Adsorption of the Tartrate Ions in the Hydroxyapatite/Aqueous Solution of NaCl System
Abstract
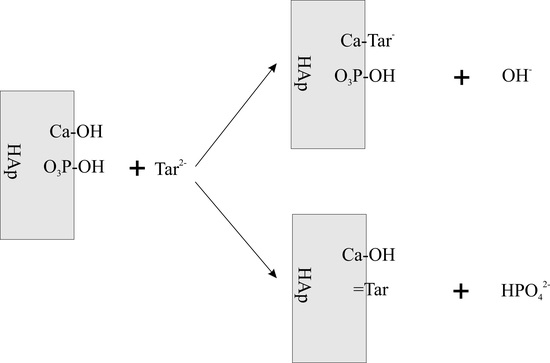
1. Introduction
2. Materials and Methods
3. Results and Discussion
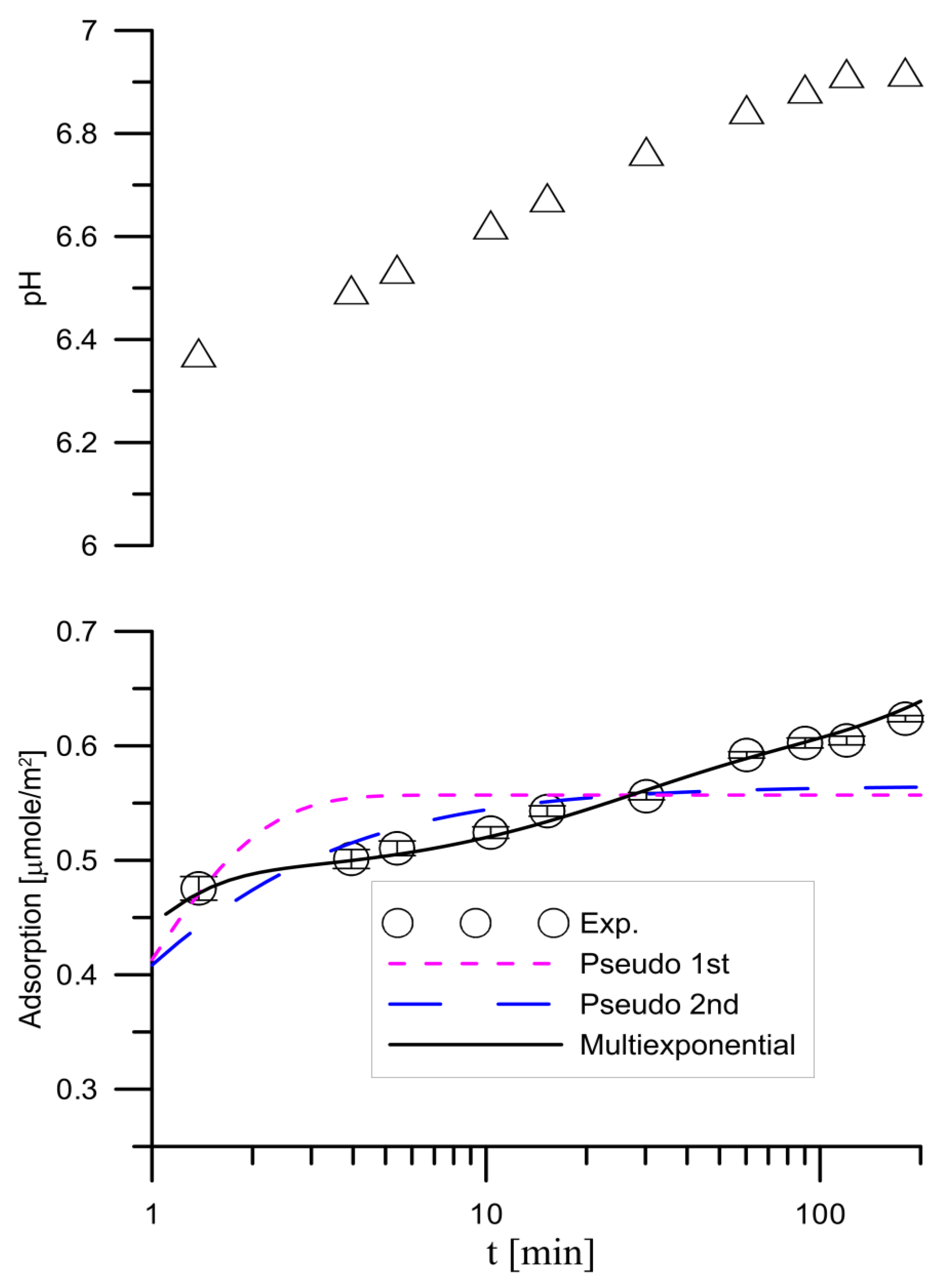
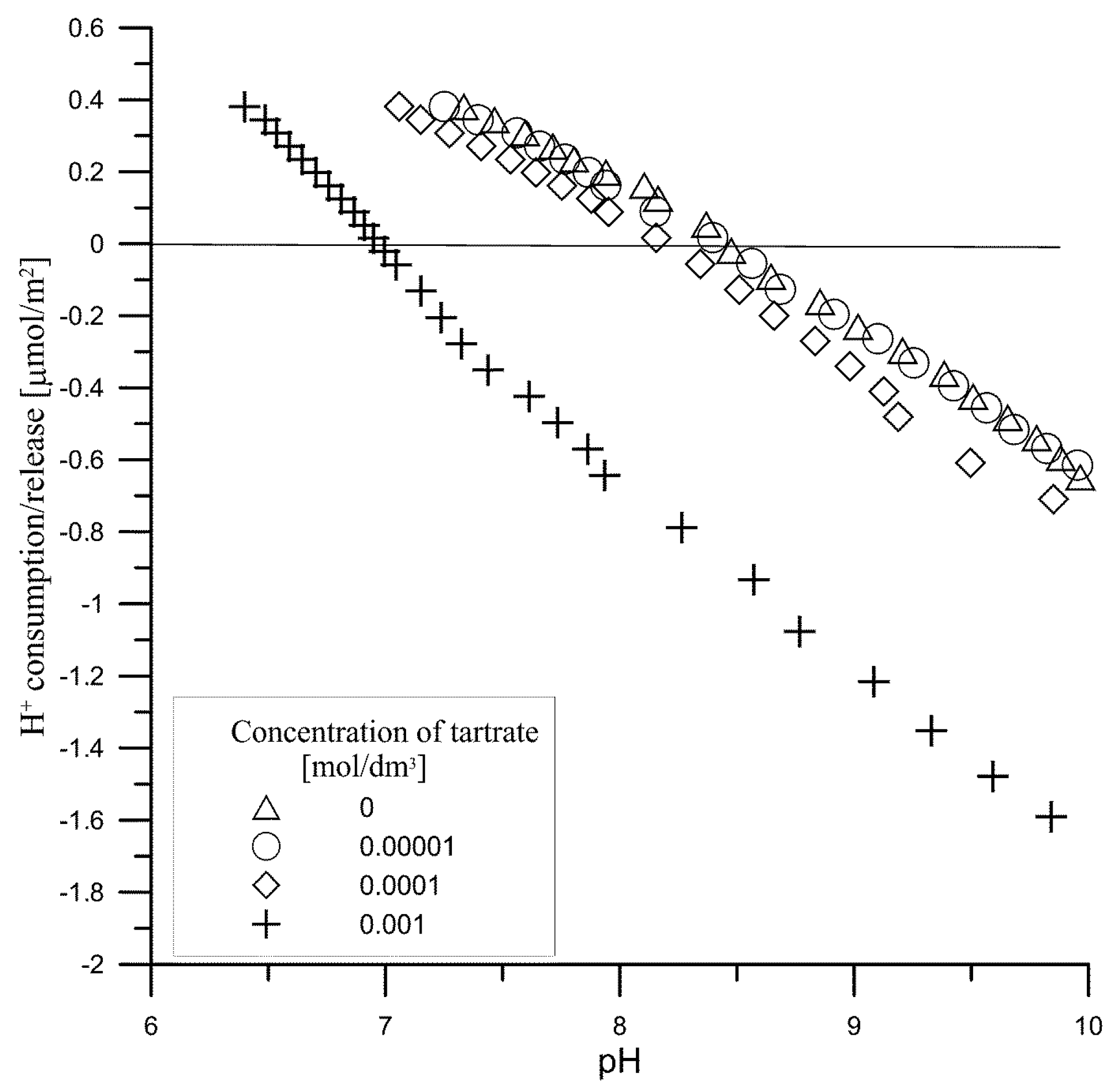
3.1. Kinetics of Tartrate Ions Adsorption Process
- a—the adsorption.
- aeq—the equilibrium adsorption.
- c0—the initial concentration.
- A0—the relative equilibrium concentration.
- Ai—describes the part of the i-th process characterized by the coefficient ki.
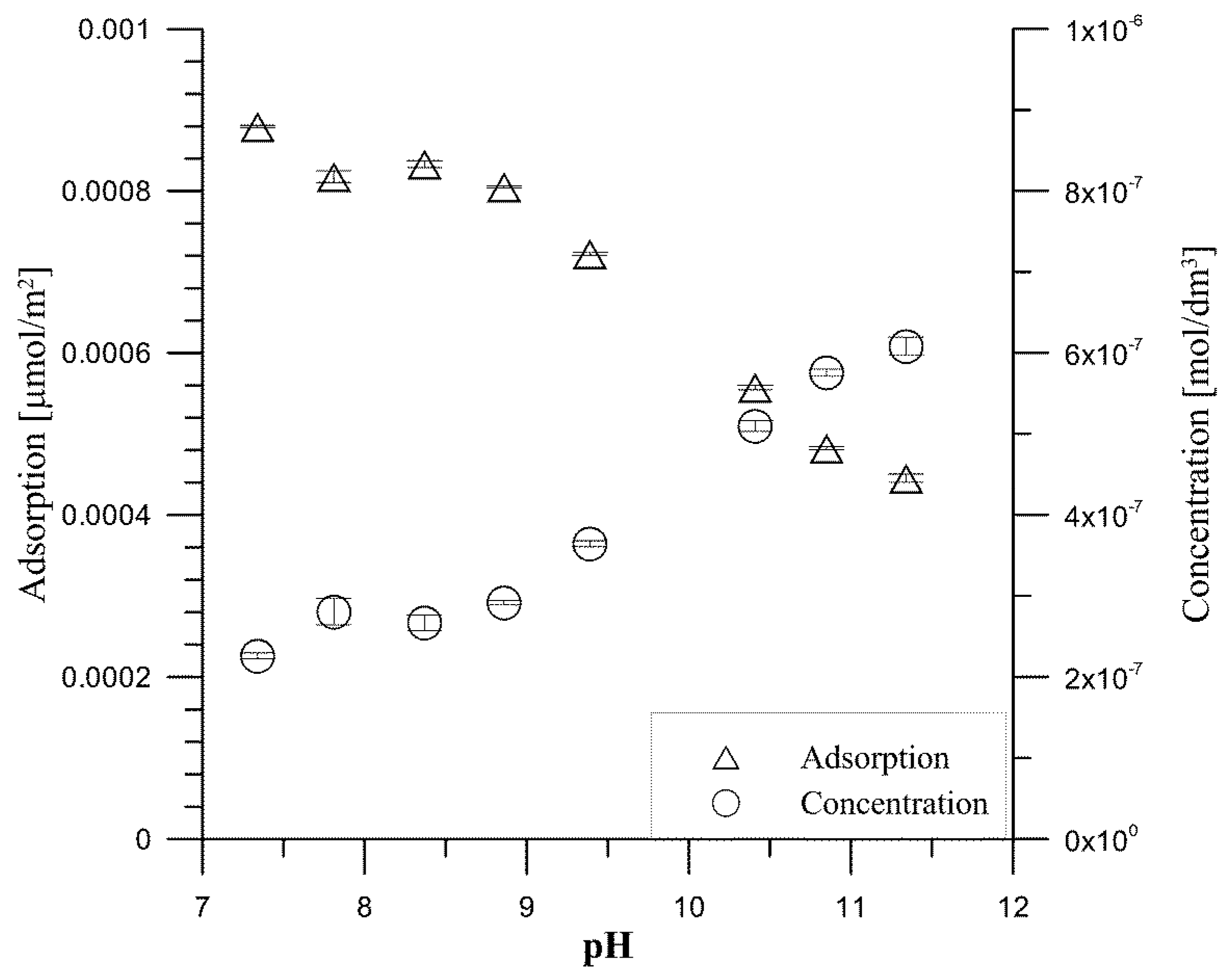
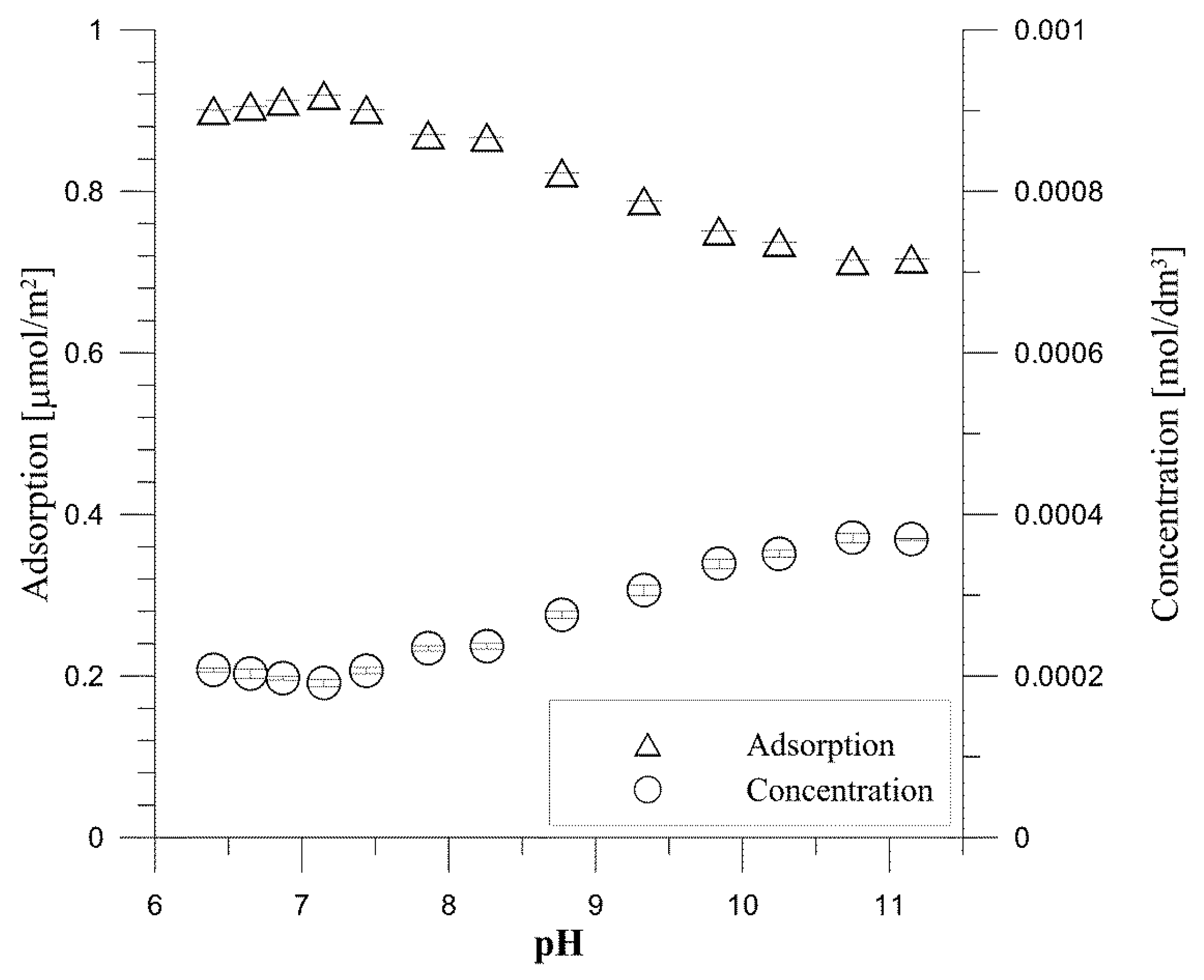
- ac (mol/m2)—the adsorption of tartrate ions.
- Ce—the equilibrium concentration of tartrate ions.
- am—the maximum amount of tartrate ions.
- KL—the constant related to the binding energy.

| Parameters | pH | ||
| 7.5 | 9 | 11 | |
| Parameters of Langmuir Isotherm | |||
| am | 2.62 × 10−6 | 1.63 × 10−6 | 8.75 × 10−7 |
| KL | 1653.7 | 1653.7 | 1039.2 |
| R2 | 0.90 | 0.77 | 0.21 |
| Parameters of Freundlich Isotherm | |||
| KF | 0.0062 | 0.0039 | 0.0077 |
| n | 0.969 | 0.968 | 0.857 |
| R2 | 0.99 | 0.99 | 0.99 |
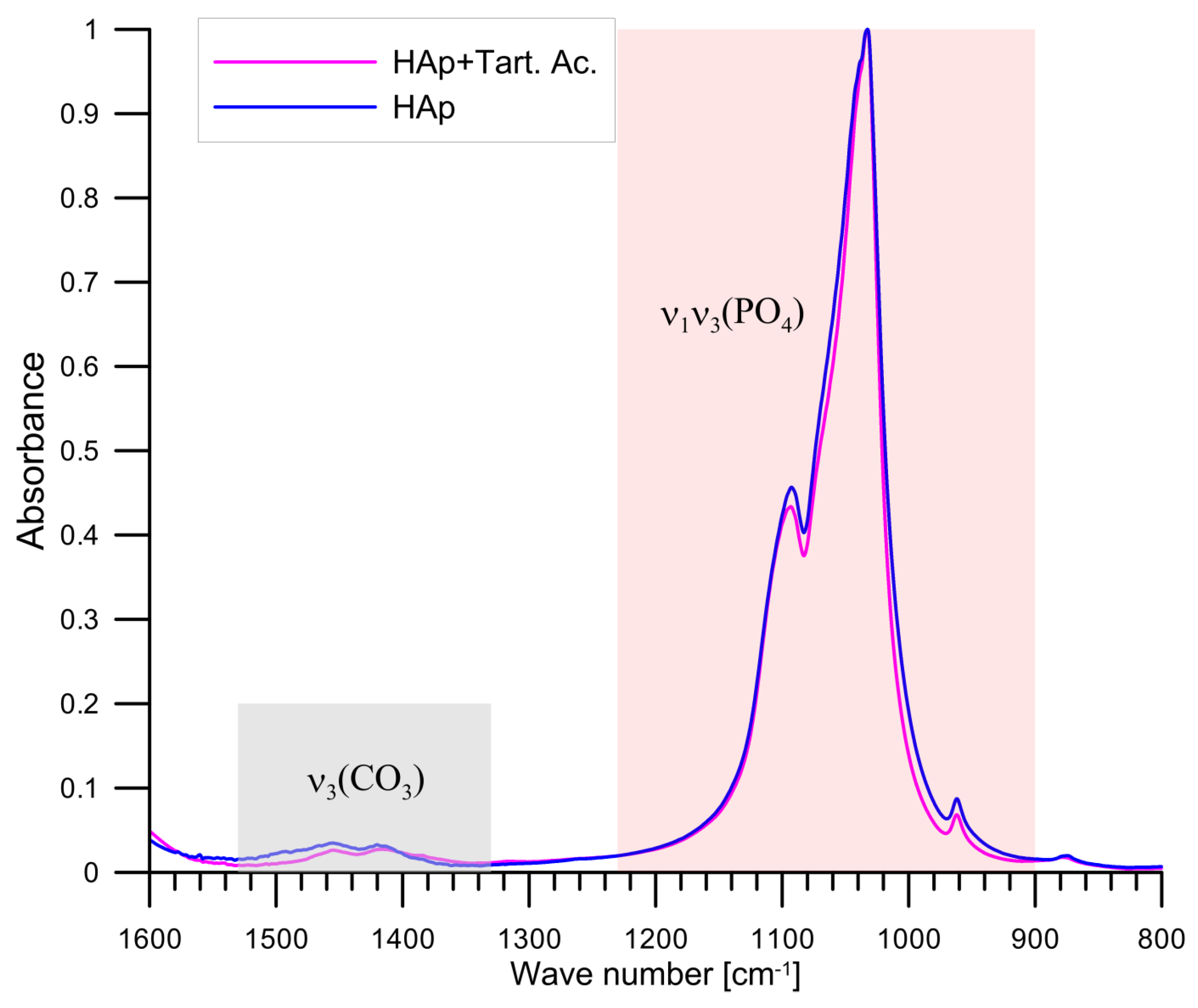
3.2. FTIR Study of Tartrate Ions Adsorption on Hydroxyapatite
3.3. PXRD Study of Hydroxyapatite Samples
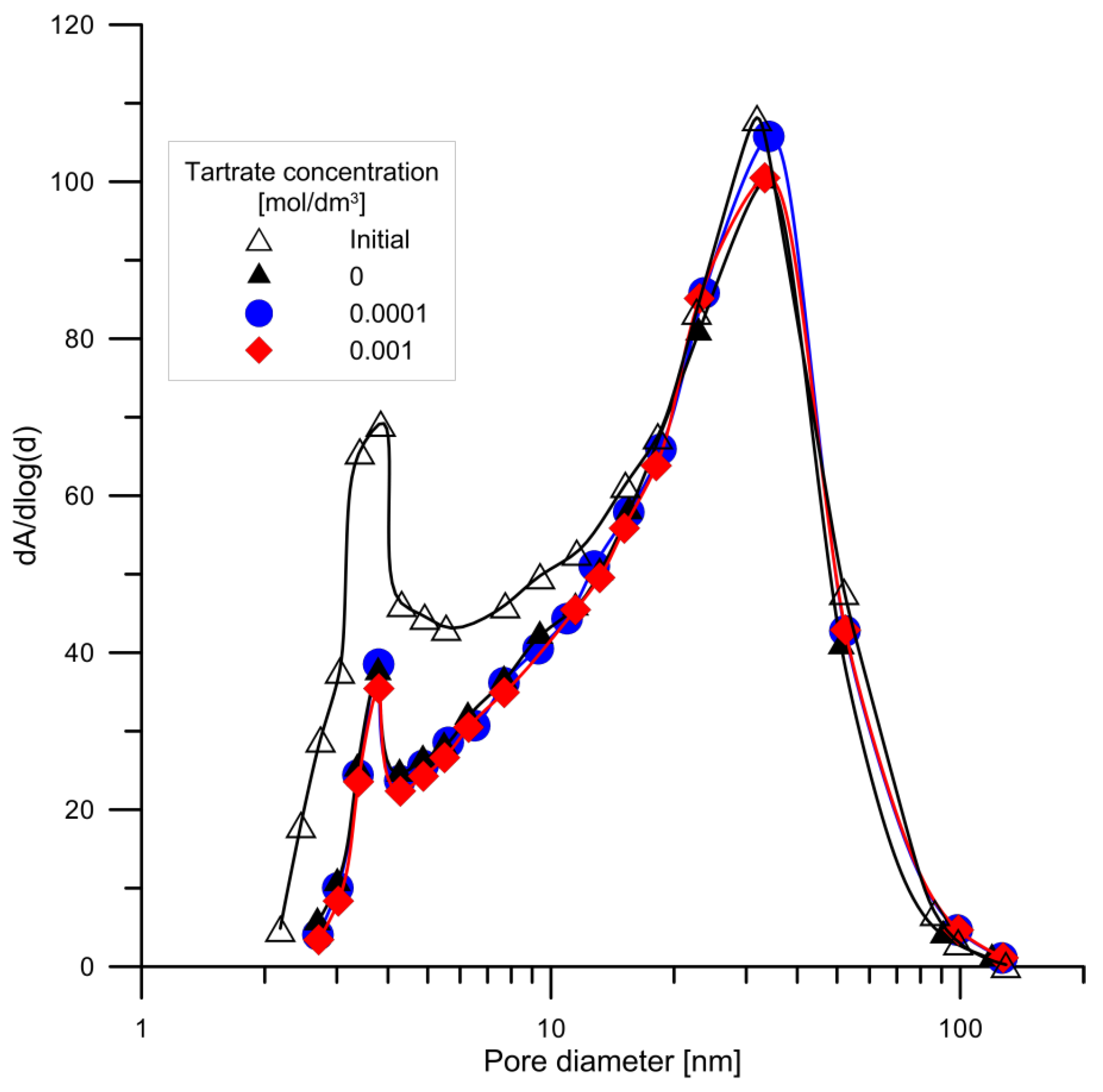
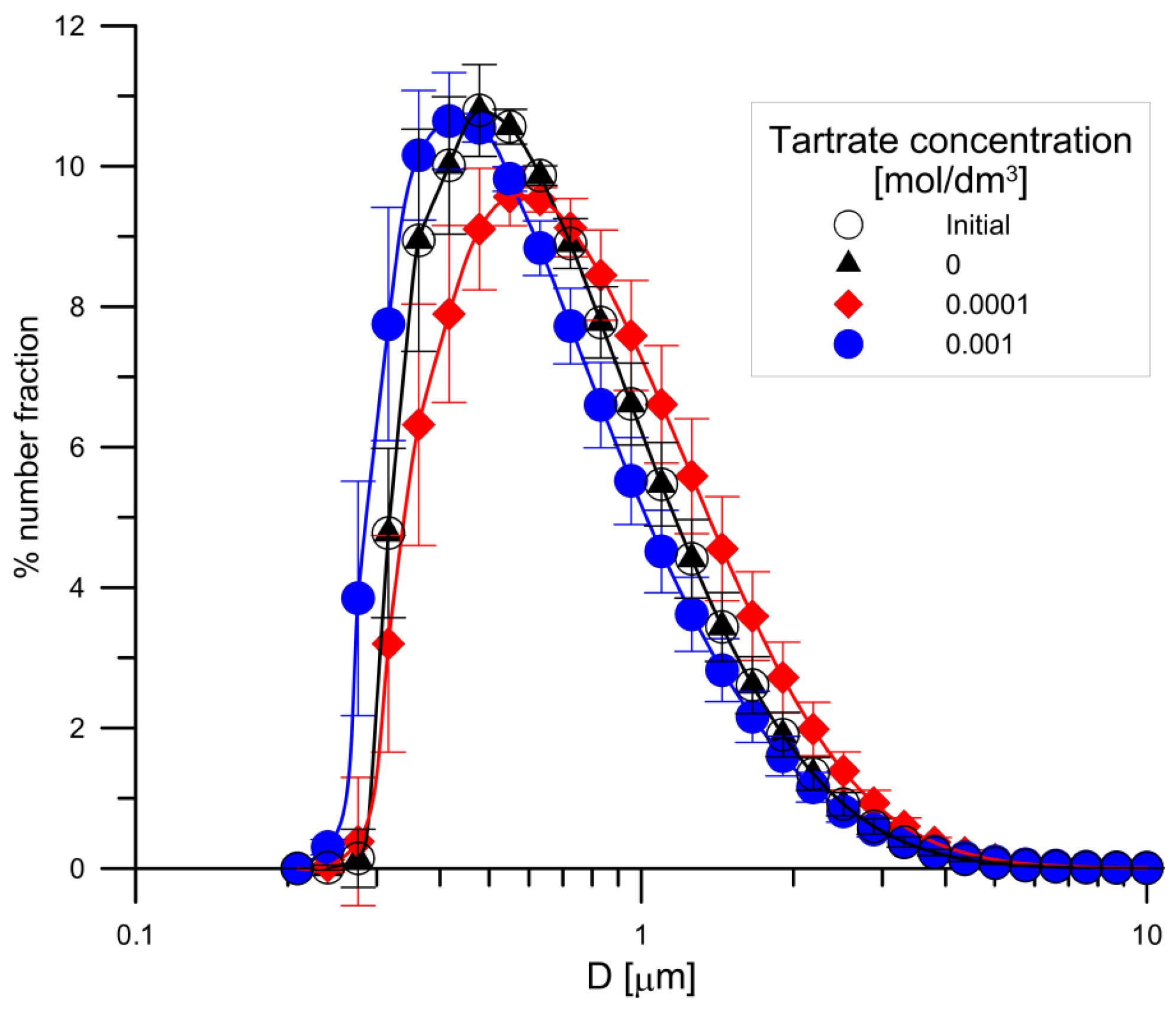
3.4. Study of the Surface Area, Porosity and Particle Size
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, F.H. Theeth and bones: Applications of surface science to dental materials and related biomaterials. Surf. Sci. Rep. 2001, 42, 75–205. [Google Scholar] [CrossRef]
- Panfilov, P.; Zaytsev, D.; Antonova, V.; Alpatova, V.; Kiselnikova, L.P. The difference of structural state and deformation behavior between teenage and mature human dentin. Int. J. Biomater. 2016, 2016, 6073051. [Google Scholar] [CrossRef]
- Featherstonea, J.D.B.; Lussi, A. Understanding the Chemistry of Dental Erosion. In Dental Erosion; Lussi, A., Ed.; Monographs in Oral Science: Basel, Karger, 2006; Volume 20, pp. 66–76. [Google Scholar]
- Li, C.; Meng, F. Nano-crystallinite hydroxyapatite synthesized by neutralization with the assist of citric acid. Mater. Lett. 2008, 62, 932–934. [Google Scholar] [CrossRef]
- Rhee, S.H.; Tanaka, J. Effect of citric acid on the nucleation of hydroxyapatite in a simulated body fluid. Biomaterials 1999, 20, 2155–2160. [Google Scholar] [CrossRef]
- Misra, D.N. Interaction of some alkali metal citrates with hydroxyapatite ion-exchange adsorption and role of charge balance. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 173–179. [Google Scholar] [CrossRef]
- Krukowski, S.; Karasiewicz, M.; Lysenko, N.; Kolmas, J. The influence of substituted hydroxyapatites heat treatment on citrate sorption behavior—Infrared spectroscopy experiments and adsorption studies. Colloids Surf. A 2018, 558, 23–32. [Google Scholar] [CrossRef]
- Lopez-Macipe, A.; Gomez-Morales, J.; Rodrıguez-Clemente, R. The role of pH in the adsorption of citrate ions on hydroxyapatite. J. Colloid Interface Sci. 1998, 200, 114–120. [Google Scholar] [CrossRef]
- Skwarek, E.; Janusz, W.; Sternik, D. Adsorption of citrate ions on hydroxyapatite synthetized by various methods. J. Radioanal. Nucl. Chem. 2014, 299, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Gácsi, A.; Kutus, B.; Csendes, Z.; Faragó, T.; Peintler, G.; Pálinkób, I.; Sipos, P. Calcium L-tartrate complex formation in neutral and in hyperalkaline aqueous solutions. Dalton Trans. 2016, 45, 17296–17303. [Google Scholar] [CrossRef]
- Kumari, S.C.; Padma, N. Stability studies on ascorbic acid content in various fruits, vegetables and their cocktail juices. Int. J. Res. Emerg. Sci. Technol. 2016, 12, 1–5. [Google Scholar]
- Hannig, C.; Hamkens, A.; Becker, K.; Attin, R.; Attin, T. Erosive effects of different acids on bovine enamel: Release of calcium and phosphate in vitro. Arch. Oral Biol. 2005, 50, 541–552. [Google Scholar] [CrossRef]
- Fu, B.; Shen, Q.; Qian, W.; Zeng, Y.; Sun, X.; Hannig, M. Interfacial interaction of tartaric acid with hydroxyapatite and enamel. J. Mater. Sci. Mater. Med. 2005, 16, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, X.; Cuia, J.; Wei, Z. Interaction between low molecular weight organic acids and hydroxyapatite with different degrees of crystallinity. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 67–75. [Google Scholar] [CrossRef]
- Vega, E.D.; Narda, G.E.; Ferretti, F.H. Adsorption of citric acid from dilute aqueous solutions by hydroxyapatite. J. Colloid Interface Sci. 2003, 268, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, M.R.T.; Mkhonto, D.; de Leeuw, N.H. Computer simulations of the adsorption of citric acid at hydroxyapatite surfaces. J. Cryst. Growth 2006, 294, 60–68. [Google Scholar] [CrossRef]
- Mostafa, N.Y. Characterization, thermal stability and sintering of hydroxyapatite powders prepared by different routes. Mater. Chem. Phys. 2005, 94, 333–341. [Google Scholar] [CrossRef]
- Skwarek, E. Thermal analysis of hydroxyapatite with adsorbed oxalic acid. J. Therm. Anal. Calorim. 2015, 122, 33–45. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Surface reactions of apatite dissolution. J. Colloid Interface Sci. 1997, 191, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Dissolution mechanism of calcium apatites in acids: A review of literature. World J. Methodol. 2012, 26, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Somasundaran, P.; Amankonah, J.O.; Ananthapadmabhan, K.P. Mineral-solution equilibria in sparingly soluble mineral systems. Colloids Surf. 1985, 15, 309–333. [Google Scholar] [CrossRef]
- Moreno, E.C.; Gregory, T.M.; Brown, W.E. Preparation and solubility of hydroxyapatite. J. Res. Natl. Bur. Stand. 1968, 72, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Linde, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Markovic, M.; Fowler, B.O.; Tung, M.S.; Lagergren, E.S. Composition and Solubility Product of a Synthetic Calcium Hydroxyapatite. In Mineral Scale Formation and Inhibition; Amjad, Z., Ed.; Springer Science Business Media: New York, NY, USA, 1995; pp. 271–282. [Google Scholar]
- Baes, C.F.; Mesmer, R.S. The Hydrolysis of Cations; John Wiley & Sons: New York, NY, USA, 1976. [Google Scholar]
- Martel, A.E.; Smit, R.M. Critical Stability Constants; Suppl. 2; Springer Science: New York, NY, USA, 1989; Volume 6. [Google Scholar]
- Greenwald, I. The dissociation of some calcium salts. J. Biol. Chem. 1938, 124, 437–452. [Google Scholar] [CrossRef]
- Marczewski, A.W. Kinetics and equilibrium of adsorption of organic solutes on mesoporous carbons. Appl. Surf. Sci. 2007, 253, 5818–5826. [Google Scholar] [CrossRef]
- Rill, C.; Kolar, Z.I.; Kickelbick, G.; Wolterbeek, H.T.; Peters, J.A. Kinetics and thermodynamics of adsorption on hydroxyapatite of the [160Tb]terbium complexes of the Bone-Targeting Ligands DOTP and BPPED. Langmuir 2009, 25, 2294–2301. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Marczewski, A.W.; Winter, S.; Sternik, D. Studies of adsorption equilibria and kinetics in the systems: Aqueous solution of dyes–mesoporous carbons. Appl. Surf. Sci. 2010, 256, 5164–5170. [Google Scholar] [CrossRef]
- Solhy, A.; Amera, W.; Karkouri, M.; Tahir, R.; El Bouarib, A.; Fihri, A.; Bousmina, M.; Zahouily, M. Bi-functional modified-phosphate catalyzed the synthesis of -α, α-(EE)-bis(benzylidene)-cycloalkanones: Microwave versus conventional-heating. J. Mol. Catal. A Chem. 2011, 336, 8–15. [Google Scholar] [CrossRef]
- Chappard, D.; Bizot, P.; Mabilleau, G.; Hubert, L. Aluminum and bone: Review of new clinical circumstances associated with Al3+ deposition in the calcified matrix of bone. Morphologie 2016, 100, 95–105. [Google Scholar] [CrossRef]
- Janusz, W.; Matysek, M. Coadsorption of Cd(II) and oxalate ions at the TiO2/electrolyte solution interface. J. Colloid Interface Sci. 2006, 296, 22–29. [Google Scholar] [CrossRef]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Yoshioka, M.; Snauwaert, J.; Abe, Y.; Lambrechts, P.; Vanherle, G.; Okazaki, M. Adhesion to and decalcification of hydroxyapatite by carboxylic acids. J. Dent. Res. 2001, 80, 1565–1569. [Google Scholar] [CrossRef]
- Kukura, M.; Bell, L.C.; Posner, A.M.; Quirk, J.P. Radioisotope determination of the surface concentrations of calcium and phosphorus on hydroxylapatite in aqueous solution. J. Phys. Chem. 1972, 76, 900–904. [Google Scholar] [CrossRef]
- Skartsila, K.; Spanos, N. Surface characterization of hydroxyapatite: Potentiometric titrations coupled with solubility measurements. J. Colloid Interface Sci. 2007, 308, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Bertinetti, L.; Tampieri, A.; Landi, E.; Ducati, C.; Midgley, P.A.; Coluccia, S.; Martra, G. Surface structure, hydration, and cationic sites of nanohydroxyapatite: UHR-TEM, IR, and Microgravimetric studies. J. