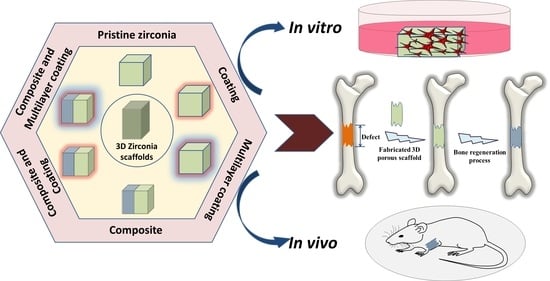
Three-Dimensional Zirconia-Based Scaffolds for Load-Bearing Bone-Regeneration Applications: Prospects and Challenges
Abstract
1. Introduction
1.1. General Overview of Zirconia Bioceramics
1.2. Inevitability of Widespread Use of Zirconia Bioceramics in Biomedical Applications
1.3. Commencement of Zirconia over Calcium-Phosphate Scaffolds in Bone-Regeneration Applications
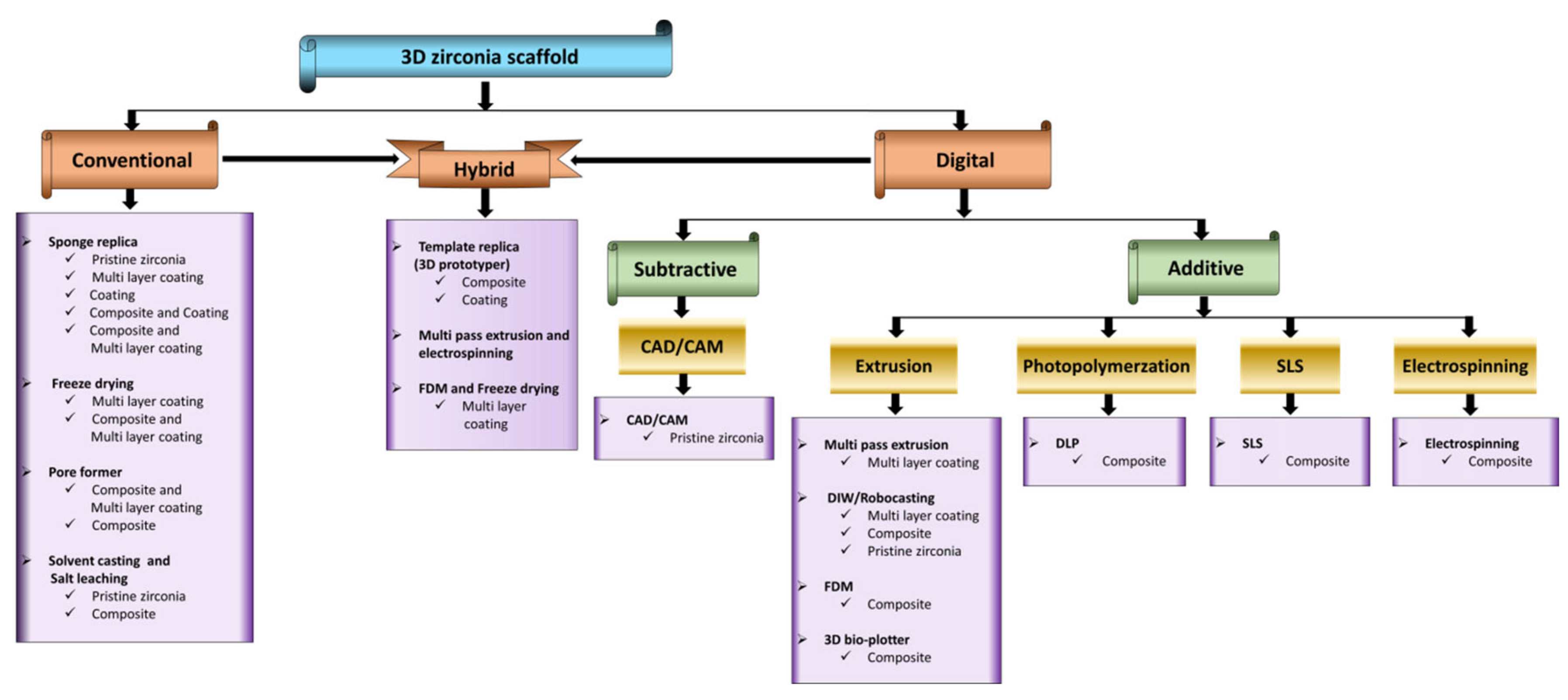
2. 3D Zirconia-Based Scaffolds via Conventional Technique
2.1. Sponge Replica Technique
2.2. Freeze-Drying Technique
2.3. Pore Former/Space Holder Technique
2.4. Solvent Casting and Salt Leaching
3. Fabrication of 3D Zirconia-Based Scaffolds via the Digital Technique
3.1. Computer-Aided Design/Computer-Aided Milling (CAD/CAM) Technique
3.2. Extrusion-Based Techniques
3.2.1. Multi-Pass Extrusion Technique
3.2.2. 3D-Bioplotter Technique
3.2.3. Fused Deposition Modelling (FDM) Technique
3.2.4. Robocasting/Direct Ink Writing (DIW) Technique
3.3. Photopolymerisation-Based Techniques
3.3.1. Digital Light Processing (DLP)
3.3.2. Selective Laser Sintering (SLS) Technique
3.4. Electrospinning
4. Hybrid Techniques
5. Prospects and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Saridag, S.; Tak, O.; Alniacik, G. Basic properties and types of zirconia: An overview. World J. Stomatol. 2013, 2, 40. [Google Scholar] [CrossRef]
- Abd El-Ghany, O.S.; Sherief, A.H. Zirconia based ceramics, some clinical and biological aspects: Review. Future Dent. J. 2016, 2, 55–64. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Christel, P.; Meunier, A.; Heller, M.; Torre, J.P.; Peille, C.N. Mechanical properties and short-term in vivo evaluation of yttrium-oxide-partially-stabilized zirconia. J. Biomed. Mater. Res. 1989, 23, 45–61. [Google Scholar] [CrossRef]
- Heuer, A.H.; Lange, F.F.; Swain, M.V.; Evans, A.G. Transformation Toughening: An Overview. J. Am. Ceram. Soc. 1986, 69, i–iv. [Google Scholar] [CrossRef]
- Zarone, F.; Russo, S.; Sorrentino, R. From porcelain-fused-to-metal to zirconia: Clinical and experimental considerations. Dent. Mater. 2011, 27, 83–96. [Google Scholar] [CrossRef]
- Madfa, A.A.; Al-Sanabani, F.A.; Al-Qudami, N.H.; Al-Sanabani, J.S.; Amran, A.G. Use of Zirconia in Dentistry: An Overview. Open Biomater. J. 2014, 1–9. [Google Scholar] [CrossRef]
- Cranin, A.N.; Schnitman, P.A.; Rabkin, M.; Dennison, T.; Onesto, E.J. Alumina and zirconia coated vitallium oral endosteal implants in beagles. J. Biomed. Mater. Res. 1975, 9, 257–262. [Google Scholar] [CrossRef]
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, S.D. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implant. Res. 2012, 23, 2–21. [Google Scholar] [CrossRef]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implant. Res. 2008, 19, 119–130. [Google Scholar] [CrossRef]
- Luthardt, R.G.; Sandkuhl, O.; Reitz, B. Zirconia-TZP and alumina—Advanced technologies for the manufacturing of single crowns. Eur. J. Prosthodont. Restor. Dent. 1999, 7, 113–119. [Google Scholar]
- Tinschert, J.; Natt, G.; Mautsch, W.; Augthun, M.; Spiekermann, H. Fracture resistance of lithium disilicate-, alumina-, and zirconia-based three-unit fixed partial dentures: A laboratory study. Int. J. Prosthodont. 2001, 14, 231–238. [Google Scholar]
- Meyenberg, K.H.; Lüthy, H.; Schärer, P. Zirconia posts: A new all-ceramic concept for nonvital abutment teeth. J. Esthet. Dent. 1995, 7, 73–80. [Google Scholar] [CrossRef]
- Kakehashi, Y.; Lüthy, H.; Naef, R.; Wohlwend, A.; Schärer, P. A new all-ceramic post and core system: Clinical, technical, and in vitro results. Int. J. Periodontics Restor. Dent. 1998, 18, 586–593. [Google Scholar]
- Christel, P.; Meunier, A.; Dorlot, J.M.; Crolet, J.M.; Witvoet, J.; Sedel, L.; Boutin, P. Biomechanical compatibility and design of ceramic implants for orthopedic surgery. Ann. N. Y. Acad. Sci. 1988, 523, 234–256. [Google Scholar] [CrossRef]
- Soon, G.; Pingguan-Murphy, B.; Lai, K.W.; Akbar, S.A. Review of zirconia-based bioceramic: Surface modification and cellular response. Ceram. Int. 2016, 42, 12543–12555. [Google Scholar] [CrossRef]
- Yin, L.; Nakanishi, Y.; Alao, A.-R.; Song, X.-F.; Abduo, J.; Zhang, Y. A Review of Engineered Zirconia Surfaces in Biomedical Applications. Procedia CIRP 2017, 65, 284–290. [Google Scholar] [CrossRef]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol. 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Al-Amleh, B.; Lyons, K.; Swain, M. Clinical trials in zirconia: A systematic review. J. Oral Rehabil. 2010, 37, 641–652. [Google Scholar] [CrossRef]
