Solvent-Free Synthesized Monolithic Ultraporous Aluminas for Highly Efficient Removal of Remazol Brilliant Blue R: Equilibrium, Kinetic, and Thermodynamic Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and UPA Monolith Synthesis
2.2. Characterization
2.3. Batch Adsorption Studies
2.4. Data Analysis
2.4.1. Adsorption Kinetic Study
2.4.2. Rate-Limiting Step Determination Study
2.4.3. Adsorption Equilibrium Study
2.4.4. Adsorption Thermodynamic Study
3. Results and Discussions
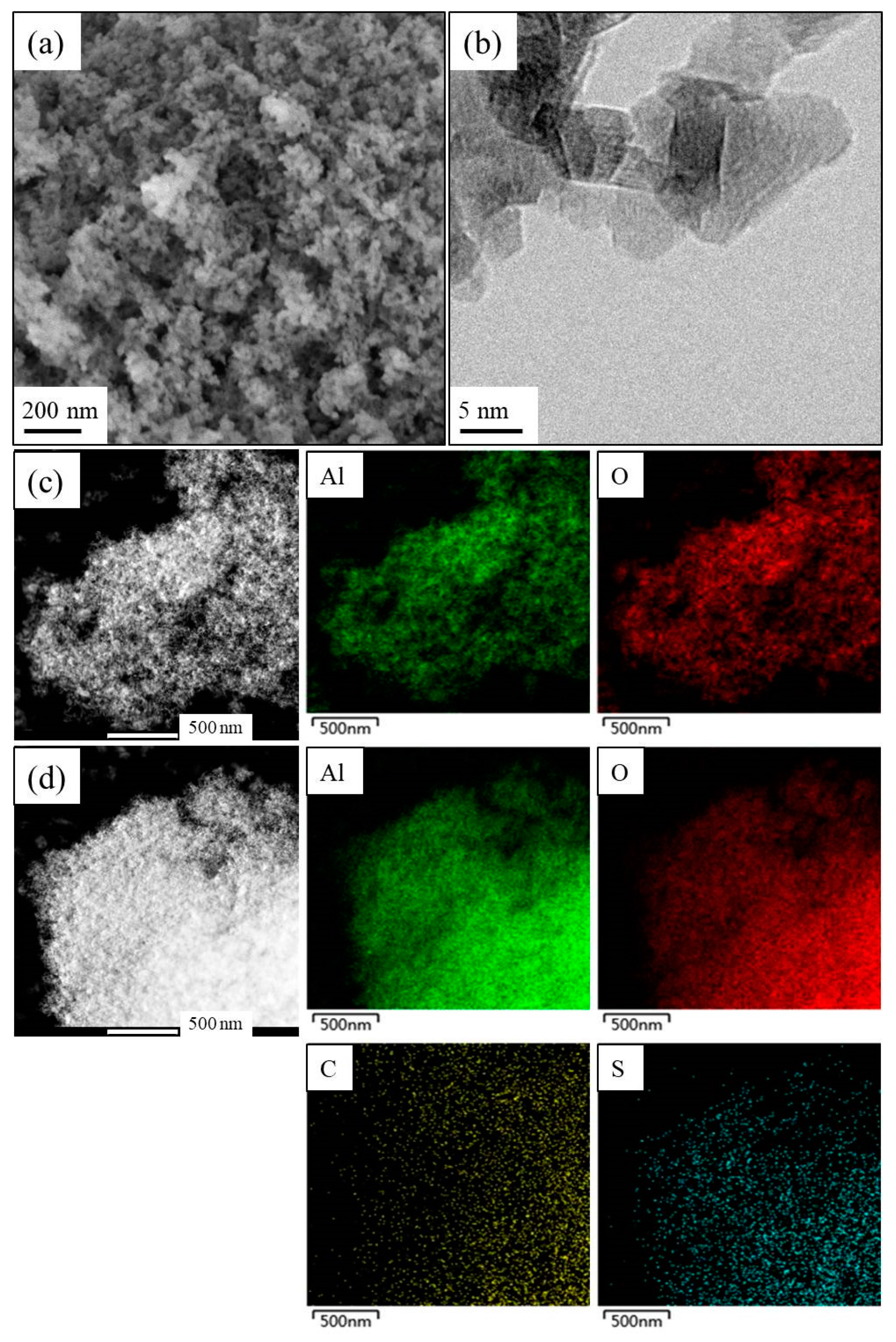
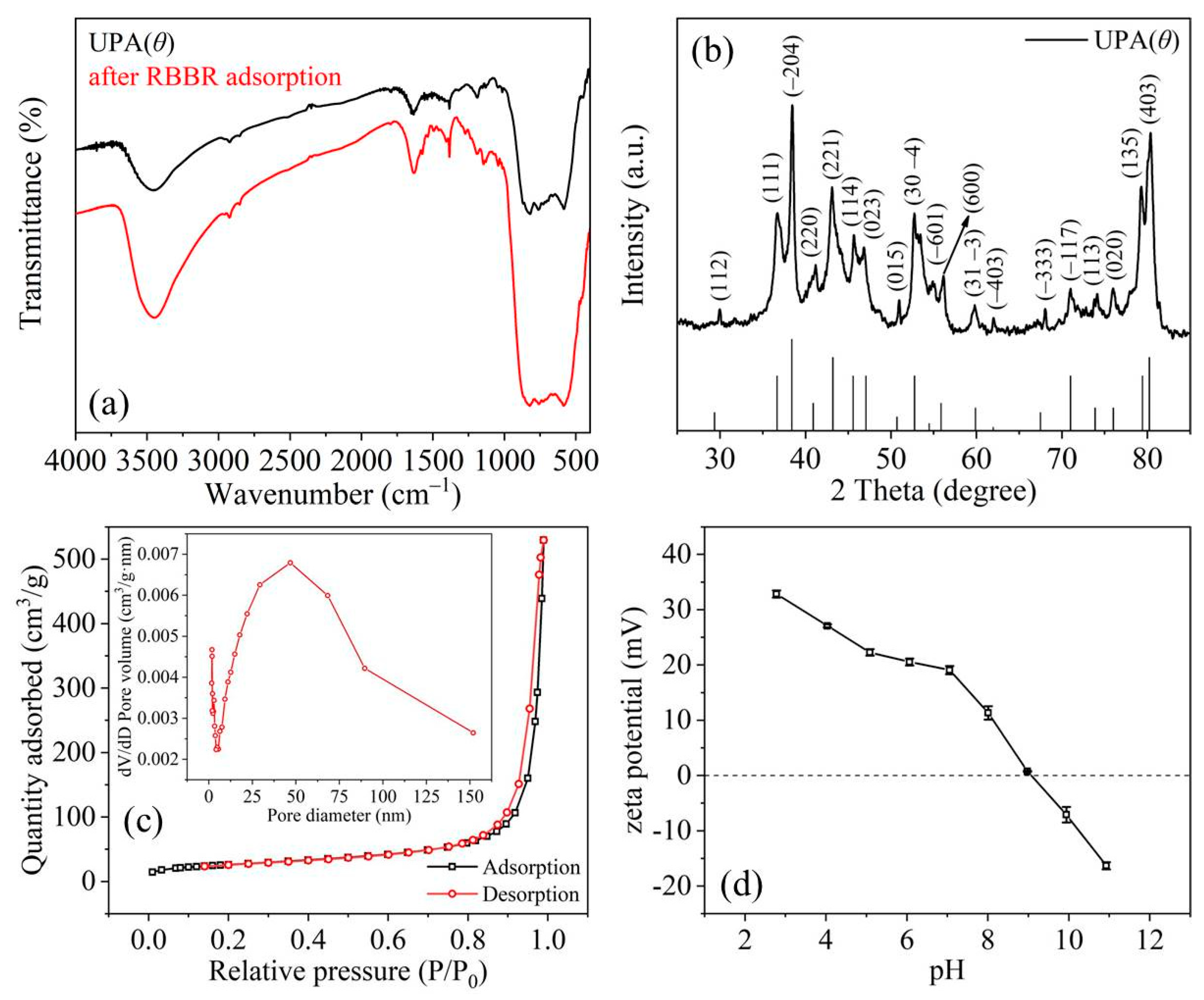
3.1. Characterization
3.2. Initial pH Effect and Adsorption Kinetics
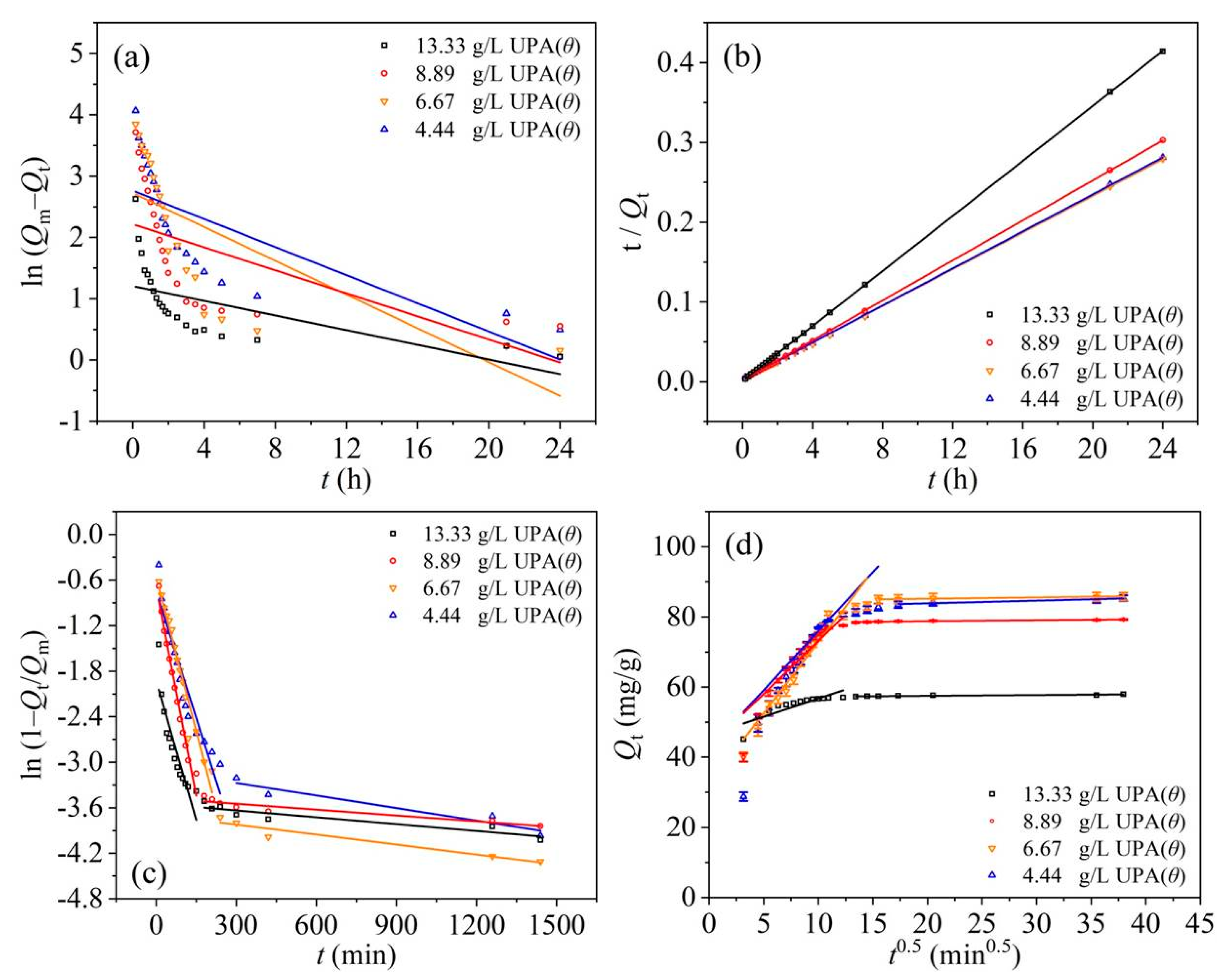
3.3. Adsorption Kinetic and Rate-Limiting Step Determination Studies
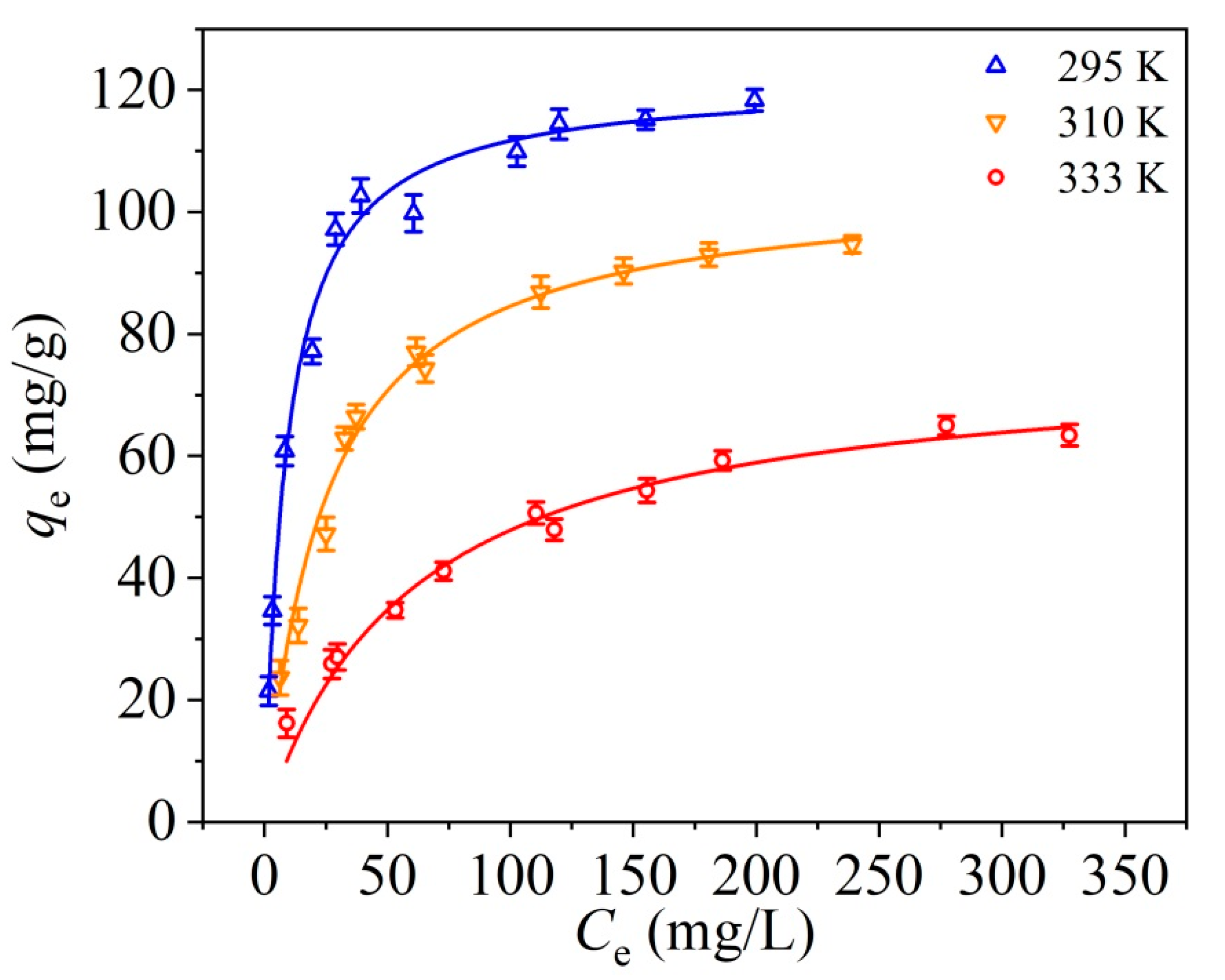
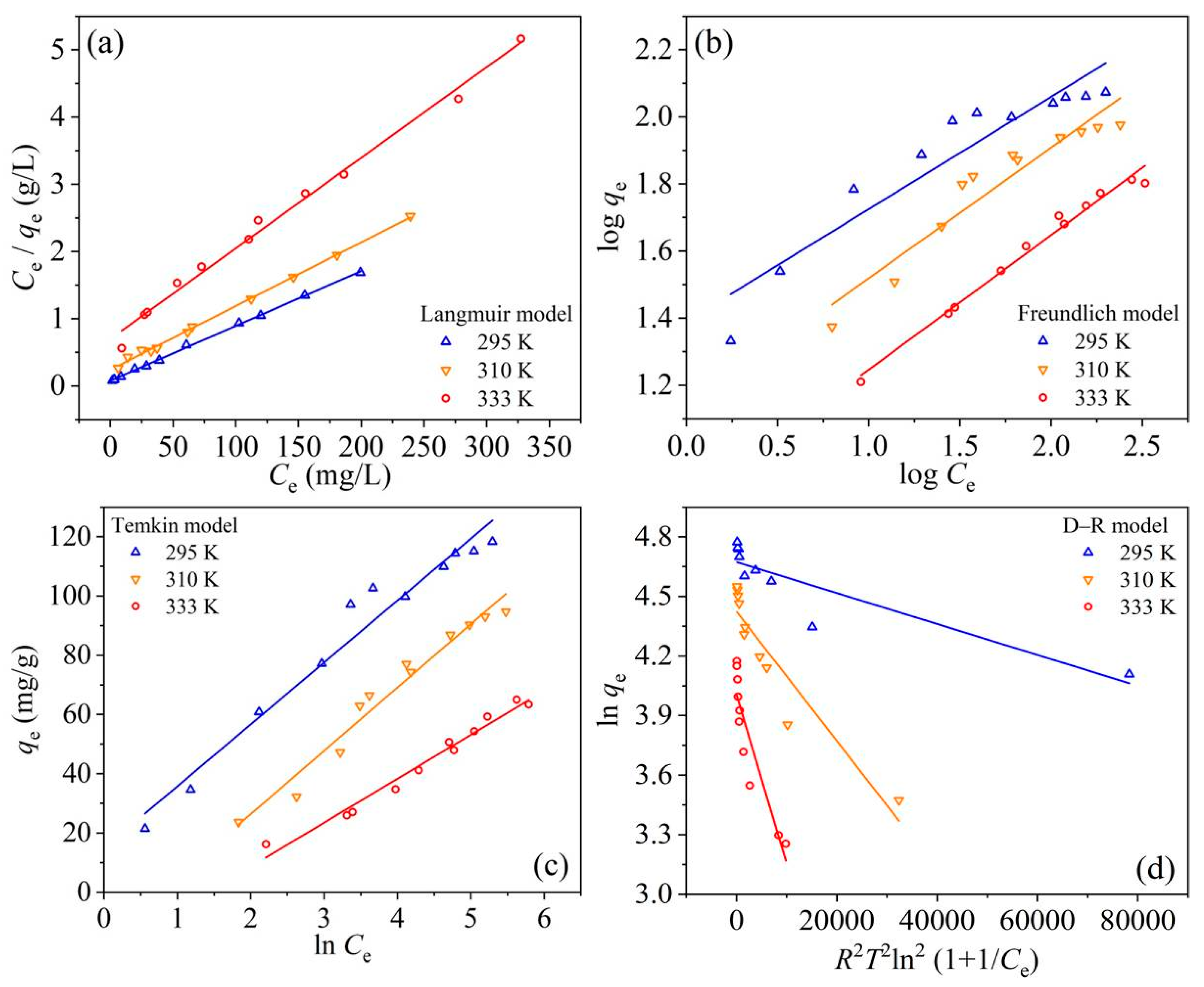
3.4. Adsorption Equilibrium Study
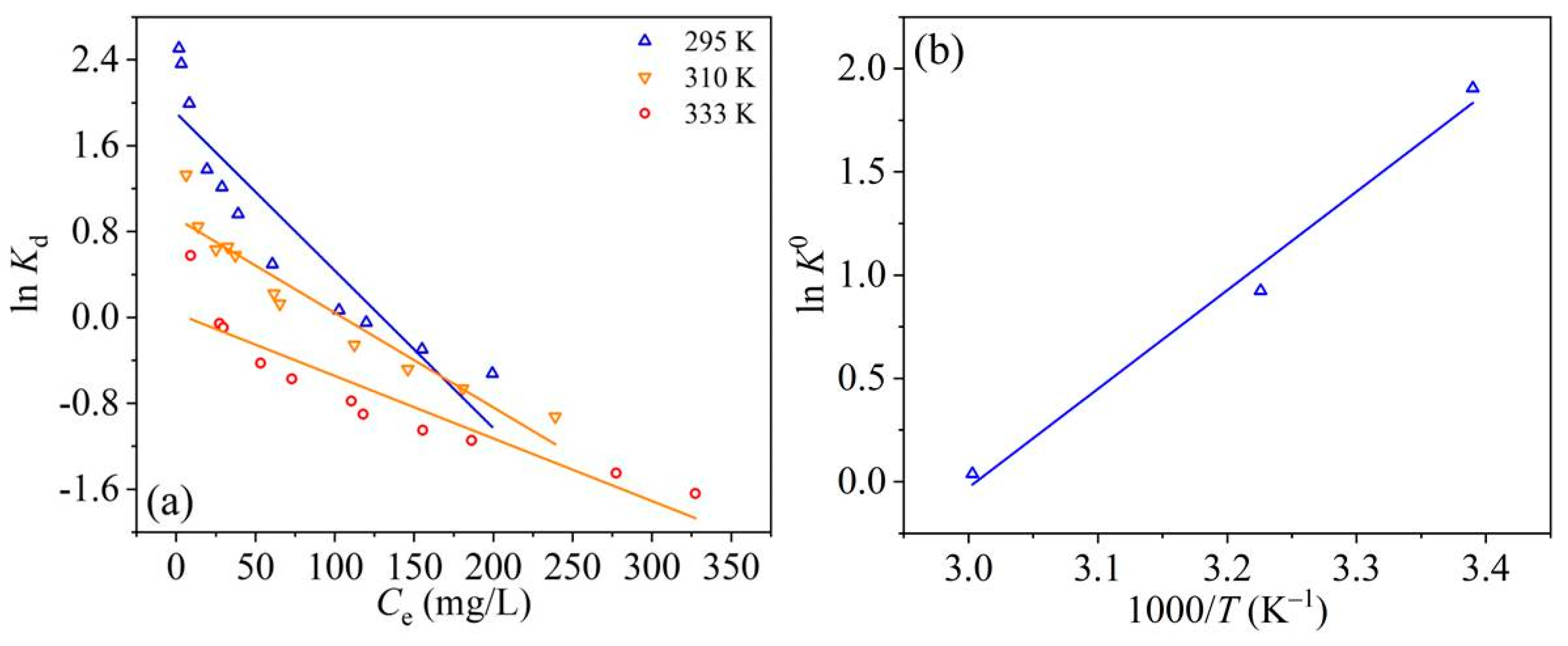
3.5. Adsorption Thermodynamic Study
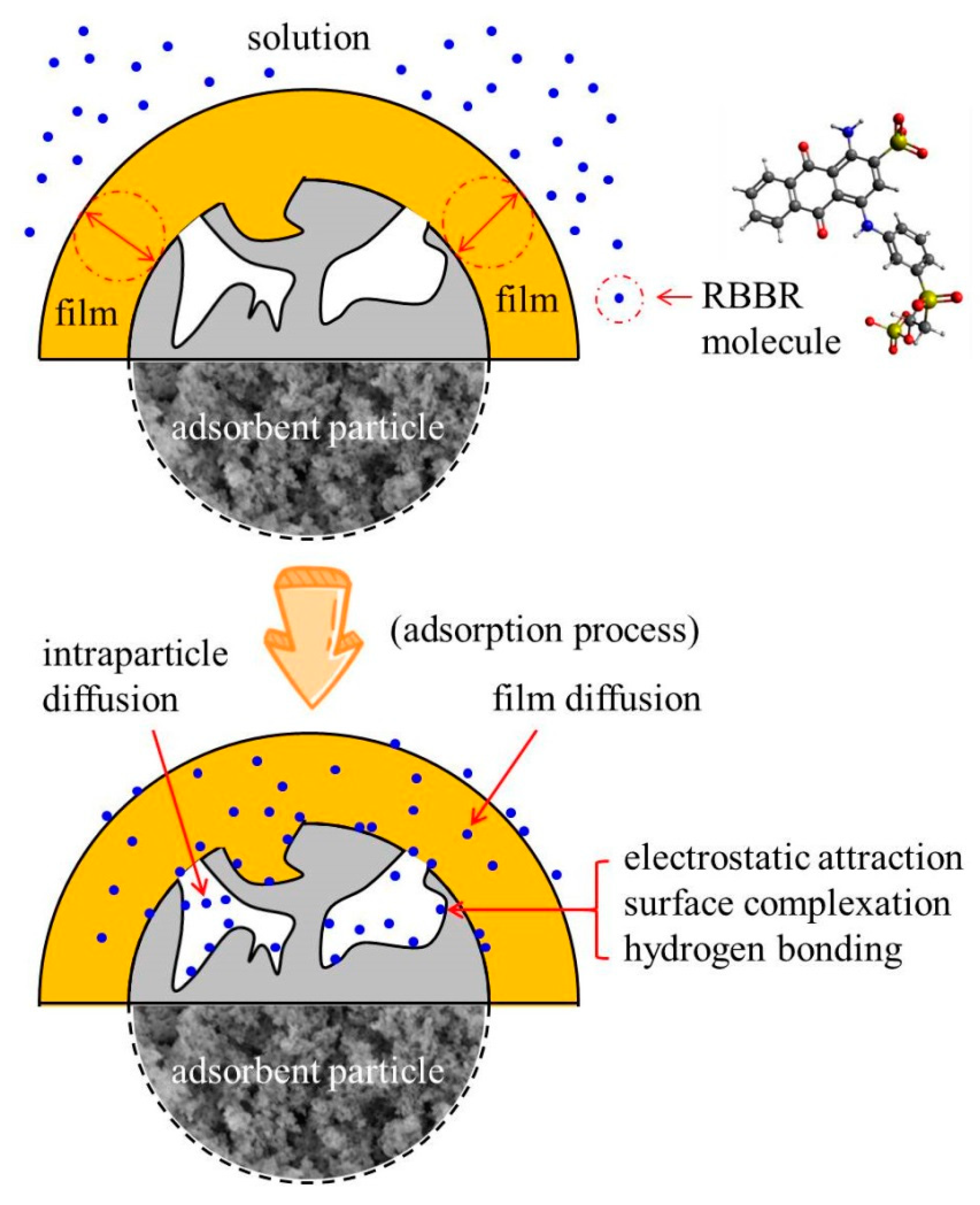
3.6. Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavis, L.D. Teaching old dyes new tricks: Biological probes built from fluoresceins and rhodamines. Annu. Rev. Biochem. 2017, 86, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Routoula, E.; Patwardhan, S.V. Degradation of anthraquinone dyes from effluents: A review focusing on enzymatic dye degradation with industrial potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Parra-Saldivar, R.; Hu, H.-B.; Wang, W.; Zhang, X.-H.; Iqbal, H.M.N. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants—A review. Sci. Total Environ. 2017, 576, 646–659. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Jalal, N.R.; Ahmadi, M. Kinetic and thermodynamic studies of the adsorption of several anionic dyes from water samples on magnetite-modified multi-walled carbon nanotubes. Sep. Purif. Technol. 2013, 48, 2638–2648. [Google Scholar] [CrossRef]
- Cicek, F.; Özer, D.; Özer, A.; Özer, A. Low cost removal of reactive dyes using wheat bran. J. Hazard. Mater. 2007, 146, 408–416. [Google Scholar] [CrossRef]
