Self-Immobilizing Metals Binder for Construction Made of Activated Metallurgical Slag, Slag from Lignite Coal Combustion and Ash from Biomass Combustion
Abstract
1. Introduction
1.1. Ground Granulated Blast Furnace Slag (GGBFS)
1.2. Fly Ash
1.3. Furnace Slag
1.4. Ashes from Biomass Combustion (BAF)
1.5. Ashes from Biomass Co-Combustion
2. Materials and Methods
- Ground granulated blast furnace slag (GGBFS) by Górażdże Cement S.A. (Chorula, Poland), which came from the Ekocem production plant (Dąbrowa Górnicza, Poland), met the standard requirements according to PN-EN 15167-1 [59]. Its specific surface area according to Blaine was 3850 cm2/g, it was delivered and stored in a sealed container;
- Fly ash from a biomass (BFA) block from a Polish power plant, taken from a silo, it was delivered and stored in a double plastic bag and a sealed container;
- Furnace slag from a lignite boiler (LFS) from a Polish power plant, taken from a slag scraper tub after the crushing process, it was delivered and stored in a double plastic bag and a sealed container;
- Distilled water with a conductivity of 0.06 μS;
- Commercial zeolite called ZeoBau 50 (Producer: ASTRA Benedykt Karczewski, Straszyn, Poland) with a specific surface area of at least 1364 m2/kg is a natural dried, ground and powdered rock from the group of aluminosilicate minerals, the main component of which is usually clinoptilolite with a skeleton structure. Zeolite is an additive to concrete that improves many of its properties. It can be dosed up to 15% of the cement mass. Zeolite, in these tests, was introduced into the mixture after being soaked with distilled water or NitCal solution;
- Water activating the slag binding, sodium metasilicate solution, was made of distilled water and sodium metasilicate pentahydrate Na2SiO3·5H2O in the weight ratio distilled water/activator = 4.174;
- CEN standard sand, according to PN-EN 196-1 [62], which was added to mixtures in the amount of 64–67% of the total mass of the mixture, in order to make mortars of beams with dimensions of 4 cm × 4 cm × 16 cm for strength tests according to PN-EN 196-1.
