Nanometer-Sized Boron Loaded Liposomes Containing Fe3O4 Magnetic Nanoparticles and Tributyl Borate and Anti-Albumin from Bovine Serum Antibody for Thermal Neutron Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Tributyl Borate
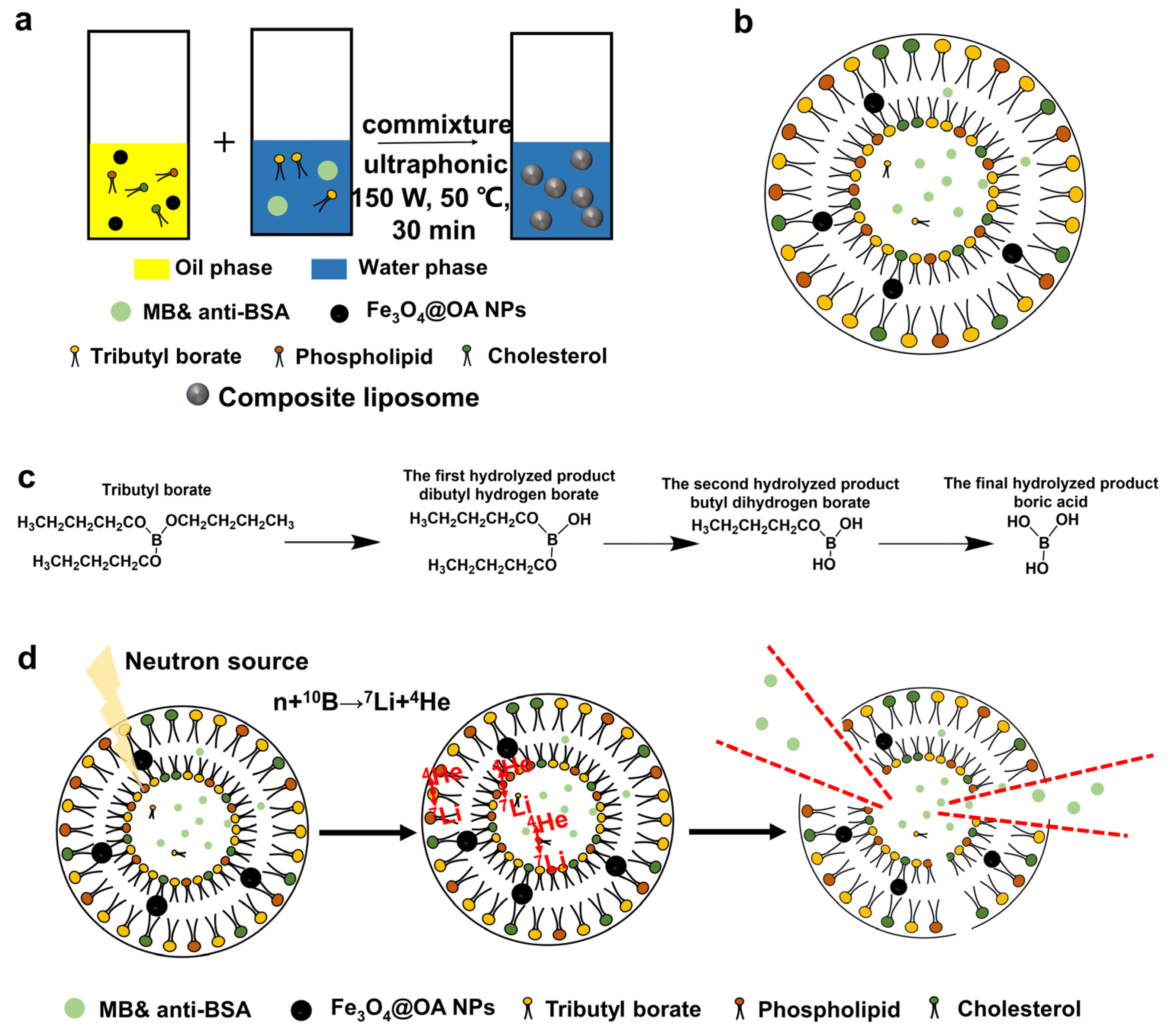
2.3. Preparation of Magnetic Liposomes Containing Boron and MB and Anti-BSA
2.4. Characterization of Materials
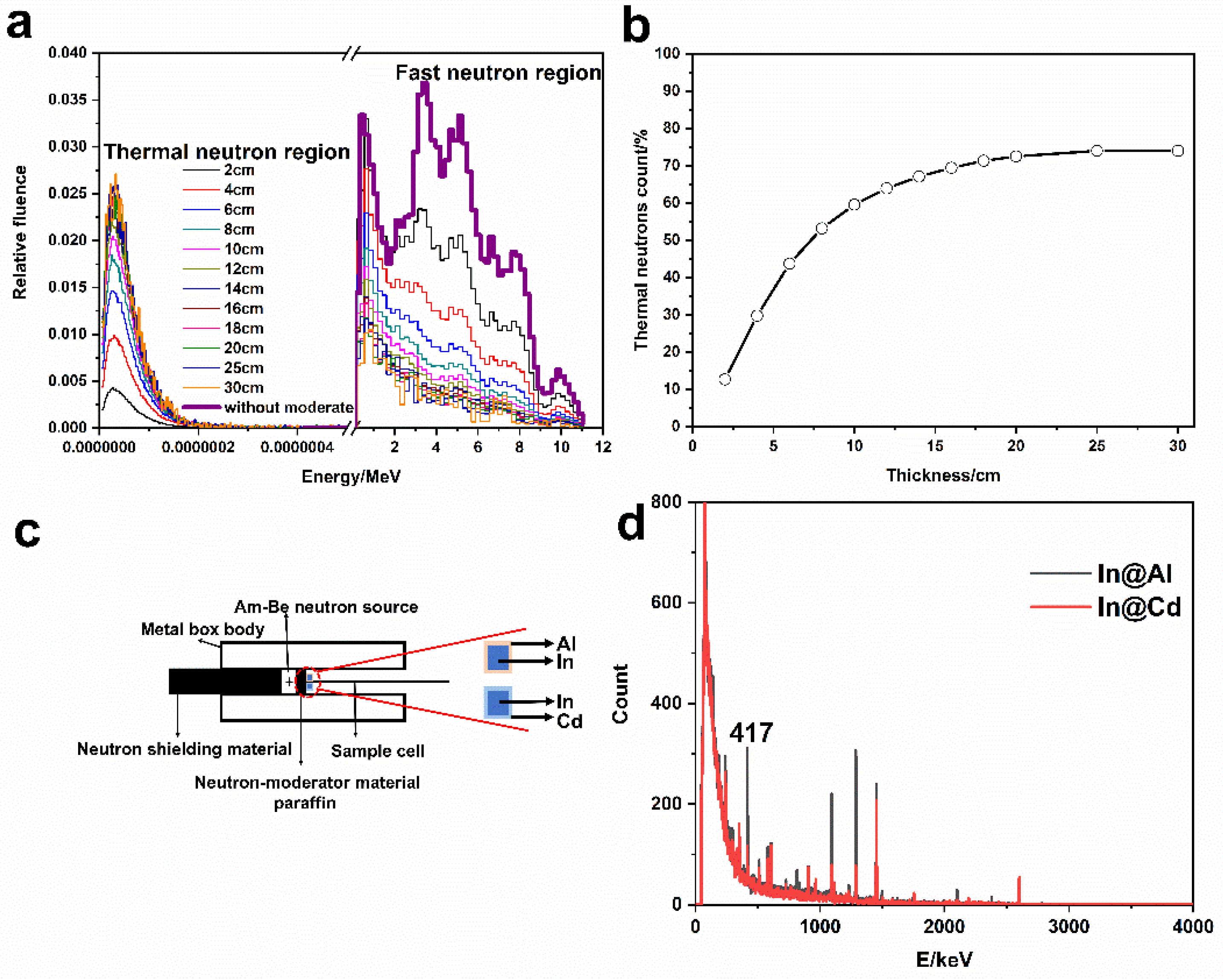
2.5. Neutron Irradiation Experiments
2.6. Thermal Neutron Detection Performance Analysis
3. Results
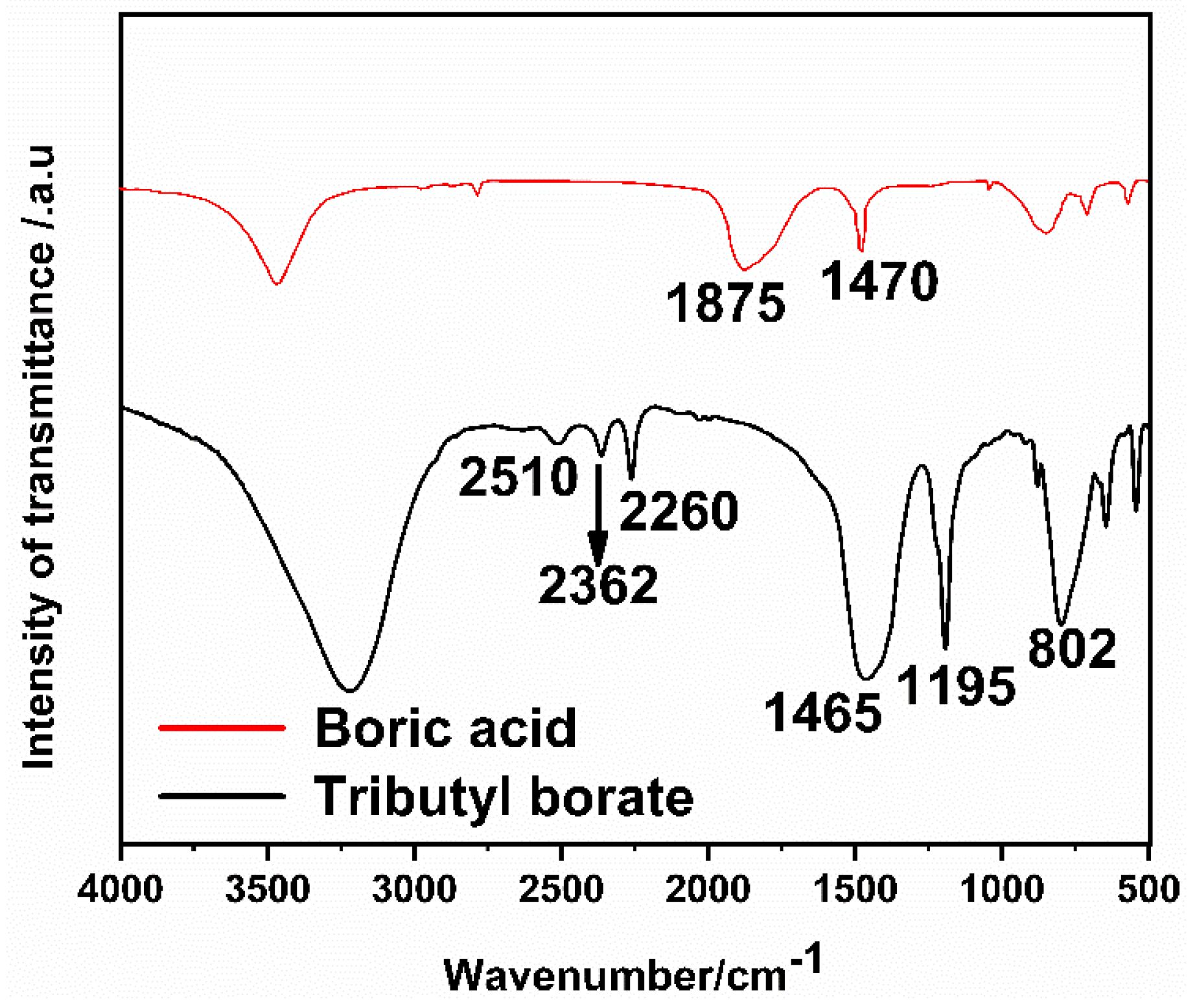
3.1. Characterization of Tributyl Borate
3.2. Process of Magnetic Liposomes Preparation
3.3. Ultrasonic Preparation of Magnetic Liposomes Containing Boron
3.4. Characterization of Magnetic Liposomes
3.5. The Measurement of Thermal Neutron
3.6. Release of MB and Anti-BSA Loaded Liposomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OA | oleic acid |
| NPs | nanoparticles |
| MB | methylene blue |
| anti-BSA | antialbumin from bovine serum |
| FT-IR | Fourier transform infrared spectroscopy |
| TEM | transmission electron microscopy |
| EDS | energy-dispersive X-ray spectroscopy |
| VSM | vibrating sample magnetometer |
| UV-vis | ultraviolet-visible |
| SPR | surface plasmon resonance |
| Li-6 | lithium-6 |
| B-10 | boron-10 |
| BN | boron nitride |
| h-BN | hexagonal BN |
| SERS | surface enhancement Raman scattering |
| 4-MPBA | 4-mercaptophenylboronic acid |
| BNCT | boron neutron capture therapy |
| BSH | B12H11SH |
| DLL | double lipidic layer |
| LSPR | localized SPR |
| HPRT | hypoxanthine phosphoribosyl transferase |
| RPEM | reverse-phase evaporation method |
| TGA | thermogravimetric analysis |
| MCNP | Monte Carlo N-Particle Transport Code System |
| TG | thermogravimetric |
| DTG | derivative of TG |
References