- Gök, Ö.; Özcan, A.S.; Özcan, A. Adsorption behavior of a textile dye of Reactive Blue 19 from aqueous solutions onto modified bentonite. Appl. Surf. Sci. 2010, 256, 5439–5443. [Google Scholar] [CrossRef]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.-X.; Zhao, G.-X.; Chen, C.-L.; Chai, Z.-F.; Alsaedi, A.; Hayat, T.; Wang, X.-K. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interf. Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.-P.; Ma, B.; Hung, C.-T.; Li, W.; Zhao, D.-Y. Spherical mesoporous materials from single to multilevel architectures. Acc. Chem. Res. 2019, 52, 2928–2938. [Google Scholar] [CrossRef] [PubMed]
- Vignes, J.-L.; Frappart, C.; Di Costanzo, T.; Rouchaud, J.-C.; Mazerolles, L.; Michel, D. Ultraporous monoliths of alumina prepared at room temperature by aluminium oxidation. J. Mater. Sci. 2008, 43, 1234–1240. [Google Scholar] [CrossRef]
- Nguyen, T.H.N. Elaboration and Modifications of Nanofibrous Al2O3. Chemical and Process Engineering. Ph.D. Thesis, Université Sorbonne, Paris, France, 2016. [Google Scholar]
- Monsef Khoshhesab, Z.; Ahmadi, M. Removal of reactive blue 19 from aqueous solutions using NiO nanoparticles: Equilibrium and kinetic studies. Desalin. Water Treat. 2015, 57, 20037–20048. [Google Scholar] [CrossRef]
- Beauvy, M.; Vignes, J.-L.; Michel, D.; Mazerolles, L.; Frappart, C.; Di Costanzo, T. Process for the Preparation of Monolithic Hydrated Alumina, Amorphous or Crystallized Alumina, Aluminates and Composite Materials by Metal Aluminum Oxidation or Aluminum Alloy. European Patent Application FR2847569, 28 May 2004. [Google Scholar]
- Beauvy, M.; Vignes, J.-L.; Michel, D.; Mazerolles, L.; Frappart, C.; Di Costanzo, T. Method for Preparing Monolithic Hydrated Aluminas and Composite Materials. European Patent Application EP1562859, 17 August 2005. [Google Scholar]
- Khatim, O.; Nguyen, T.H.N.; Amamra, M.; Museur, L.; Khodan, A.; Kanaev, A. Synthesis and photoluminescence properties of nanostructured mullite/α-Al2O3. Acta Mater. 2014, 71, 108–116. [Google Scholar] [CrossRef]
