Abstract
This study is focused on the kinetics and adsorption isotherms of amine-functionalized magnesium ferrite (MgFe2O4) for treating the heavy metals in wastewater. A sol-gel route was adopted to produce MgFe2O4 nanoparticles. The surfaces of the MgFe2O4 nanoparticles were functionalized using primary amine (ethanolamine). The surface morphology, phase formation, and functionality of the MgFe2O4 nano-adsorbents were studied using the SEM, UV-visible, FTIR, and TGA techniques. The characterized nanoparticles were tested on their ability to adsorb the Pb2+, Cu2+, and Zn2+ ions from the wastewater. The kinetic parameters and adsorption isotherms for the adsorption of the metal ions by the amine-functionalized MgFe2O4 were obtained using the pseudo-first-order, pseudo-second-order, Langmuir, and Freundlich models. The pseudo-second order and Langmuir models best described the adsorption kinetics and isotherms, implying strong chemisorption via the formation of coordinative bonds between the amine groups and metal ions. The Langmuir equation revealed the highest adsorption capacity of 0.7 mmol/g for the amine-functionalized MgFe2O4 nano-adsorbents. The adsorption capacity of the nanoadsorbent also changed with the calcination temperature. The MgFe2O4 sample, calcined at 500 °C, removed the most of the Pb2+ (73%), Cu2+ (59%), and Zn2+ (62%) ions from the water.
1. Introduction
Water contamination with heavy metals mainly occurs due to anthropogenic or natural processes. Since the production of metals from industrial activities is growing over time, the issue of waste management is worsening; dedicated efforts are needed to find economical solutions [1]. The heavy metals in wastewater are treated through chemical coagulation, chemical precipitation, photocatalytic degradation, flocculation, electrochemical routes, ion exchange, adsorption, membrane filtration, and bioremediation [2,3,4,5]. Most accessible technologies, on the other hand, may have technical and economic limitations, i.e., high capital and operating costs, sensitivity to operational conditions, large energy use, or sludge formation. Taking these considerations into account, adsorption is considered an economical and effective approach to eliminating heavy metal ions from water [6,7,8]. Surface atoms govern the adsorption of heavy metals by an adsorbent. The adsorbent surface becomes unstable and active as the number of surface atoms rises and, therefore, produces many unsaturated bonds [9].
Conventional adsorbents provide a limited number of active sites, a small surface area, and complex adsorption kinetics and post-adsorption separation mechanisms. The difficulty of separating the adsorbents from the treated solution limits their use in wastewater treatment technology [10]. On the other hand, magnetic nanoparticles become supermagnetic under 25 nm. These materials offer a large active surface area and can be separated from the water by subjection to an external magnetic field. However, magnetic adsorbents may undergo surface poisoning and a reduction in magnetic field strength due to the dissolution of the magnetic core in acidic environments and the agglomeration of magnetic adsorbent particles. This issue can be addressed by modifying the adsorbent surface and preventing the dissolution of the magnetic core in the solution. In many cases, modified surfaces negatively affect the activity of the adsorbent nanoparticles. To develop good synergy between the physiochemical characteristics of the adsorbent and the adsorption capacity, the modification approaches should be researched further to improve understanding the mechanism involved. This discussion suggests that surface modification is critical for increasing selectivity and removal efficiency [11].
The level of surface modification also depends on the pre-functionalization and calcination of the magnetic adsorbent nanoparticles. The effect of calcination temperature on the surface functionalization of magnetic adsorbent nanoparticles and MgFe2O4 nanoparticles, in particular, is not reported in the published literature. Herein, the role of the calcination temperature in the amine functionalization of MgFe2O4 nanoparticles in the context of the adsorption of heavy metals is assessed. An increase in calcination temperature may influence the particle size and exothermic oxidation of the adsorbent particles and, consequently, their level of functionalization. The density of the adsorbent nanoclusters may also change on heating due to oxidation, the recrystallization of the clusters, and the dissolution of impure products. The oxidation and crystal reformation govern the inter-grain spacing, surface activation, and surface porosity of the adsorbent particles. The sol-gel technique produces more remarkable results when developing ultrafine particles at reasonably low temperatures than the other available methods [12]. It is applied due to its unmatchable benefits, such as its short rate of reaction, energy efficiency, nano-sized powders, ease of operation, and limited aggregation of particles [13]. In the reported work, MgFe2O4 was produced using the sol-gel method and functionalized by utilizing ethanolamine as a surface modifier. This study aimed to investigate the characteristics of the absorption of heavy metals from wastewater by magnesium ferrite nanoparticles.
2. Materials and Methods
2.1. Synthesis of Ferrite Nanoparticles
The analytical-grade reagents used in this work were procured from Sigma-Aldrich. These reagents were used as supplied without any processing or additional treatment. First of all, 1.6 g of iron(III) nitrate nonahydrate and 0.5 g of magnesium nitrate hexahydrate [Mg(NO3)2.6H2O] were added to 40 mL of DI water. Next, 1.8 g of citric acid monohydrate [C6H8O7.H2O] was added to 40 mL of DI water and added into the initial aqueous solution. The solution was then stirred continuously at 60 °C. The solution pH was adjusted to 7 by dropwise addition of sodium hydroxide (NaOH) to the mixture. The mixture converted into sol on heating, which changed to gel after stirring for 3 h. The obtained gel was dried in an oven at 90 °C for 7 h. Subsequently, the dry gel was calcined in a furnace at 500 °C, 600 °C, and 700 °C, labeled as samples A, B, and C. After calcination, the samples were ground into a fine powder. The ferrite powder was characterized and tested for adsorption of heavy metals.
2.2. Characterization Techniques
FTIR, SEM, TGA, and UV-vis techniques were used to analyze the pristine and amine-functionalized adsorbents. An Agilent Cary-630 spectrometer was employed to produce FTIR spectra of the adsorbents in the range of 4000–500 cm−1. A scanning electron microscope (Nano-SEM 450) was used to produce SEM micrographs for evaluating the surface morphology of the adsorbents. A SEM microscope, featuring a STEM detector with a standard TEM grid, was used for transmission electron microscopy analysis. STEM analysis was used to determine particle size and morphology in support of SEM analysis. A TGA/DSC instrument was operated to get thermogravimetry profiles of pristine and functionalized adsorbents for the analysis of the amount of amine coating during the functionalization of the adsorbent.
2.3. Amine Functionalization of Ferrite Nanoparticles
The ethanolamine was used as a surface modifier for the functionalization of MgFe2O4 nanoparticles. About 1.5 g of MgFe2O4 and 2 mL ethanolamine were added to 20 mL of distilled water. After 30 min of stirring and heating, the aqueous liquid was cooled to room temperature. To wash the precipitates and maintain a pH of 7, deionized water was used. The washed sample was dried in an oven for 2 h. All samples were amine-functionalized using the same procedure. The functionalization procedure was completed by following the catalyst-free method reported by Gu et al. [14] and Nonkumwong et al. [15] in different studies.
2.4. Adsorption of Heavy Metals
Precisely 0.02 g of lead nitrate, 0.02 g of copper chloride dehydrate, and 0.02 g of zinc acetate dehydrate were added to deionized water (50 mL) to simulate wastewater. The adsorption experiments were conducted by dissolving 0.25 g of MgFe2O4-NH2 adsorbent in the prepared wastewater. The pH of the aqueous mixture was maintained at 7. This aqueous mixture was stirred for 90 min and supernatants were filtered. The residual metal content in supernatants was measured using atomic absorption spectroscopy of the samples. The agitation period and initial concentrations of the heavy metals might be varied to have a good understanding of the adsorption response of the magnetic nano-adsorbent. The kinetic parameters and adsorption isotherms for the selected heavy metals onto the amine-functionalized MgFe2O4 were calculated using different chemical models [16].
3. Results and Discussion
3.1. Metal Ion Adsorption Mechanism
Tetraethyl orthosilicate (TEOS) silane was used for the direct silanization of the surface of the magnetic adsorbent. On the surface, the silica produces a highly robust covalent coating with grafted functional groups. It enables the additional functionalization of the core functional groups, resulting in a wide range of surface functionalities for adsorption applications [17]. The silica coating also prevents the agglomeration of the adsorbent particles. The silica-coated adsorbent was amino-functionalized using ethanolamine. A reaction scheme involving the amine functionalization of the MgFe2O4 adsorbent for the adsorption of the heavy metal ions is presented as Scheme 1. The mechanism of metal ions’ adsorption onto the adsorbent heavily depends on functional groups, which give binding affinity to the metal ions. The deposition of pollutants on the MgFe2O4-NH2 adsorbent in a multicomponent solution may occur through a physical process or the formation of chemical bonds. The −NH2 groups on the functionalized MgFe2O4 nanoparticles can be protonated and deprotonated, depending on the acidic and basic environment.