- An, S.-H.; Matsumoto, T.; Miyajima, H.; Nakahira, A.; Kim, K.-H.; Imazato, S. Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent. Mater. 2012, 28, 1221–1231. [Google Scholar] [CrossRef]
- Parfenov, V.A.; Mironov, V.A.; Koudan, E.V.; Nezhurina, E.K.; Karalkin, P.A.; Pereira, F.D.A.S.; Petrov, S.V.; Krokhmal, A.A.; Aydemir, T.; Vakhrushev, I.V.; et al. Fabrication of calcium phosphate 3D scaffolds for bone repair using magnetic levitational assembly. Sci. Rep. 2020, 10, 4013. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Yoon, Y.-J. A review on 3D printed bioimplants. Int. J. Precis. Eng. Manuf. 2015, 16, 1035–1046. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Li, B. Direct write printing of three-dimensional ZrO2 biological scaffolds. Mater. Des. 2015, 72, 16–20. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef]
- Kondo, N.; Ogose, A.; Tokunaga, K.; Ito, T.; Arai, K.; Kudo, N.; Inoue, H.; Irie, H.; Endo, N. Bone formation and resorption of highly purified beta-tricalcium phosphate in the rat femoral condyle. Biomaterials 2005, 26, 5600–5608. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Liu, S.; Dong, X.; Li, Y.; Zhang, H.; Yang, Z.; Su, Q.; Huang, W.; Zheng, W.; et al. Zirconia toughened hydroxyapatite biocomposite formed by a DLP 3D printing process for potential bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110054. [Google Scholar] [CrossRef]
- Kim, I.-J.; Shin, S.-Y. Comparative study of new bone formation capability of zirconia bone graft material in rabbit calvarial. J. Adv. Prosthodont. 2018, 10, 167–176. [Google Scholar] [CrossRef]
- Shang, L.; Yu, Y.; Liu, Y.; Chen, Z.; Kong, T.; Zhao, Y. Spinning and Applications of Bioinspired Fiber Systems. ACS Nano 2019, 13, 2749–2772. [Google Scholar] [CrossRef]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef]
- Service, R.F. Tissue engineers build new bone. Science 2000, 289, 1498–1500. [Google Scholar] [CrossRef]
- Jakus, A.E.; Rutz, A.L.; Jordan, S.W.; Kannan, A.; Mitchell, S.M.; Yun, C.; Koube, K.D.; Yoo, S.C.; Whiteley, H.E.; Richter, C.-P.; et al. Hyperelastic “bone”: A highly versatile, growth factor–free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci. Transl. Med. 2016, 8, 358ra127. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, R.; Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng. 2017, 45, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; He, R.; Xie, C.; Wang, G.; Ding, G.; Wang, M.; Song, W.; Fang, D. Photosensitive ZrO2 suspensions for stereolithography. Ceram. Int. 2019, 45, 12189–12195. [Google Scholar] [CrossRef]
- Muhamad Nor, M.A.A.; Hong, L.C.; Arifin Ahmad, Z.; Md Akil, H. Preparation and characterization of ceramic foam produced via polymeric foam replication method. J. Mater. Process. Technol. 2008, 207, 235–239. [Google Scholar] [CrossRef]
- Jayakumar, R.; Ramachandran, R.; Sudheesh Kumar, P.T.; Divyarani, V.V.; Srinivasan, S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V. Fabrication of chitin–chitosan/nano ZrO2 composite scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2011, 49, 274–280. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, H.; Lee, J. Various preparation methods of highly porous hydroxyapatite/polymer nanoscale biocomposites for bone regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef]
- Sulaiman, S.B.; Keong, T.K.; Cheng, C.H.; Saim, A.B.; Idrus, R.B.H. Tricalcium phosphate/hydroxyapatite (TCP-HA) bone scaffold as potential candidate for the formation of tissue engineered bone. Indian J. Med. Res. 2013, 137, 1093–1101. [Google Scholar]
- Deschamps, I.S.; Magrin, G.L.; Magini, R.S.; Fredel, M.C.; Benfatti, C.A.M.; Souza, J.C.M. On the synthesis and characterization of β-tricalcium phosphate scaffolds coated with collagen or poly (D, L-lactic acid) for alveolar bone augmentation. Eur. J. Dent. 2017, 11, 496–502. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, K.-L.; Chun, H.-H.; Kim, T.-W.; Park, H.-C.; Yoon, S.-Y. In vitro biodegradable and mechanical performance of biphasic calcium phosphate porous scaffolds with unidirectional macro-pore structure. Ceram. Int. 2014, 40, 8293–8300. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Oudadesse, H.; Mohamed, M.B.; Foad, E.S.; Le Gal, Y.; Cathelineau, G. Convenient approach of nanohydroxyapatite polymeric matrix composites. Chem. Eng. J. 2009, 153, 187–192. [Google Scholar] [CrossRef]