2.1. General Characteristics of Ash and Slag (ad.2 and ad.3 above) from the Polish Power Plant
2.2. Tests of the Chemical Composition of the Materials Used for Specimens Preparation
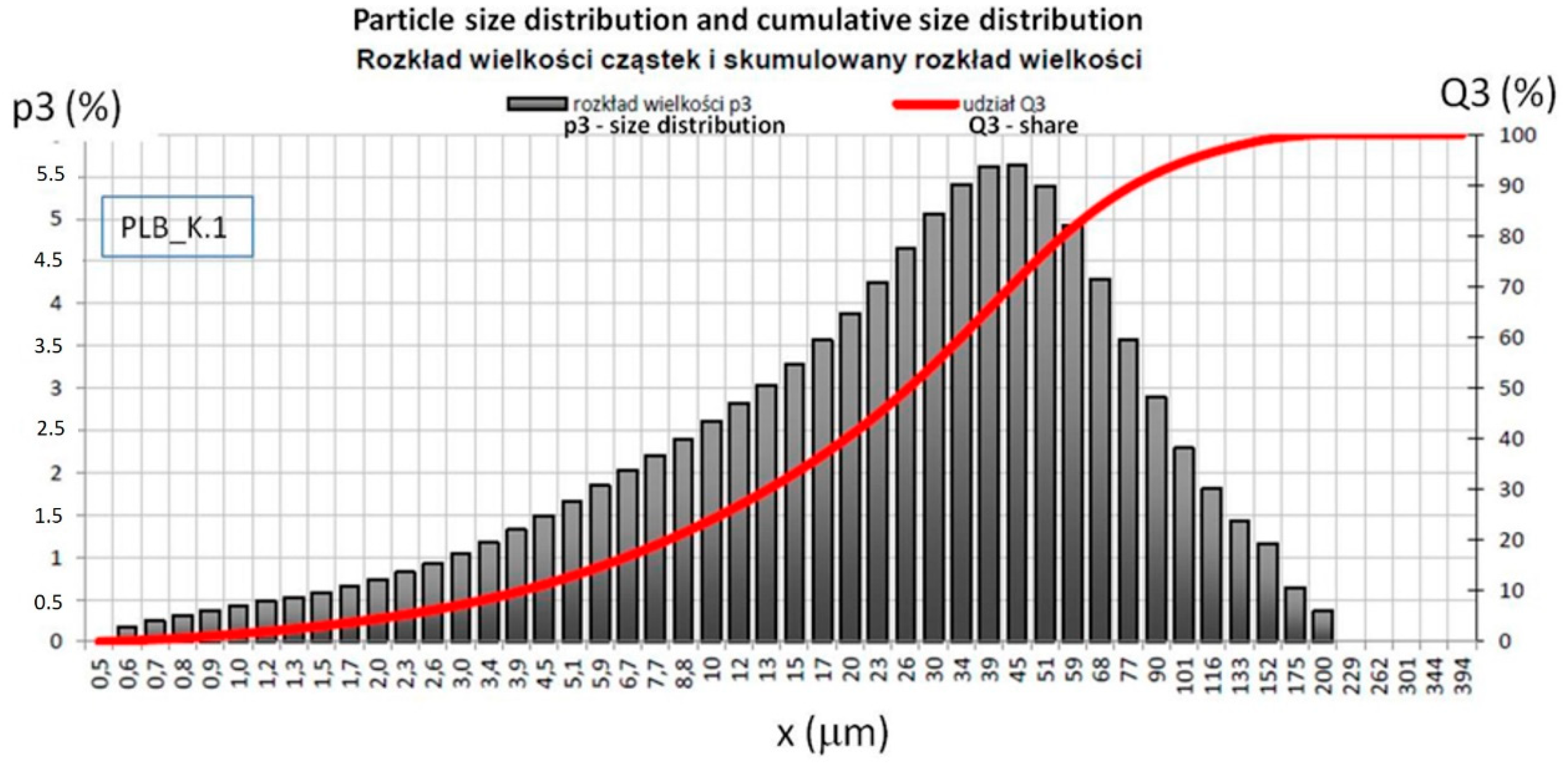
2.3. Particle Size Tests of Combustion Products of the Materials Used for Specimens Preparation
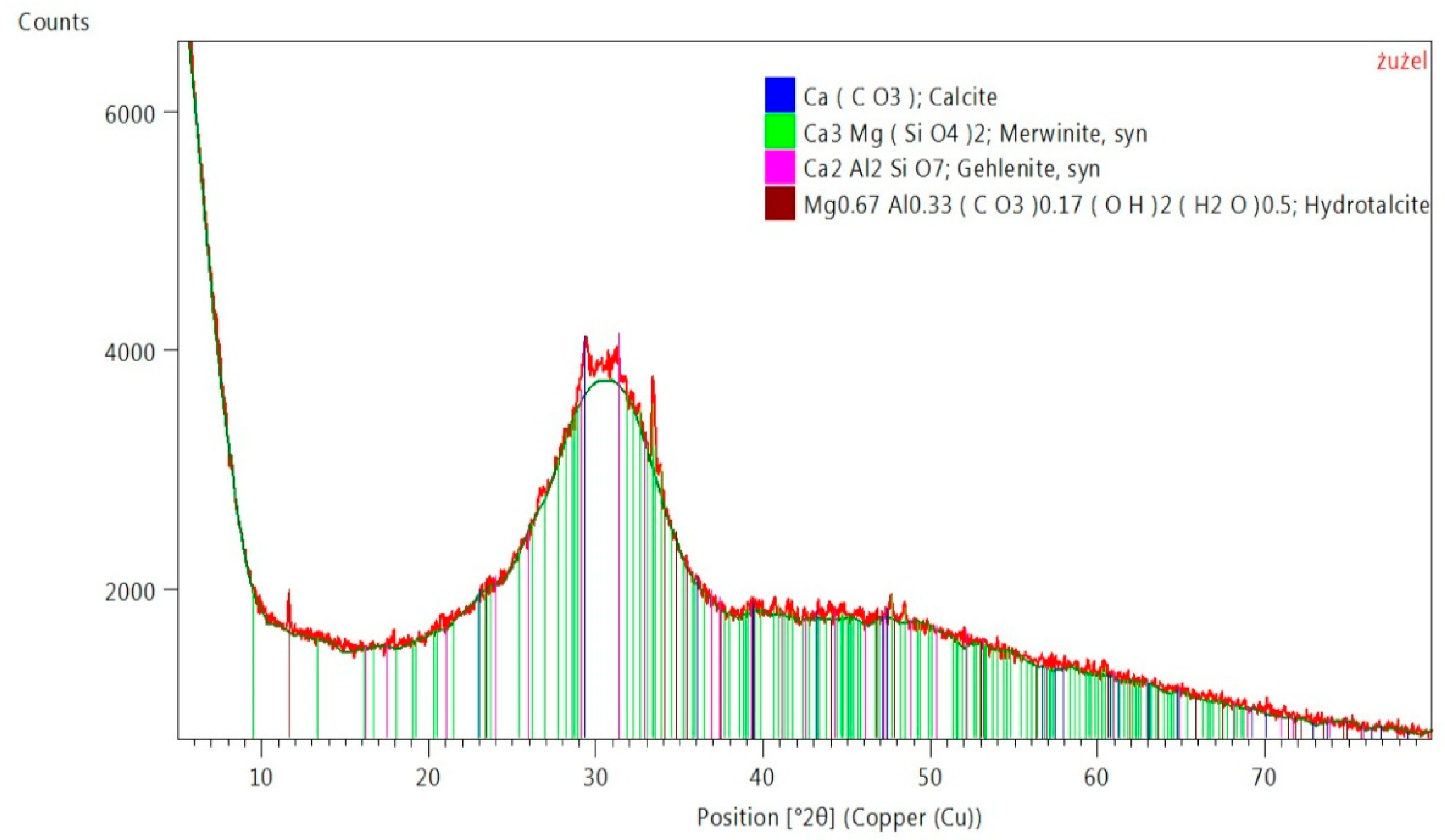
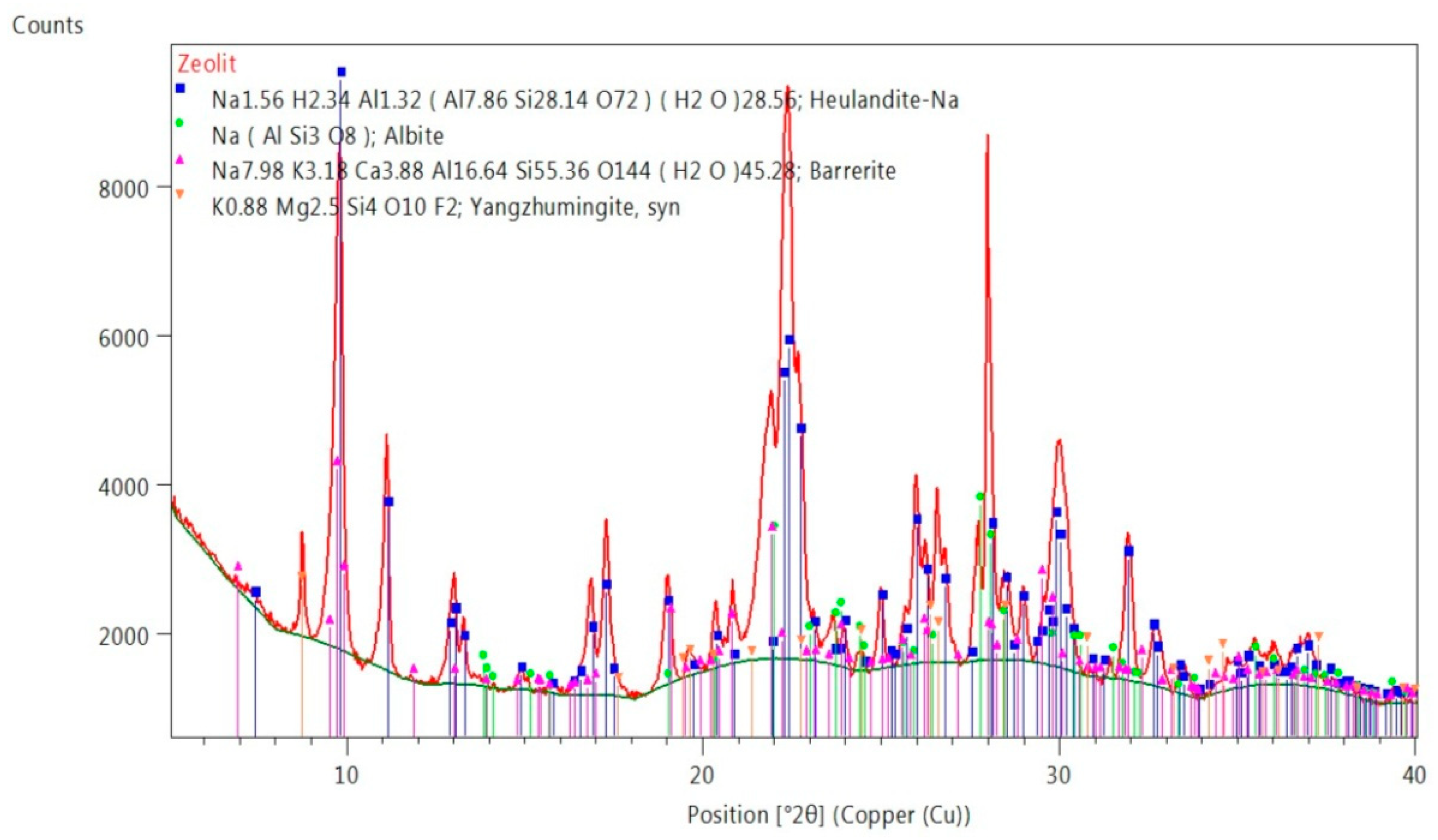
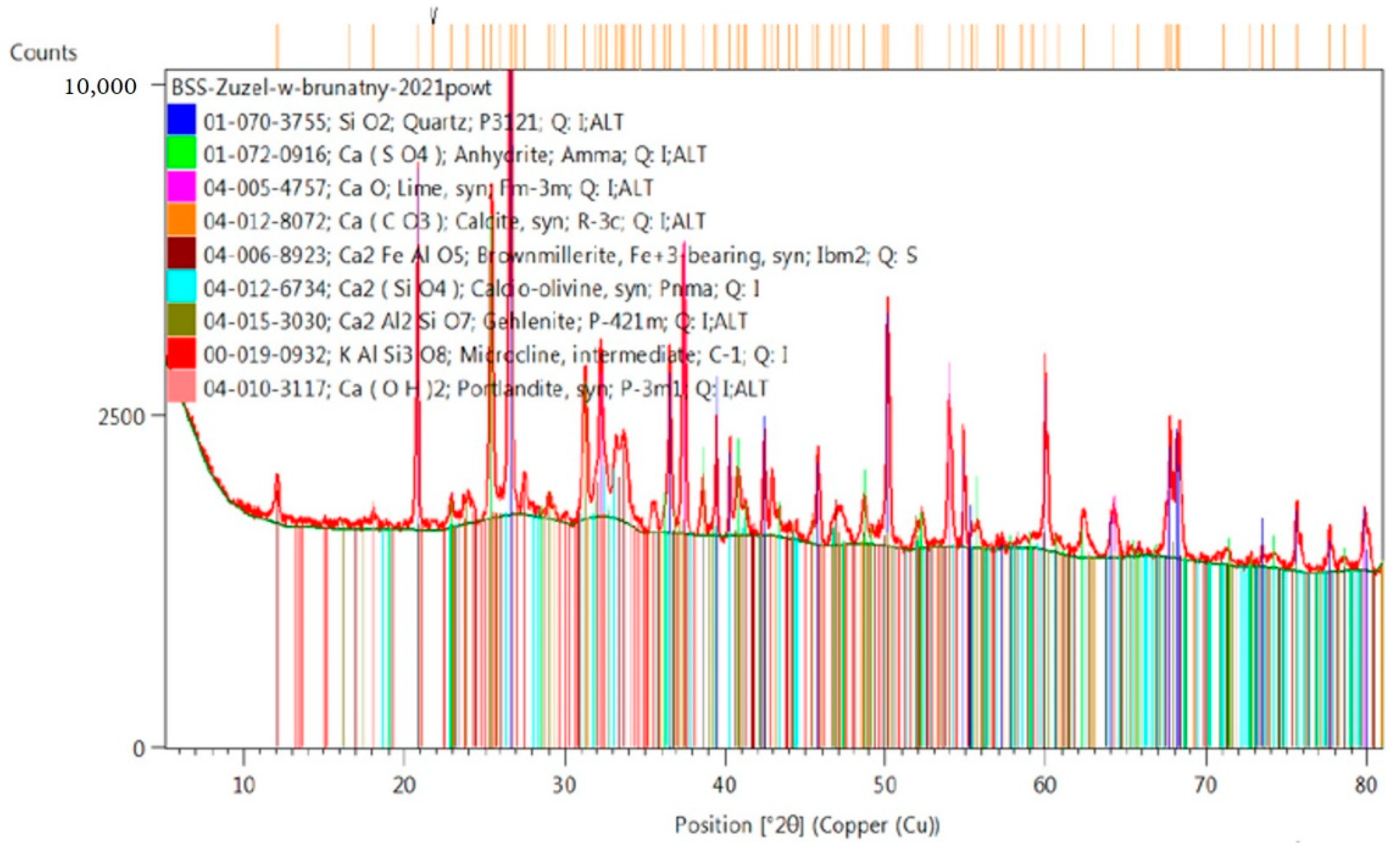
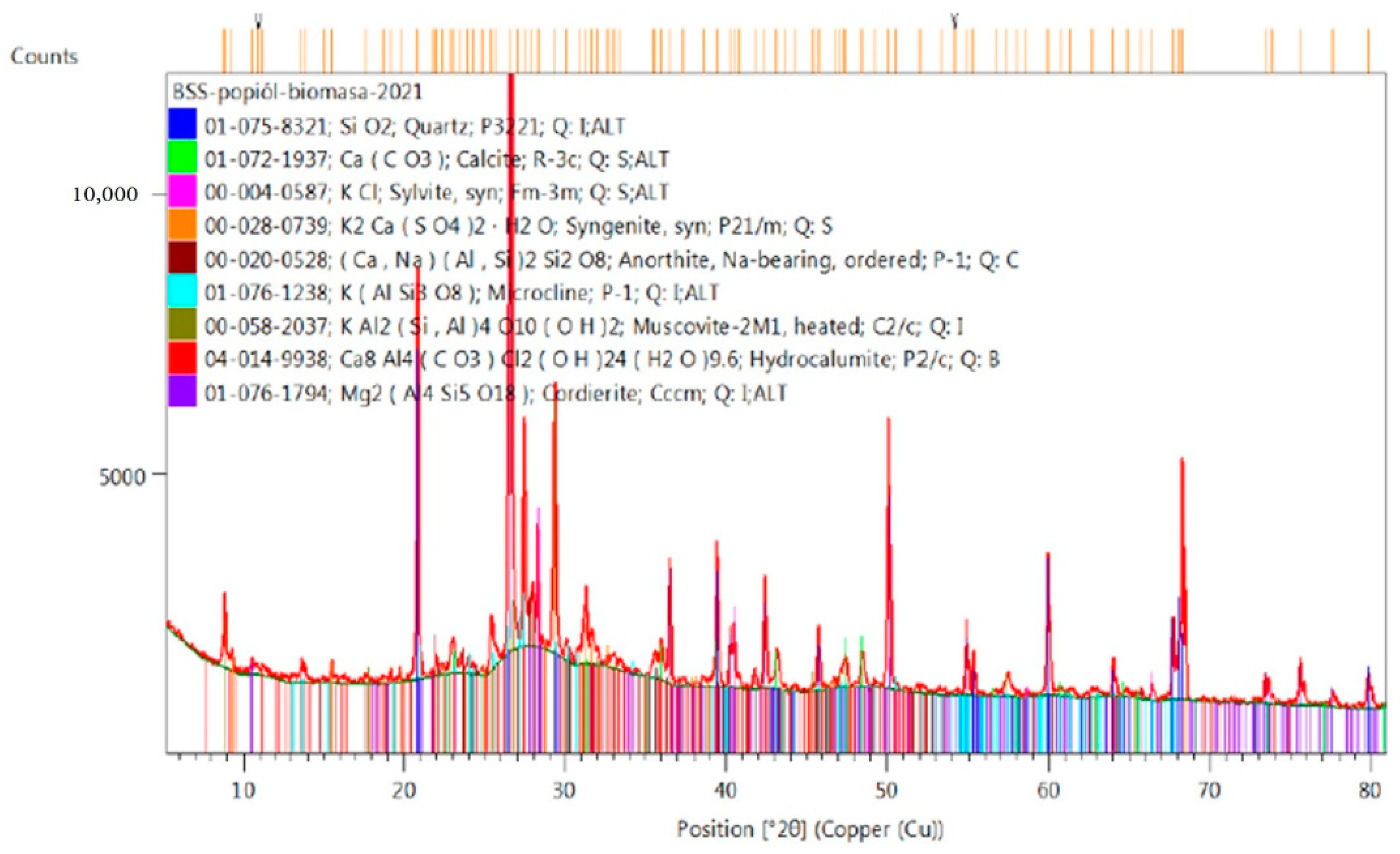
2.4. Analysis of Phase Composition of Materials Used for Testing, XRD (X-ray Diffraction) Examination
2.5. Preparation of Samples
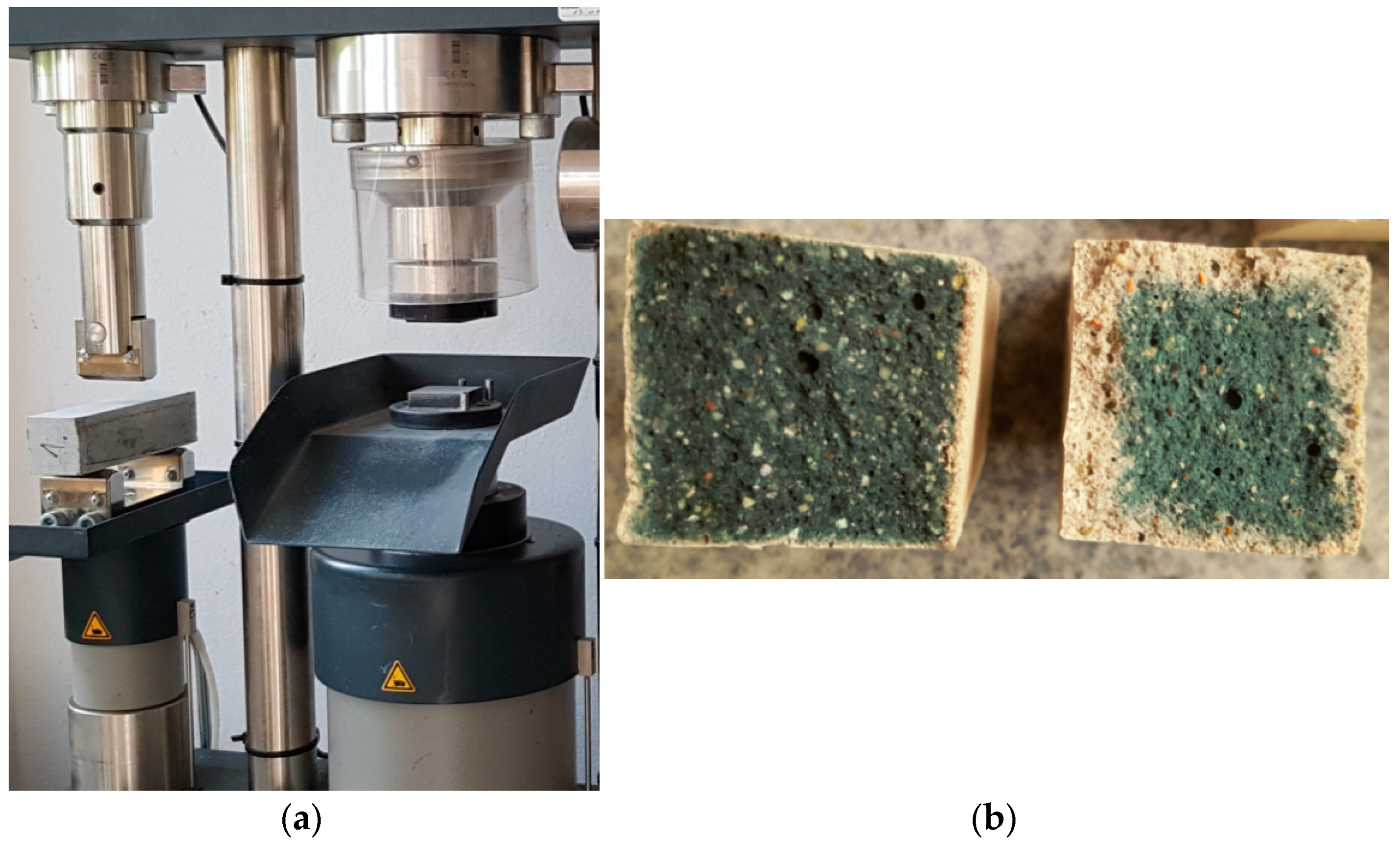
2.6. Strength Tests of the Prepared Samples
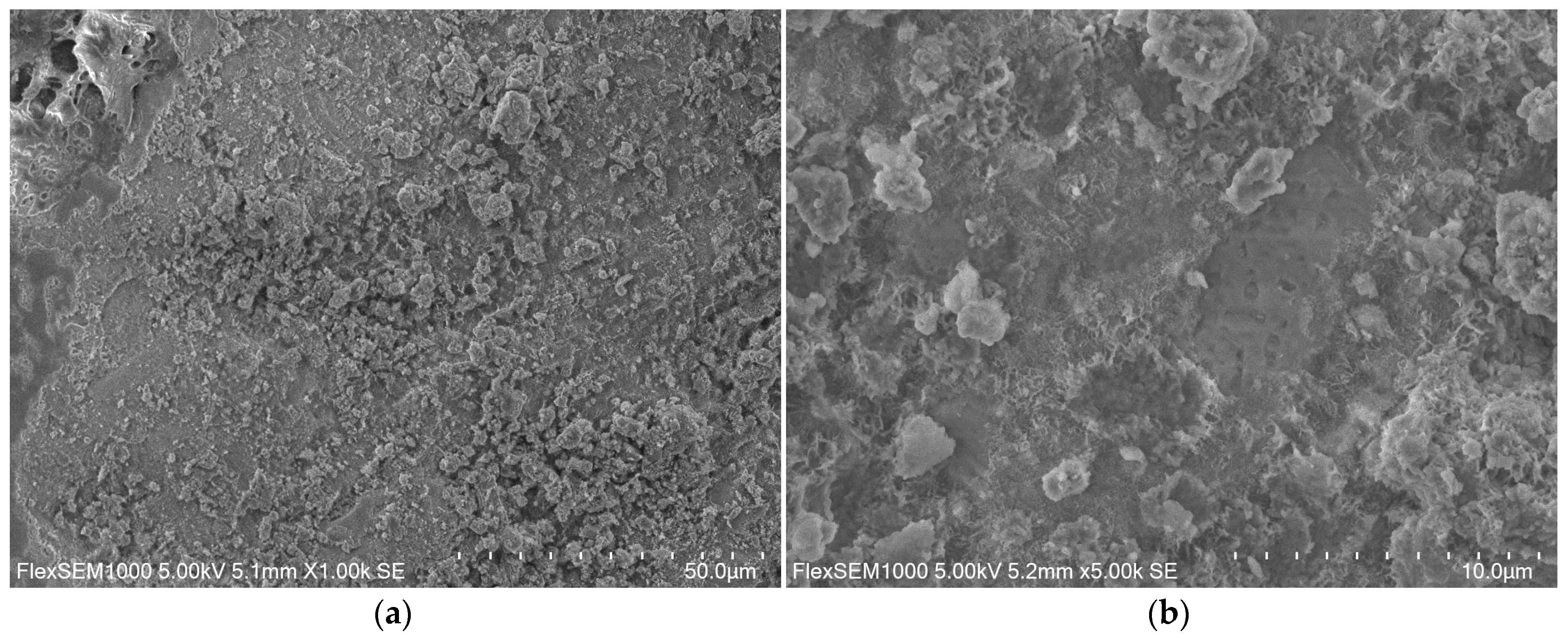
2.7. Microscopic Examination Using SEM
2.8. Metal Leaching Tests
3. Results and Discussion
3.1. Strength Tests
3.2. Microscopic Observations
3.2.1. ZK1 Examination
3.2.2. ZK1z10 Examination
3.2.3. ZK1z10nc Examination
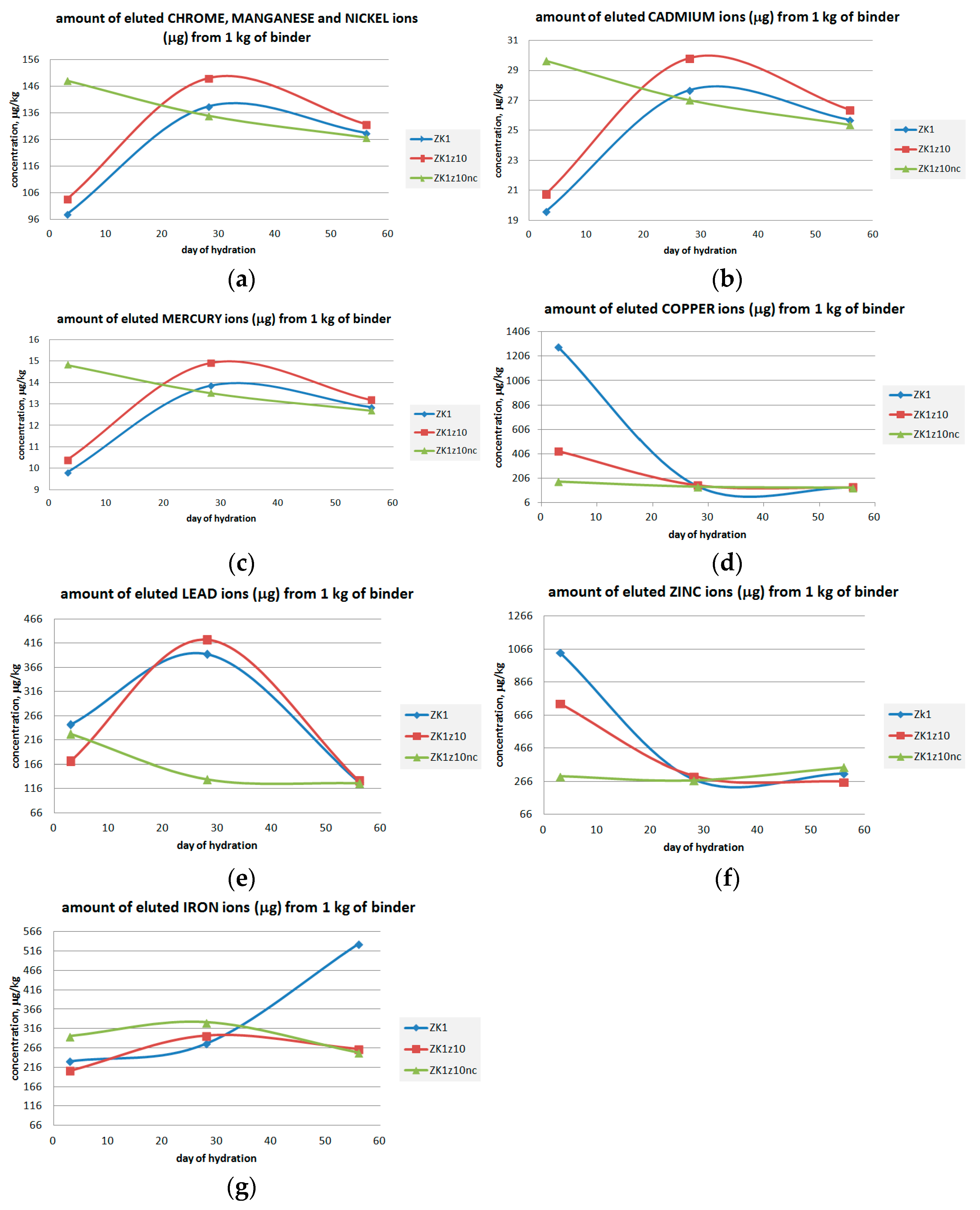
3.3. Metals Leaching
4. Conclusions
5. Patents
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pietrzyński, P. Cementownie są stałym i ważnym elementem polskiego system gospodarki odpadami (Cement plants are a permanent and important element of the Polish waste management system). Mater. Bud. 2021, 582, 42–43. Available online: https://www.materialybudowlane.info.pl/pl/13282 (accessed on 1 February 2021).
- Kurdowski, W. Cement and Concrete Chemistry; Springer Nature Switzerland AG: Cham, Switzerland, 2014. [Google Scholar]
- Solski, A. Wpływ toksyczny substancji wyługowanych z żużla wielkopiecowego huty “Florian” w Świętochłowicach na organizmy wodne (Toxic effect of substances leached from blast furnace slag "Florian" smelter in Świętochłowice on aquatic organisms). Ochr. Sr. 1983, 1, 17–22. [Google Scholar]
- BN-79/6722-09 Popioły lotne i żużle z kotłów opalanych węglem kamiennym i brunatnym. In Podział, Nazwy i Określenia (Fly Ash and Slag from Coal and Lignite-Fired Boilers. Division, Names and Terms); Standard Publishing House: Warsaw, Poland, 1980.