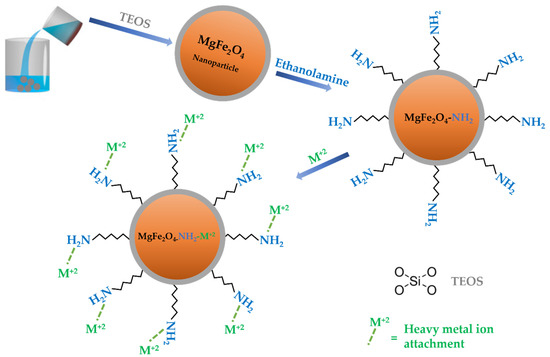
Scheme 1.
Illustration of amine-functionalization and metal ions adsorption onto MgFe2O4-NH2 adsorbent.
Since −NH2 groups possess a hard basic nature, they can bind strongly with heavy metal cations. As shown by the processes below, the ions in the aqueous solution can be in the form of hydrolysis or solvation species:
The mass transfer may occur due to pore filling, ion exchange, hydrophobic interaction, and H-bonding. The adsorption by the MgFe2O4-NH2 adsorbent increases as the number of surface atoms increases, making them more active and unstable, as well as providing more unsaturated bonds. The M2+ ions dominate when the solution pH is below 5, while M(OH)+ tends to initiate when the solution pH is greater than 5. The M(OH)2 is present when the solution pH exceeds 6.5. Since the solution pH was maintained at 7 in this study, the formation of the M(OH)2 was anticipated. The rapid adsorption of the M2+ ions onto MgFe2O4-NH2 reveals that chemical adsorption dominates the physical adsorption, most likely due to the complexation of the -NH2 groups and M2+ ions. The pseudo-second order described the adsorption of the heavy metal ions onto the adsorbent surface due to the formation of chemical bonding between the polar functional groups and the metal ions. The chemical bonding resulted in an adsorbent with a high capacity for cation exchange. The findings suggested that the adsorption of heavy metal ions onto MgFe2O4-NH2 adsorbent is driven by the formation of coordinate bonds or chemisorption.
3.2. FTIR and TGA Analysis of Amine-Functionalized Adsorbent
The attachment of the amine groups onto the MgFe2O4 adsorbent was characterized by the production of the FTIR spectra of the pristine and amine-treated adsorbents. Figure 1a reports the FTIR profiles of the pristine adsorbents calcined at 500 °C, 600 °C, and 700 °C. The FTIR spectra did not vary significantly with the change in calcination temperature. Three curves at 500 °C, 600 °C, and 700 °C show the same number of peaks. However, the intensity of the characteristic absorption peaks of the M–O band slightly increased with the calcination temperature. The formation of the spinel structure of the MgFe2O4 adsorbent with cation and anion distribution on the octahedral and tetrahedral lattice sites was confirmed by the FTIR analysis. Two absorption bands were noticed in the spinel structure of the MgFe2O4 adsorbent, at 570 cm−1 and 450 cm−1. The high-frequency band revealed the M–O bond stretching vibration at the tetrahedral sites (A), while the low-frequency band revealed metal-oxygen stretching vibration at the octahedral sites (B). These vibrations were attributed to the shorter bond length of the metal–oxygen link in the tetrahedral sites and the larger metal–oxygen bond length in the octahedral sites. A bending vibration mode of O–H was seen at 1630 cm−1, indicating both free and absorbed water [18]. The length of the metal oxide bonds in each site caused a shift in the placement of the tetrahedral and octahedral bands. The presence of Fe2+ cations on the octahedral sites was confirmed by a shoulder near the octahedral absorption band. The cations caused the absorption band to split due to the Jahn–Teller phenomenon, which occurred due to local deformations in the lattice [19].
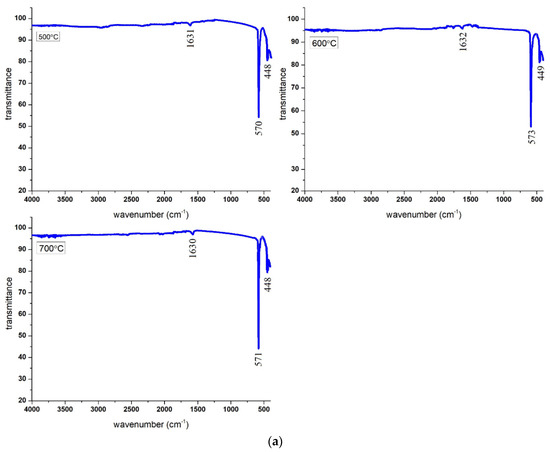
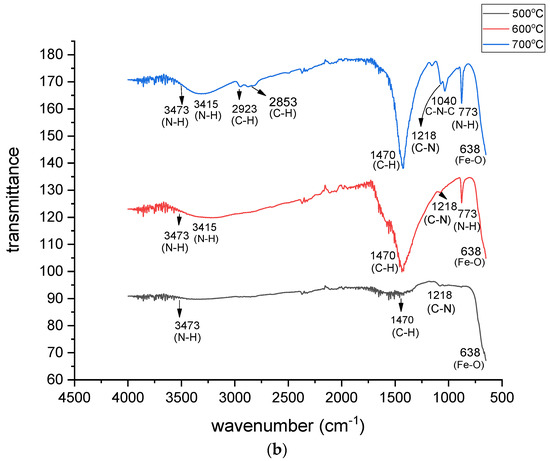
Figure 1.
(a) FTIR spectra of pristine MgFe2O4 adsorbent calcined at different temperatures. (b) FTIR spectra of amine-functionalized magnesium ferrite nanoparticles calcined at different temperatures.
The MgFe2O4 samples were amine-functionalized after calcination at different temperatures. No calcination was performed after the amine functionalization of the adsorbent samples. Therefore, the FTIR spectra of the functionalized adsorbents and the level of functionalization are discussed based on the calcination of the pristine samples. Figure 1b shows that there was a small difference between the FTIR spectra of the MgFe2O4-NH2 samples obtained after the calcination of the pristine MgFe2O4 at 600 °C and at 700 °C. However, the FTIR spectrum of the MgFe2O4-NH2 nanoparticles at 500 °C was different from the other two spectra. The FTIR spectra of the MgFe2O4-NH2 nanoparticles and the pristine calcined at 500 °C, differed because at low temperatures, the active sites on the surfaces of the particles were low in number and no definite sharp peaks were formed. As the temperature increased, more active sites were produced on the nanoadsorbent surface, revealing the attachment of the functional groups. A rise in calcination temperature might have impacted the particle size and exothermic reactions of the oxidation of the adsorbent particles and, consequently, the level of functionalization. The density of the adsorbent nanoclusters increased on heating due to the occurrence of oxidation, the recrystallization of the clusters, and the removal of the impure products at high temperatures. The oxidation and crystal reformation enhanced the inter-grain spacing and surface porosity of the adsorbent particles. Therefore, as the calcination temperature of the pristine adsorbent increased, extra active sites were produced on the adsorbent surface, revealing the attachment of an excessive number of functional groups.
The creation of the Fe-O stretching mode by the MgFe2O4 is marked by a spectral line at 638 cm−1, which confirms the spinel structure of the adsorbent. An FTIR peak at 773 cm−1 indicates the N-H wagging mode. The Fe-O vibrations masked the Fe–O–Si bonds in the FTIR spectrum. Similarly, an FTIR peak at 1040 cm−1 shows the formation of the C-N-C asymmetric stretching mode. This mode also shows C−O overlapping with C−N stretching vibration, which offers confirmation of the attachment of the amine-functional groups on the adsorbent. The peak around 1218 cm−1 reflects the C-N stretching mode. The peak around 1470 cm−1 indicates the C-H scissoring mode. The pair of peaks at 3415 and 3473 cm−1 represents the N-H stretching of athe mine, which confirms the grafting of the amine onto the MgFe2O4 nanoparticles. These peaks are missing in the FTIR spectra of the pristine MgFe2O4 nanoparticles, as shown in Figure 1a. The peaks at 2923 cm−1 and 2853 cm−1 show the C−H stretching vibration bond in the ethanolamine. The low-frequency peaks in the 500−850 cm−1 range show the stretching modes of the Fe−O and Mg−O in the spinel MgFe2O4.