- Bouslama, M.; Amamra, M.C.; Jia, Z.-X.; Ben Amar, M.; Chhor, K.; Brinza, O.; Abderrabba, M.; Vignes, J.-L.; Kanaev, A. Nanoparticulate TiO2-Al2O3 photocatalytic media: Effect of particle size and polymorphism on photocatalytic activity. ACS Catal. 2012, 2, 1884–1892. [Google Scholar] [CrossRef]
- Bouslama, M.; Amamra, M.C.; Brinza, O.; Tieng, S.; Chhor, K.; Abderrabba, M.; Vignes, J.-L.; Kanaev, A. Isolation of titania nanoparticles in monolithic ultraporous alumina: Effect of nanoparticle aggregation on anatase phase stability and photocatalytic activity. Appl. Catal. A 2011, 402, 156–161. [Google Scholar] [CrossRef]
- Tchieda, V.K.; D’Amato, E.; Chiavola, A.; Parisi, M.; Chianese, A.; Amamra, M.; Kanaev, A. Removal of arsenic by alumina: Effects of material size, additives, and water contaminants. Clean 2016, 44, 496–505. [Google Scholar] [CrossRef]
- Chiavola, A.; Tchieda, V.K.; D’Amato, E.; Chianese, A.; Kanaev, A. Synthesis and characterization of nanometric titania coated on granular alumina for arsenic removal. Chem. Eng. Trans. 2016, 47, 331–336. [Google Scholar]
- Özsoy, H.D.; Ünyayar, A.; Mazmanci, M.A. Decolourisation of reactive textile dyes Drimarene Blue X3LR and Remazol Brilliant Blue R by Funalia trogii ATCC 200800. Biodegradation 2005, 16, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Khodan, A.; Nguyen, T.H.N.; Esaulkov, M.; Kiselev, M.R.; Amamra, M.; Vignes, J.-L.; Kanaev, A. Porous monoliths consisting of aluminum oxyhydroxide nanofibrils: 3D structure, chemical composition, and phase transformations in the temperature range 25–1700 °C. J. Nanopart. Res. 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S.J. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Pur. Met. 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption of carbon from solutions. J. Sanit. Eng. Div. 1963, 89, 31–63. [Google Scholar] [CrossRef]