- Kouzes, R.T.; Ely, J.H.; Erikson, L.E.; Kernan, W.J.; Lintereur, A.T.; Siciliano, E.R.; Stephens, D.L.; Stromswold, D.C.; Ginhoven, R.M.V.; Woodring, M.L. Neutron detection alternatives to 3He for national security applications. Nucl. Instrum. Methods Phys. Res. A 2010, 623, 1035–1045. [Google Scholar] [CrossRef]
- Kouzes, R.T.; Lintereur, A.T.; Siciliano, E.R. Progress in alternative neutron detection to address the helium-3 shortage. Nucl. Instrum. Methods Phys. Res. A 2015, 784, 172–175. [Google Scholar] [CrossRef]
- Britvich, G.I.; Chenko, V.G.; Gilitsky, Y.V.; Chubenko, A.P.; Shepetov, A.L. Prototype of a neutron detector based on a boron-containing plastic scintillator. Instrum. Exp. Tech. 2004, 47, 571–584. [Google Scholar] [CrossRef]
- Ryzhikov, V.D.; Desenko, S.M.; Kopina, I.V.; Afanasiadi, L.S.; Chernikov, V.V.; Onyshchenko, G.M. Scintillation neutron detector on the basis of boron-containing plastics. Porblems At. Sience Technol. 2004, 2, 169–170. [Google Scholar]
- Kiaguchi, H.; Izumi, S.; Kitaguchi, K.; Kaihara, A.; Nakamura, T. Energy response of a neutron area monitor with a silicon semiconductor detector. J. Nucl. Sci. Technol. 1993, 30, 1283–1287. [Google Scholar] [CrossRef]
- Kumashiro, Y. Refractory semiconductor of boron phosphide. J. Mater. Res. 1990, 5, 2933–2947. [Google Scholar] [CrossRef]
- Li, J.; Dahal, R.; Majety, S.; Lin, J.Y.; Jiang, H.X. Hexagonal boron nitride epitaxial layers as neutron detector materials. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 654, 417–420. [Google Scholar] [CrossRef]
- Doan, T.C.; Majety, S.; Grenadier, S.; Li, J.; Lin, J.Y.; Jiang, H.X. Fabrication and characterization of solid-state thermal neutron detectors based on hexagonal boron nitride epilayers. Nucl. Instrum. Methods Phys. Res. A 2014, 748, 84–90. [Google Scholar] [CrossRef]