- Kim, H.-W.; Lee, S.-Y.; Bae, C.-J.; Noh, Y.-J.; Kim, H.-E.; Kim, H.-M.; Ko, J.S. Porous ZrO2 bone scaffold coated with hydroxyapatite with fluorapatite intermediate layer. Biomaterials 2003, 24, 3277–3284. [Google Scholar] [CrossRef]
- Kim, H.-W.; Kim, H.-E.; Salih, V.; Knowles, J.C. Dissolution control and cellular responses of calcium phosphate coatings on zirconia porous scaffold. J. Biomed. Mater. Res. A 2004, 68, 522–530. [Google Scholar] [CrossRef]
- Kim, H.-W.; Kim, H.-E.; Knowles, J.C. Hard-tissue-engineered zirconia porous scaffolds with hydroxyapatite sol-gel and slurry coatings. J. Biomed. Mater. Res. B. Appl. Biomater. 2004, 70, 270–277. [Google Scholar] [CrossRef]
- Kim, H.-W.; Shin, S.-Y.; Kim, H.-E.; Lee, Y.-M.; Chung, C.-P.; Lee, H.-H.; Rhyu, I.-C. Bone formation on the apatite-coated zirconia porous scaffolds within a rabbit calvarial defect. J. Biomater. Appl. 2008, 22, 485–504. [Google Scholar] [CrossRef]
- Latifi, M.; Talaei-Khozani, T.; Mehraban-Jahromi, H.; Sani, M.; Sadeghi-Atabadi, M.; Fazel-Anvari, A.; Kabir-Salmani, M. Fabrication of platelet-rich plasma heparin sulfate/hydroxyapatite/zirconia scaffold. Bioinspired Biomim. Nanobiomater. 2018, 7, 122–130. [Google Scholar] [CrossRef]
- Shahsavari-Pour, S.; Aliabadi, E.; Latifi, M.; Zareifard, N.; Namavar, M.R.; Talaei-Khozani, T. Evaluation of the Possible Synergic Regenerative Effects of Platelet-Rich Plasma and Hydroxyapatite/Zirconia in the Rabbit Mandible Defect Model. Iran. J. Med. Sci. 2018, 43, 633–644. [Google Scholar]
- Alizadeh, A.; Moztarzadeh, F.; Ostad, S.N.; Azami, M.; Geramizadeh, B.; Hatam, G.; Bizari, D.; Tavangar, S.M.; Vasei, M.; Ai, J. Synthesis of calcium phosphate-zirconia scaffold and human endometrial adult stem cells for bone tissue engineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 66–73. [Google Scholar] [CrossRef]
- Mohammad, M.; Taha, A.; Shahatha, S. Preparation of hydroxyapatite/zirconia porous composites via polymeric sponge method and study the physical and bioactivity properties. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Istanbul, Turkey, 20–25 September 2019; IOP Publishing: Bristol, UK, 2020; Volume 757, p. 012046. [Google Scholar] [CrossRef]
- Kim, M.; Franco, R.A.; Lee, B.-T. Synthesis of functional gradient BCP/ZrO2 bone substitutes using ZrO2 and BCP nanopowders. J. Eur. Ceram. Soc. 2011, 31, 1541–1548. [Google Scholar] [CrossRef]
- Song, Y.-G.; Cho, I.-H. Characteristics and osteogenic effect of zirconia porous scaffold coated with β-TCP/HA. J. Adv. Prosthodont. 2014, 6, 285–294. [Google Scholar] [CrossRef][Green Version]
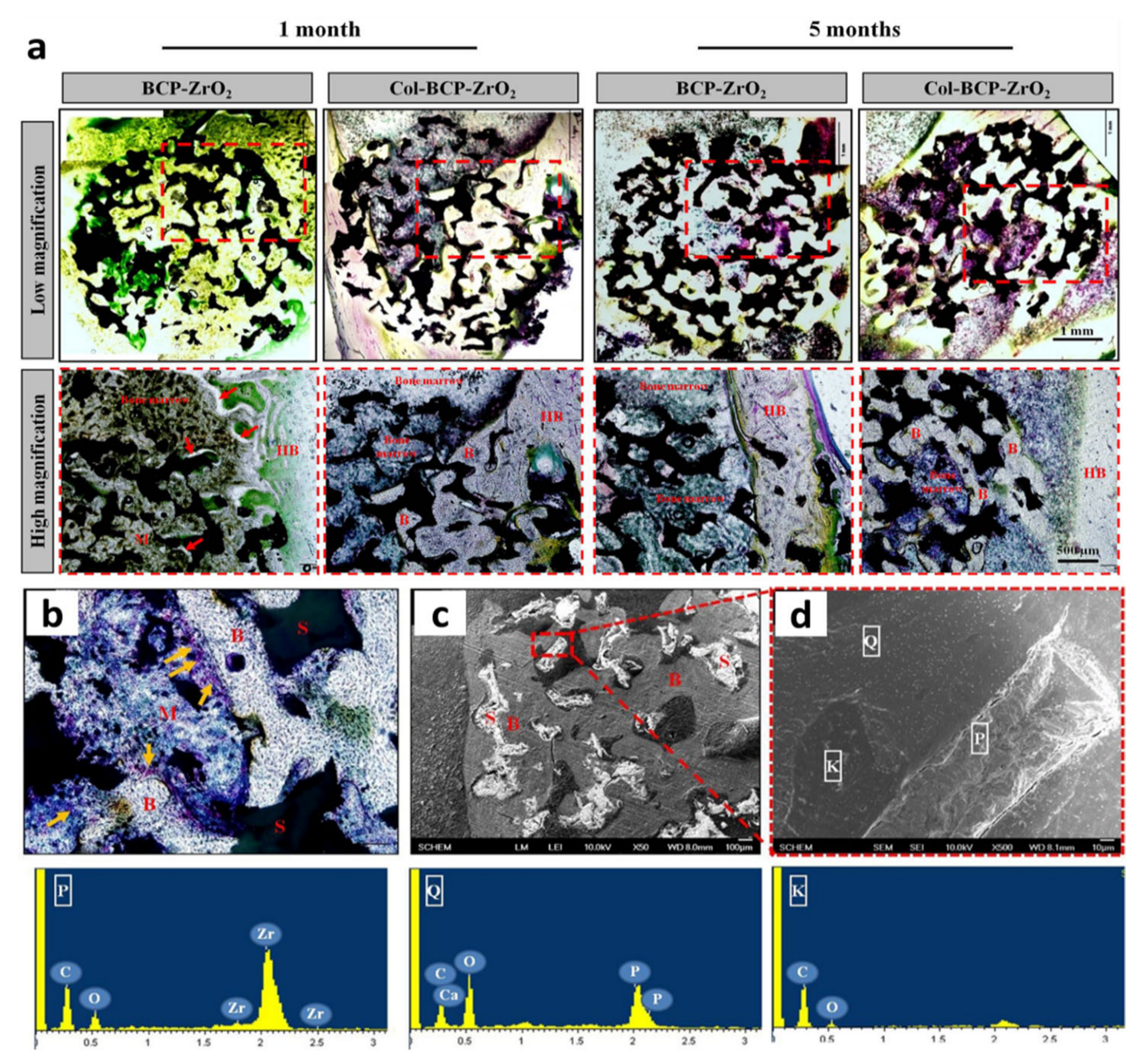
- Linh, N.T.B.; Jang, D.-W.; Lee, B.-T. Collagen immobilization of multi-layered BCP-ZrO2 bone substitutes to enhance bone formation. Appl. Surf. Sci. 2015, 345, 238–248. [Google Scholar] [CrossRef]
- Jones, A.C.; Arns, C.H.; Sheppard, A.P.; Hutmacher, D.W.; Milthorpe, B.K.; Knackstedt, M.A. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials 2007, 28, 2491–2504. [Google Scholar] [CrossRef]
- Mačković, M.; Hoppe, A.; Detsch, R.; Mohn, D.; Stark, W.J.; Spiecker, E.; Boccaccini, A.R. Bioactive glass (type 45S5) nanoparticles: In vitro reactivity on nanoscale and biocompatibility. J. Nanopart. Res. 2012, 14, 966. [Google Scholar] [CrossRef]
- Mesquita-Guimarães, J.; Ramos, L.; Detsch, R.; Henriques, B.; Fredel, M.C.; Silva, F.S.; Boccaccini, A.R. Evaluation of in vitro properties of 3D micro-macro porous zirconia scaffolds coated with 58S bioactive glass using MG-63 osteoblast-like cells. J. Eur. Ceram. Soc. 2019, 39, 2545–2558. [Google Scholar] [CrossRef]
- Gouveia, P.F.; Mesquita-Guimarães, J.; Galárraga-Vinueza, M.E.; Souza, J.C.M.; Silva, F.S.; Fredel, M.C.; Boccaccini, A.R.; Detsch, R.; Henriques, B. In-vitro mechanical and biological evaluation of novel zirconia reinforced bioglass scaffolds for bone repair. J. Mech. Behav. Biomed. Mater. 2021, 114, 104164. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Ma, J.; Weng, Z.; Wang, Y.; Shi, X.; Li, Y.; Yan, X.; Dong, Z.; Xu, J.; et al. In vitro cell proliferation evaluation of porous nano-zirconia scaffolds with different porosity for bone tissue engineering. Biomed. Mater. 2015, 10, 55009. [Google Scholar] [CrossRef]
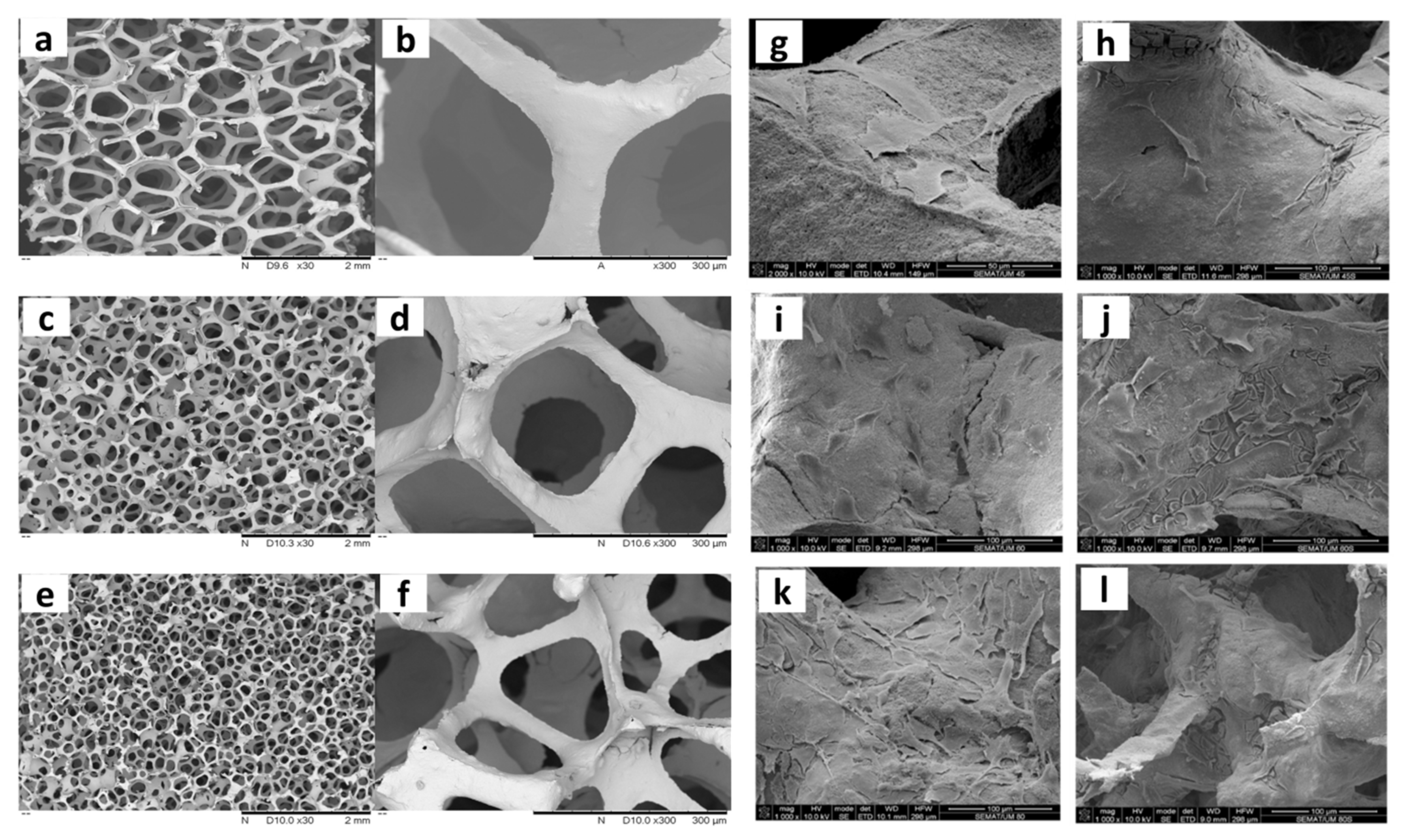
- Askari, E.; Cengiz, I.F.; Alves, J.L.; Henriques, B.; Flores, P.; Fredel, M.C.; Reis, R.L.; Oliveira, J.M.; Silva, F.S.; Mesquita-Guimarães, J. Micro-CT based finite element modelling and experimental characterization of the compressive mechanical properties of 3-D zirconia scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 102, 103516. [Google Scholar] [CrossRef]