- Fan, H.-T.; Sun, W.; Jiang, B.; Wang, Q.-J.; Li, D.-W.; Huang, C.-C.; Wang, K.-J.; Zhang, Z.-G.; Li, W.-X. Adsorption of antimony(III) from aqueous solution by mercapto-functionalized silica-supported organic-inorganic hybrid sorbent: Mechanism insights. Chem. Eng. J. 2016, 286, 128–138. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundamen. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Webber, T.W.; Chakkravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AlChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen (Over the adsorption in solution). J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Haghseresht, F.; Lu, G.-Q. Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Temkin, M.I.; Pyzhev, V.P. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of the activated charcoal. Proc. USSR Acad. Sci. 1947, 55, 331–337. [Google Scholar]
- Hobson, J.P. Physical adsorption isotherms extending from ultra high vacuum to vapor pressure. J. Phys. Chem. 1969, 73, 2720–2727. [Google Scholar] [CrossRef]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloid. Surf. A 2006, 277, 90–97. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. Wastewater treatment by physical-chemical technologies. In Wastewater Engineering: Advanced Wastewater Treatment Systems; Aziz, H.A., Mojiri, A., Eds.; IJSR Publications: Ahmedabad, India, 2014; pp. 5–48. [Google Scholar]
- Souza Santos, P.; Souza Santos, H.; Toledo, S.P. Standard transition aluminas. Electron microscopy studies. J. Mater. Res. 2000, 3, 104–114. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Storck, S.; Bretinger, H.; Maier, W.F. Characterization of micro- and mesoporous solids by physisorption methods and pore-size analysis. Appl. Catal. A 1998, 174, 137–146. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A. Biosorption of reactive dye by loofa sponge-immobilized fungal biomass of Phanerochaete chrysosporium. Process Biochem. 2007, 42, 1160–1164. [Google Scholar] [CrossRef]
- O’Mahony, T.; Guibal, E.; Tobin, J.M. Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme Microb. Technol. 2002, 31, 456–463. [Google Scholar] [CrossRef]