- Maity, A.; Doan, T.C.; Li, J.; Li, J.; Lin, J.Y.; Jiang, H.X. Realization of highly efficient hexagonal boron nitride neutron detectors. Appl. Phys. Lett. 2016, 109, 072101. [Google Scholar] [CrossRef]
- Zheng, H.; Ramalingam, B.; Mukherjee, S.; Zhou, Y.; Gangopadhyay, K.; Brockman, J.D.; Lee, M.W.; Gangopadhyay, S. Neutron detection with integrated sub-2 nm Pt nanoparticles and 10B enriched dielectrics—A direct conversion device. Sens. Bio-Sens. Res. 2016, 9, 1–6. [Google Scholar] [CrossRef]
- Koirala, M.; Wu, J.W.; Weltz, A.; Dahal, R.; Danon, Y.; Bhat, I. Electrophoretic deposition of 10B nano/micro particles in deep silicon trenches for the fabrication of solid state thermal neutron detectors. Int. J. High Speed Electron. Syst. DevicesIntegr. Circuits Syst. Opt. Quantum Electron. 2018, 27, 184002. [Google Scholar]
- Amaro, F.D.; Monteiro, C.M.B.; Santos, J.M.; Antognini, A. Novel concept for neutron detection: Proportional counter filled with 10B nanoparticle aerosol. Sci. Rep. 2017, 7, 46942–46948. [Google Scholar] [CrossRef]
- Sun, J.; Wang, K.; Han, S.; Zhang, M.; Wang, Y.; Qian, W.; Zhang, H.; Dong, J. A SERS method for thermal neutron detection. J. Raman Spectrosc. 2018, 49, 1190–1197. [Google Scholar] [CrossRef]
- Wieland, K.; Ramer, G.; Weiss, V.U.; Allmaier, G.; Lendl, B.; Centrone, A. Nanoscale chemical imaging of individual chemotherapeutic cytarabine-loaded liposomal nanocarriers. Nano Res. 2019, 12, 197–203. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, D.; Lin, Y. Glucose encapsulating liposome for signal amplification for quantitative detection of biomarkers with glucometer readout. Biosens. Bioelectron. 