- Jiang, B.; Hu, X.; Huang, Z. Porous bio-ceramic coating on zirconia formed through freeze-drying. Mater. Lett. 2013, 109, 66–69. [Google Scholar] [CrossRef]
- Teimouri, A.; Ghorbanian, L.; Salavati, H.; Chermahini, A.N. Fabrication and characterization of POM/ZrO2/silk fibroin composite scaffolds. Mater. Lett. 2015, 157, 85–88. [Google Scholar] [CrossRef]
- Teimouri, A.; Ebrahimi, R.; Emadi, R.; Beni, B.H.; Chermahini, A.N. Nano-composite of silk fibroin–chitosan/Nano ZrO2 for tissue engineering applications: Fabrication and morphology. Int. J. Biol. Macromol. 2015, 76, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, B.; Jayasuriya, A.C. Comparative investigation of porous nano-hydroxyapaptite/chitosan, nano-zirconia/chitosan and novel nano-calcium zirconate/chitosan composite scaffolds for their potential applications in bone regeneration. Mater. Sci. Eng. C 2018, 91, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, Z.; Miao, X. Porous Alumina/Zirconia Composite Scaffold with Bioactive Glass 58S33C Coating. J. Biomim. Biomater. Tissue Eng. 2010, 6, 87–104. [Google Scholar] [CrossRef]
- Hadjicharalambous, C.; Mygdali, E.; Prymak, O.; Buyakov, A.; Kulkov, S.; Chatzinikolaidou, M. Proliferation and osteogenic response of MC3T3-E1 pre-osteoblastic cells on porous zirconia ceramics stabilized with magnesia or yttria. J. Biomed. Mater. Res. A 2015, 103, 3612–3624. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs 2020, 43, 69–86. [Google Scholar] [CrossRef]
- Ziaee, F.; Zebarjad, S.M.; Javadpour, S. Compressive and flexural properties of novel polylactic acid/hydroxyapatite/yttria-stabilized zirconia hybrid nanocomposite scaffold. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 229–238. [Google Scholar] [CrossRef]
- Mokhtar, K.E.; Elmahallawy, A.S.; Elashwah, A.A. Evaluation of the Use of Zirconia Porous Scaffold in the Repair of Oroantral Fistula. Alex. Dent. J. 2018, 43, 87–93. [Google Scholar] [CrossRef]
- Aftan, K.T. 3D Reconstruction of Mandibular Defects using CAD/CAM Designed Zirconia Prosthesis. Al Anbar Med. J. 2019, 15, 24–29. [Google Scholar] [CrossRef]
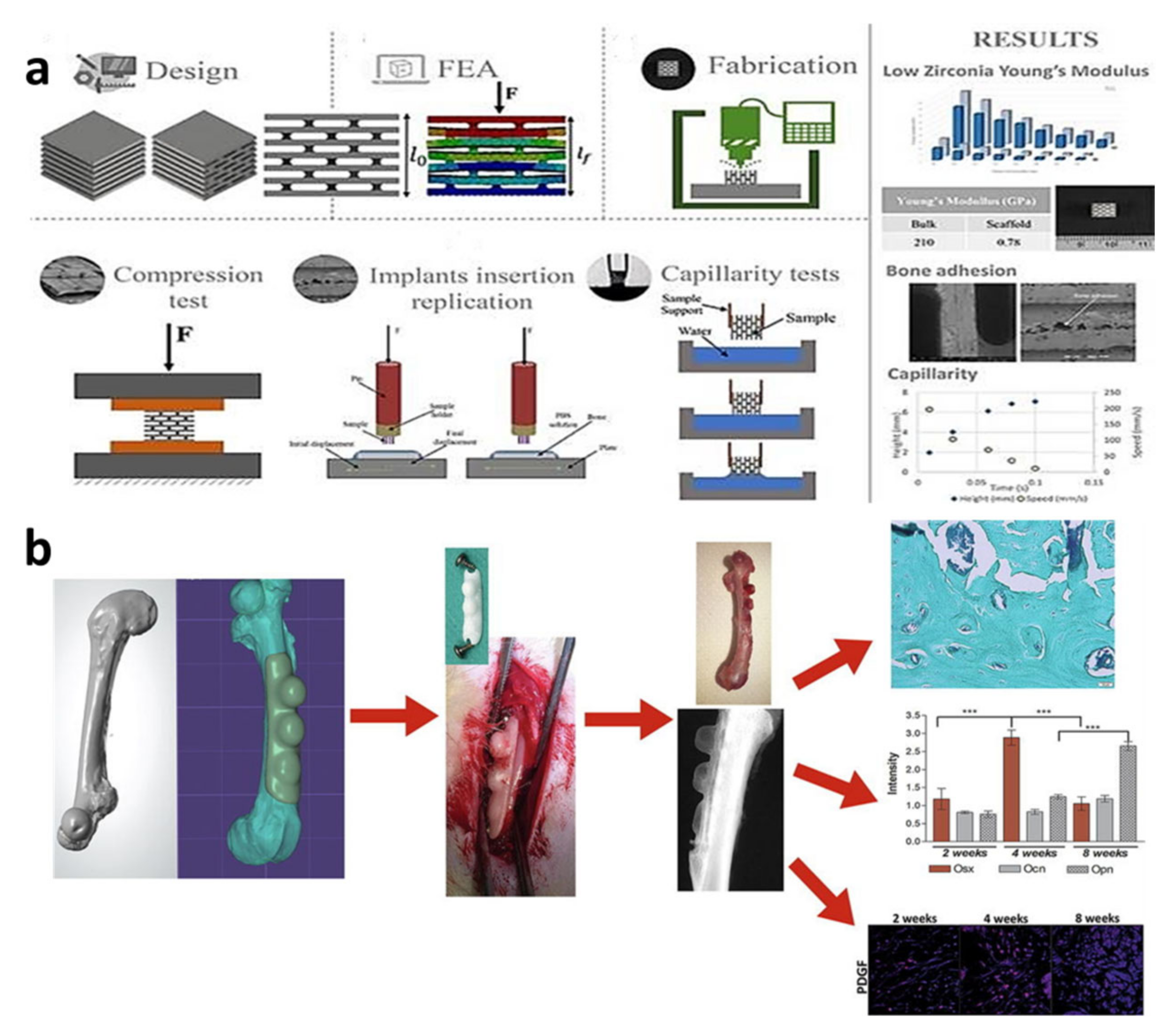
- Marques, A.; Miranda, G.; Faria, D.; Pinto, P.; Silva, F.; Carvalho, Ó. Novel design of low modulus high strength zirconia scaffolds for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 97, 375–384. [Google Scholar] [CrossRef]
- Petre, A.; Balta, C.; Herman, H.; Gharbia, S.; Codreanu, A.; Onita-Mladin, B.; Anghel-Zurbau, N.; Hermenean, A.-G.; Ignat, S.-R.; Dinescu, S.; et al. A novel experimental approach to evaluate guided bone regeneration (GBR) in the rat femur using a 3D-printed CAD/CAM zirconia space-maintaining barrier. J. Adv. Res. 2021, 28, 221–229. [Google Scholar] [CrossRef]
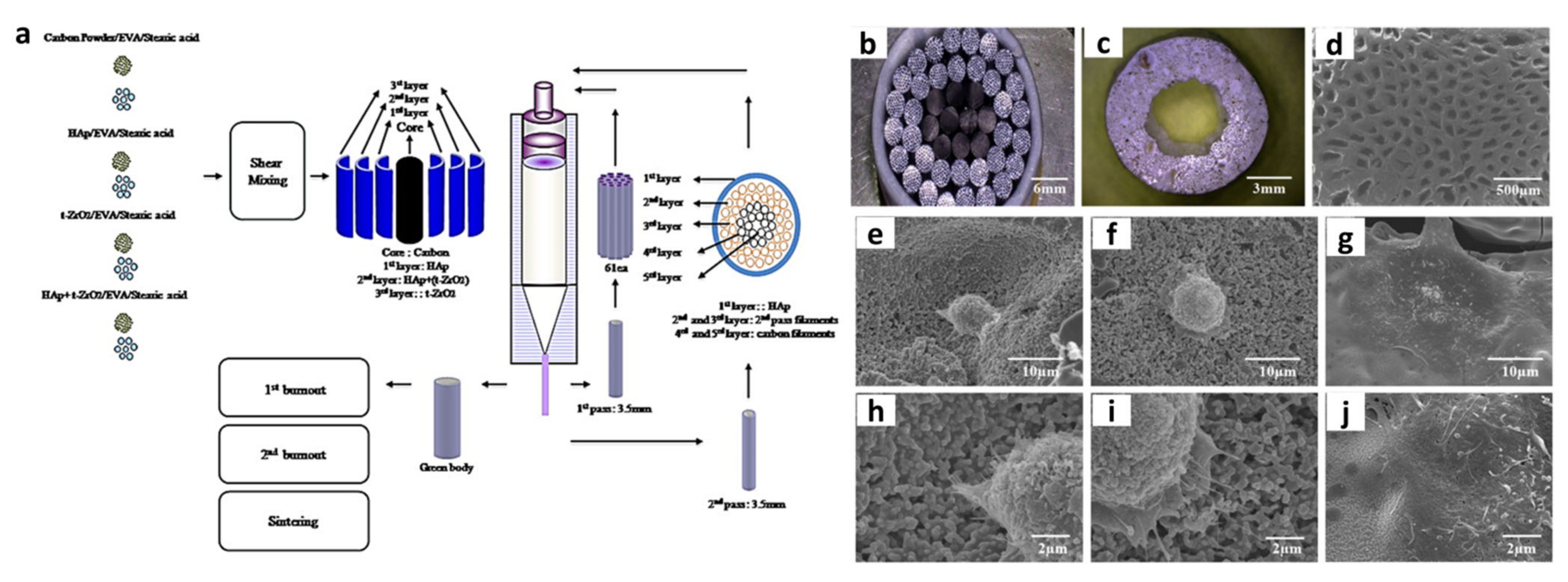
- Kumar Gain, A.; Lee, B.-T. Fabrication of HAp-Coated Micro-Channelled t-ZrO2 Bodies by the Multi-Pass Extrusion Process. J. Am. Ceram. Soc. 2006, 89, 2051–2056. [Google Scholar] [CrossRef]
- Lee, B.-T.; Kim, K.-H.; Han, J.-K. Microstructures and material properties of fibrous Al2O3–(m-ZrO2)/t-ZrO2 composites fabricated by a fibrous monolithic process. J. Mater. Res. 2004, 19, 3234–3241. [Google Scholar] [CrossRef]
- Lee, B.-T.; Kang, I.C.; Gain, A.K.; Kim, K.-H.; Song, H.-Y. Fabrication of pore-gradient Al2O3–ZrO2 sintered bodies by fibrous monolithic process. J. Eur. Ceram. Soc. 2006, 26, 3525–3530. [Google Scholar] [CrossRef]
- Gain, A.K.; Lee, B.-T. Microstructure control of continuously porous t-ZrO2 bodies fabricated by multi-pass extrusion process. Mater. Sci. Eng. A 2006, 419, 269–275. [Google Scholar] [CrossRef]
- Lee, B.-T.; Kang, I.-C.; Cho, S.-H.; Song, H.-Y. Fabrication of a Continuously Oriented Porous Al2O3 Body and Its In Vitro Study. J. Am. Ceram. Soc. 2005, 88, 2262–2266. [Google Scholar] [CrossRef]
- Jang, D.-W.; Kim, Y.-H.; Lee, B.-T. Microstructure control of TCP/TCP-(t-ZrO2)/t-ZrO2 composites for artificial cortical bone. Mater. Sci. Eng. C 2011, 31, 1660–1666. [Google Scholar] [CrossRef]
- Jang, D.-W.; Nguyen, T.-H.; Sarkar, S.K.; Lee, B.-T. Microwave sintering and in vitro study of defect-free stable porous multilayered HAp–ZrO2 artificial bone scaffold. Sci. Technol. Adv. Mater. 2012, 13, 35009. [Google Scholar] [CrossRef]