- Shanehsaz, M.; Seidi, S.; Ghorbani, Y.; Shoja, S.M.; Rouhani, S. Polypyrrole-coated magnetic nanoparticles as an efficient adsorbent for RB19 synthetic textile dye: Removal and kinetic study. Spectrochim. Acta A 2015, 149, 481–486. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Delacroix, A. Removal of Cd(II) and Pb(II) ions, from aqueous solutions, by adsorption onto sawdust of Pinus sylvestris. J. Hazard. Mater. 2003, 105, 121–142. [Google Scholar] [CrossRef]
- Monsef Khoshhesab, Z.; Souhani, S. Adsorptive removal of reactive dyes from aqueous solutions using zinc oxide nanoparticles. J. Chin. Chem. Soc. 2018, 65, 1482–1490. [Google Scholar] [CrossRef]
- Monsef Khoshhesab, Z.; Modaresnia, N. Adsorption of Acid Black 210 and Remazol Brilliant Blue R onto magnetite nanoparticles. Inorg. Nano Met. Chem. 2019, 49, 231–239. [Google Scholar] [CrossRef]
- Ijagbemi, C.O.; Baek, M.H.; Kim, D.S. Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J. Hazard. Mater. 2009, 166, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.C.; Swamy, M.M.; Mall, I.D.; Prasad, B.; Mishra, I.M. Adsorptive removal of phenol by bagasse fly ash and activated carbon: Equilibrium, kinetics and thermodynamics. Colloid. Surf. A 2006, 272, 89–104. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Choi, I.; Rengraj, S.; Yi, J. Arsenic removal using mesoporous alumina prepared via a templating method. Environ. Sci. Technol. 2004, 38, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Ouali, M.S.; Elandaloussi, E.H.; De Menorval, L.C.; Lindheimer, M. Chemically modified olive stone: A low-cost sorbent for heavy metals and basic dyes removal from aqueous solutions. J. Hazard. Mater. 2009, 163, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Anayurt, R.A.; Sari, A.; Tuzen, M. Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem. Eng. J. 2009, 151, 255–261. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S. Insight into adsorption thermodynamics. In Thermodynamics; Mizutani, T., Ed.; InTech: London, UK, 2011; pp. 349–364. ISBN 978-953-307-544-0. [Google Scholar]