2015, 72, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Steffes, V.M.; Zhang, Z.; MacDonald, S.; Crowe, J.; Safinya, C.R. PEGylation of Paclitaxel-Loaded Cationic Liposomes Drives Steric Stabilization of Bicelles and Vesicles thereby Enhancing Delivery and Cytotoxicity to Human Cancer Cells. ACS Appl. Mater. Interfaces 2019, 12, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ai, J.; Dong, Y.; Zhang, S.; Gao, Q.; Qi, H.L.; Zhang, C.X.; Cheng, Z.L. Combining 3D graphene-like screen-printed carbon electrode with methylene blue-loaded liposomal nanoprobes for phospholipase A(2) detection. Biosens. Bioelectron. 2019, 126, 255–260. [Google Scholar] [CrossRef]
- Dutt, C.A.; Park, E.Y. Methylene blue-encapsulated liposomal biosensor for electrochemical detection of sphingomyelinase enzyme. Sens. Actuators B-Chem. 2019, 301, 127153. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, A.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 40–57. [Google Scholar] [CrossRef]
- Tian, L.; Wang, Y.; Kang, X. Target-controlled liposome amplification for versatile nanopore analysis. Chem. Commun. 2019, 55, 5159–5162. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Shan, G. Preparation of Liposome@ Ag/Au Nanocomposites and Their Interaction with H2O2. Chem. J. Chin. Univ. Chin. 2019, 40, 639–644. [Google Scholar]
- Zhang, Y.; Xue, Q.; Liu, J.; Wang, H. Magnetic bead-liposome hybrids enable sensitive and portable detection of DNA methyltransferase activity using personal glucose meter. Biosens. Bioelectron. 2017, 87, 537–544. [Google Scholar] [CrossRef]
- Xue, Q.; Kong, Y.; Wang, H.; Jiang, W. Liposome-encoded magnetic beads initiated by padlock exponential rolling circle amplification for portable and accurate quantification of microRNAs. Chem. Commun. 2017, 53, 10772–10775. [Google Scholar] [CrossRef]
- Galindo, S.; Urena-Nunez, F. Electron paramagnetic resonance (EPR) study of free radicals in irradiated zinc poly methacrylate. Radiat. Phys. Chem. 2015, 111, 67–73. [Google Scholar] [CrossRef]