- Mondal, D.; So-Ra, S.; Sarkar, S.K.; Min, Y.K.; Yang, H.M.; Lee, B.T. Fabrication of multilayer ZrO2–biphasic calcium phosphate–poly-caprolactone unidirectional channeled scaffold for bone tissue formation. J. Biomater. Appl. 2012, 28, 462–472. [Google Scholar] [CrossRef]
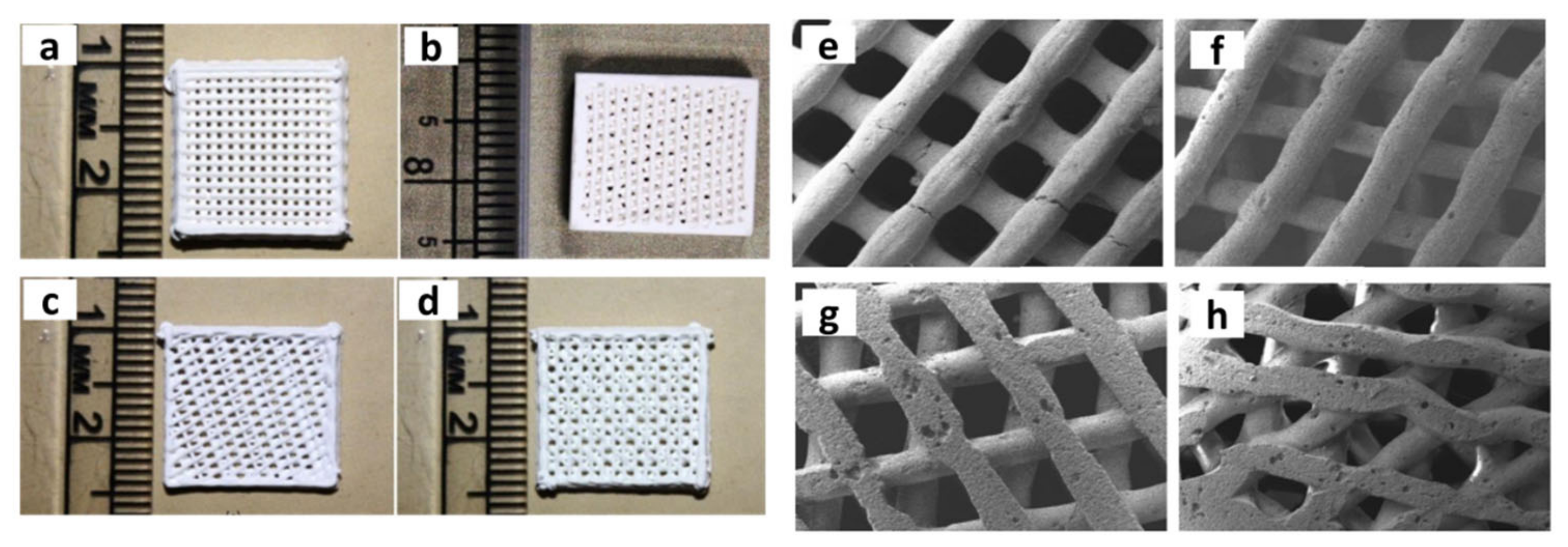
- Sapkal, P.S.; Kuthe, A.M.; Mathankar, S.; Deshmukh, A.A. 3D Bio-Plotted Tricalcium Phosphate/Zirconia Composite Scaffolds to Heal Large Size Bone Defects. Mol. Cell. Biomech. 2017, 14, 125–136. [Google Scholar] [CrossRef]
- Fu, S.Y.; Yu, B.; Ding, H.F.; Shi, G.D.; Zhu, Y.F. Zirconia Incorporation in 3D Printed β-Ca2SiO4 Scaffolds on Their Physicochemical and Biological Property. J. Inorg. Mater. 2019, 34, 444–454. [Google Scholar] [CrossRef]
- Sa, M.-W.; Nguyen, B.-N.B.; Moriarty, R.A.; Kamalitdinov, T.; Fisher, J.P.; Kim, J.Y. Fabrication and evaluation of 3D printed BCP scaffolds reinforced with ZrO2 for bone tissue applications. Biotechnol. Bioeng. 2018, 115, 989–999. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Mozetic, P.; Rainer, A.; Trombetta, M. Biological response of human mesenchymal stromal cells to titanium grade 4 implants coated with PCL/ZrO2 hybrid materials synthesized by sol–gel route: In vitro evaluation. Mater. Sci. Eng. C 2014, 45, 395–401. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Z.; Wang, Y.; Zhong, L.; Xie, W. Fabrication and characterization of 3D printed biocomposite scaffolds based on PCL and zirconia nanoparticles. Bio Des. Manuf. 2021, 4, 60–71. [Google Scholar] [CrossRef]
- Peng, E.; Wei, X.; Garbe, U.; Yu, D.; Edouard, B.; Liu, A.; Ding, J. Robocasting of dense yttria-stabilized zirconia structures. J. Mater. Sci. 2018, 53, 247–273. [Google Scholar] [CrossRef]
- Brazete, D.; Neto, A.S.; Ferreira, J.M.F. Optimization of zirconia inks to fabricate 3D porous scaffolds by robocasting. Lek. Tech. Clin. Technol. 2019, 49, 5–10. [Google Scholar] [CrossRef]
- Kocyło, E.; Franchin, G.; Colombo, P.; Chmielarz, A.; Potoczek, M. Hydroxyapatite-coated ZrO2 scaffolds with a fluorapatite intermediate layer produced by direct ink writing. J. Eur. Ceram. Soc. 2021, 41, 920–928. [Google Scholar] [CrossRef]
- Stanciuc, A.-M.; Sprecher, C.M.; Adrien, J.; Roiban, L.I.; Alini, M.; Gremillard, L.; Peroglio, M. Robocast zirconia-toughened alumina scaffolds: Processing, structural characterisation and interaction with human primary osteoblasts. J. Eur. Ceram. Soc. 2018, 38, 845–853. [Google Scholar] [CrossRef]
- Gabriel, S.; Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4575330A, 8 August 1984. [Google Scholar]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, H.; Wu, J.-M.; Chen, S.; Cheng, L.-J.; Shi, Y.-S.; Mo, Y.-C.; Li, C.-H.; Xiao, J. Preparation and biological evaluation of ZrO2 all-ceramic teeth by DLP technology. Ceram. Int. 2020, 46, 11268–11274. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, T.; Jiao, C.; Liang, H.; Chen, R.; Tian, Z.; Zou, A.; Yang, Y.; Wei, Z.; Wang, C.; et al. Fabrication and properties of zirconia/hydroxyapatite composite scaffold based on digital light processing. Ceram. Int. 2020, 46, 2300–2308. [Google Scholar] [CrossRef]
- Savalani, M.M.; Hao, L.; Zhang, Y.; Tanner, K.E.; Harris, R.A. Fabrication of porous bioactive structures using the selective laser sintering technique. Proc. Inst. Mech. Eng. Part. H J. Eng. Med. 2007, 221, 873–886. [Google Scholar] [CrossRef]
- Shuai, C.; Feng, P.; Yang, B.; Cao, Y.; Min, A.; Peng, S. Effect of Nano-Zirconia on the Mechanical and Biological Properties of Calcium Silicate Scaffolds. Int. J. Appl. Ceram. Technol. 2015, 12, 1148–1156. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, B.; Zhou, H.; Feng, C.; Wang, X.; Yuan, K.; Gan, X.; Zhu, L.; Zhang, G.; Xu, D. Mesoporous ZrO2 fibers with enhanced surface area and the application as recyclable absorbent. Appl. Surf. Sci. 2017, 399, 288–297. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef]
- Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Cadafalch Gazquez, G.; Chen, H.; Veldhuis, S.A.; Solmaz, A.; Mota, C.; Boukamp, B.A.; van Blitterswijk, C.A.; ten Elshof, J.E.; Moroni, L. Flexible Yttrium-Stabilized Zirconia Nanofibers Offer Bioactive Cues for Osteogenic Differentiation of Human Mesenchymal Stromal Cells. ACS Nano 2016, 10, 5789–5799. [Google Scholar] [CrossRef]
- Thakare, V.G.; Joshi, P.A.; Godse, R.R.; Bhatkar, V.B.; Wadegaokar, P.A.; Omanwar, S.K. Fabrication of polycaprolactone/zirconia nanofiber scaffolds using electrospinning technique. J. Polym. Res. 2017, 24, 232. [Google Scholar] [CrossRef]