- PKN. PN-EN 197-1:2012 Cement. In Part 1: Composition, Requirements and Compliance Criteria for Common Cements; PKN: Warsaw, Poland, 2013. [Google Scholar]
- Giergiczny, Z. Fly Ash in Cement and Concrete Composition; Publishing House of the Silesian University of Technology: Gliwice, Poland, 2013; ISBN 978-83-7880-174-0. (In Polish) [Google Scholar]
- Czech, T.; Jaworek, A.; Marchewicz, A.; Krupa, A.; Sobczyk, A.T. Zawartość metali ciężkich w popiołach lotnych (Heavy metal content in fly ash). In Proceedings of the XXIV International Conference “Ashes From Energy”, Sopot, Poland, 25–27 October 2017; Available online: http://unia-ups.pl/wp-content/uploads/2016/03/Czech.pdf (accessed on 16 November 2017). (In Polish).
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Fernando, R. Cofiring High Ratios of Biomass with Coal; CCC/194; IEA Clean Coal Centre: London, UK; ISBN 978-92-9029-514-3. Available online: https://www.iea-coal.org/report/cofiring-high-ratios-of-biomass-with-coal-ccc-194/ (accessed on 1 January 2012).
- Diatta, J.; Kowalski, M. Popioły z biomasy–ich potencjał agrochemiczny jako szansa, a nie ograniczenie (Ashes from biomass-their agrochemical potential as an opportunity, not a limitation.). In Proceedings of the XXIV International Conference "“Ashes From Energy”, Sopot, Poland, 25–27 October 2017; Available online: http://unia-ups.pl/materialy-konferencyjne-2017 (accessed on 16 November 2017). (In Polish).
- Carević, I.; Štirmer, N.; Serdar, M.; Ukrainczyk, N. Effect of Wood Biomass Ash Storage on the Properties of Cement Composites. Materials 2021, 14, 1632. [Google Scholar] [CrossRef]
- Terazśrodowisko. Available online: https://www.teraz-srodowisko.pl/slownik-ochrona-srodowiska/definicja/rdf.html (accessed on 22 April 2021).
- Rozporządzenie Ministra Środowiska w Sprawie Sposobu Prowadzenia Oceny Zanieczyszczenia Powierzchni Ziemi (Regulation of the Minister of Environment on the Method of Assessing the Pollution of the Earth’s Surface.). Dz.U. 1 September 2016, Item 1395 Status: Effective from 5 September 2016. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 1 September 2016).
- Pourret, O.; Bollinger, J.C.; Hursthouse, A. Heavy metal: A misused term? Acta Geochim. 2021. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic Polymeric New Materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Why Alkali-Activated Materials (AAM) are Not Geopolymers. Publ. Tech. Paper 2018, 25. [Google Scholar] [CrossRef]
- Głuchowski, W.D. Własności alkaliczno-glinokrze8,9,mianowych tworzyw wiążących i betonów (Properties of alkali-aluminosilicate binders and concretes). Cem. Wapno Gips 1976, 4, 93–96. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Con. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 2008, 12, 3864–3869. [Google Scholar] [CrossRef]
- Keeley, P.M.; Rowson, N.A.; Johnson, T.P.; Deegan, D.E. The effect of the extent of polymerisation of a slag structure on the strength of alkali-activated slag binders. Int. J. Miner. Process. 2017, 164, 37–44. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Con. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Vishnu, N.; Kolli, R.; Ravella, D.P. Studies on Self-Compacting geopolymer concrete containing flyash, GGBS, wollastonite and graphene oxide. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Ferreira, S.R.; Ukrainczyk, N.; Silva, K.D.C.; Silva, L.E.; Koenders, E. Effect of microcrystalline cellulose on geopolymer and Portland cement pastes mechanical performance. Con. Build. Mater. 2021, 288, 123053. [Google Scholar] [CrossRef]
- Krzywoń, R.; Dawczyński, S. Strength Parameters of Foamed Geopolymer Reinforced with GFRP Mesh. Materials 2021, 14, 689. [Google Scholar] [CrossRef] [PubMed]
- Cifrian, E.; Dacuba, J.; Llano, T.; Díaz-Fernández, M.d.C.; Andrés, A. Coal Fly Ash–Clay Based Geopolymer-Incorporating Electric Arc Furnace Dust (EAFD): Leaching Behavior and Geochemical Modeling. Appl. Sci. 2021, 11, 810. [Google Scholar] [CrossRef]
- Milad, A.; Ali, A.S.B.; Babalghaith, A.M.; Memon, Z.A.; Mashaan, N.S.; Arafa, S.; Md. Yusoff, N.I. Utilisation of Waste-Based Geopolymer in Asphalt Pavement Modification and Construction—A Review. Sustainability 2021, 13, 3330. [Google Scholar] [CrossRef]
- Azad, N.M.; Samarakoon, S.M. Utilization of Industrial By-Products/Waste to Manufacture Geopolymer Cement/Concrete. Sustainability 2021, 13, 873. [Google Scholar] [CrossRef]
- Sivakrishna, A.; Adesina, A.; Awoyera, P.O.; Kumar, K.R. Green concrete: A review of recent developments. Mater. Today Proc. 2020, 27, 54–58. [Google Scholar] [CrossRef]
- Abbas, R.; Aly, M.; Ghorab, H.Y. Spoiwo geopolimerowe z prażonej gliny o umiarkowanej zawartości kaolinu (Alkali activated clay of moderate kaolin content). Cem. Wapno Beton 2019, 24. [Google Scholar] [CrossRef]
- Vishwakarma, V.; Ramachandran, D. Green Concrete mix using solid waste and nanoparticles as alternatives—A review. Con. Build. Mater. 2018, 162, 96–103. [Google Scholar] [CrossRef]
- Liew, K.M.; Sojobi, A.O.; Zhang, L.W. Green concrete: Prospects and challenges. Con. Build. Mater. 2017, 156, 1063–1095. [Google Scholar] [CrossRef]
- Suhendro, B. Toward Green Concrete for Better Sustainable Environment. Procedia Eng. 2014, 95, 305–320. [Google Scholar] [CrossRef]
- Garg, C.; Jain, A. Green Concrete: Efficient & Eco-Friendly Construction Materials. Int. J. Res. Eng. Technol. 2014, 2, 259–264. [Google Scholar]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef]
- Glavind, M.; Munch-Petersen, C. Green Concrete–A Life Cycle Approach. In Challenges of Concrete Construction: Vol. 5. In Sustainable Concrete Construction, Proceedings of the International Conference held at the University of Dundee, Scotland, UK, 9–11 September 2002; Thomas Telford Publishing: London, UK, 2015; pp. 771–786. [Google Scholar] [CrossRef]
- Król, M.; Błaszczyński, T.Z. Ekobetony geopolimerowe (Geopolymer eco-concrete). Materiały Bud. 2013, 11, 23–26. [Google Scholar]
- Słomka-Słupik, B.; Podwórny, J.; Trybalska, B. Korozja zaczynu z żużla wielkopiecowego w wodnym roztworze NH4Cl (Corrosion of blastfurnace slag in aqueous NH4Cl solution). Cem. Wap. Beton 2019, 24, 202–214. [Google Scholar] [CrossRef]
- Mazur, P.; Mikuła, J.; Kowalski, J.S. Odporność na korozję geopolimeru na bazie popiołu lotnego (Corrosion resistance of fly ash based geopolymer). Arch. Foundry Eng. 2013, 13, 83–86. [Google Scholar]
- Figiela, B.; Korniejenko, K. The possibility of using waste materials as raw materials for the production of geopolymers. Acta Innov. 2020, 48, 48–56. [Google Scholar] [CrossRef]
- Paszek, N.; Górski, M. The basic mechanical properties of the fluidised bed combustion fly ash-based geopolymer. Tech. Trans. 2019, 9, 107–118. [Google Scholar] [CrossRef]
- Anaszewicz, Ł.; Stolarski, A. The basic strength tests of geopolymer mortar. Materiały Bud. 2014, 12, 8–10. [Google Scholar] [CrossRef]