- Azizi, A.; Alavi Moghaddam, M.R.; Arami, M. Applications of wood waste for removal of reactive blue 19 from aqueous solutions: Optimization through response surface methodology. Environ. Eng. Manag. J. 2012, 11, 795–804. [Google Scholar]
- Ayazi, Z.; Khoshhesab, Z.M.; Norouzi, S. Modeling and optimizing of adsorption removal of Reactive Blue 19 on the magnetite/graphene oxide nanocomposite via response surface methodology. Desalin. Water Treat. 2016, 57, 25301–25316. [Google Scholar] [CrossRef]
- Hu, C.-W.; Hu, N.-T.; Li, X.-L.; Shen, H.-L.; Zhao, Y.-J. Adsorption of remazol brilliant blue R by carboxylated multi-walled carbon nanotubes. Desalin. Water Treat. 2017, 62, 282–289. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Mahmood-Kashani, H.; Ahmadi, M. Adsorption of some cationic and anionic dyes on magnetite nanoparticles-modified activated carbon from aqueous solutions: Equilibrium and kinetics study. J. Iran. Chem. Soc. 2012, 10, 481–489. [Google Scholar] [CrossRef]
- Liao, M.-H.; Chen, W.-C.; Lai, W.-C. Magnetic nanoparticles assisted low-molecular weight polyethyleneimine for fast and effective removal of reactive blue 19. Fresen. Environ. Bull. 2006, 15, 609–613. [Google Scholar]

| UPA(θ) Dosage (g·L−1) | ||||
|---|---|---|---|---|
| Kinetic Models | 4.44 | 6.67 | 8.89 | 13.33 |
| Lagergren pseudo-first-order | ||||
| k′ (h−1) | 0.115 | 0.138 | 0.094 | 0.060 |
| Qmc (mg·g−1) a | 15.844 | 15.243 | 9.171 | 3.337 |
| R2 | 0.521 | 0.511 | 0.336 | 0.332 |
| Pseudo-second-order | ||||
| k″ (g·mg−1·h−1) | 0.049 | 0.046 | 0.102 | 0.422 |
| Qmc (mg·g−1) a | 86.207 | 86.957 | 79.745 | 57.803 |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 |
| Qme (mg·g−1) b | 87.385 | 87.934 | 81.116 | 59.987 |
| First-Curved Adsorption Part | Second-Linear Adsorption Part | |||||||
|---|---|---|---|---|---|---|---|---|
| Diffusion Models | 4.44 | 6.67 | 8.89 | 13.33 | 4.44 | 6.67 | 8.89 | 13.33 |
| Film diffusion | ||||||||
| kFD (min−1) | 0.0111 | 0.0136 | 0.0184 | 0.0123 | 0.0006 | 0.0004 | 0.0003 | 0.0003 |
| Intercept on Y axis a | −0.746 | −0.556 | −0.690 | −1.922 | −3.108 | −3.691 | −3.471 | −3.545 |