- Esfahani, H.; Darvishghanbar, M.; Farshid, B. Enhanced bone regeneration of zirconia-toughened alumina nanocomposites using PA6/HA nanofiber coating via electrospinning. J. Mater. Res. 2018, 33, 4287–4295. [Google Scholar] [CrossRef]
- Ghelich, R.; Mehdinavaz Aghdam, R.; Sadat Torknik, F.; Reza Jahannama, M. Synthesis and characterization of biocompatible zirconia nanofibers based on electrospun PVP/Zr(OPr)4. Mater. Today Proc. 2018, 5, 15733–15738. [Google Scholar] [CrossRef]
- Castkova, K.; Maca, K.; Sekaninova, J.; Nemcovsky, J.; Cihlar, J. Electrospinning and thermal treatment of yttria doped zirconia fibres. Ceram. Int. 2017, 43, 7581–7587. [Google Scholar] [CrossRef]
- Rodaev, V.V.; Razlivalova, S.S.; Zhigachev, A.O.; Vasyukov, V.M.; Golovin, Y.I. Preparation of Zirconia Nanofibers by Electrospinning and Calcination with Zirconium Acetylacetonate as Precursor. Polymers 2019, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Yan, C.; Zheng, W.; Fan, W.; Adam, C.; Oloyede, A. Preparation of Mesoporous Bioglass Coated Zirconia Scaffold for Bone Tissue Engineering. Adv. Mater. Res. 2012, 365, 209–215. [Google Scholar] [CrossRef]
- Sapkal, P.S.; Kuthe, A.M.; Kashyap, R.S.; Nayak, A.R.; Kuthe, S.A.; Kawle, A.P. Indirect casting of patient-specific tricalcium phosphate zirconia scaffolds for bone tissue regeneration using rapid prototyping methodology. J. Porous Mater. 2017, 24, 1013–1023. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lee, B.-T. Novel approach to the fabrication of an artificial small bone using a combination of sponge replica and electrospinning methods. Sci. Technol. Adv. Mater. 2011, 12, 35002. [Google Scholar] [CrossRef]
- Sakthiabirami, K.; Kang, J.-H.; Jang, J.-G.; Soundharrajan, V.; Lim, H.-P.; Yun, K.-D.; Park, C.; Lee, B.-N.; Yang, Y.P.; Park, S.-W. Hybrid porous zirconia scaffolds fabricated using additive manufacturing for bone tissue engineering applications. Mater. Sci. Eng. C 2021, 123, 111950. [Google Scholar] [CrossRef]
- Weng, W.; Wu, W.; Hou, M.; Liu, T.; Wang, T.; Yang, H. Review of zirconia-based biomimetic scaffolds for bone tissue engineering. J. Mater. Sci. 2021, 56, 8309–8333. [Google Scholar] [CrossRef]
- Feng, P.; He, J.; Peng, S.; Gao, C.; Zhao, Z.; Xiong, S.; Shuai, C. Characterizations and interfacial reinforcement mechanisms of multicomponent biopolymer based scaffold. Mater. Sci. Eng. C 2019, 100, 809–825. [Google Scholar] [CrossRef]
- Sakthiabirami, K.; Vu, V.T.; Kim, J.W.; Kang, J.H.; Jang, K.J.; Oh, G.J.; Fisher, J.G.; Yun, K.D.; Lim, H.P.; Park, S.W. Tailoring interfacial interaction through glass fusion in glass/zinc-hydroxyapatite composite coatings on glass-infiltrated zirconia. Ceram. Int. 2018, 44, 16181–16190. [Google Scholar] [CrossRef]
- Asadian, M.; Chan, K.V.; Norouzi, M.; Grande, S.; Cools, P.; Morent, R.; De Geyter, N. Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds. Nanomaterials 2020, 10, 119. [Google Scholar] [CrossRef]
- Yang, J.-Z.; Sultana, R.; Hu, X.-Z.; Ichim, P. Novel Layered Hydroxyapatite/Tri-Calcium Phosphate–Zirconia Scaffold Composite with High Bending Strength for Load-Bearing Bone Implant Application. Int. J. Appl. Ceram. Technol. 2014, 11, 22–30. [Google Scholar] [CrossRef]
- Hadjicharalambous, C.; Buyakov, A.; Buyakova, S.; Kulkov, S.; Chatzinikolaidou, M. Porous alumina, zirconia and alumina/zirconia for bone repair: Fabrication, mechanical and in vitro biological response. Biomed. Mater. 2015, 23, 025012. [Google Scholar] [CrossRef]
- Malmström, J.; Slotte, C.; Adolfsson, E.; Norderyd, O.; Thomsen, P. Bone response to free form-fabricated hydroxyapatite and zirconia scaffolds: A histological study in the human maxilla. Clin. Oral Implants Res. 2009, 20, 379–385. [Google Scholar] [CrossRef] [PubMed]




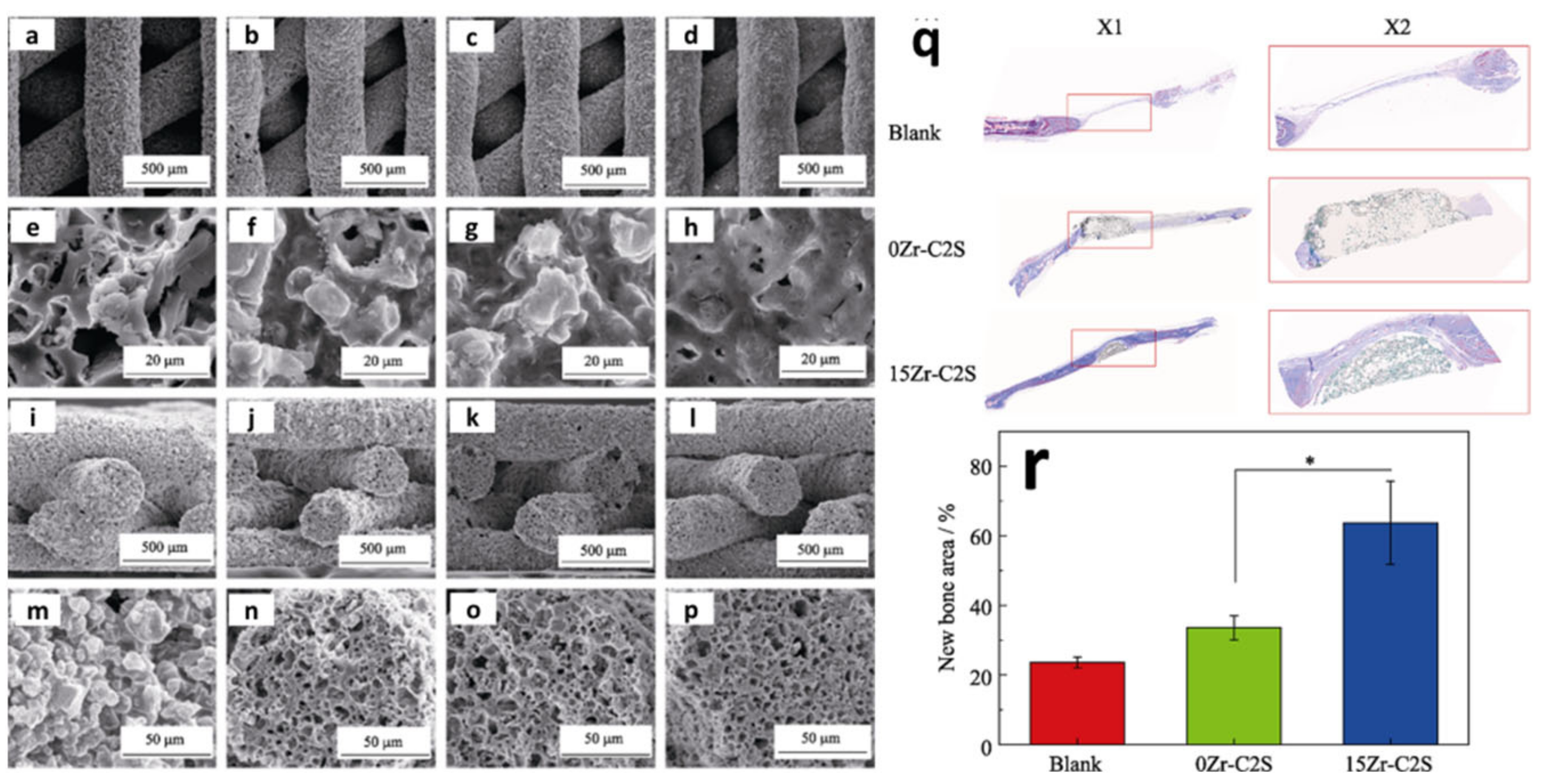




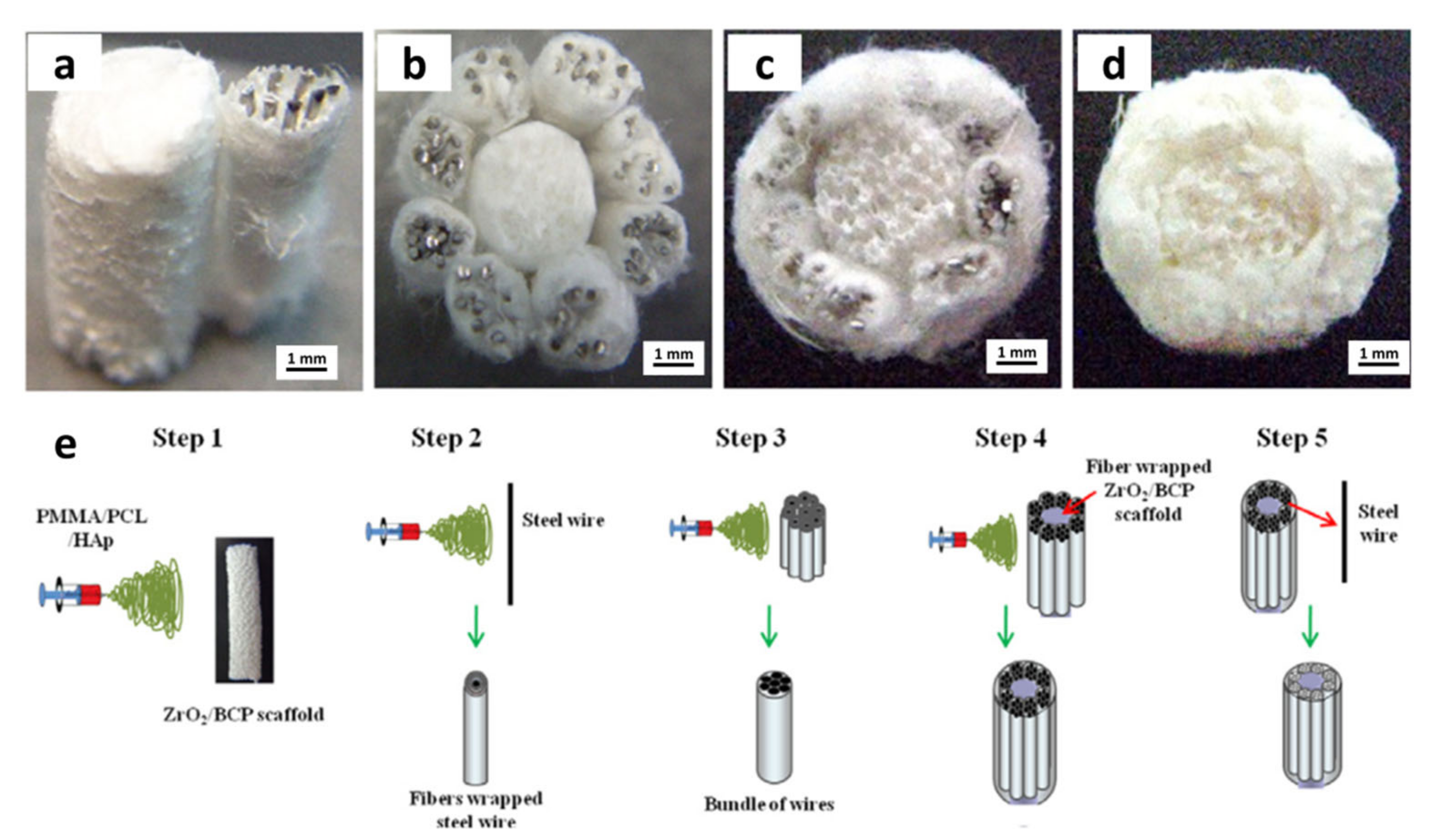
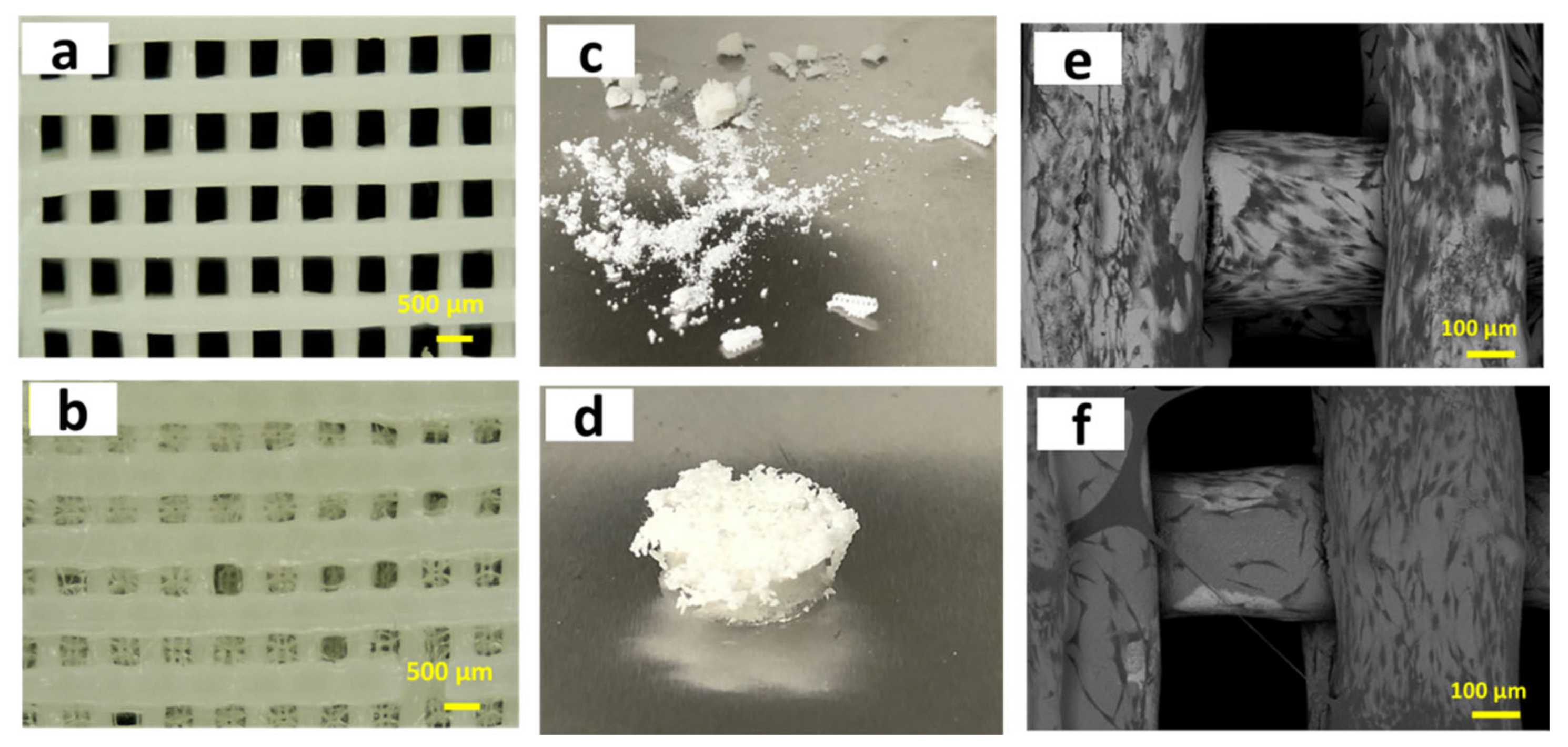
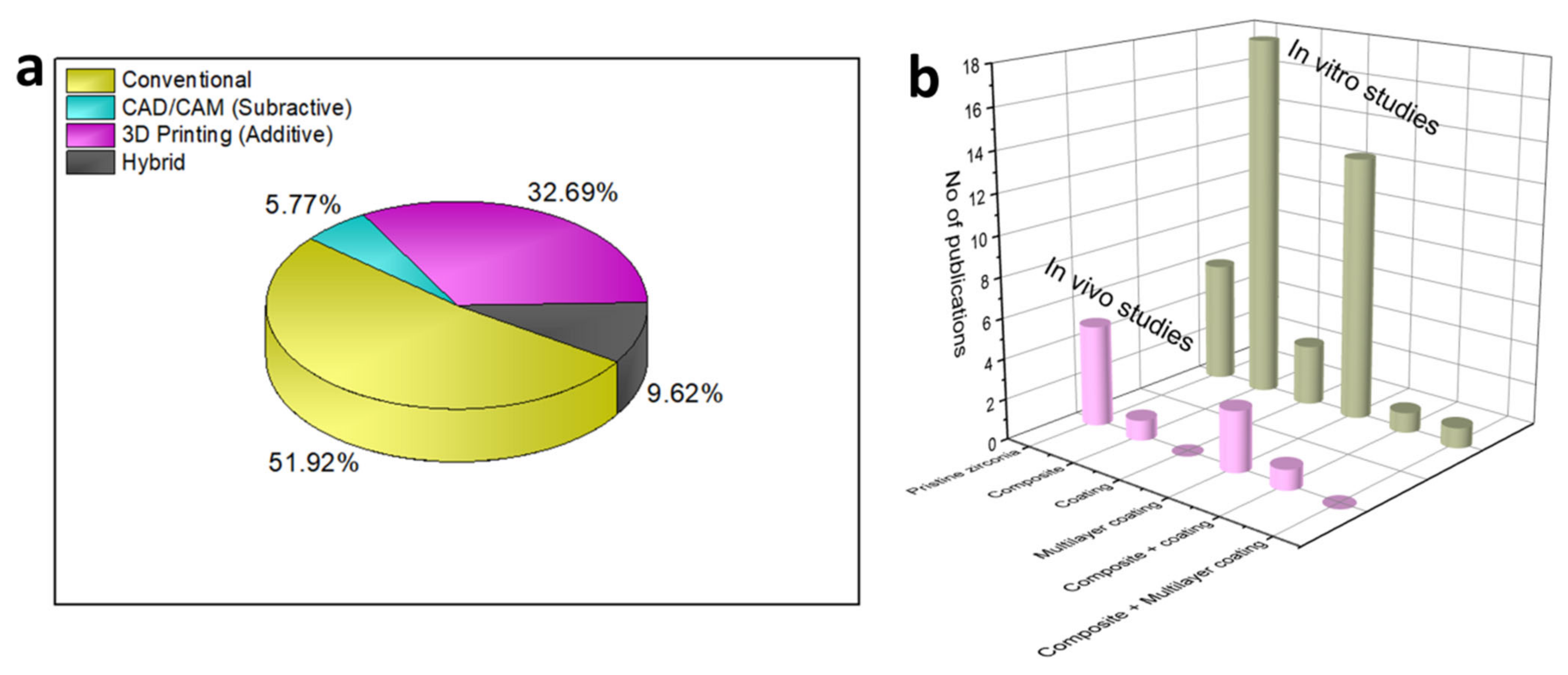
| Year | Materials | Ceramic Content | Fabrication Techniques | Composite Materials | Infiltration or Intermediate Layer | Coating Materials | Porosity (%) | Pore Size(µm) | Mechanical Properties | Biological Properties | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CS (MPa) | Modulus | Others | In Vitro | In Vivo (weeks) | ||||||||||
| Conventional Technique | ||||||||||||||
| 2003 | YSZ-FA-HA | – | Sponge replica | – | FA ( ̴ 5µm) | HA ( ̴ 20µm) | 74–92 | ̴ 600 | 1.6–35 | - | Adhesion strength ̴22MPa | HOS cells | – | [42] |