- Sitarz-Palczak, E.; Kalembkiewicz, J.; Galas, D. Comparative study on the characteristics of coal fly ash and biomass ash geopolymers. Arch. Environ. Prot. 2019, 45, 126–135. [Google Scholar]
- Sikora, S.; Hołuj, B.; Michałowski, B.; Hynowski, M. The influence of ground blast furnace slag addition on the mechanical properties of fly ash-based geopolymer slurries. In Prace Instytutu Ceramiki i Materiałów Budowlanych; Instytut Śląski Sp. z o.o.: Opole, Poland, 2017; Volume 10, pp. 70–80. [Google Scholar]
- Zarębska, K.; Klima, K.; Złotkowski, A.Z.; Kamienowska, M.; Czuma, N.K.; Baran, P.T. Synteza geopolimerów z wykorzystaniem żużla wielkopiecowego (Synthesis of geopolymers by using blast furnace Slag). Przemysł Chem. 2019, 2, 298–301. [Google Scholar] [CrossRef]
- Rajczyk, K.; Giergiczny, E.; Szota, M. Microstructure and properties of geopolymeric binders prepared using fly ash. In Prace Instytutu Ceramiki i Materiałów Budowlanych; Instytut Śląski Sp. z o.o.: Opole, Poland, 2015; Volume 8, pp. 79–89. [Google Scholar]
- Mikuła, J.; Łach, M. Wytwarzanie i właściwości geopolimerów na bazie tufu wulkanicznego (Production and properties of geopolymers based on volcanic tuff). Inż. Mater. 2014, 35, 270–276. [Google Scholar]
- Rosiek, G.; Wala, D. Właściwości mechaniczne i odporność korozyjna geopolimerowych kompozytów (Mechanical properties and corrosion resistance of geopolymer composites). Inż. Mater. 2007, 28, 857–862. [Google Scholar]
- Derdacka, A.; Małolepszy, J. Zastosowanie granulowanych żużli wielkopiecowych do wytwarzania bezklinkierowego, hydraulicznego spoiwa wiążącego (The use of granulated blast furnace slags for the production of clinker-free, hydraulic binder). Cem. Wapno Gips 1975, 10, 291–295. [Google Scholar]
- Derdacka–Grzymek, A.; Małolepszy, J.; Brylicki, W.; Deja, J. Spoiwo Żużlowo-Alkaliczne (Slag-Alkali Binder). Patent 284873, 18 April 1990. [Google Scholar]
- Deja, J. Skład fazowy zaczynów żużlowych aktywowanych alkaliami (Phase composition of slag pastes activated with alkali). Cem. Wapno Beton 2005, 3, 127–137. [Google Scholar]
- Łach, M.; Mierzwiński, D.; Mikuła, J. Synteza zeolitów z popiołów i żużli ze spalarni odpadów (Synthesis of zeolites from ashes and slags from waste incineration plants). Inż. Ekolog. 2017, 1, 196–201. [Google Scholar] [CrossRef][Green Version]
- Mierzwiński, D.; Łach, M.; Mikuła, J. Alkaliczna obróbka i immobilizacja odpadów wtórnych ze spalania odpadów (Alkaline treatment and immobilization of secondary waste from waste incineration). Inż. Ekolog. 2017, 2, 102–108. [Google Scholar] [CrossRef]
- Mikuła, J.; Łach, M.; Mierzwiński, D. Sposoby zagospodarowania popiołów i żużli ze spalarni odpadów (Ways of management of ashes and slags from waste incineration plants). Inż. Ekolog. 2017, 3, 37–46. [Google Scholar] [CrossRef]
- Górski, M.; Wielgus, N.; Loska, K.; Kozioł, M.; Landrat, M.; Ścierski, W.; Pikoń, K. Characteristics of Metakaolin-Based Geopolymer with Cathode Ray Tube Glass. Polymers 2021, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Economic Development, Labour and Technology. Available online: https://www.gov.pl/web/rozwoj-praca-technologia/rada-ministrow-przyjela-projekt-mapy-drogowej-goz (accessed on 10 September 2019).
- ITB Raport. Wykonanie Analizy Cyklu Życia (LCA) w Celu Określenia Śladu Węglowego dla Średnich Cementów z Grupy CEM I, CEM II i CEM III produkowanych w Polsce, zgodnie z PN-EN 15804: 2012 (Performing a Life Cycle Analysis (LCA) to Determine the Carbon Footprint for Medium Cements from the CEM I, CEM II and CEM III Groups Produced in Poland, in Accordance with PN-EN 15804: 2012). No. 01 929/1 2/ZOONF.; Warsaw, September 2013. Available online: https://www.polskicement.pl/wp-content/uploads/2019/12/Analiza-cyklu-zycia-LCA.pdf (accessed on 17 August 2018).
- Huang, X.; Hu, S.; Wang, F.; Liu, Y.; Mu, Y. Properties of alkali-activated slag with addition of cation exchange material. Con. Build. Mater. 2017, 146, 321–328. [Google Scholar] [CrossRef]
- Azad, A.; Saeedian, A.; Mousavi, S.-F.; Karami, H.; Farzin, S.; Singh, V.P. Effect of zeolite and pumice powders on the environmental and physical characteristics of green concrete filters. Con. Build. Mater. 2020, 240, 117931. [Google Scholar] [CrossRef]
- PKN. PN-EN 15167-1:2007. In Mielony Granulowany Żużel Wielkopiecowy do Stosowania w Betonie, Zaprawie i Zaczynie—Część 1: Definicje, Specyfikacje i Kryteria Zgodności (Ground Granular Blast Furnace Slag for Use in Concrete, Mortar and Paste-Part 1: Definitions, Specifications and Compliance Criteria); PKN: Warsaw, Poland, 2007. [Google Scholar]
- Karagöl, F.; Demirboğa, R.; Kaygusuz, M.A.; Yadollahi, M.M.; Polat, R. The influence of calcium nitrate as antifreeze admixture on the compressive strength of concrete exposed to low temperatures. Cold Reg. Sci. Technol. 2013, 89, 30–35. [Google Scholar] [CrossRef]
- Kumar, M.P.; Mini, K.M.; Rangarajan, M. Ultrafine GGBS and calcium nitrate as concrete admixtures for improved mechanical properties and corrosion resistance. Con. Build. Mater. 2018, 182, 249–257. [Google Scholar] [CrossRef]
- PKN. PN-EN 196-2:2005. In Metody Badania Cementu—Część 2: Analiza Chemiczna Cementu (Cement Test Methods-Part 2: Chemical Analysis of Cement); PKN: Warsaw, Poland, 2005. [Google Scholar]
- PN-EN ISO 12677:2011. In Chemical Analysis of Refractory Products By X-Ray Fluorescence (XRF)-Fused Cast-Bead Method (in pol.:Analiza Chemiczna Wyrobów Ogniotrwałych Techniką Fluorescencji (XRF)-Metoda Perły); PKN: Warsaw, Poland, 2011.
- Słomka-Słupik, B.; Podwórny, J.; Polus, M. An attempt to apply a slag binder with zeolite in mining construction. Durability and hardness. IOP Conf. Ser. Earth Environ. Sci. 2019, 261, 012046. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/261/1/012046/meta (accessed on 26 April 2019). [CrossRef]
- ISO 13320:2009 Particle Size Analysis-Laser Diffraction Methods. Publication Date: 2009-10. Technical Committee: ISO/TC 24/SC 4 Particle Characterization. ICS: 19.120 Particle Size Analysis. Sieving. Available online: https://www.iso.org/standard/44929.html (accessed on 6 January 2020).
- PN-EN 450-1:2012. In Popiół Lotny do Betonu—Część 1: Definicje, Specyfikacje i Kryteria Zgodności (Fly Ash for Concrete-Part 1: Definitions, Specifications and Compliance Criteria); PKN: Warsaw, Poland, 2014.
- Giergiczny, Z.; Ostrowski, M.; Baran, T. Wpływ popiołów lotnych krzemionkowych kategorii S na wybrane właściwości kompozytów cementowych (Influence of S-category silica fly ash on selected properties of cement composites). In Proceedings of the International Conference Ashes from Energy, Zakopane, Poland, 19–21 September 2016; Available online: http://unia-ups.pl/wp-content/uploads/2016/12/Wp%C5%82yw-popio%C5%82%C3%B3w-lotnych-krzemionkowych-kategorii-S-na-wybrane-w%C5%82a%C5%9Bciwo%C5%9Bci-kompozyt%C3%B3w-cementowych.pdf (accessed on 12 December 2016).