- Lee, J.D.; Ueno, M.; Miyajima, Y.; Nakamura, H. Synthesis of boron cluster lipids: Closo-dodecaborate as an alternative hydrophilic function of boronated liposomes for neutron capture therapy. Org. Lett. 2007, 9, 323–326. [Google Scholar] [CrossRef]
- Maruyama, K.; Ishida, O.; Kasaoka, S.; Takizawa, T.; Utoguchi, N.; Shinohara, A.; Chiba, M.; Kobayashi, H.; Eriguchi, M.; Yanagie, H. Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferrin-PEG liposomes, for boron neutron-capture therapy (BNCT). J. Control. Release 2004, 98, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Altieri, S.; Balzi, M.; Bortolussi, S.; Bruschi, P.; Ristori, S. Carborane Derivatives Loaded into Liposomes as Efficient Delivery Systems for Boron Neutron Capture Therapy. J. Med. Chem. 2009, 52, 7829–7835. [Google Scholar] [CrossRef]
- Bignell, L.J.; Mume, E.; Jackson, T.W.; Lee, G.P. Plasmonic light yield enhancement of a liquid scintillator. Appl. Phys. Lett. 2013, 102, 647–656. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, Z.; Cheng, C.; Wu, Q.; Gu, M.; Xu, J.; Chen, H.; Liu, J.L.; Chen, L.; Ouyang, X.P. Plasmonic lattice resonance-enhanced light emission from plastic scintillators by periodical Ag nanoparticle arrays. Appl. Phys. Lett. 2017, 110, 31–36. [Google Scholar] [CrossRef]
- Janos, T.P.; Quentin, Q.; Julie, B.; Roxana, J.J.; Sundh, H.; Boukherroub, R.; Szunerits, S.; Linden, S.K. Mucin modified SPR interfaces for studyeing the effect of flow on pathogen binding to Atlantic salmon mucins. Biosens. Bioelectron. 2019, 146, 111736–111744. [Google Scholar]
- Wang, B.T.; Wang, Q. Sensitivity-Enhanced optical fiber biosensor based on coupling effect between SPR and LSPR. IEEE Sens. J. 2018, 18, 8303–8310. [Google Scholar] [CrossRef]
- Tao, Y.; Qing, S.W. Plasmonic molecular assays:Recent advances and applications for mobilee health. Nano Res. 2018, 12274, 2094–2129. [Google Scholar]