| 2004 | YSZ-Ca P | – | Sponge replica | – | FA | Ca P (HA,FA,TCP,HA+FA,HA+TCP) | ̴ 90 | 500–700 | – | – | – | SBF, MG-63 and HOS cells | – | [43] |
| 2004 | YSZ (5.2 wt% Y)-FA-HA | – | Sponge replica | – | FA | HA | 90 | ̴ 600 | – | – | Adhesion strength ̴33MPa | Saline and MG-63 cells | – | [44] |
| 2008 | YSZ-FA-HA | – | Sponge replica | – | FA | HA | ̴ 84–87 | ̴ 500–700 and ̴ 150–200 | ̴ 7-8 | – | – | – | Rabbit calvarial defect (4,12) | [45] |
| 2010 | YSZ—MgO doped alumina | 60 wt% ( ̴ 20 vol%) | Pore former (Expanded polystyrene beads) and vacuum slip casting | YSZ (5, 10, 20 vol%) | – | 58S33C or 58S bioglass (15–20µm) | – | 262–121 | 7.52–5.42 | 1.64–1.17 GPa | – | SBF | – | [63] |
| 2011 | YSZ-BCP | YSZ (10 vol%) | Sponge replica | – | BCP/YSZ (1:1) | BCP (top layer) | 68.3 | 100–250 | 7.2 | – | – | MG-63 cells | – | [50] |
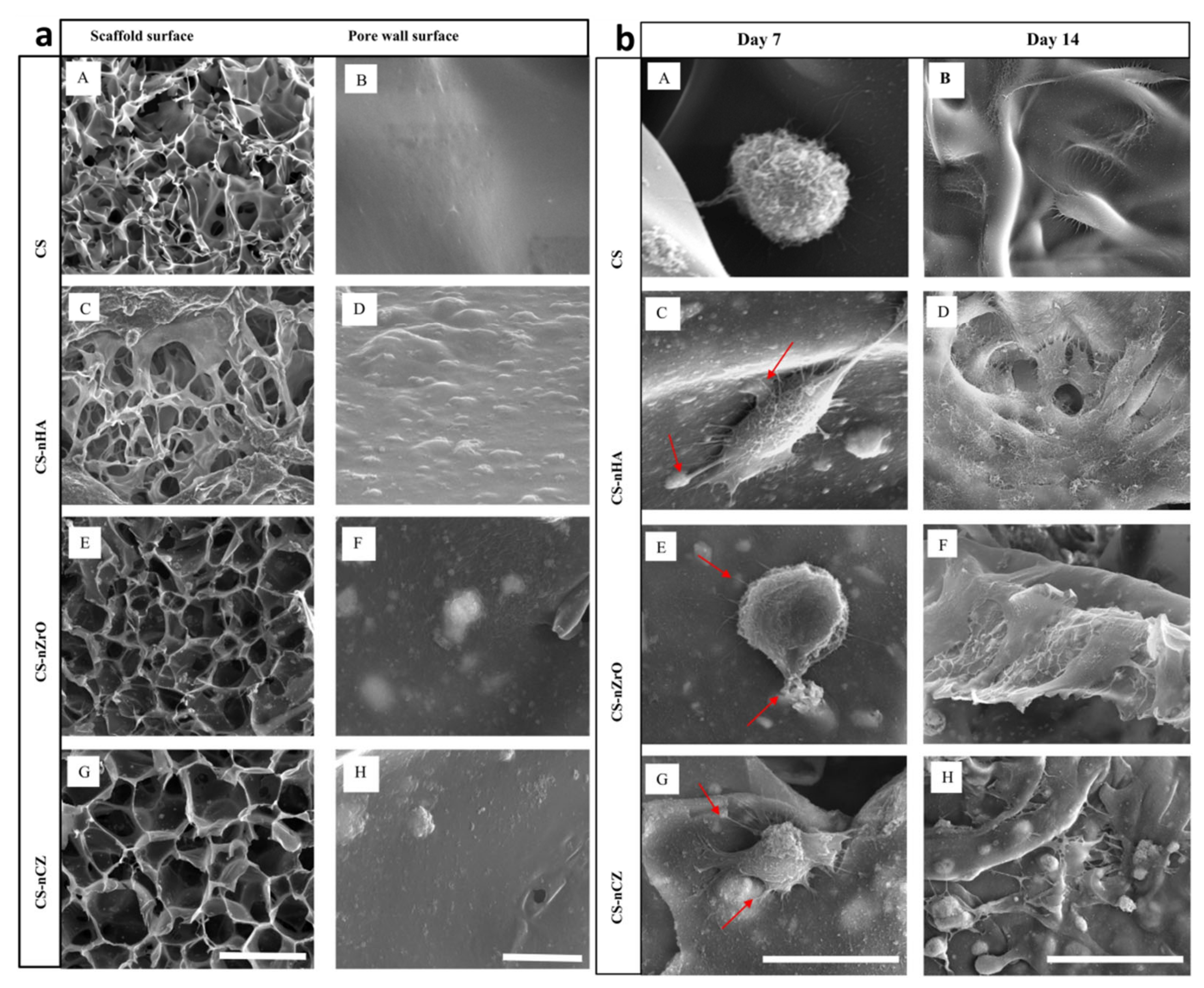
| 2012 | YSZ-HA | – | Sponge replica | HA (20, 30, 40, 50 wt%) | - | Fibrin gel (with BMSCs encapsulation) | 72–91 | – | 2.5–13.8 | – | – | MCT3-E1 and BMSC cells | Rat calvarial defect (3,6) | [20] |
| 2013 | YSZ-bioglass-HA | – | Freeze drying | HA (18%) | 45S5 bioglass | HA ( ̴ 20µm) | 68.2 | 100-500 | 2.11 | – | Flexural strength 343 MPa; Vickers hardness (substrate) 5.22 GPa | – | – | [59] |
| 2013 | YSZ-HA | – | Pore former (PMMA beads and starch powder) | HA (30 vol%) | 1st layer-YSZ (20 and 10 vol%) and 2nd layer-HA+YSZ(5 vol.%) +Al2O3 (5 vol.%) | HA (pure) | – | 1–10 and 20–50 | – | – | Bending strength 320–390 Mpa and Adhesion strength 24.5MPa | L929 cells | – | [111] |
| 2014 | YSZ-Ca P | – | Sponge replica | β-TCP (50, 60, 70 wt%) | – | – | 65–84 | – | 4.95–6.25 | 48–63 MPa | – | h-ESC cells | – | [48] |
| 2014 | YSZ- β-TCP-HA | – | Sponge replica | – | – | β-TCP, HA and BCP | – | 200–500 | – | – | – | MC3T3-E1 cells | – | [51] |
| 2015 | YSZ-BCP-Collagen | – | Sponge replica | – | BCP/YSZ (1:1) ( ̴ 10–15µm) | BCP (top layer) ( ̴ 10–15µm) and collagen | – | 150–500 | 6.8 | – | – | MC3T3-E1 cells | Rabbit femoral defect (1 and 5 months) | [52] |
| 2015 | YSZ | – | Sponge replica | – | – | – | 92.7–68 | 830–577 | 0.6–4.4 | – | – | SBF and BMSC cells | – | [57] |
| 2015 | YSZ -Al2O3 | – | Pore former (polyethylene beads) | Al2O3 (20wt%) | – | – | 50–61 | 113–185 | 60–240 | 3–10 GPA | – | MC3T3-E1 cells | – | [112] |
| 2015 | YSZ and MgSZ | – | Pore former (polyethylene beads) | Y2O3 (8, 3 wt%) and MgO (8, 3 wt%) | – | – | 50 and 57 | 167 and 144 | 210 and 120 | 10 and 6 | – | MC3T3-E1 cells | – | [64] |
| 2015 | ZrO2-CS-SF | – | Freeze drying | ZrO2 (1)-CS (2)-SF(2.5)wt% | – | – | >75 | 50–150 | – | – | – | PBS and HGF cells | – | [61] |
| 2015 | ZrO2- POM-SF | – | Freeze drying | POM-ZrO2-SF | – | – | – | 100–200 | – | – | – | SBF and HGF cells | – | [60] |
| 2017 | YSZ-HA-PLA | – | Solvent casting and salt-leaching | PLA-HA (10, 15, 20 wt%)-YSZ (10, 15, 20 wt%) | – | – | – | – | 0.8–6.9 | 0.7–5.1 MPa | Flexural strength 0.09–0.47 MPa; Flexural strain 17.5–8.2% | SBF | – | [66] |
| 2018 | YSZ | – | Sponge replica(crushed into granules after sintering) | – | – | – | – | 300–400 | – | – | – | – | Rabbit calvarial defect (2, 4, 8) | [27] |
| 2018 | YSZ-FA-HA | 55 | Sponge replica | – | FA ( ̴ 45.7µm) | HA-PRP/HS | 71.6 | 310 150 | 2.98–18.8 | – | – | MG-63cells | – | [46] |
| 2018 | ZrO2- HA-PRP/HS | – | Sponge replica | – | FA | HA and Loaded with PRP gel/HS | – | – | – | – | – | – | Rabbit mandible defect (6,8) | [47] |
| 2018 | ZrO2 | – | Solvent casting and Salt-leaching | – | – | – | 79 | 100 | – | – | – | – | Case study (repair of oroantral fistula) | [67] |
| 2018 | ZrO2-CS-HA -CZ | – | Freeze drying | CS(2.5wt%)-HA- ZrO2-CZ | – | – | 85–94 | 10–100 | 0.3–1 | CM-0.24–0.97 MPa;EM- 3–4 GPa | – | PBS and OB-6 cells | – | [62] |
| 2019 | YSZ | 50–70 wt% | Sponge replica | – | – | 58S bioglass ( ̴ 2µm) | 94–85 | 700–322 | – | – | – | MG-63cells | – | [55] |
| 2020 | YSZ | 60wt% | Sponge replica | – | – | – | 94–85 | 700–120 | 0.2–0.8 | 9–39 MPa | FEA, hardness (387 MPa) | – | – | [58] |
| 2020 | YSZ-58S | 60 wt% | Sponge replica | – | 58S bioglass (infiltration) | 58S bioglass | 93–89 | – | 0.33–0.44 | – | – | SBF and MG-63 cells | – | [56] |
| 2020 | YSZ-HA-TCP | 55 wt% | Sponge replica | YSZ (5, 10, 15, 20 and 25 wt%) | – | – | 65.7–84.4 | – | 13.2–4.5 | – | – | SBF | – | [49] |
| Extrusion Based Printing | ||||||||||||||
| 2011 | YSZ—HA(α –TCP) | 48–43 vol% | Multipass extrusion | – | HA (α-TCP)—YSZ | HA | 77 | 86 | 53 | – | – | MG-63 cells | – | [79] |