The TGA plots in Figure 2 were analyzed to calculate the amount of amine on the surface of the functionalized adsorbent. The sample was heat-treated by raising the temperature from ambient to 600 °C at a fixed rate of 10 °C/min. To quantify the decomposition of the amine coating on the adsorbent surface, the weight loss of the pristine adsorbent was compared to that of the functionalized adsorbent during heating. The weight loss began at 100 °C and continued until 600 °C. The weight loss was rapid between 200 °C and 400 °C, before gradually decreasing to a steady state. The pristine and amine-functionalized adsorbents exhibited weight losses of 4.7% and 6.2%, respectively. The weight loss of the pristine adsorbent was mainly due to evaporation of moisture content in the adsorbent through chemical and physical processes and the stabilizer used to stabilize the particle size. The weight loss of the functionalized adsorbent was higher than that of the pristine adsorbent due to the decomposition of the functional groups conjugated on the adsorbent surface [20]. The amount of amine groups attached to the adsorbent was measured at about 1.5%. The amount of amine groups was measured from the difference in the initial weight losses (%) of the pristine and functionalized adsorbent nanoparticles at a temperature below 300 °C. The difference in weight loss values was equal to the amount of functional groups and bound water. Thus, the FTIR and TGA analyses confirmed that the magnetic adsorbent was functionalized with amine groups. The functionalized adsorbent was highly stable in water due to the H-bonding between the water and the amine groups of the functionalized adsorbent [21].
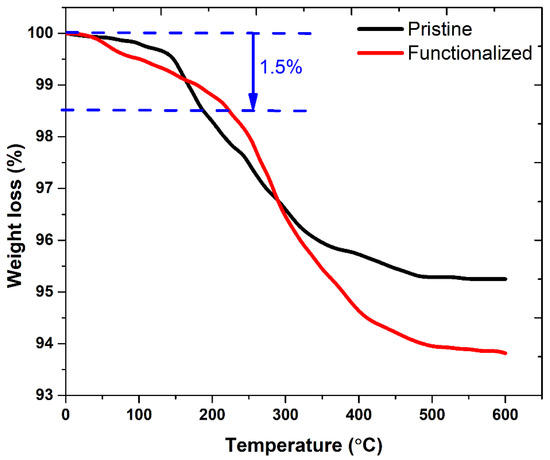
Figure 2.
TGA profiles of pristine and amine-functionalized adsorbents.
3.3. UV-Visible Analysis
UV-visible spectroscopy was conducted to study the optical properties of the synthesized sample. The UV-visible absorption spectra of the MgFe2O4 and MgFe2O4-NH2 nanoparticles, calcined at different temperatures, are reported in Figure 3 and Figure 4, respectively. These spectra reveal high absorption in the ultraviolet and visible regions. The bandgap energy was determined from the expression of the absorption coefficient near the band edge by supposing a direct bandgap using Tauc relation, given as follows:
where, h is the Plank constant, λmax is the wavelength at the absorption edge, and c is the speed of light. The Eg values of the pristine MgFe2O4 nanoparticles, calcined at 500 °C, 600 °C, and 700 °C, were calculated at around 2.05 eV, 2.03 eV, and 2.00 eV, respectively. The Eg values of the MgFe2O4-NH2 nanoparticles were calculated at around 2.09 eV, 2.08 eV, and 2.06 eV, respectively. The bandgap energy decreased as the temperature of the calcination increased in both the pristine and amine-functionalized adsorbents. The crystallite growth of the synthesized products was most probably due to the lowering of the bandgap energy at high calcination temperatures. The UV spectra were measured by preparing the suspensions of the pristine and functionalized nanoparticles. The bandgap energy and UV absorption of the adsorbent nanoparticles were found nearly in the visible region. This showed that the light absorption partly dominated the light-scattering part, since scattering is inversely proportional to the wavelength. The longer the wavelength, the lower the scattering factor.
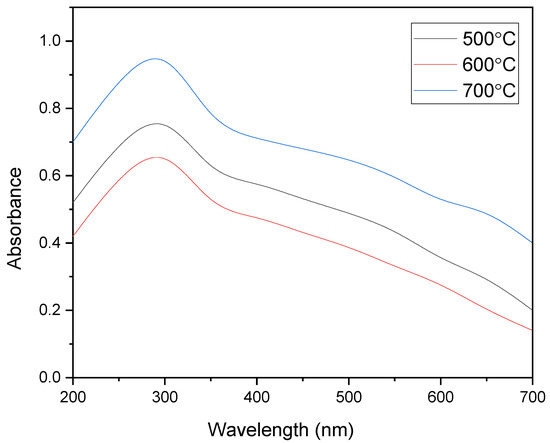
Figure 3.
UV absorption profiles of pristine MgFe2O4 nanoparticles calcined at different temperatures.
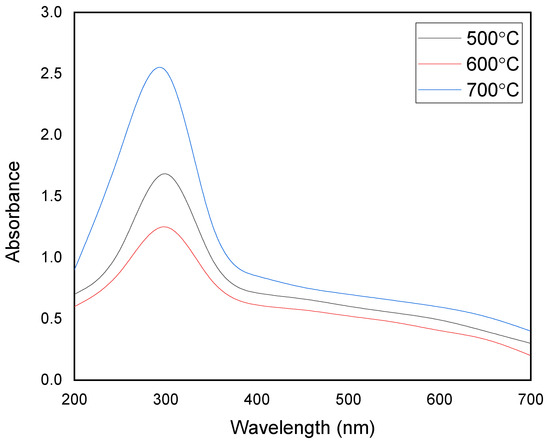
Figure 4.
UV absorption profiles of MgFe2O4-NH2 samples calcined at different temperatures.
The MgFe2O4-NH2 samples showed a stronger absorbance peak than the pristine MgFe2O4 nanoparticles. The ethanolamine-functionalized MgFe2O4 nanoparticles were expected to enhance the absorbance peak intensity due to the deposition of the nonmagnetic amino groups on the adsorbent. It was revealed that the absorbance intensity depends on the number of reactive −NH2 groups attached to the surface. The amount of −NH2 group on the nanoparticle surface increases with the calcination temperature due to increased surface roughness and the porosity of the nanoparticles.
4. Morphology and Size Distribution
SEM images were produced to elaborate the morphology and size of the spinel MgFe2O4 nanoparticles. Figure 5a,b compares the SEM morphologies of the pristine and amine-functionalized MgFe2O4 nanoparticles at 700 °C. The pristine MgFe2O4 nanoparticles were uniformly dispersed, less agglomerated, and homogeneous, with an average particle size slightly larger than that of the functionalized nanoparticles. The silanization and functionalization processes prevented the agglomeration of the nanoparticles. The calcination temperature did not cause significant changes in surface morphology. As shown in Figure 6, the particle size of the pristine MgFe2O4 at 500 °C, 600 °C, and 700 °C was measured at about 527.41 nm, 620.5 nm, and 762.32 nm, respectively. The particle size slightly decreased after the amine functionalization. The pre- and post-functionalization morphologies of MgFe2O4 were assessed further by producing STEM images, as shown in Figure 5c,d. The STEM images showed a slight change in morphology after functionalization. The shapes and boundaries of the nanoparticles were clearer compared to the pristine nanoparticles. A thin coating of functional material on the nanoparticle surface can also be seen in the form of light shade circling the nanoparticles.
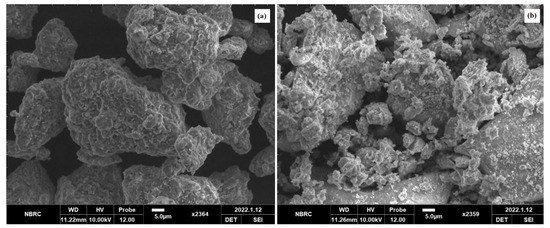
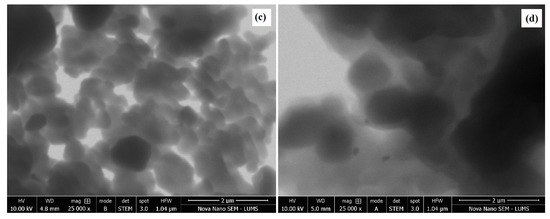
Figure 5.
(a) SEM micrograph of pristine MgFe2O4, (b) SEM micrograph of amine-functionalized MgFe2O4, (c) STEM micrograph of pristine MgFe2O4, (d) STEM micrograph of amine-functionalized MgFe2O4.
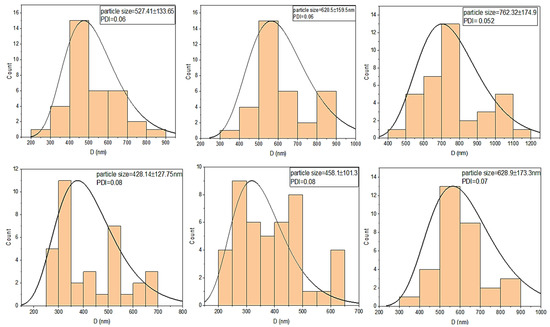
Figure 6.
Particle-size histograms of pristine and amine-functionalized MgFe2O4 nanoparticles calcined at different temperatures.