- Nath, S.K.; Kumar, S. Reaction kinetics of fly ash geopolymerization: Role of particle size controlled by using ball mill. Adv. Powder Technol. 2019, 30, 1079–1088. [Google Scholar] [CrossRef]
- Bolewski, A.; Manecki, A. Mineralogia Szczegółowa (Detailed Mineralogy); Publishing House PAE: Warsaw, Ploand, 1993; ISBN 83-85636-03-X. [Google Scholar]
- Rackley, S.A. 7-Adsorption capture systems. In Rackley, Carbon Capture and Storage, 2nd ed.; Stephen, A., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 151–185. ISBN 9780128120415. [Google Scholar] [CrossRef]
- Słomka-Słupik, B.; Podwórny, J. Wpływ dodatku zeolitu na mikrostrukturę zaczynu aktywowanego żużla wielkopiecowego (Influence of zeolite addition on the microstructure of activated blast furnace slag paste). In Proceedings of the VIII Scientific Conference “Energy and Environment in the Technologies of Building, Ceramic and Refractory Materials”, Szczyrk, Poland, 25–27 September 2017; Instytut Śląski: Opole, Poland, 2017; ISBN 978-83-7511-254-2. [Google Scholar]
- Yao, G.; Wang, Z.; Yao, J.; Cong, X.; Anning, C.; Lyu, X. Pozzolanic activity and hydration properties of feldspar after mechanical activation. Powder Technol. 2021, 383, 167–174. [Google Scholar] [CrossRef]
- Kinuthia, J.M. 22-Sustainability of wastepaper in construction. In Woodhead Publishing Series in Civil and Structural Engineering, Sustainability of Construction Materials, 2nd ed.; Jamal, M.K., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 567–596. ISBN 9780081009956. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing, Thomas Telford Services Ltd: London UK, 1997. [Google Scholar]
- Appelo, C.A.J. The anion exchange properties of AFm (hydrocalumite-group) minerals defined from solubility experiments and crystallographic information. Cem. Con. Res. 2021, 140, 106270. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Chen, Z.; Ji, R.; Liu, L.; Wang, X. Fabrication and characterization of porous cordierite ceramics prepared from fly ash and natural minerals. Ceram. Int. 2019, 45, 18306–18314. [Google Scholar] [CrossRef]
- PKN. PN-EN 196-1:2016-07. In Metody badania cementu -Część 1: Oznaczanie Wytrzymałości (Cement Test Methods-Part 1: Determination of Strength); PKN: Warsaw, Poland, 2018. [Google Scholar]
- PN-EN ISO 11885:2009 Jakość wody. In Oznaczanie Wybranych Pierwiastków Metodą Optycznej Spektrometrii Emisyjnej z Plazmą Wzbudzoną Indukcyjnie (ICP-OES) (Water Quality. Determination of Selected Elements by Means of Optical Emission Spectrometry with Inductively Excited Plasma); PKN: Warsaw, Poland, 2009.
- Wang, L.; Geddes, D.A.; Walkley, B.; Provis, J.L.; Mechtcherine, V.; Tsang, D.C. The role of zinc in metakaolin-based geopolymers. Cem. Con. Res. 2020, 136, 106194. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhou, Y.L.; Chang, Q.; Qu, H.Q. Study on the factors of affecting the immobilization of heavy metals in fly ash-based geopolymers. Mater. Lett. 2006, 60, 820–822. [Google Scholar] [CrossRef]
- Palomo, A.; Palacios, M. Alkali-activated cementitious materials: Alternative matrices for the immobilisation of hazardous wastes: Part II. Stabilisation of chromium and lead. Cem. Con. Res. 2003, 33, 289–295. [Google Scholar] [CrossRef]
- Medina, T.J.; Arredondo, S.P.; Corral, R.; Jacobo, A.; Zárraga, R.A.; Rosas, C.A.; Cabrera, F.G.; Bernal, J.M. Microstructure and Pb2+ Adsorption Properties of Blast Furnace Slag and Fly Ash based Geopolymers. Minerals 2020, 10, 808. [Google Scholar] [CrossRef]
- Ariffin, N.; Abdullah, M.M.; Zainol, R.R.; Murshed, M.F. Geopolymer as An Adsorbent of Heavy Metal: A Review. In Proceedings of the AIP Conference Proceedings, Krabi, Thailand, 29–30 April 2017; Volume 1885, p. 020030, ISBN 978-0-7354-1565-2. Available online: https://aip.scitation.org/doi/abs/10.1063/1.5002224 (accessed on 26 September 2017). [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Con. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]




| Oxide | Chemical Formula | Unit | April/May 2015 (Range of 4 Tests) | June 2015 | June 2019 | July 2020 |
|---|---|---|---|---|---|---|
| Silica | SiO2 | % | 64.6–72.2 | 54.5–56.4 | 40.3 | 64.1 |
| Iron | Fe2O3 | % | 1.17–1.65 | 1.35–2.06 | 3.31 | 3.86 |
| Aluminum | Al2O3 | % | 3.30–2.60 | 3.45–3.46 | 6.22 | 3.60 |
| Manganese | Mn3O4 | % | 0.33–0.38 | 0.48–0.50 | 2.95 | 0.65 |
| Titanium | TiO2 | % | 0.17–0.23 | 0.23 | 0.69 | 0.34 |
| Calcium | CaO | % | 11.4–14.5 | 19.4–20.2 | 22.4 | 13.5 |
| Magnesium | MgO | % | 1.41–1.68 | 2.04–2.37 | 2.67 | 1.27 |
| Sulfur | SO3 | % | 1.33–1.57 | 3.09–2.90 | 2.87 | 2.41 |
| Phosphorus | P2O5 | % | 1.35–1.57 | 2.16–2.24 | 2.87 | 0.47 |
| Sodium | Na2O | % | 0.36–0.47 | 0.43–0.45 | 0.79 | 0.22 |
| Potassium | K2O | % | 4.30–3.90 | 3.89–5.94 | 4.55 | 1.32 |
| Barium | BaO | % | 0.05–0.06 | 0.07 | - | - |
| Strontium | SrO | % | 0.03 | 0.04 | - | - |
| Loss on ignition-LOI | (550 °C) | % | 0.22–1.07 | 0.01 | - | - |
