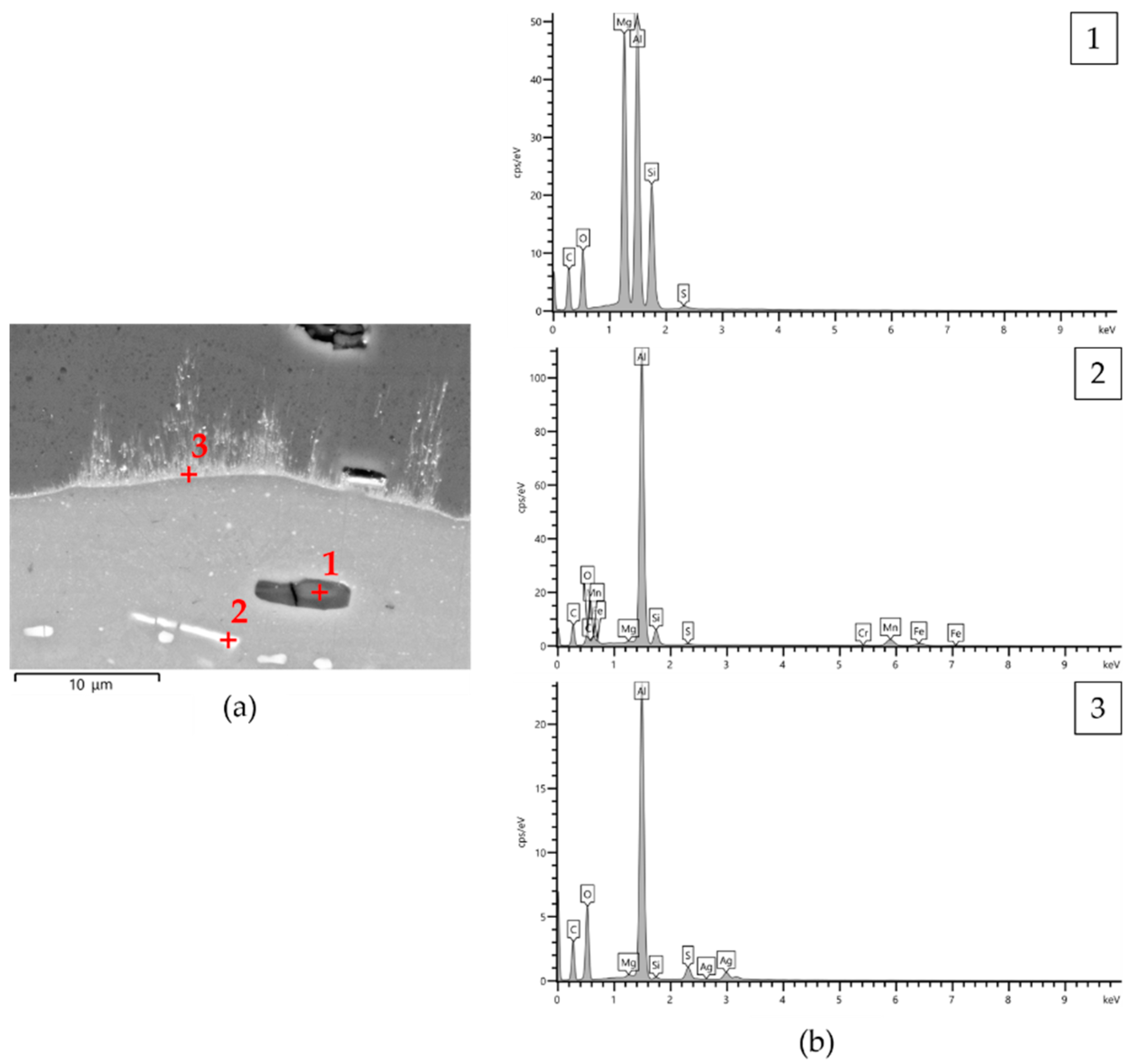
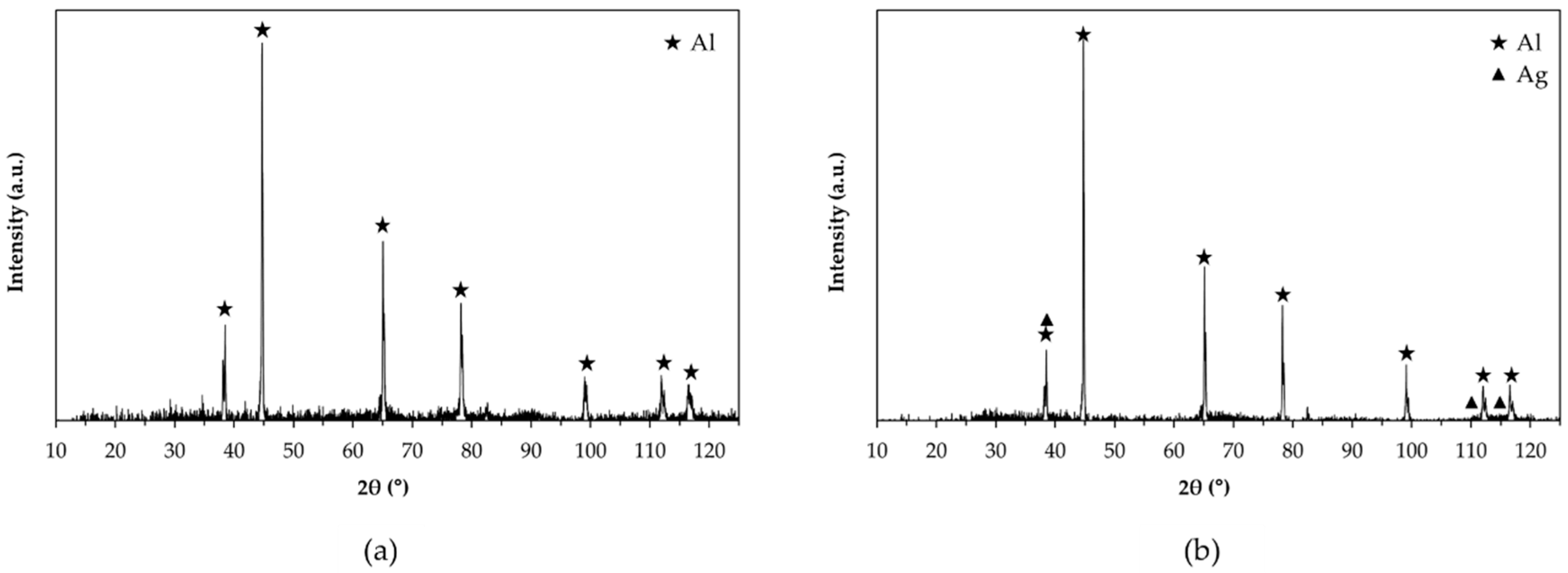
- All AAO layers show the typical microstructure of anodic aluminum oxides with very fine-scale and Ag-rich dendrites that appear only at the coating/aluminum substrate interfaces of G- and GP-treated surfaces. In agreement with the phase compositions analysis, all AAO layers exhibit amorphous structures with some boehmite deriving from the sealing in hot deionized water;
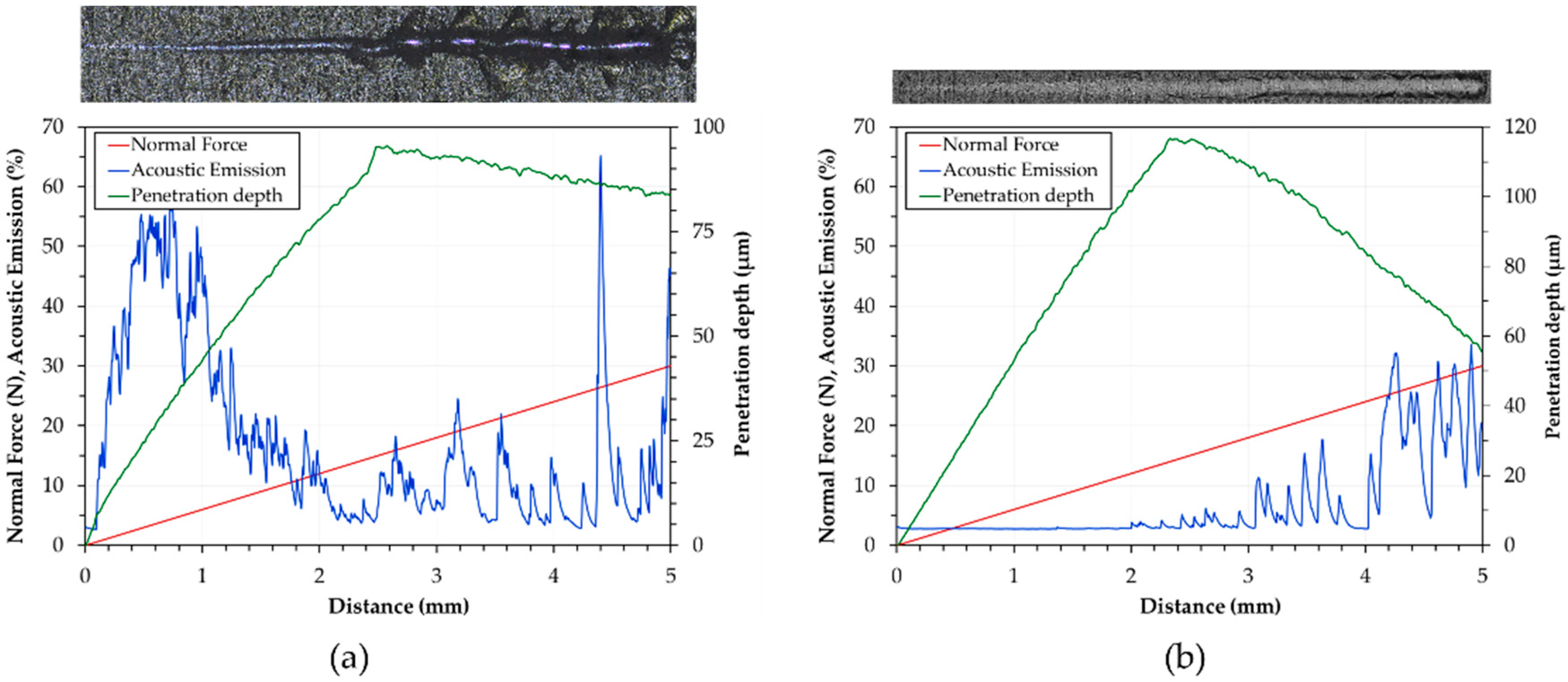