These results show that the functionalization of MgFe2O4 nanoparticles can reduce the agglomeration of pristine ferrite nanoparticles. The grain sizes of the amine-functionalized MgFe2O4 at different temperatures were measured at about 428.14 nm, 458.1 nm, and 628.9 nm, respectively. Some of the histograms, presented in Figure 6, showed a slightly bimodal distribution in the particle sizes, as depicted by major and minor modes. The bimodal distribution was not important in our case, since we determined the average size of one class of particles. The bimodal distribution is only important when the sizes of different species or types of particles are being studied. This distribution is commonly used to examine individual size subpopulations to better understand individual distributions.
4.1. Adsorption Kinetics
The pseudo models were developed to understand the adsorption kinetics. In reversible reactions in which an equilibrium is maintained between the solid and liquid phases, the pseudo-first-order kinetics are utilized, implying physisorption instead of chemisorption. The kinetic process of heavy metal ions’ adsorption onto the adsorbents is explained through the pseudo-second-order model. The cation exchange capacity of adsorbents is influenced by a chemical interaction among the surface functional groups and heavy metal ions.
For the study of kinetic parameters, the adsorbent is added to heavy water and the resulting solution is stirred. The agitation time is changed and the corresponding initial and final metal ion concentration in the aqueous solution is monitored. At a certain point in time, the concentration does not change further. At this point, the final concentration is equal to the equilibrium concentration. The adsorption capacity (qe) is determined by defining Cf as Ce in the following formula:
where is the quantity of metal ions adsorbed by the adsorbent, is the initial concentration of heavy metals in the aqueous solution, and is the final concentration of heavy metals. The volume of heavy metals in the solution is represented by , while m represents the mass of the dry adsorbent in grams. For the pseudo-first-order model, the graph is plotted between t and log(qe − qt), and the resulting line equation is compared with the integrated linear form of the pseudo-first-order model. The slope and intercept of the line is used to calculate the pseudo-first-order parameters. For the pseudo-second-order model, the graph is plotted between t and t/qt and the resulting line equation is compared with the integrated linear form of the pseudo-second-order model. The slope and intercept of the line is used to calculate the pseudo-first-order parameters.
For plotting isotherm models, the parameters Ce and qe are used. Ce is the concentration of the adsorbate in the aqueous solution after adsorption. From Ce, qe is calculated by using the above equation. For Langmuir isotherms, the graph is plotted between Ce and Ce/qe, and the resulting line equation is compared with the linear form of the Langmuir isotherm model. The slope and intercept of the line is used to calculate the isotherm parameters. For Freundlich model, the graph is plotted between log Ce and logqe, and the resulting line equation is compared with the linear form of the Freundlich model. The slope and intercept of the line is used to calculate the Freundlich parameters. In the present work, the pseudo-second-order model fit the best. This model suggests chemisorption, i.e., chemical changes occur when the adsorbate binds with the adsorbent. As a result, heavy metal ion adsorption onto the prepared sample occurred via chemisorption rather than physisorption.
4.2. Pseudo-First-Order Kinetics
The pseudo-first-order model is premised on the idea that a change in the solute take-up rate can have a direct relationship with the difference in the saturation concentrations and the amount of solid take-up with time, and it is widely applicable during the first phase of adsorption processes. For adsorption, occuring via diffusion through an interface, the kinetics are typically observed to fit pseudo-first-order rate equation. The linear form of the pseudo-first-order model is as follows.
where qt and qe (milligram/gram) represent the quantity adsorbed at “t” time and at equilibrium, respectively, while k1 denotes the pseudo-first-order model’s constant at equilibrium. After applying the integration and boundary conditions, the above equation becomes:
The linear graphs of the log(qe − qt) over time are used to find the k1 value. If adsorption obeys first-order kinetics, the intercept of the log(qe − qt) against the t plot is equal to log(qe). For slower adsorption, the correct equilibrium condition can be difficult to attain, making a precise determination of qe extremely difficult [22].
4.3. Pseudo-Second-Order Kinetics
The pseudo-second-order kientics are premised on the idea that chemisorption is a rate-limiting phase, which predicts adsorption behavior throughout the adsorption process. The adsorption capacity, rather than the adsorbate concentration, determines the rate of adsorption in this situation. The main benefit of this model over the pseudo-first-order model is that the adsorption capacity at equilibrium may be derived from this model, eliminating the requirement of measuring the equilibrium adsorption capacity via experiment. For pseudo-second-order kinetics, the differential expression is as follows:
After the mathematical elaboration and consideration of the boundary condition, the pseudo-second-order model is:
with
where h denotes the initial sorption rate, qe (milligram/gram) represents the equilibrium quantity of the adsorbed dye, and k2 represents the pseudo-second-order model rate-constant (gram/milligram.minute). A direct relationship should emerge when plotting t/qt against t. We can determine the quantity of theadsorbed adsorbate at the equilibrium using the slope (1/qe) and intercept (1/h or 1/k2qe2), which yields the second-order rate constant, k2 [22].
4.4. Pseudo Models for Adsorption of Cu2+ onto MgFe2O4-NH2 Nanoparticles
It is reported that the amount of copper adsorbed on magnetic ferrite nanoparticles at equilibrium is about 2.32 mg/g. The pseudo-first-order kinetics were used in these calculations [23]. The value of qe, obtained using pseudo-second-order kinetics, is around 149.25 mg/g. Based on the pseudo-first-order model, the plots of the adsorption of the Cu+2 onto the MgFe2O4-NH2 nanoparticles are reported in Figure 7, and the subsequent computed kinetic parameters are reported in Table 1. It is shown that the kinetic parameters of the adsorbent, calcined at different temperatures, did not differ notably. For the second-order model, the plots of the Cu2+ adsorption onto the MgFe2O4-NH2 nanoparticles are shown in Figure 8, and the subsequent computed kinetic parameters are reported in Table 1. The pseudo-second-order relation yielded a better-fitting straight line when the correlation coefficient (R2) was taken into account. These results indicated that Cu2+ adsorption on amine-treated ferrite nanoparticles occurs through the chemisorption process.
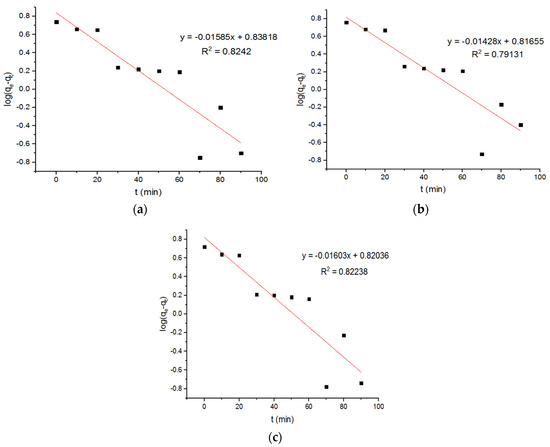
Figure 7.
Pseudo-first-order adsorption plots of Cu+2 ions onto MgFe2O4-NH2 nanoparticle pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.

Table 1.
Pseudo-first- and second-order-based adsorption rate constant for Cu+2 ions.
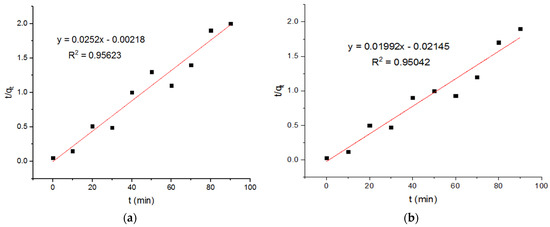
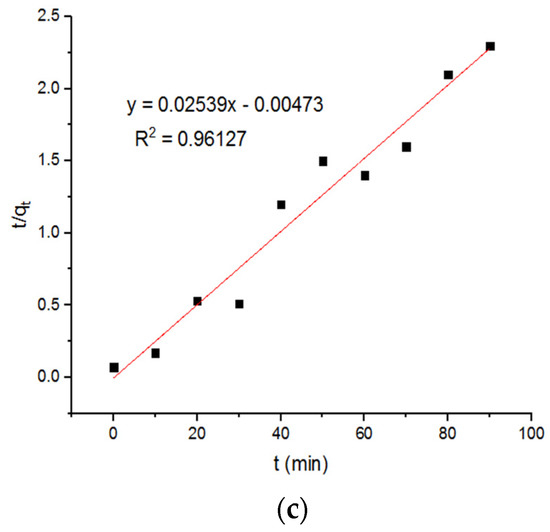
Figure 8.
Pseudo-second-order adsorption plots of Cu+2 ions onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.