- Zhang, Y.; Wang, F.; Qian, S.; Liu, Z.; Qiao, W.; Gu, Y.; Wu, Z.; Jing, Z.; Sun, C.; Wei, P. A Novel Fiber Optic Surface Plasmon Resonance Biosensors with Special Boronic Acid Derivative to Detect Glycoprotein. Sensors 2017, 17, 2259. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, Y.; Zhang, D.; Li, S.; Wang, X.; Song, D.Q. Magnetic field-assisted SPR biosensor based on carboxyl-functionalized graphene oxide sensing film and Fe3O4-hollow gold nanohybrids probe. Biosens. Bioelectron. 2016, 5663, 30654–30658. [Google Scholar] [CrossRef]
- Zhang, X.H.; Lou, Z.C.; Wang, A.L.; Zhang, H.Q. Detection of Wild-Type Hypoxanthine Guanine Phosphoribosyl Transferase of Lymphocytes in GammA-Irradiated Mice with Surface Plasmon Resonance. Anal. Lett. 2012, 45, 850–861. [Google Scholar] [CrossRef]
- Wang, K.K.; Zhang, W.; Zhang, X.H.; Hu, X.D.; Zhang, H.Q. Highly sensitive gold nanoparticles-DNA nanosensor for γ-radiation detection. Appl. Mater. Interfaces 2020, 12, 42403–42409. [Google Scholar] [CrossRef] [PubMed]
- Paff, M.G.; Clarke, S.D.; Kouzes, R.T.; Pozzi, S.A. IEEE, Fast and thermal neutron detectors for radiation portal monitors. In Proceedings of the 2017 IEEE Nuclear Science Symposium and Medical Imaging Conference, Atlanta, GA, USA, 21–28 October 2017; pp. 978–979. [Google Scholar]
- Desai, S.S.; Rao, M.N. Drift of Electrons and Performance of BF3 Filled Neutron Proportional Counters. In Proceedings of the 61st Dae-Solid State Physics Symposium, Mumbai, India, 26–30 December 2017; pp. 1832–1836. [Google Scholar]
- Wang, Q.; Zheng, G.; Ai, J.; Wei, X. The Synthesis of High Yield Diglycerin Borate. Appl. Machanics Mater. 2011, 55, 312–316. [Google Scholar] [CrossRef]
- Barth, R.F. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef]