| R2 | 0.911 | 0.956 | 0.976 | 0.807 | 0.885 | 0.909 | 0.866 | 0.826 |
| Intraparticle diffusion | ||||||||
| kIPD (mg·g−1·min−0.5) | 3.3628 | 4.0538 | 3.0117 | 1.0385 | 0.0769 | 0.0374 | 0.0281 | 0.0217 |
| CIPD | 42.309 | 32.299 | 43.003 | 46.347 | 82.332 | 84.429 | 78.199 | 57.072 |
| R2 | 0.708 | 0.904 | 0.873 | 0.704 | 0.899 | 0.916 | 0.875 | 0.838 |
| Isotherm Models | 295 K | 310 K | 333 K |
|---|---|---|---|
| Langmuir | |||
| qe,max (mg·g−1) | 122.549 | 105.485 | 74.184 |
| KL (L·mg−1) | 0.107 | 0.039 | 0.019 |
| R2 | 0.999 | 0.998 | 0.991 |
| RL | 0.011–0.078 | 0.030–0.188 | 0.056–0.275 |
| Freundlich | |||
| KF (mg(1−1/n)·L1/n·g−1) | 24.548 | 13.461 | 6.991 |
| 1/n | 0.335 | 0.390 | 0.401 |
| R2 | 0.880 | 0.903 | 0.982 |
| Temkin | |||
| B (J·mol−1) | 20.892 | 21.376 | 14.824 |
| KT (L·g−1) | 2.036 | 0.464 | 0.242 |
| R2 | 0.960 | 0.961 | 0.973 |
| D–R | |||
| qe,max (mg·g−1) | 107.037 | 83.502 | 54.976 |
| β (mol2·kJ−2) | 7.795 × 10−6 | 3.257 × 10−5 | 8.496 × 10−5 |
| R2 | 0.784 | 0.866 | 0.823 |
| T (K) | ΔG0 (KJ·Mol−1) | ΔH0 (kJ·Mol−1) | ΔS0 (J·Mol−1·K−1) |
|---|---|---|---|
| 295 | −4.673 | ||
| 310 | −2.383 | −39.711 | −119.372 |
| 333 | −0.107 | ||
| Adsorbents | Experimental Conditions a | qe,max (Mg·g−1) b | Ref. |
|---|---|---|---|
| Mazandaran wood waste (WW) | pH = 1.72, T = ND | 4.75 | [57] |
| ZnO nanoparticles (ZnO NPs) | pH = 3.0, T = 298 K | 38.02 | [49] |
| Commercial NiO | pH = ND, T = 298 K | 38.62 | [13] |
| NiO nanoparticles | pH = ND, T = 298 K | 98.83 | [13] |
| Magnetite/GO (MGO) nanocomposite | pH = 3.0, T = 298 K | 62.50 | [58] |
| Magnetite nanoparticles (MNPs) | pH = ND, T = 298 K | 74.40 | [50] |
| Free fungal biomass (FFB) | pH = 2.0, T = 303 K | 80.91 | [45] |
| Loofa sponge-immobilized fungal biomass (LSIFB) | pH = 2.0, T = 303 K | 98.90 | [45] |
| Magnetite-modified MWCNTs (MMMCNTs) | pH = 4.0, T = 298 K | 88.80 | [4] |
| Rhizopus arrhizus biomass | pH = 2.0, T = 298 K | 90.00 | [46] |
| Carboxylated MWCNTs | pH = ND, T = 298 K | 95.24 | [59] |
| Wheat bran | pH = 1.5, T = 293 K | 97.10 | [5] |