| Chlorides | as Cl | % | 0.41–0.58 | 0.62–1.05 | - | - |
| Carbonates | as CO2 | % | 1.98–3.10 | 4.63–4.66 | 6.15 | 2.47 |
| Free CaO | % | 0.85–1.64 | 2.90–3.41 | - | - | |
| Total Organic Carbon | as C | % | <0.34 | 0.15–0.35 | - | - |
| Oxide | Chemical Formula | Unit | 2008 | 2011 | 2014 | 2019 |
|---|---|---|---|---|---|---|
| Silica | SiO2 | % | 79.5 | 81.03 | 78.00 | 75.8 |
| Iron | Fe2O3 | % | 8.33 | 4.81 | 7.30 | 7.50 |
| Aluminum | Al2O3 | % | 3.54 | 5.29 | 5.87 | 4.51 |
| Manganese | Mn3O4 | % | 0.11 | 0.07 | 0.04 | 0.07 |
| Titanium | TiO2 | % | 0.23 | 0.42 | 0.04 | 0.23 |
| Calcium | CaO | % | 5.48 | 3.88 | 3.17 | 6.50 |
| Magnesium | MgO | % | 0.94 | 1.06 | 0.69 | 0.76 |
| Sulfur | SO3 | % | 0.91 | 0.44 | 1.48 | 1.07 |
| Phosphorus | P2O5 | % | 0.05 | 0.10 | 0.05 | 0.04 |
| Sodium | Na2O | % | 0.08 | 0.08 | 0.05 | 0.12 |
| Potassium | K2O | % | 0.25 | 0.52 | 0.05 | 0.52 |
| Barium | BaO | % | 0.03 | 0.04 | 0.02 | - |
| Strontium | SrO | % | 0.04 | 0.03 | 0.02 | - |
| Carbon | C | % | TOC: 0.33 | TOC: 2.81 | TOC: 1.87 | CO2: 7.2% |
| LOI Loss on ignition in 550 °C | % | 0.36 | 1.94 | 2.10 | - | |
| Content (% by Mass) | ||||
|---|---|---|---|---|
| Oxide | GGBFS | Zeolite | LFS | BFA |
| CaO | 43.27 | 3.08 | 23.53 | 11.93 |
| SiO2 | 40.43 | 70.77 | 53.42 | 64.96 |
| Fetot. as Fe2O3 | 0.81 | 1.50 | 5.51 | 2.50 |
| Al2O3 | 7.88 | 12.56 | 6.24 | 4.16 |
| MgO | 6.97 | 0.65 | 3.14 | 1.79 |
| SO3 | 0.50 | - | 0.18 | 0.21 |
| Na2O | 0.46 | 0.59 | 0.11 | 0.49 |
| K2O | 0.29 | 3.40 | 0.41 | 3.92 |
| TiO2 | 0.28 | 0.15 | 0.81 | 0.48 |
| MnO | 0.16 | 0.04 | 0.21 | 0.40 |
| P2O5 | 0.02 | 0.03 | 0.09 | 1.57 |
| Cr2O3 | 0.01 | 0.01 | 0.03 | 0.03 |
| ZrO2 | <0.01 | <0.01 | 0.04 | 0.04 |
| HfO2 | <0.01 | <0.01 | <0.01 | <0.01 |
| loss on ignition (LOI) | 0.46 | 7.27 | 0.84 | 7.25 |
| Material | Share, % | Fraction, mm | Standard Deviation | Coefficient of Variation |
|---|---|---|---|---|
| LFS | D10 | 7.27 | 0.066 | 0.91 |
| D50 | 39.51 | 0.231 | 0.58 | |
| D90 | 128.27 | 2.908 | 2.27 | |
| BFA | D10 | 3.96 | 0.088 | 2.23 |
| D50 | 27.11 | 0.414 | 1.53 | |
| D90 | 81.80 | 1.694 | 2.07 |
| Series | GGBFS | Activating Water | Sand | LFS | BFA | Zeolite | Soaking the Zeolite | |
|---|---|---|---|---|---|---|---|---|
| NitCal | Deionized Water | |||||||
| ZK1 | 2700 | 1755 | 10,530 | 540 | 270 | - | - | - |
| ZK1z10 | 2700 | 1755 | 10,530 | 540 | 270 | 270 | - | 126.9 |
| ZK1z10nc | 2700 | 1755 | 10,530 | 540 | 270 | 270 | 216 | - |
| Series | GGBFS | Activating Water | Sand | LFS | BFA | Zeolite | Soaking the Zeolite | |
|---|---|---|---|---|---|---|---|---|
| NitCal | Deionized Water | |||||||
| ZK1 | 17.29 | 11.24 | 66.28 | 3.46 | 1.73 | - | - | - |
| ZK1z10 | 16.86 | 10.96 | 64.64 | 3.37 | 1.69 | 1.69 | - | 0.79 |
| ZK1z10nc | 16.77 | 10.90 | 64.28 | 3.35 | 1.68 | 1.68 | 1.34 | - |
| Hydration Time, Week | ZK1 | ZK1z10 | ZK1z10nc | |||
|---|---|---|---|---|---|---|
| Bending, MPa | Compressing, MPa | Bending, MPa | Compressing, MPa | Bending, MPa | Compressing, MPa | |
| 1 | 3.6 | 16.0 | 3.6 | 18.7 | 3.0 | 9.8 |
| 2 | - | - | 3.0 | 24.7 | 2.7 | 12.0 |
| 3 | 3.2 | 24.0 | 3.5 | 26.5 | 2.2 | 12.4 |
| 8 | 2.9 | 31.0 | 2.7 | 30.3 | 1.0 | 15.6 |
| Curing, 28d | ZK1 | ZK1z10 | ZK1z10nc | |||
|---|---|---|---|---|---|---|
| Bending, MPa | Compressing, MPa | Bending, MPa | Compressing, MPa | Bending, MPa | Compressing, MPa | |
| Wet | 7.5 | 25.5 | 7.8 | 26.3 | 5.5 | 13.8 |
| Dry | 3.5 | 27.5 | 2.8 | 28.2 | 2.5 | 14.0 |
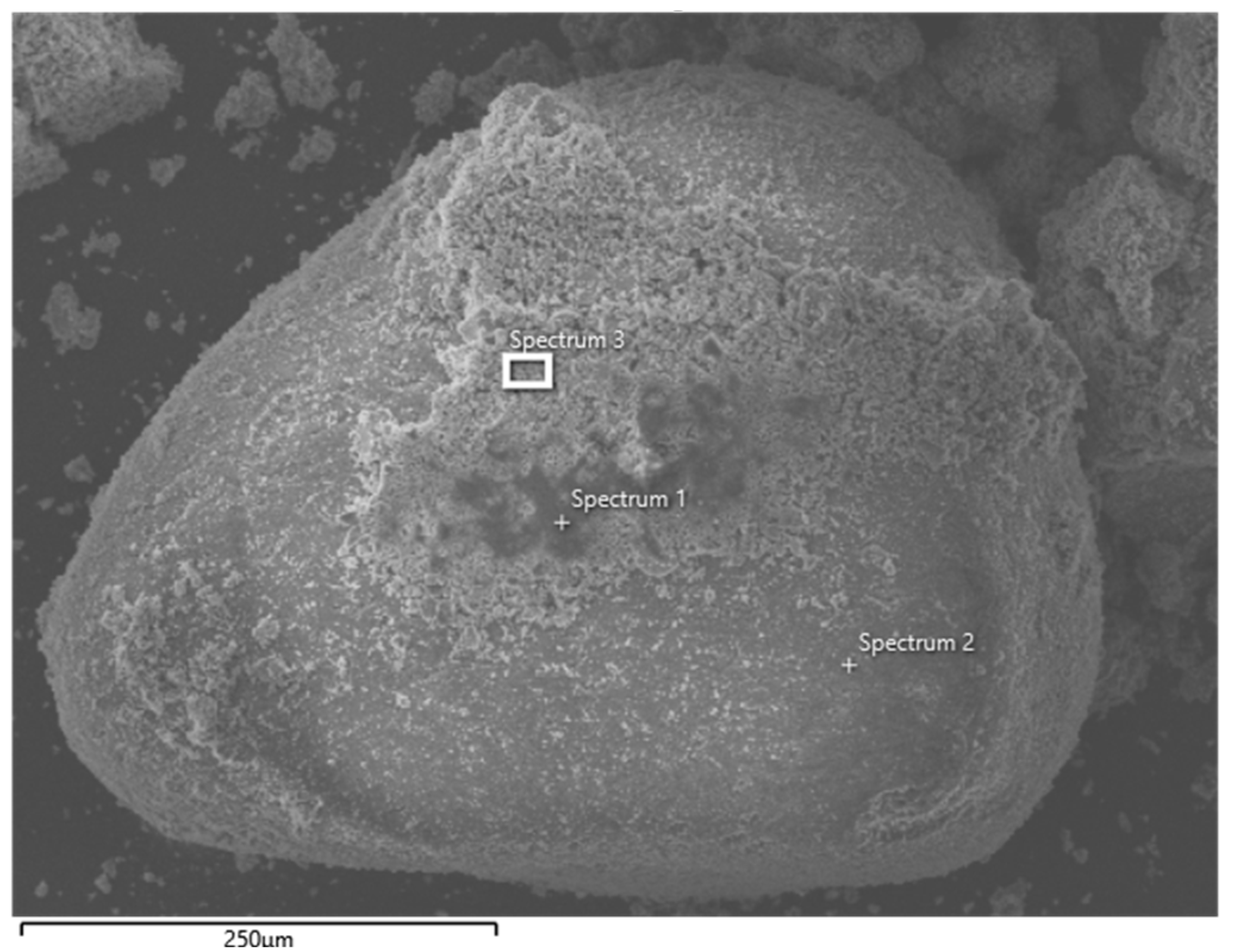
| Element | Weight % | ||
|---|---|---|---|
| Spectrum 1 | Spectrum 2 | Spectrum 3 | |
| Si | 11.76 | 40.04 | 12.42 |
| Ca | 15.27 | 0.22 | 21.48 |
| O | 45.11 | 58.50 | 43.47 |
| Mg | 2.68 | 0.12 | 2.23 |
| Al | 2.70 | 0.15 | 2.43 |
| S | 0.78 | 0.99 | |
| Zn | 4.73 | 4.24 | |
| Na | 4.69 | 0.96 | 3.16 |
| K | 0.28 | 0.44 | |
| Ti | 0.54 | 0.09 | |
| C | 11.16 | 7.51 | |
| Fe | 0.31 | 1.55 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słomka-Słupik, B. Self-Immobilizing Metals Binder for Construction Made of Activated Metallurgical Slag, Slag from Lignite Coal Combustion and Ash from Biomass Combustion. Materials 2021, 14, 3101. https://doi.org/10.3390/ma14113101
Słomka-Słupik B. Self-Immobilizing Metals Binder for Construction Made of Activated Metallurgical Slag, Slag from Lignite Coal Combustion and Ash from Biomass Combustion. Materials. 2021; 14(11):3101. https://doi.org/10.3390/ma14113101
Chicago/Turabian StyleSłomka-Słupik, Barbara. 2021. "Self-Immobilizing Metals Binder for Construction Made of Activated Metallurgical Slag, Slag from Lignite Coal Combustion and Ash from Biomass Combustion" Materials 14, no. 11: 3101. https://doi.org/10.3390/ma14113101
APA StyleSłomka-Słupik, B. (2021). Self-Immobilizing Metals Binder for Construction Made of Activated Metallurgical Slag, Slag from Lignite Coal Combustion and Ash from Biomass Combustion. Materials, 14(11), 3101. https://doi.org/10.3390/ma14113101