- McGregor, D.S.; Hamming, M.D.; Yang, Y.H.; Gersch, H.K.; Klann, R.T. Design considerations for thin film coated semiconductor thermal neutron detectors—I: Basics regarding alpha particle emitting neutron reactive films. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 500, 272–308. [Google Scholar] [CrossRef]







| Elements | Fe | O | C | H |
|---|---|---|---|---|
| Wt% | 51 | 15 | 31 | 3 |
| Elements | B | C | N | O | P | Fe | H | S | Cl |
|---|---|---|---|---|---|---|---|---|---|
| Wt% | 14 | 40 | 1 | 23 | 4 | 7 | 9 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, K.; Hu, X.; Zhang, X.; Chang, S.; Zhang, H. Nanometer-Sized Boron Loaded Liposomes Containing Fe3O4 Magnetic Nanoparticles and Tributyl Borate and Anti-Albumin from Bovine Serum Antibody for Thermal Neutron Detection. Materials 2021, 14, 3040. https://doi.org/10.3390/ma14113040
Zhang W, Wang K, Hu X, Zhang X, Chang S, Zhang H. Nanometer-Sized Boron Loaded Liposomes Containing Fe3O4 Magnetic Nanoparticles and Tributyl Borate and Anti-Albumin from Bovine Serum Antibody for Thermal Neutron Detection. Materials. 2021; 14(11):3040. https://doi.org/10.3390/ma14113040
Chicago/Turabian StyleZhang, Wei, Kaikai Wang, Xiaodan Hu, Xiaohong Zhang, Shuquan Chang, and Haiqian Zhang. 2021. "Nanometer-Sized Boron Loaded Liposomes Containing Fe3O4 Magnetic Nanoparticles and Tributyl Borate and Anti-Albumin from Bovine Serum Antibody for Thermal Neutron Detection" Materials 14, no. 11: 3040. https://doi.org/10.3390/ma14113040
APA StyleZhang, W., Wang, K., Hu, X., Zhang, X., Chang, S., & Zhang, H. (2021). Nanometer-Sized Boron Loaded Liposomes Containing Fe3O4 Magnetic Nanoparticles and Tributyl Borate and Anti-Albumin from Bovine Serum Antibody for Thermal Neutron Detection. Materials, 14(11), 3040. https://doi.org/10.3390/ma14113040