4.5. Pseudo Models for Adsorption of Pb2+ onto MgFe2O4-NH2 Nanoparticles
Tran et al. [24] reported that the amount of lead adsorbed on Cu0.5Mg0.5Fe2O4 at equilibrium is about 3.66 mg/g. They used pseudo-first-order kinetics and calculated qe at around 38.2 mg/g. The plots of adsorption the Pb2+ ions onto the MgFe2O4-NH2 nanoparticles, based on pseudo-first-order kinetics at pre-functionalization calcination temperatures, are reported in Figure 9, and the subsequent computed kinetic parameters are reported in Table 2. It is shown that the kinetic parameters at 500 °C and 700 °C did not differ significantly. Three pseudo-second-order plots for the adsorption of Pb2+ ions onto MgFe2O4-NH2 nanoparticles are reported in Figure 10, and the subsequent computed kinetic parameters are reported in Table 2. It is shown that the kinetic parameters at different calcination temperatures did not differ significantly.
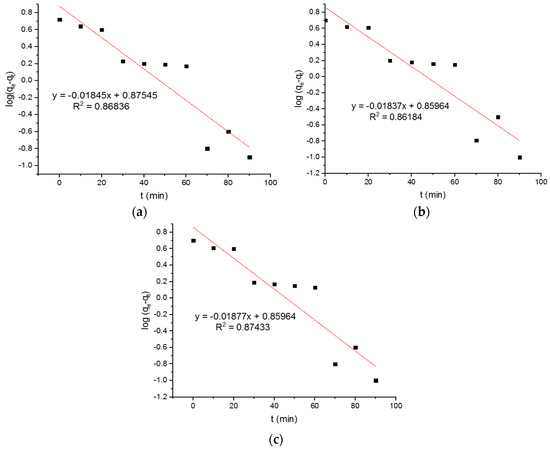
Figure 9.
Pseudo-first-order plots for Pb2+ adsorption onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.

Table 2.
Pseudo-first- and second-order-based adsorption rate constant.
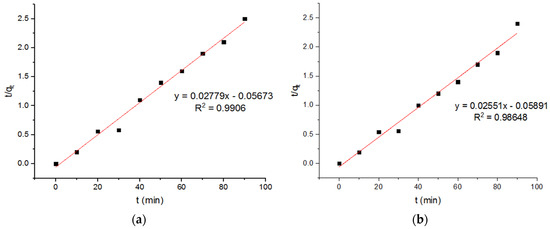
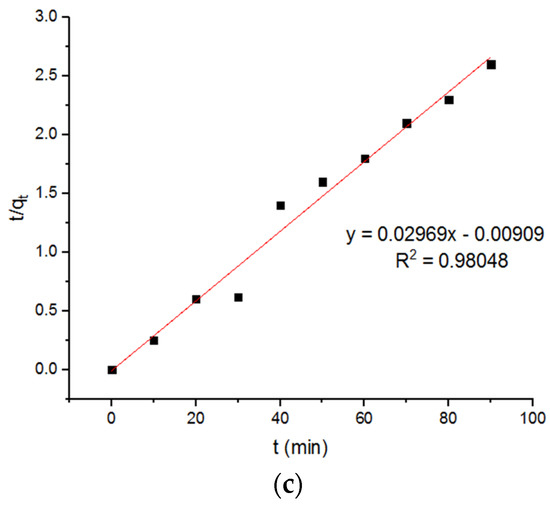
Figure 10.
Pseudo-second-order plots of adsorption of Pb2+ onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C (b) 600 °C, and (c) 700 °C.
4.6. Pseudo Models for Adsorption of Zn2+ onto MgFe2O4-NH2 Nanoparticles
The plots of the pseudo-first-order and second-order adsorption of the Zn2+ ions onto the MgFe2O4-NH2 nanoparticles, pre-functionalization-calcined at different temperatures, are presented in Figure 11 and Figure 12, respectively. The subsequent kinetic parameters are reported in Table 3. It is shown that the kinetic parameters at different calcination temperatures did not differ significantly.
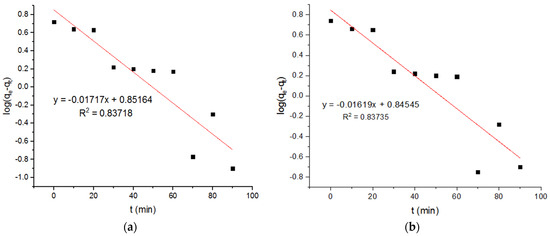
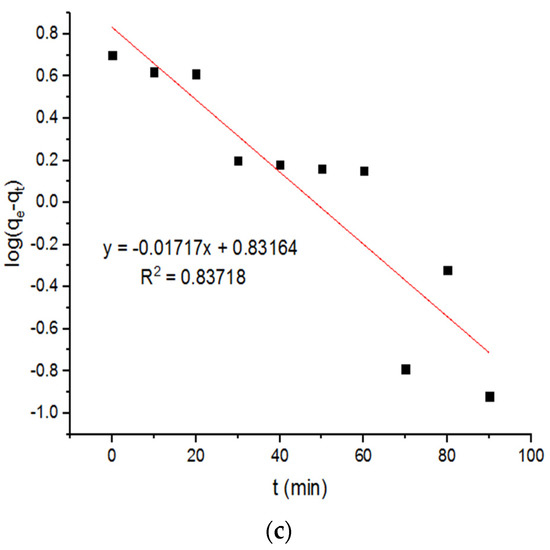
Figure 11.
Pseudo-first-order plots for adsorption of Zn2+ ions onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.
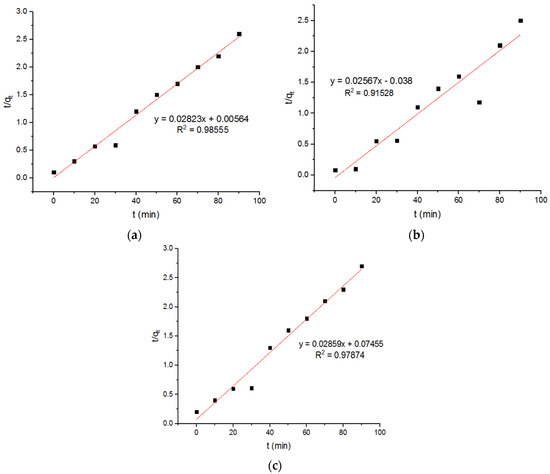
Figure 12.
Pseudo-second-order plots for adsorption of Zn2+ ions onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.

Table 3.
Adsorption rate constant calculated from pseudo models.
5. Adsorption Isotherms
Th adsorption isotherms were investigated utilizing both Freundlich and Langmuir isotherms. It was demonstrated how the adsorbent interacted with the adsorbates. The Langmuir model involves the homogeneous monolayered adsorption of pollutants onto the adsorbent by excluding the multilayer capillary-condensation. It simply indicates that the more binding sites are present on the surface of adsorbent, the more effective the adsorption. The Freundlich isotherm is well described for the surface of heterogeneous adsorbents, with many types of adsorption site.
5.1. Langmuir Model
The adsorbate and adsorbent remain in dynamic equilibrium in monolayer adsorptions and the Langmuir model. The Langmuir adsorption isotherm was created to explain gas-to-solid-phase adsorption, but it was later used to examine the solid–liquid interface. Fractional coverage is a measure of how much of the surface is covered, and it is determined by the adsorbate concentration. The mathematical evolution is based on a physical simplification of the mechanism regarding certain assumptions: (1) the surfaces are homogeneous, implying that almost all the sites are equivalent energetically, (2) adsorption is the monolayer procedure in which every site may only adsorb one adsorbate molecule, (3) adsorbed molecules do not network with each other laterally, and (4) adsorption can be reversed.
The Langmuir adsorption isotherm is expressed as:
where Ce (mole/litter) and Qm (milligram/gram or mole/gram) indicate the equilibrium concentration and maximum adsorption capacity, respectively, and the Langmuir constant ‘KL’ is the adsorption energy or adsorbate–adsorbent constant at equilibrium. This equation can be transformed into linear form as:
Langmuir constants can be determined by plotting 1/qe against 1/Ce, or the Ce/qe against Ce [22].
5.2. Freundlich Model
Reversible, non-ideal, multilayer adsorption at heterogeneous surfaces can be described by the Freundlich adsorption isotherm. The binding energies of adsorption sites differs according to the isotherm. Instead of uniform energy, the energy distributions at the adsorbing sites have spectra with varied binding energy values and obey the exponential function that is closer to the actual situation [25].
where Ce (mole/litter) is the adsorbate concentration under equilibrium. The dsorption intensity and the capacity of the adsorption are represented by the Freundlich constants 1/n and KF, respectively. By applying logarithms, the above equation can indeed be represented as:
where the n and KF parameters can be accessed by representing log(qe) as the function of Ce. The 1/n value provides useful information about the adsorption processes. A 1/n value of less than one indicates chemisorption or a typical Langmuir adsorption isotherm, and a 1/n value greater than one indicates cooperative adsorption [22].
log(qe) = logKF + (1/n) logCe
5.3. Isotherm Models for Cu2+ Adsorption onto MgFe2O4-NH2 Nanoparticles
Ivanets et al. [7] reported that the magnesium ferrite nano-adsorbents possessed a maximum capacity for Cu2+, calculated from the Langmuir equation, around 0.49 mmol/g. Liu et al. [23] found that the maximum adsorption capacity (qm) of magnetic ferrite nanoparticles for Cu2+ is about 124.8 mg/g. The value of R2 for the Langmuir isotherm is greater than the R2 value for the Freundlich adsorption isotherm. This indicates that the Langmuir isotherm is best fitted to the adsorption of copper (II) ions.
Three Langmuir plots for the adsorption of the Cu2+ ions onto the MgFe2O4-NH2 nanoparticles, calcined at 500 °C, 600 °C, and 700 °C, are shown in Figure 13, and the subsequent computed isotherm parameters are reported in Table 4. It is shown that the isotherm parameters at different calcination temperatures did not differ significantly. Three Freundlich plots for the adsorption of the Cu2+ ions onto the MgFe2O4-NH2 nanoparticles, pre-functionalization-calcined at 500 °C, 600 °C, and 700 °C, are shown in Figure 14, and the subsequent computed isotherm parameters are reported in Table 4, which indicates that the isotherm parameters at different calcination temperatures did not differ significantly. The results show that the Langmuir model has a higher R2 value than the Freundlich model, indicating that it fits better.
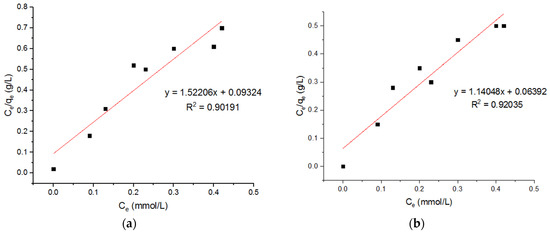
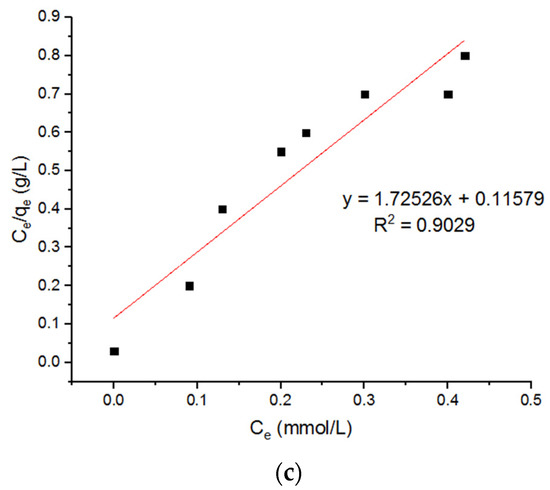
Figure 13.
Langmuir plots for adsorption of Cu2+ ions onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.

Table 4.
Isotherm parameters calculated from Langmuir and Freundlich model.
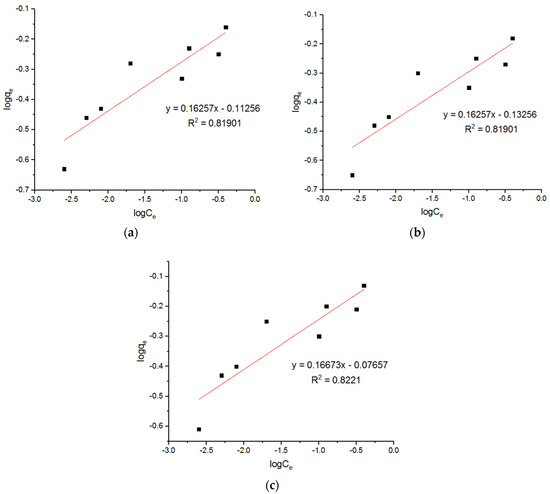
Figure 14.
Freundlich plots for adsorption of Cu2+ onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.
5.4. Isotherm Models for Pb2+ Adsorption onto MgFe2O4-NH2 Nanoparticles
Tran et al. [24] reported that a Cu0.5Mg0.5Fe2O4 nano-adsorbent possessed a maximum capacity of 57.44 mg/g, as calculated from the Langmuir equation. The Langmuir plots for the adsorption of the Pb2+ ions onto the MgFe2O4-NH2 nanoparticles are shown in Figure 15, and the subsequent computed isotherm parameters are reported in Table 5. This shows that the isotherm parameters of the adsorbent, pre-functionalization-calcined at 500 °C and 700 °C, did not differ significantly. Three Freundlich plots for the adsorption of the Pb2+ onto the MgFe2O4-NH2 nanoparticles are shown in Figure 16, and subsequent computed isotherm parameters are reported in Table 5, which indicates that the isotherm parameters at different calcination temperatures are not significantly different.
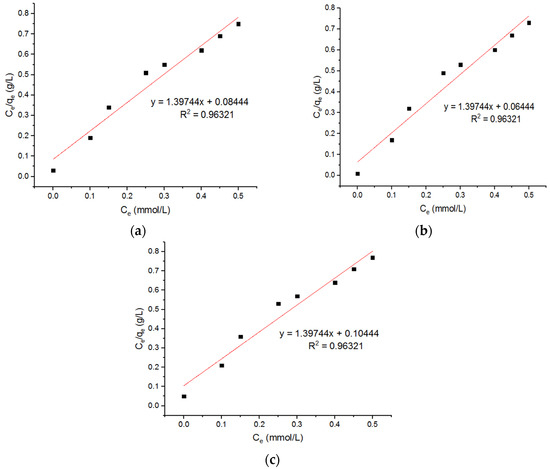
Figure 15.
Langmuir plots for adsorption of Pb2+ onto MgFe2O4-NH2 NPs pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.

Table 5.
Isotherm parameters calculated from Langmuir model.
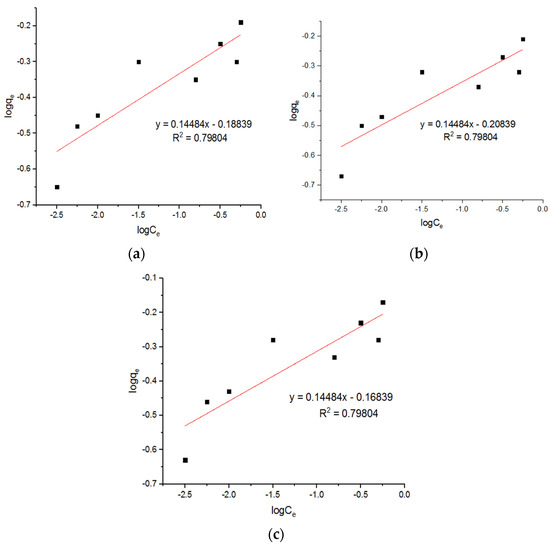
Figure 16.
Freundlich plots for adsorption of Pb2+ onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.
5.5. Isotherm Models for Zn2+ Adsorption onto MgFe2O4-NH2 Nanoparticles
Three Langmuir plots for the adsorption of the Zn2+ ions onto the MgFe2O4-NH2 nanoparticles are shown in Figure 17, and the subsequent computed isotherm parameters are reported in Table 6. It is shown that the isotherm parameters at 500 °C and 700 °C did not change significantly. The Freundlich plots for the adsorption of the Zn2+ onto the MgFe2O4-NH2 nanoparticles, pre-functionalization-calcined at 500 °C, 600 °C, and 700 °C are shown in Figure 18, and the subsequent isotherm parameters are reported in Table 6. It is shown that the isotherm parameters at different calcination temperatures did not differ significantly. The results indicate that the value of R2 for the Langmuir isotherm was greater than the R2 value for the Freundlich adsorption isotherm. This confirms that the Langmuir isotherm is best fitted to heavy metal ion adsorption.
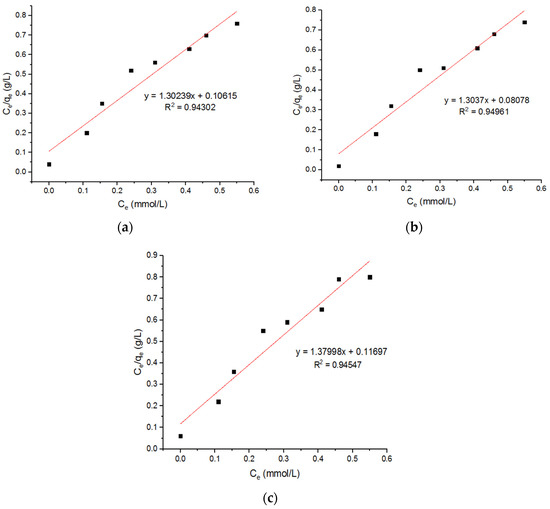
Figure 17.
Langmuir plots for adsorption of Zn2+ ions onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.

Table 6.