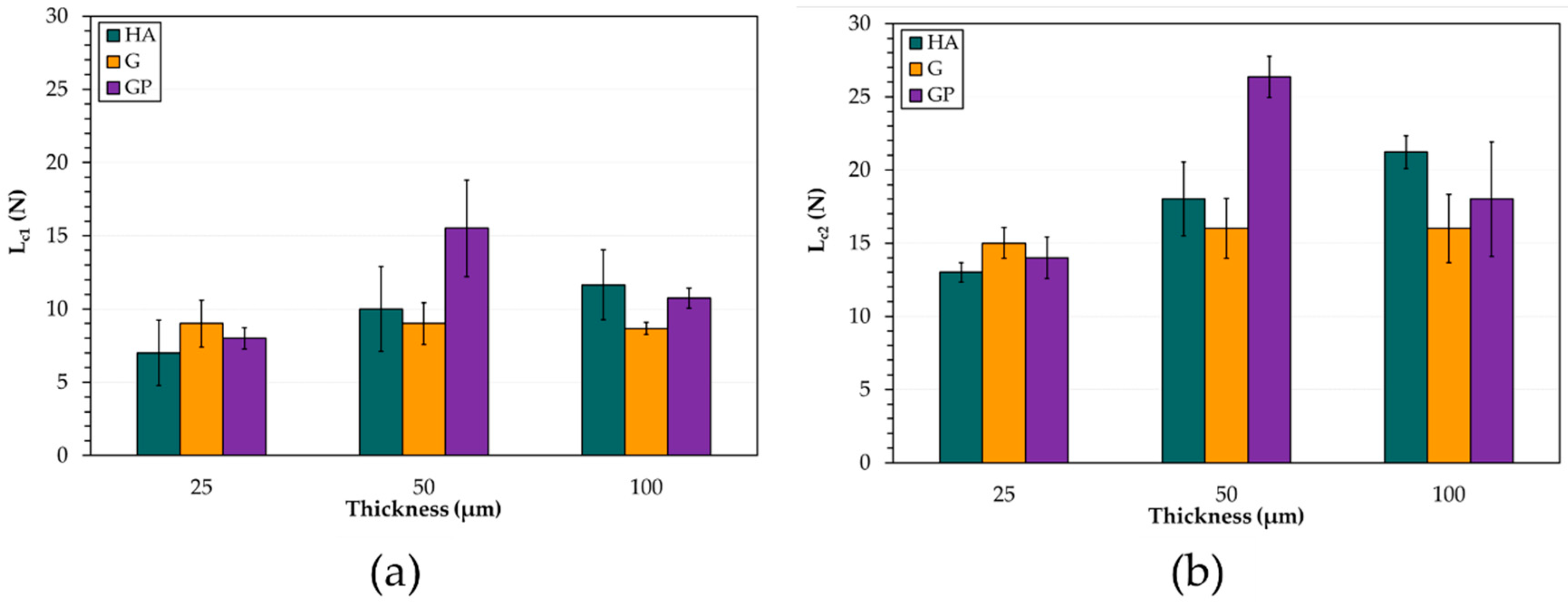
- The HA treatment generates the hardest anodic aluminum oxides, while GP produces the softest. The examination of scratches reveals two main failure modes: wedge spallation for the coatings 25 μm thick and conformal cracking for those 50 and 100 μm thick. Finally, all anodic aluminum oxides with a thickness of 25 μm display the lowest adhesion strength, whereas the GP-treated surfaces show the highest one;
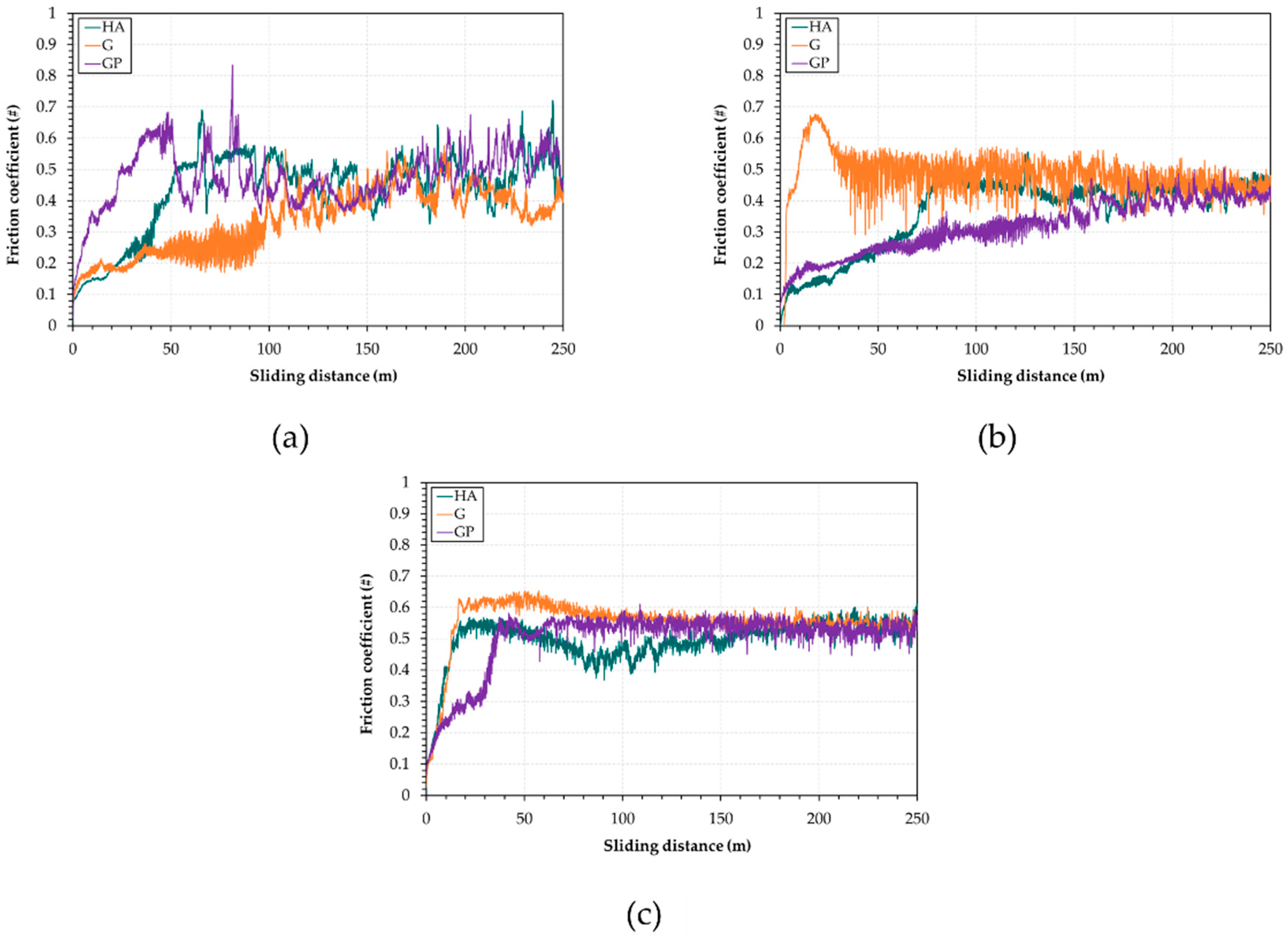
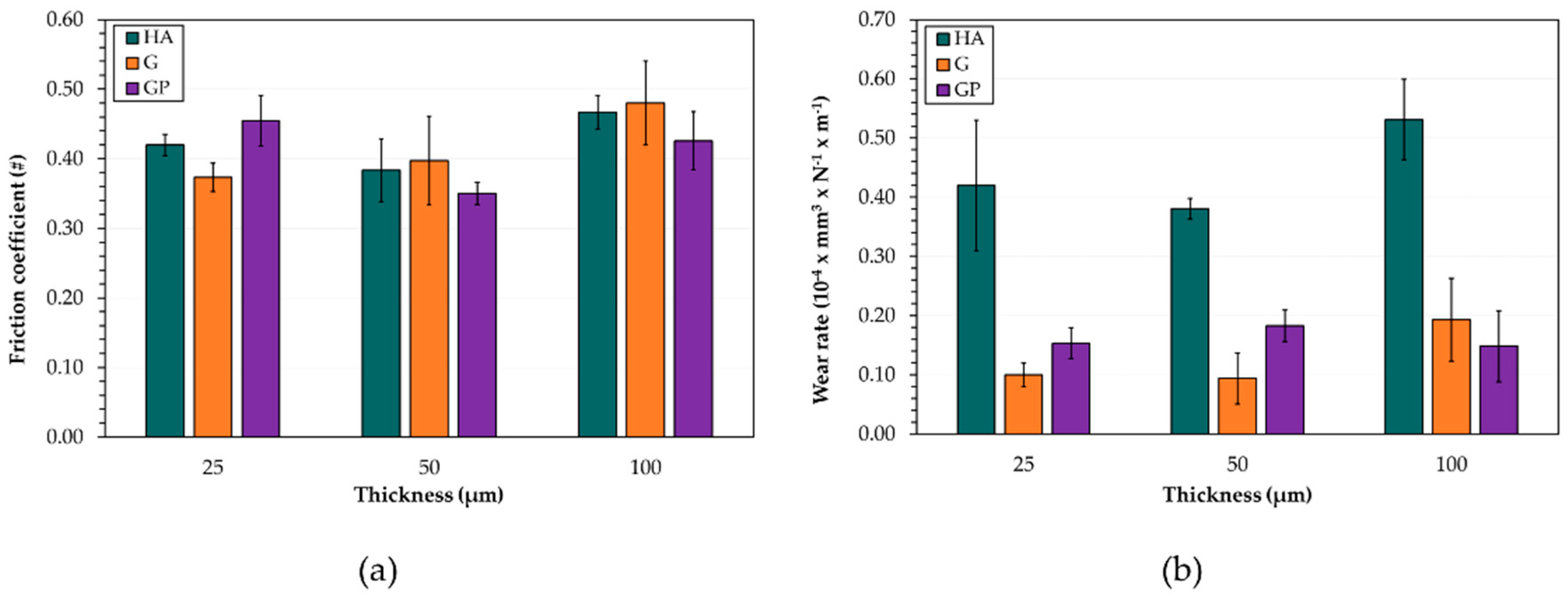
- The friction coefficient variations with sliding distance are influenced by changes in wear morphology and the extent of oxidation. The GP-treated surfaces with a thickness of 50 μm exhibit the lowest friction coefficients and wear rates. Their improved wear resistance may be related to the increased adhesion strength compared to those of the other anodized surfaces;
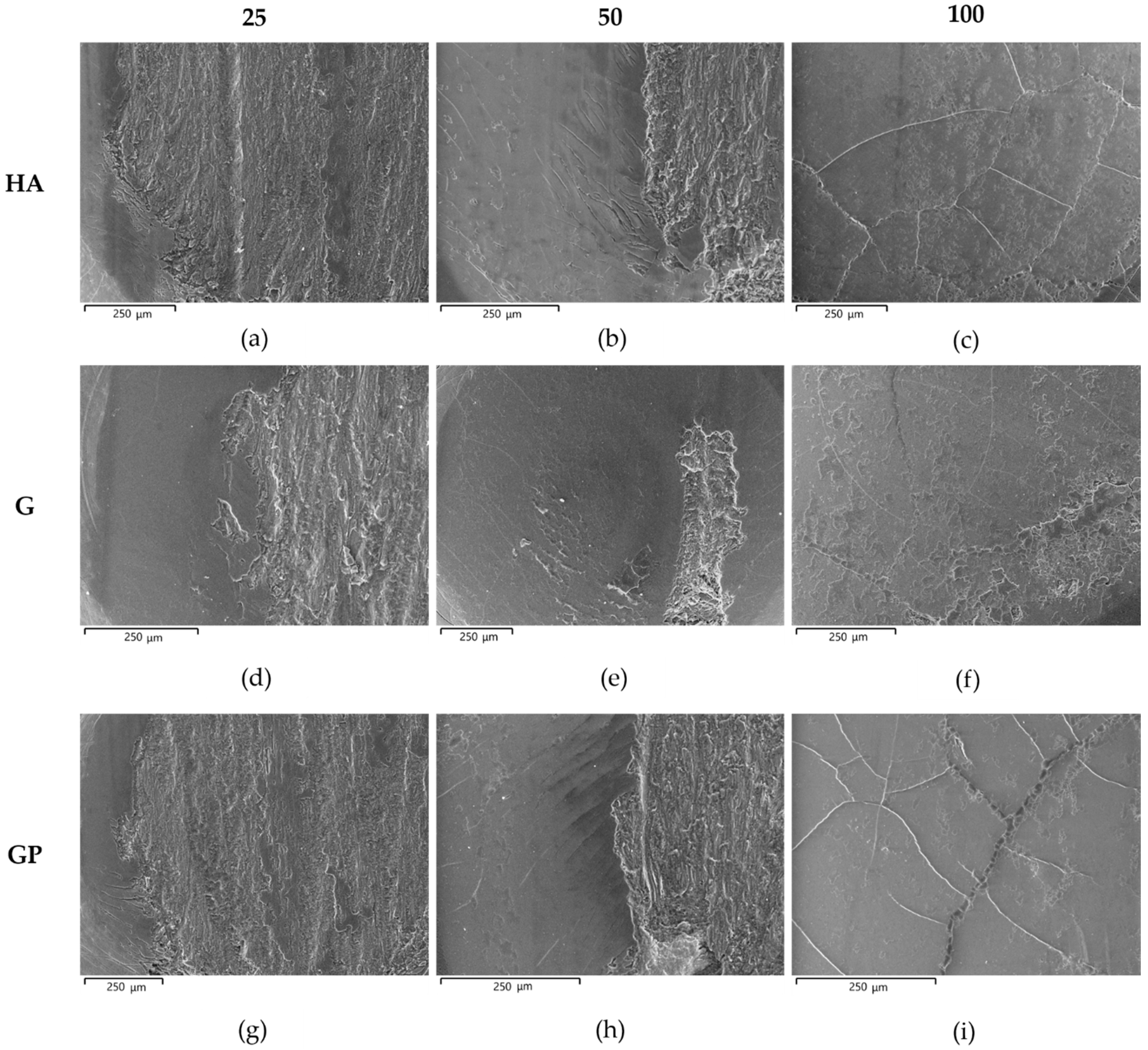
- In any case, a well-adherent tribofilm consisting of iron oxide is observed on the wear tracks as a result of tribochemical reaction and metal transfer between the mating materials. During sliding, its detachment leads to delamination of the underlying anodic aluminum oxides and to the subsequent abrasion of the aluminum substrate. Despite the presence of abraded surfaces, the best tribological performance of G- and GP-treated surfaces may be related to the existence of a thin Ag-rich film at the coating/aluminum substrate interfaces.
Author Contributions
Funding
Acknowledgments
Data Availability Statement
Conflicts of Interest
References
- King, F. Aluminium and Its Alloys; Halsted Press: Sidney, Australia, 1987. [Google Scholar]
- Anderson, K.; Weritz, J.; Gilbert Kaufman, J. ASM Handbook, Volume 2A: Aluminum Science and Technology; ASM International: Materials Park, OH, USA, 2018. [Google Scholar]
- Soffritti, C.; Fortini, A.; Merlin, M.; Garagnani, G.L.; Vazquez Aguilar, R. Wear behaviour of a plasma-sprayed Al2O3-13%TiO2 ceramic coating/steel couple under high constant and variable loads. Metall. Ital. 2019, 111, 21–32. [Google Scholar]
- Soffritti, C.; Merlin, M.; Vazquez, R.; Fortini, A.; Garagnani, G.L. Failure analysis of worn valve train components of a four-cylinder diesel engine. Eng. Fail. Anal. 2018, 92, 528–538. [Google Scholar] [CrossRef]
- Soffritti, C.; Merlin, M.; Vazquez, R.; Garagnani, G.L. Tribological behavior of a Cr2O3 ceramic coating/steel couple under dry sliding and heavy loading conditions. J. Mater. Eng. Perform. 2018, 27, 3699–3708. [Google Scholar] [CrossRef]
- Merlin, M.; Soffritti, C.; Vazquez, R. Effect of relative humidity and applied loads on the tribological behaviour of a steel/Cr2O3-ceramic coupling. Wear 2013, 303, 371–380. [Google Scholar] [CrossRef]
- Merlin, M.; Soffritti, C.; Vazquez, R.; Garagnani, G.L. Friction and wear behaviour of APS and HVOF advanced ceramic coatings. Metall. Ital. 2011, 103, 17–23. [Google Scholar]
- Merlin, M.; Soffritti, C.; Vazquez, R.; Garagnani, G.L. Effect of varying load on the tribological behaviour of a steel/ Al2O3-TiO2 ceramic coating. In Proceedings of the 5th World Tribology Congress, WTC 2013, Turin, Italy, 8–13 September 2013; pp. 1592–1595. [Google Scholar]
- Bensalah, W.; Elleuch, K.; Feki, M.; DePetris-Wery, M.; Ayedi, H.F. Comparative study of mechanical and tribological properties of alumina coatings formed on aluminium in various conditions. Mater. Des. 2009, 30, 3731–3737. [Google Scholar] [CrossRef]
- Bolelli, G.; Lusvarghi, L.; Barletta, M. HVOF-sprayed WC–CoCr coatings on Al alloy: Effect of the coating thickness on the tribological properties. Wear 2009, 267, 944–953. [Google Scholar] [CrossRef]
- Sabatini, G.; Ceschini, L.; Martini, C.; Williams, J.A.; Hutchings, I.M. Improving sliding and abrasive wear behaviour of cast A356 and wrought AA7075 aluminium alloys by plasma electrolytic oxidation. Mater. Des. 2010, 31, 816–828. [Google Scholar] [CrossRef]
- Young, L. Anodic Oxide Films; Academic Press: Cambridge, MA, USA, 1961. [Google Scholar]
- Thompson, G. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Mezlini, S.; Elleuch, K.; Kapsa, P. The effect of sulphuric anodisation of aluminium alloys on contact problems. Surf. Coat. Technol. 2006, 200, 2852–2856. [Google Scholar] [CrossRef]
- López, V.; Otero, E.; Bautista, A.; González, J. Sealing of anodic films obtained in oxalic acid baths. Surf. Coat. Technol. 2000, 124, 76–84. [Google Scholar] [CrossRef]
- Jagminas, A.; Bigelien, D.; Mikulskas, I.; Tomašiūnas, R. Growth peculiarities of aluminum anodic oxide at high voltages in diluted phosphoric acid. J. Cryst. Growth 2001, 233, 591–598. [Google Scholar] [CrossRef]
- Lunder, O.; Walmsley, J.C.; Mack, P.; Nisancioglu, K. Formation and characterisation of a chromate conversion coating on AA6060 aluminium. Corros. Sci. 2005, 47, 1604–1624. [Google Scholar] [CrossRef]
- Shih, H.-H.; Tzou, S.-L. Study of anodic oxidation of aluminum in mixed acid using a pulsed current. Surf. Coat. Technol. 2000, 124, 278–285. [Google Scholar] [CrossRef]
- Sulka, G.D.; Parkoła, K.G. Anodising potential influence on well-ordered nanostructures formed by anodisation of aluminium in sulphuric acid. Thin Solid Films 2006, 515, 338–345. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.; Pagetti, J.; Normand, B. Influence of oxalic acid addition to chromic acid on the anodising of Al 2024 alloy. Surf. Coat. Technol. 2004, 182, 117–123. [Google Scholar] [CrossRef]
- Bensalah, W.; Elleuch, K.; Feki, M.; Wery, M.; Ayedi, H.F. Optimization of anodic layer properties on aluminium in mixed oxalic/sulphuric acid bath using statistical experimental methods. Surf. Coat. Technol. 2007, 201, 7855–7864. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Lee, W.; Cho, S.J.; Hahn, J.-H.; Ahn, H.-S. Tribological properties of nanoporous anodic aluminum oxide film. Surf. Coat. Technol. 2010, 205, 1431–1437. [Google Scholar] [CrossRef]
- Guezmil, M.; Bensalah, W.; Khalladi, A.; Elleuch, K.; De-Petris Wery, M.; Ayedi, H.F. Effect of test parameters on the friction behaviour of anodized aluminium alloy. Int. Sch. Res. Not. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Malayoglu, U.; Tekin, K.C.; Malayoglu, U.; Shrestha, S. An investigation into the mechanical and tribological properties of plasma electrolytic oxidation and hard-anodized coatings on 6082 aluminum alloy. Mater. Sci. Eng. A 2011, 528, 7451–7460. [Google Scholar] [CrossRef]
- Tsyntsaru, N.; Kavas, B.; Sort, J.; Urgen, M.; Celis, J.-P. Mechanical and frictional behaviour of nano-porous anodised aluminium. Mater. Chem. Phys. 2014, 148, 887–895. [Google Scholar] [CrossRef]
- Guezmil, M.; Bensalah, W.; Khalladi, A.; Elleuch, K.; Depetris-Wery, M.; Ayedi, H.F. Friction coefficient and microhardness of anodized aluminum alloys under different elaboration conditions. Trans. Nonferrous Met. Soc. China 2015, 25, 1950–1960. [Google Scholar] [CrossRef]
- Vengatesh, P.; Kulandainathan, M.A. Hierarchically ordered self-lubricating superhydrophobic anodized aluminum surfaces with enhanced corrosion resistance. ACS Appl. Mater. Interfaces 2015, 7, 1516–1526. [Google Scholar] [CrossRef]
- Dejun, K.; Jinchun, W.; Hao, L. Friction and wear performances of 7475 aluminium alloy after anodic oxidation. Rare Met. Mater. Eng. 2016, 45, 1122–1127. [Google Scholar] [CrossRef]
- Lu, J.; Wei, G.; Yu, Y.; Guo, C.; Jiang, L. Aluminum alloy AA2024 anodized from the mixed acid system with enhanced mechanical properties. Surf. Interfaces 2018, 13, 46–50. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Z.; Wang, B.; Song, Q.; Wan, Y.; Chen, L. Surface characterization and tribological performance of anodizing micro-textured aluminum-silicon alloys. Materials (Basel) 2019, 12, 1862. [Google Scholar] [CrossRef] [PubMed]
- Benea, L.; Dumitrascu, V. Enhancement in sustained friction and wear resistance of nanoporous aluminum oxide films obtained by controlled electrochemical oxidation process. RSC Adv. 2019, 9, 25056–25063. [Google Scholar] [CrossRef]
- Wernick, S.; Pinner, R.; Mason, R.B. The surface treatment and finishing of aluminium and its alloys. J. Electrochem. Soc. 1957, 104, 180C. [Google Scholar] [CrossRef]
- Hu, N.; Ge, S.; Fang, L. Tribological properties of nano-porous anodic aluminum oxide template. J. Cent. South Univ. Technol. 2011, 18, 1004–1008. [Google Scholar] [CrossRef]
- Lee, G.-S.; hoon Choi, J.; Choi, Y.C.; Bu, S.D.; Lee, Y.-Z. Tribological effects of pores on an anodized Al alloy surface as lubricant reservoir. Curr. Appl. Phys. 2011, 11, S182–S186. [Google Scholar] [CrossRef]
- Xu, T.; Chen, J.; Zhao, J.; Dang, H. The pore-enlargement and self-lubrication treatment of anodic oxide film of aluminum. Wear 1996, 196, 214–218. [Google Scholar]
- Takaya, M.; Hashimoto, K.; Toda, Y.; Maejima, M. Novel tribological properties of anodic oxide coating of aluminum impregnated with iodine compound. Surf. Coat. Technol. 2003, 169–170, 160–162. [Google Scholar] [CrossRef]
- Tu, J.P.; Jiang, C.X.; Guo, S.Y.; Zhao, X.B.; Fu, M.F. Tribological properties of aligned film of amorphous carbon nanorods on AAO membrane in different environments. Wear 2005, 259, 759–764. [Google Scholar] [CrossRef]
- Skeldon, P.; Wang, H.; Thompson, G. Formation and characterization of self-lubricating MoS2 precursor films on anodized aluminium. Wear 1997, 206, 187–196. [Google Scholar] [CrossRef]
- Maejima, M.; Saruwatari, K.; Takaya, M. Friction behaviour of anodic oxide film on aluminum impregnated with molybdenum sulfide compounds. Surf. Coat. Technol. 2000, 132, 105–110. [Google Scholar] [CrossRef]
- Liew, K.W.; Chia, S.Y.; Kok, C.K.; Low, K.O. Evaluation on tribological design coatings of Al2O3, Ni–P–PTFE and MoS2 on aluminium alloy 7075 under oil lubrication. Mater. Des. 2013, 48, 77–84. [Google Scholar] [CrossRef]
- Jiang, C.X.; Tu, J.P.; Guo, S.Y.; Fu, M.F.; Zhao, X.B. Friction properties of oil-infiltrated porous AAO film on an aluminum substrate. Acta Metall. Sin. (Engl. Lett.) 2005, 18, 249–253. [Google Scholar]
- Mitani, M. Method for Surface Treatment of Aluminum or Aluminum Alloy. European Patent No EP 1 207 220 B1, 16 January 2008. [Google Scholar]
- Santecchia, E.; Cabibbo, M.; Hamouda, A.M.S.; Musharavati, F.; Popelka, A.; Spigarelli, S. Dry sliding tribological properties of a hard anodized AA6082 aluminum alloy. Metals (Basel) 2020, 10, 207. [Google Scholar] [CrossRef]
- Ellingham Diagrams. Standard Gibb’s Energies of Formation for Chlorides, Nitrades, Oxides and other Compounds. Free Energies of Formation of Binary Compounds; Howard, S.M., Ed.; MIT Press: Cambridge, USA, 2006. [Google Scholar]
- Zhu, B.; Seifeddine, S.; Persson, P.O.Å.; Jarfors, A.E.W.; Leisner, P.; Zanella, C. A study of formation and growth of the anodised surface layer on cast Al-Si alloys based on different analytical techniques. Mater. Des. 2016, 101, 254–262. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.; Tichelaar, F.D.; Thompson, G.E.; Terryn, H.; Skeldon, P.; Duszczyk, J.; Katgerman, L. A transmission electron microscopy study of hard anodic oxide layers on AlSi(Cu) alloys. Electrochim. Acta 2004, 49, 3169–3177. [Google Scholar] [CrossRef]
- Alemi, A.; Hosseinpour, Z.; Dolatyari, M.; Bakhtiari, A. Boehmite (γ-AlOOH) nanoparticles: Hydrothermal synthesis, characterization, pH-controlled morphologies, optical properties, and DFT calculations. Phys. Status Solidi 2012, 249, 1264–1270. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ruan, H.D.; Frost, R.L. Thermal decomposition of bauxite minerals: Infrared emission spectroscopy of gibbsite, boehmite and diaspore. J. Mater. Sci. 2002, 37, 1121–1129. [Google Scholar] [CrossRef]
- Russell, J.D.; Farmer, V.C.; Lewis, D.G. Lattice vibrations of boehmite (γ-AlOOH): Evidence for a C122v rather than a D172h space group. Spectrochim. Acta Part A Mol. Spectrosc. 1978, 34, 1151–1153. [Google Scholar] [CrossRef]
- Kiss, A.B.; Keresztury, G.; Farkas, L. Raman and i.r. spectra and structure of boehmite (γ-AlOOH). evidence for the recently discarded D172h space group. Spectrochim. Acta Part A Mol. Spectrosc. 1980, 36, 653–658. [Google Scholar] [CrossRef]
- Farmer, C. The Infrared Spectra of Minerals; Mineralogical Society of Great Britain and Ireland: London, UK, 1974. [Google Scholar]
- Van Der Marel, R.; Van Der Marel, H.W.; Beutelspacher, H. Atlas of Infrared Spectroscopy of Clay Minerals and Thesir Admixtures; ElsevierScientific Publishing Company: Amsterdam, The Netherlands, 1974. [Google Scholar]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Ofoegbu, S.U.; Fernandes, F.A.O.; Pereira, A.B. The sealing step in aluminum anodizing: A focus on sustainable strategies for enhancing both energy efficiency and corrosion resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef]
- Bull, S.J.; Berasetegui, E.G. An overview of the potential of quantitative coating adhesion measurement by scratch testing. Tribol. Int. 2006, 39, 99–114. [Google Scholar] [CrossRef]
- Barletta, M.; Rubino, G.; Tagliaferri, V.; Trovalusci, F.; Vesco, S. Wood-reinforced polyphthalamide resins: Multifunctional composite coating for metal substrates. Int. J. Polym. Sci. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Bull, S.J. Failure mode maps in the thin film scratch adhesion test. Tribol. Int. 1997, 30, 491–498. [Google Scholar] [CrossRef]
- Burnett, P.J.; Rickerby, D.S. The relationship between hardness and scratch adhesion. Thin Solid Films 1987, 154, 403–416. [Google Scholar] [CrossRef]
- Sola, R.; Tonelli, L.; Shashkov, P.; Bogdanoff, T.H.; Martini, C. Anodizing of AA6082-T5 by conventional and innovative treatments: Microstructural characterization and dry sliding behaviour. Wear 2020, 458–459, 203423. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Zhu, S.; Cheng, J.; Qiao, Z.; Yang, J. High temperature solid-lubricating materials: A review. Tribol. Int. 2019, 133, 206–223. [Google Scholar] [CrossRef]
- Lu, J.; Xue, Q.; Zhang, G. Effect of silver on the sliding friction and wear behavior of CeF3 compact at elevated temperatures. Wear 1998, 214, 107–111. [Google Scholar] [CrossRef]


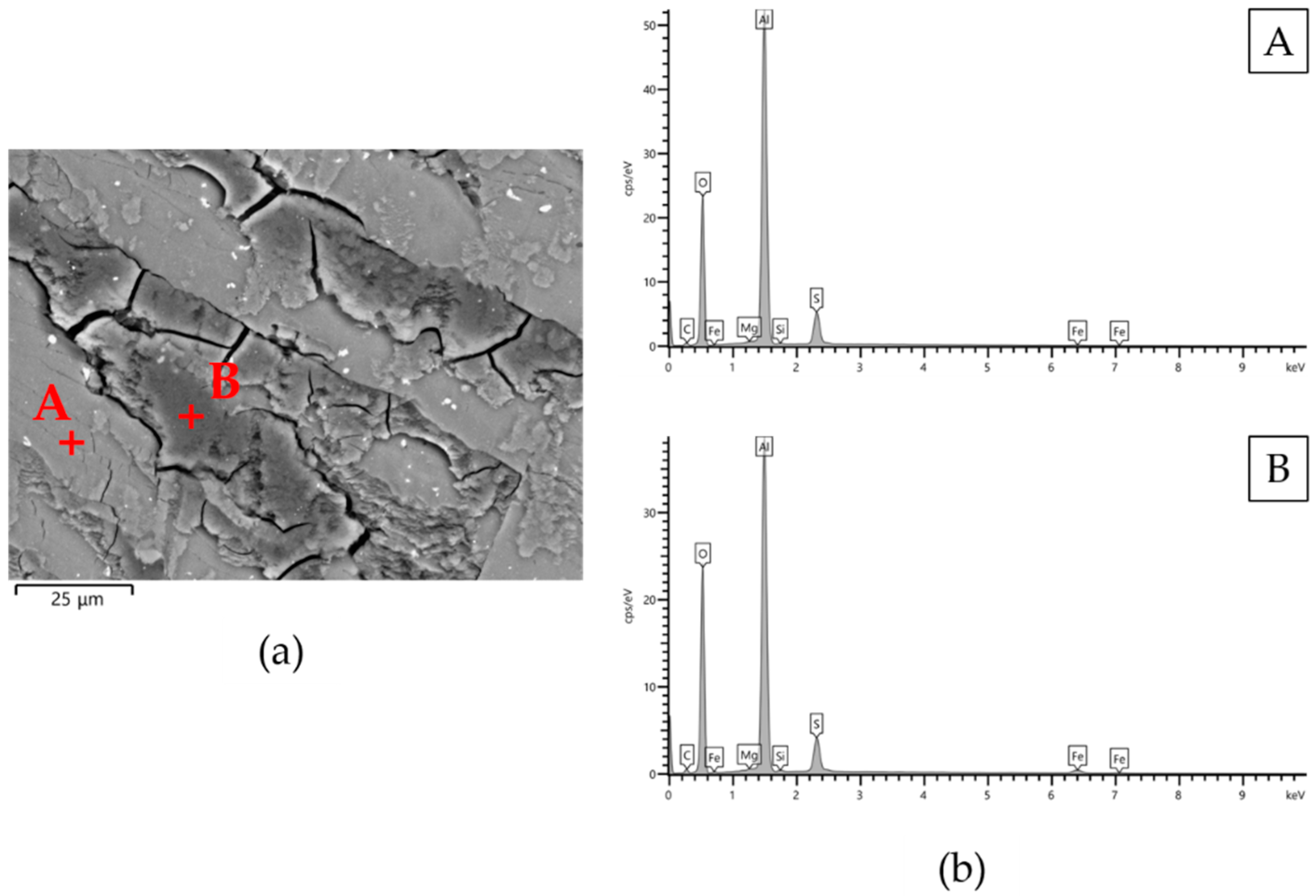
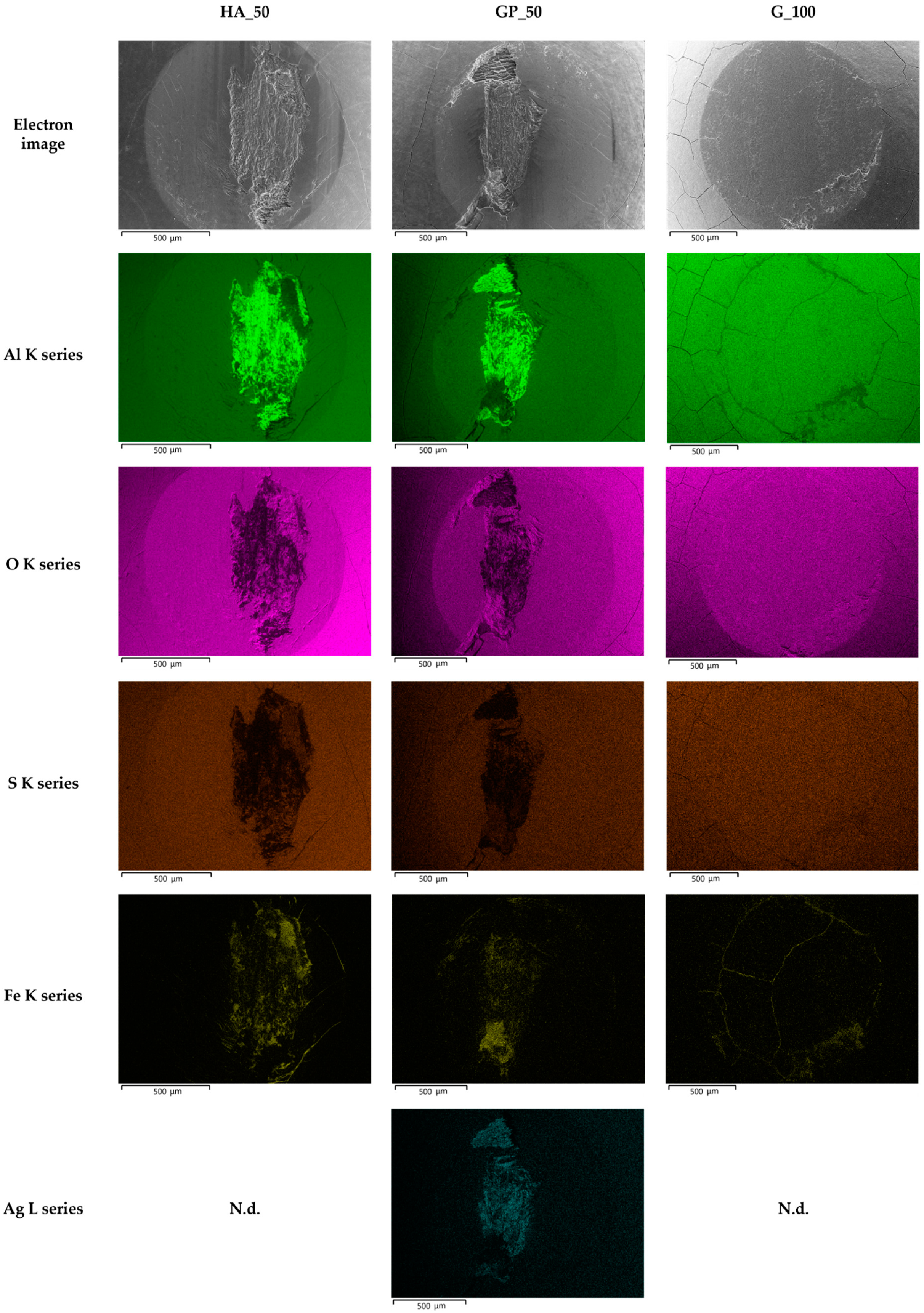
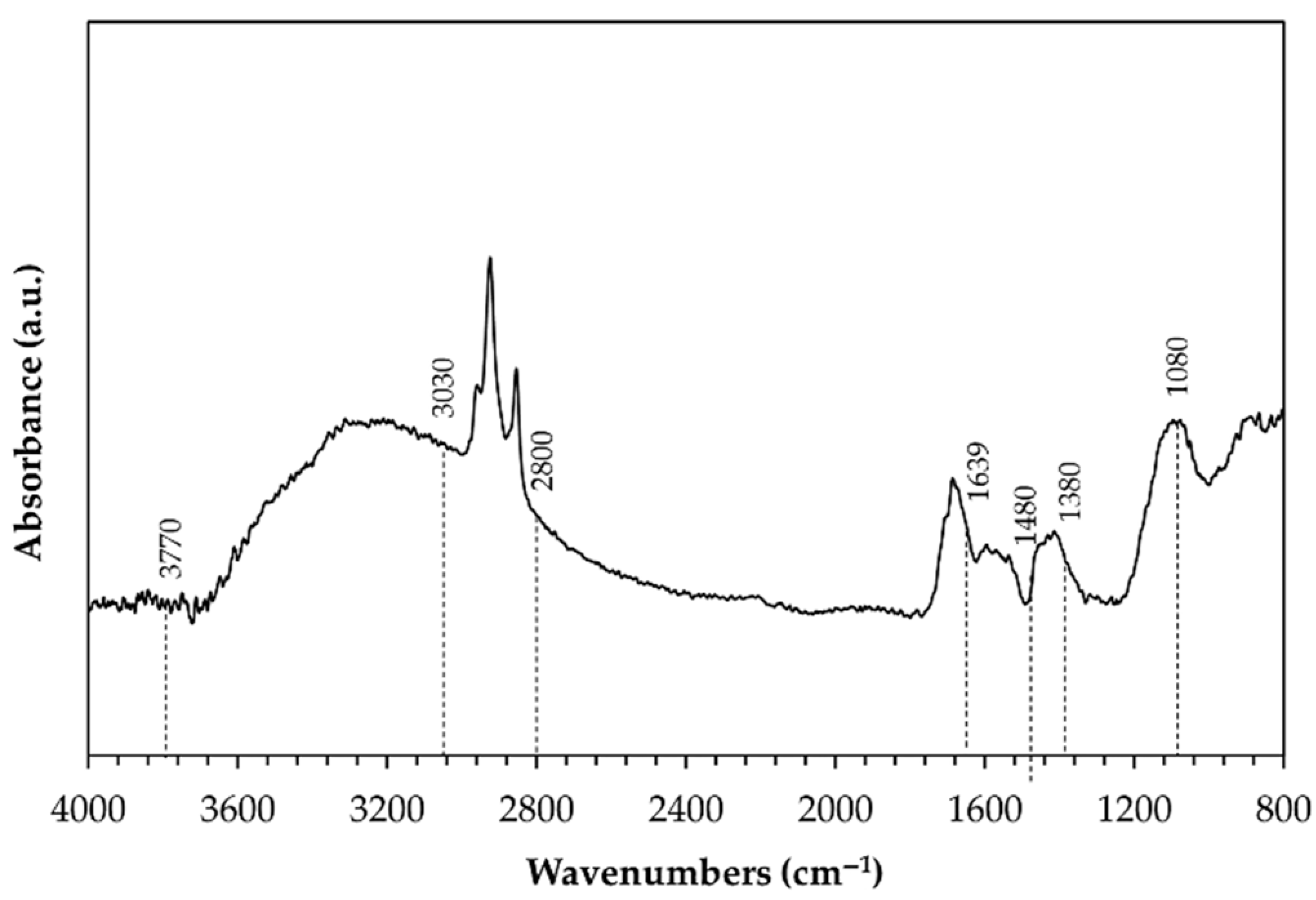
| Types of Anodizing Treatment | Designation | Thicknesses of AAO Layers (μm) | Samples |
|---|---|---|---|
| Hard anodizing treatment | HA | 25 | HA_25 |
| 50 | HA_50 | ||
| 100 | HA_100 | ||
| Golden hard anodizing treatment | G | 25 | G_25 |
| 50 | G_50 | ||
| 100 | G_100 | ||
| Golden hard anodizing treatment_plus | GP | 25 | GP_25 |
| 50 | GP_50 | ||
| 100 | GP_100 |
| 25 | 50 | 100 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ra (μm) | Rz (μm) | HV0.01 | Ra (μm) | Rz (μm) | HV0.01 | Ra (μm) | Rz (μm) | HV0.01 | |
| HA | 0.18 ± 0.02 | 2.09 ± 0.40 | 450 ± 20 | 0.22 ± 0.02 | 1.98 ± 0.33 | 458 ± 10 | 0.17 ± 0.01 | 1.81 ± 0.27 | 438 ± 11 |
| G | 0.19 ± 0.01 | 2.13 ± 0.37 | 427 ± 18 | 0.19 ± 0.05 | 2.06 ± 0.53 | 435 ± 12 | 0.17 ± 0.02 | 1.65 ± 0.13 | 398 ± 15 |
| GP | 0.23 ± 0.02 | 2.16 ± 0.35 | 374 ± 31 | 0.21 ± 0.05 | 2.14 ± 0.53 | 412 ± 17 | 0.18 ± 0.05 | 2.09 ± 0.48 | 356 ± 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soffritti, C.; Fortini, A.; Nastruzzi, A.; Sola, R.; Merlin, M.; Garagnani, G.L. Dry Sliding Behavior of an Aluminum Alloy after Innovative Hard Anodizing Treatments. Materials 2021, 14, 3281. https://doi.org/10.3390/ma14123281
Soffritti C, Fortini A, Nastruzzi A, Sola R, Merlin M, Garagnani GL. Dry Sliding Behavior of an Aluminum Alloy after Innovative Hard Anodizing Treatments. Materials. 2021; 14(12):3281. https://doi.org/10.3390/ma14123281
Chicago/Turabian StyleSoffritti, Chiara, Annalisa Fortini, Anna Nastruzzi, Ramona Sola, Mattia Merlin, and Gian Luca Garagnani. 2021. "Dry Sliding Behavior of an Aluminum Alloy after Innovative Hard Anodizing Treatments" Materials 14, no. 12: 3281. https://doi.org/10.3390/ma14123281
APA StyleSoffritti, C., Fortini, A., Nastruzzi, A., Sola, R., Merlin, M., & Garagnani, G. L. (2021). Dry Sliding Behavior of an Aluminum Alloy after Innovative Hard Anodizing Treatments. Materials, 14(12), 3281. https://doi.org/10.3390/ma14123281