| Magnetite nanoparticles-modified AC (MMAC) | pH = 4.0, T = 298 K | 104.60 | [60] |
| Polypyrrole-coated Fe3O4 (Ppy@Fe3O4 MNPs) | pH = 3.0, T = 298 K | 112.36 | [47] |
| Ultraporous alumina(γ) (UPA(α)) | pH = 4.0, T = 295 K | 17.42 | This study |
| Ultraporous alumina(θ) (UPA(θ)) | pH = 4.0, T = 295 K | 122.55 | This study |
| Ultraporous alumina(α) (UPA(γ)) | pH = 4.0, T = 295 K | 212.31 | This study |
| Modified polyethyleneimine (LMW–PEI) | pH = 10.0, T = 298 K | 121.00 | [61] |
| Modified bentonite (DAH–bentonite) | pH = 1.5, T = 293 K | 134.71 | [6] |
| MgO nanoparticles (Nano-MgO) | pH = 8.0, T = 298 K | 166.70 | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Boeuf, G.; Jia, Z.; Zhu, K.; Nikravech, M.; Kanaev, A.; Azouani, R.; Traore, M.; Elm’selmi, A. Solvent-Free Synthesized Monolithic Ultraporous Aluminas for Highly Efficient Removal of Remazol Brilliant Blue R: Equilibrium, Kinetic, and Thermodynamic Studies. Materials 2021, 14, 3054. https://doi.org/10.3390/ma14113054
Xu H, Boeuf G, Jia Z, Zhu K, Nikravech M, Kanaev A, Azouani R, Traore M, Elm’selmi A. Solvent-Free Synthesized Monolithic Ultraporous Aluminas for Highly Efficient Removal of Remazol Brilliant Blue R: Equilibrium, Kinetic, and Thermodynamic Studies. Materials. 2021; 14(11):3054. https://doi.org/10.3390/ma14113054
Chicago/Turabian StyleXu, Huan, Guilhem Boeuf, Zixian Jia, Kairuo Zhu, Mehrdad Nikravech, Andrei Kanaev, Rabah Azouani, Mamadou Traore, and Abdellatif Elm’selmi. 2021. "Solvent-Free Synthesized Monolithic Ultraporous Aluminas for Highly Efficient Removal of Remazol Brilliant Blue R: Equilibrium, Kinetic, and Thermodynamic Studies" Materials 14, no. 11: 3054. https://doi.org/10.3390/ma14113054
APA StyleXu, H., Boeuf, G., Jia, Z., Zhu, K., Nikravech, M., Kanaev, A., Azouani, R., Traore, M., & Elm’selmi, A. (2021). Solvent-Free Synthesized Monolithic Ultraporous Aluminas for Highly Efficient Removal of Remazol Brilliant Blue R: Equilibrium, Kinetic, and Thermodynamic Studies. Materials, 14(11), 3054. https://doi.org/10.3390/ma14113054