| 2012 | YSZ—HA | 45–40 vol% | Multipass extrusion | – | HA—YSZ | HA | – | – | 7–20 | – | – | MG-63 cells | – | [77] |
| 2012 | YSZ—BCP | 46–41 vol% | Multipass extrusion | – | YSZ—BCP | PCL/BCP | 92–78 | – | 8.27–12.7 | – | – | MG-63 cells | – | [78] |
| 2014 | YSZ | 70 wt% | Direct ink writing (DIW) | – | – | – | 55 and 63 | – | 8 and 10 | – | – | HCT116 cells | – | [23] |
| 2017 | ZrO2—β-TCP | – | 3D Bio-plotter | ZrO2 (30wt%) | – | – | 60–76.46 | 160–226 | 7–12.025 | – | – | MG-63 cells | – | [79] |
| 2018 | YSZ-Al2O3(ZTA) | 70wt% (35.5 vol%) | Robocasting | ZTA (YSZ-16 wt.%) | – | – | 50 | 245 | – | – | – | HOB cells | – | [87] |
| 2018 | ZrO2-BCP | – | FDM | ZrO2 (10 wt%) | – | – | – | 350 | 0.5 | – | – | MG-63 and hMSCs cells | – | [81] |
| 2019 | YSZ | 48 vol% | Robocasting | – | – | – | – | 200–500 | – | – | – | – | – | [85] |
| 2019 | ZrO2—β-Ca2SiO4 | – | 3D Bio-plotter | ZrO2 (5, 10, 15 wt%) | – | – | ̴ 67 | – | 3.9–6.1 | – | – | SBF and BMSC cells | Rat calvarial defect (8) | [80] |
| 2020 | ZrO2—PCL | – | FDM | ZrO2 (5, 10, 20 wt%) | – | – | 46.2–47 | 459.2–462.7 | 5.5–7.9 | 43–67 MPa | – | MC3T3-E1 cells | – | [83] |
| 2021 | YSZ-FA-HA | 39.5 vol% | Direct ink writing (DIW) | – | FA | HA ( ̴ 20µm) | 61.1–75.3 | – | 20.8–62.9 | – | – | SBF | – | [86] |
| Polymerization Based Printing Technique | ||||||||||||||
| 2019 | ZrO2 -HA | 60 wt% | DLP | ZrO2 (1, 3, 6 wt%) | – | – | – | – | – | – | Tensile strength (29.4%); Bending strength (23.9%) | BMSC cells | – | [26] |
| 2020 | YSZ-HA | – | DLP | HA (10, 20, 30 wt%) | – | – | 54.6 | – | 52.25 | – | CS after soaking in SBF (25 MPa) | SBF and MC3T3-E1 cells | – | [91] |
| CAD/CAM Technique | ||||||||||||||
| 2019 | YSZ | – | CAD/CAM (5-axis milling) | – | – | – | 45.06 | – | 52.25 | – | FEA | SBF | – | [69] |
| 2019 | YSZ | – | CAD/CAM | – | – | – | – | – | – | – | – | – | Case study (mandibu-lar defects) | [68] |
| 2021 | YSZ | – | CAD/CAM | – | – | – | – | – | – | – | – | MC3T3-E1 cells | Rat femur (2, 4, 8) | [70] |
| Electrospinning | ||||||||||||||
| 2016 | YSZ- PVP | – | Electrospinning | – | – | – | – | – | – | 1.11MPa | – | HMSCcells | – | [97] |
| 2017 | ZrO2-PCL | 6–30 wt.% | Electrospinning | – | – | – | – | – | – | – | – | 3T3 cells | – | [98] |
| SLS Technique | ||||||||||||||
| 2014 | ZrO2-CaSiO3 | – | SLS | ZrO2 (10, 20, 20, 40 wt%) | – | – | 70 | 1600 | 17.9–44.1 | – | Fracture toughness 1.14–1.66 MPa.m1/2 | SBF and MG-63 cells | – | [93] |
| Hybrid Technique | ||||||||||||||
| 2011 | YSZ-TCP | – | 3D Rapid Prototyper (ABS template)followed by slurry impergation | – | – | Mesoporous bioglass | 63–68 | 500–800 | 44.35–123.32 | – | – | SBF and BMSC cells | – | [103] |
| 2011 | YSZ-BCP and PMMA-PCL-HA (Fiber) | 10 vol% | Sponge replicaAnd Electrospinning | – | YSZ-BCP | BCP | 67.68–69.65 | – | 4.83–4.97 | – | – | MG-63 cells | – | [105] |
| 2012 | ZrO2 | 50 vol.% | Free-form | – | – | – | 40 | 350 | – | – | – | – | Case study (maxilla) | [113] |
| 2016 | ZrO2—β-TCP | – | 3D Rapid Prototyper (ABS template) followed by impergation | ZrO2 (10, 20, 30, 40, 50 wt%) | – | – | 68.5–82.5 | – | 3–15 | 184–396 MPa | – | PBS and MG-63 cells | – | [104] |
| 2020 | YSZ-bioglass-Zn-HA-Biopolymer | 40 Vol% | FDM and Freeze drying | – | Glass (Infiltration) | Glass/Zn-HA(̴ 1µm) and Gelatin/alginate | ̴ 40% | 300–450 | 68.2–89.8 | 1.7–2.6 GPa | Strain energy density 1.8-4.2 MJ/m3 | DPCs cells | – | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakthiabirami, K.; Soundharrajan, V.; Kang, J.-H.; Yang, Y.P.; Park, S.-W. Three-Dimensional Zirconia-Based Scaffolds for Load-Bearing Bone-Regeneration Applications: Prospects and Challenges. Materials 2021, 14, 3207. https://doi.org/10.3390/ma14123207
Sakthiabirami K, Soundharrajan V, Kang J-H, Yang YP, Park S-W. Three-Dimensional Zirconia-Based Scaffolds for Load-Bearing Bone-Regeneration Applications: Prospects and Challenges. Materials. 2021; 14(12):3207. https://doi.org/10.3390/ma14123207
Chicago/Turabian StyleSakthiabirami, Kumaresan, Vaiyapuri Soundharrajan, Jin-Ho Kang, Yunzhi Peter Yang, and Sang-Won Park. 2021. "Three-Dimensional Zirconia-Based Scaffolds for Load-Bearing Bone-Regeneration Applications: Prospects and Challenges" Materials 14, no. 12: 3207. https://doi.org/10.3390/ma14123207
APA StyleSakthiabirami, K., Soundharrajan, V., Kang, J.-H., Yang, Y. P., & Park, S.-W. (2021). Three-Dimensional Zirconia-Based Scaffolds for Load-Bearing Bone-Regeneration Applications: Prospects and Challenges. Materials, 14(12), 3207. https://doi.org/10.3390/ma14123207