Isotherm parameters calculated from Langmuir model.
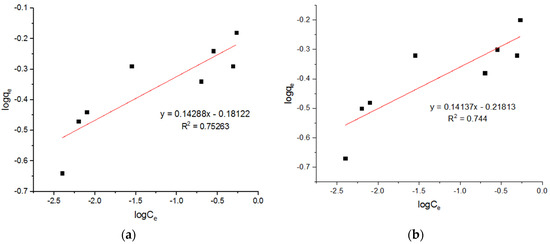
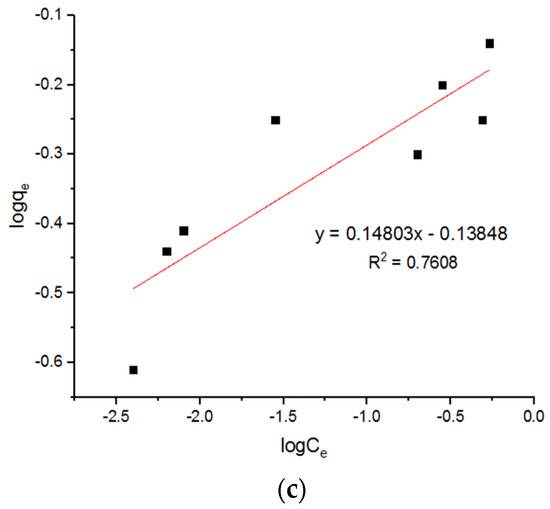
Figure 18.
Freundlich plots for adsorption of Zn2+ ions onto MgFe2O4-NH2 nanoparticles pre-functionalization-calcined at (a) 500 °C, (b) 600 °C, and (c) 700 °C.
It can be seen that this adsorption process followed the pseudo-second-order model and the Langmuir model, as in the process reported by Nonkumwong et al. [15] and Fan et al. [26]. The present work shows that the pseudo-second-order model and Langmuir model were better fitted the adsorption of different metal ions. There was no difference between different metal ions when the adsorption was taken into account. Table 7 summarizes a comparison of the qm values achieved in this study with those in other published literature.
The published literature shows greatly varying qm values for removing heavy metal ions. Some studies revealed a high adsorption of Pb2+ ions on nano-adsorbents, while others showed a high adsorption of Cu2+ ions. Ren et al. [27] used magnetic porous ferrospinel MnFe2O4 to adsorb Pb2+ and Cu2+ ions. They reported qm values of 69 and 37, respectively. Our study revealed qm values of 145.04 and 55.7 for the Pb2+ and Cu2+ ions, respectively. Although MnFe2O4 and MnFe2O4 magnetic adsorbents fall in the same class of spinel ferrites, the high adsorbtion of metal ions by the latter is attributed to ethanolamine functionalization of the adsorbent. The presence of −NH2 groups on the surfaces MnFe2O4 nanoparticles enhances their adsorption capacity [28]. The heat treatment of the magnetic adsorbent also affects the level of functionalization and, consequently, the adsorption capacity. The number of −NH2 groups attached to the surface increases with an increase in calcination temperature and surface roughness.
Tran et al. [24] used a Mg0.5Cu0.5Fe2O4 composite magnetic adsorbent to remove Pb2+ ions. They revealed a qm value of 57.7, which was significantly lower than the values reported by the current study. Duan et al. [29] used a Co0.6Fe2.4O4 magnetic adsorbent and reported qm values for Pb2+ ions in the range of 44.58 to 70.22, depending on the adsorbent synthesis conditions. Tamez et al. [30] used magnetic Fe3O4 adsorbent for the removal of heavy metals from aqueous medium. They reported qm values in the range of 47.62 to 166.67 and 19.61 to 37.04 for Pb2+ and Cu2+ ions, respectively. All these studies revealed much lower adsorption capacities than the current study. The low adsorption was attributed the pristine nature of the adsorbent. However, some other researchers composited the magnetic materials with non-magnetic materials for the construction of efficient adsorbents for heavy metals. For instance, Kalantari et al. [31] used Fe3O4/montmorillonite (Fe3O4/MMT NC) for the removal of Pb2+, Ni2+, and Cu2+ ions from aqueous solutions. They measured qm values of 263.15 and 70.92 for the Pb2+ and Cu2+ ions, respectively. Mittal et al. [32] reported a poly(methyl methacrylate)-decorated alginate/Fe3O4 composite as a novel adsorbent for heavy metals. The adsorption data for Pb2+ and Cu2+ ions were analyzed using the Freundlich, Langmuir, Sips, and Temkin models. The experimental data were fitted best to the Freundlich model. The adsorption capacities were 62.5 mg/g and 35.71 mg/g for the Pb2+ and Cu2+ ions, respectively. Lasheen et al. [33] used magnetite chitosan (NMag–CS) films for the adsorption of heavy metals. They reported adsorption capacities of 114.9 mg/g and 123.4 mg/g for Pb2+ and Cu2+ ions, respectively. For all the metals, the adsorption kinetics followed a pseudo-second-order equation. The nanomagnetic materials showed better finding capacities for the heavy metal ions when combined with magnetic materials.

Table 7.
A comparison of maximum adsorption capacities of different adsorbents.
Table 7.
A comparison of maximum adsorption capacities of different adsorbents.
| Adsorbent | qm (mg/g) | References | |
|---|---|---|---|
| Pb2+ | Cu2+ | ||
| Magnetic porous ferrospinel MnFe2O4 | 69 | 37 | [27] |
| EDTA-modified chitosan/SiO2/Fe3O4 | 12.5 | 44 | [34] |
| Fe3O4@2,3-diaminophenol and formaldehyde nanorods | 83 | - | [35] |
| Chitosan-coated bentonite beads | - | 12 | [36] |
| Fe3O4-NH2 nanoparticles | 40 | - | [6] |
| Fe3O4@SiO2-NH2 nanoparticles | 76 | - | [37] |
| Mg0.5Cu0.5Fe2O4 | 57.7 | - | [24] |
| Fe3O4/montmorillonite | 263.15 | 70.92 | [31] |
| PMMA-gft-Alg/Fe3O4 | 62.5 | 35.71 | [32] |
| Co0.6Fe2.4O4 | 44.58 to 70.22 | - | [29] |
| NMag–CS | 114.9 | 123.4 | [33] |
| Fe3O4 | 47.62 to 166.67 | 19.61 to 37.04 | [30] |
| MgFe2O4-NH2 nanoparticles | 145.04 | 55.7 | Present work |
6. Removal Efficiency
The removal efficiencies of different cations achieved by utilizing MgFe2O4-NH2 nanoparticles at different calcination temperatures are given in Table 8, which shows that the metal ions were largely removed by amine-functionalized nanoparticles pre-functionalization-calcined at 500 °C. Tan et al. [6] reported that about 98% of lead ions could be removed from tap water and wastewater by amino-functionalized Fe3O4 magnetic nanoparticles. The reported adsorbent cannot selectively capture different metal ions.

Table 8.
Metal ion removal efficiency of adsorbent pre-functionalization thermally treated at 500 °C, 600 °C, and 700 °C.
7. Conclusions
Magnesium ferrite (MgFe2O4) was successfully prepared by using the sol-gel method. The amine functionalization of the ferrite nanoparticles was carried out by using ethanolamine as a surface modifier. The kinetic and isotherm analysis of Pb2+, Cu2+, and Zn2+ adsorption on the surface of the MgFe2O4-NH2 was carried out after carefully optimizing the adsorption conditions. The synthesized materials had good removal efficiency and a fast adsorption rate. These results suggest a process for industrial wastewater treatment. The kinetic parameters and adsorption isotherms for the adsorption of the metal ions onto the amine-functionalized MgFe2O4 were obtained using the pseudo-first-order, pseudo-second-order, Langmuir, and Freundlich models. The pseudo-second-order and Langmuir models best described the adsorption kinetics and isotherms, implying strong chemisorption via the formation of coordinative bonds between the amine groups and metal ions. The Langmuir equation revealed the highest adsorption capacity of 0.7 mmol/g for the amine-functionalized MgFe2O4 nano-adsorbent. The calcination temperature also had an effect on the adsorption capacity of the nano-adsorbent. The MgFe2O4 sample, which was calcined at 500 °C, removed the most Pb2+ (73%), Cu2+ (59%), and Zn2+ (62%) from the effluent.
Author Contributions
F.Z., H.H., M.I., M.Y.N. and S.S. were responsible for the data collection, software, methodology, visualization, analysis, and writing of the draft. S.L., I.K., D.G.-K. and M.I. performed the editing, validation, analysis, and resource management. M.H.M., M.A.A., A.A.J.G., S.R., O.A., F.S.A. and M.K.A.K. performed the editing, management, and final draft approval. M.I., M.H.M., and S.R. performed project management, funding acquisition, and validation. All authors have read and agreed to the published version of the manuscript.
Funding
The paper fee was paid through the Poznan University of Technology—project no. 0713/SBAD/0958.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support from the Deanship of Scientific Research, Najran University, Kingdom of Saudi Arabia, for funding this work under the National Research Priorities funding program, code number (NU/NRP/SERC/11/26).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. CHAPTER 1 Contamination of Heavy Metals in Aquatic Media: Transport, Toxicity and Technologies for Remediation. In Heavy Metals in Water: Presence, Removal and Safety; The Royal Society of Chemistry: London, UK, 2015; pp. 1–24. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.-M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandy, S.; Bossart, K.; Mueller, R.; Ritschel, J.; Hauser, L.; Schulin, R.; Nowack, B. Extraction of Heavy Metals from Soils Using Biodegradable Chelating Agents. Environ. Sci. Technol. 2004, 38, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, M.; Hao, Y. High efficient removal of Pb (II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2012, 191, 104–111. [Google Scholar] [CrossRef]
- Ivanets, A.; Srivastava, V.; Roshchina, M.Y.; Sillanpää, M.; Prozorovich, V.; Pankov, V. Magnesium ferrite nanoparticles as a magnetic sorbent for the removal of Mn2+, Co2+, Ni2+ and Cu2+ from aqueous solution. Ceram. Int. 2018, 44, 9097–9104. [Google Scholar] [CrossRef]
- Rauret, G. Extraction procedures for the determination of heavy metals in contaminated soil and sediment. Talanta 1998, 46, 449–455. [Google Scholar] [CrossRef]
- Hu, H.; Xu, K. Chapter 8—Physicochemical technologies for HRPs and risk control. In High-Risk Pollutants in Wastewater; Ren, H., Zhang, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–207. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, R. Simultaneous fluorescence response and adsorption of functionalized Fe3O4@SiO2 nanoparticles to Cd2+, Zn2+ and Cu2+. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 240–246. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Bringas, E.; Ortiz, I. Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem. Eng. J. 2014, 256, 187–204. [Google Scholar] [CrossRef]
- Pradeep, A.; Priyadharsini, P.; Chandrasekaran, G. Sol–gel route of synthesis of nanoparticles of MgFe2O4 and XRD, FTIR and VSM study. J. Magn. Magn. Mater. 2008, 320, 2774–2779. [Google Scholar] [CrossRef]
- Rashad, M.; El-Shaarawy, M.; Shash, N.; Maklad, M.; Afifi, F. Controlling the composition, microstructure, electrical and magnetic properties of LiFe5O8 powders synthesized by sol gel auto-combustion method using urea as a fuel. J. Magn. Magn. Mater. 2015, 374, 495–501. [Google Scholar] [CrossRef]
- Gu, W.; Li, X.; Xing, M.; Fang, W.; Wu, D. Removal of phosphate from water by amine-functionalized copper ferrite chelated with La (III). Sci. Total Environ. 2018, 619, 42–48. [Google Scholar] [CrossRef]
- Nonkumwong, J.; Ananta, S.; Srisombat, L. Effective removal of lead (II) from wastewater by amine-functionalized magnesium ferrite nanoparticles. RSC Adv. 2016, 6, 47382–47393. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Gaur, R.; Yadav, M.; Goswami, A.; Zbořil, R.; Gawande, M.B. An efficient copper-based magnetic nanocatalyst for the fixation of carbon dioxide at atmospheric pressure. Sci. Rep. 2018, 8, 1901. [Google Scholar] [CrossRef] [PubMed]
- Naaz, F.; Dubey, H.K.; Kumari, C.; Lahiri, P. Structural and magnetic properties of MgFe2O4 nanopowder synthesized via co-precipitation route. SN Appl. Sci. 2020, 2, 808. [Google Scholar] [CrossRef] [Green Version]
- Aliyan, N.; Mirkazemi, S.M.; Masoudpanah, S.M.; Akbari, S. The effect of post-calcination on cation distributions and magnetic properties of the coprecipitated MgFe2O4 nanoparticles. Appl. Phys. A 2017, 123, 446. [Google Scholar] [CrossRef]
- Wang, Q.; Puerto, M.C.; Warudkar, S.; Buehler, J.; Biswal, S.L. Recyclable amine-functionalized magnetic nanoparticles for efficient demulsification of crude oil-in-water emulsions. Environ. Sci. Water Res. Technol. 2018, 4, 1553–1563. [Google Scholar] [CrossRef] [Green Version]
- Oddo, E.; Pesce, R.M.; Derudi, M.; Magagnin, L. Amino-functionalized magnetic nanoparticles for CO2 capture. Int. J. Smart Nano Mater. 2021, 12, 472–490. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Adsorption processes for the removal of contaminants from wastewater: The perspective role of nanomaterials and nanotechnology. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar]
- Huang, J.H.; Dong, J.; Liu, Z.L.; Liu, Y.P.; Wu, D.Y. Degradation of Dyes by H2O2 with Activated Charcoal Supported MgFe2O4 under Microwave Irradiation. Adv. Mater. Res. 2014, 1004–1005, 972–977. [Google Scholar] [CrossRef]
- Tran, C.V.; Quang, D.V.; Nguyen Thi, H.P.; Truong, T.N.; La, D.D. Effective Removal of Pb(II) from Aqueous Media by a New Design of Cu–Mg Binary Ferrite. ACS Omega 2020, 5, 7298–7306. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Sultan, N.; Ali, M.; Naz, M.Y.; AbdEl-Salam, N.M.; Ibrahim, K.A. Thermochemical conversion of waste glass and mollusk shells into an absorbent material for separation of direct blue 15 azo dye from industrial wastewater. ACS Omega 2020, 5, 18114–18122. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; Li, J.; Ying, D.; Wang, Y.; Jia, J. Comparative and competitive adsorption of Pb (II) and Cu (II) using tetraethylenepentamine modified chitosan/CoFe2O4 particles. J. Hazard. Mater. 2017, 326, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, N.; Feng, J.; Luan, T.; Wen, Q.; Li, Z.; Zhang, M. Adsorption of Pb (II) and Cu (II) from aqueous solution on magnetic porous ferrospinel MnFe2O4. J. Colloid Interface Sci. 2012, 367, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.H.K.; Wei, W.; Shuo, L.; Song, M.H.; Yun, Y.S. Fabrication of Stable and Regenerable Amine Functionalized Magnetic Nanoparticles as a Potential Material for Pt(IV) Recovery from Acidic Solutions. ACS Appl. Mater. Interfaces 2017, 9, 18650–18659. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Tang, R.; Xue, Z.; Zhang, X.; Zhao, Y.; Zhang, W.; Zhang, J.; Wang, B.; Zeng, S.; Sun, D. Effective removal of Pb(II) using magnetic Co0.6Fe2.4O4 micro-particles as the adsorbent: Synthesis and study on the kinetic and thermodynamic behaviors for its adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 211–223. [Google Scholar] [CrossRef]
- Tamez, C.; Hernandez, R.; Parsons, J.G. Removal of Cu (II) and Pb (II) from aqueous solution using engineered iron oxide nanoparticles. Microchem. J. 2016, 125, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantari, K.; Ahmad, M.B.; Fard Masoumi, H.R.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid and high capacity adsorption of heavy metals by Fe3O4/montmorillonite nanocomposite using response surface methodology: Preparation, characterization, optimization, equilibrium isotherms, and adsorption kinetics study. J. Taiwan Inst. Chem. Eng. 2015, 49, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Ahmad, R.; Hasan, I. Poly (methyl methacrylate)-grafted alginate/Fe3O4 nanocomposite: Synthesis and its application for the removal of heavy metal ions. Desalination Water Treat. 2016, 57, 19820–19833. [Google Scholar] [CrossRef]
- Lasheen, M.R.; El-Sherif, I.Y.; Tawfik, M.E.; El-Wakeel, S.T.; El-Shahat, M.F. Preparation and adsorption properties of nano magnetite chitosan films for heavy metal ions from aqueous solution. Mater. Res. Bull. 2016, 80, 344–350. [Google Scholar] [CrossRef]
- Ren, Y.; Abbood, H.A.; He, F.; Peng, H.; Huang, K. Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: Preparation, characterization, and application in heavy metal adsorption. Chem. Eng. J. 2013, 226, 300–311. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yoon, M. Core–shell ferromagnetic nanorod based on amine polymer composite (Fe3O4@ DAPF) for fast removal of Pb (II) from aqueous solutions. ACS Appl. Mater. Interfaces 2015, 7, 25362–25372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yang, N.; Zhang, D. Poly (N, N-dimethylaminoethyl methacrylate) modification of activated carbon for copper ions removal. Mater. Chem. Phys. 2009, 113, 784–789. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-functionalized Fe3O4@ SiO2 core–shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).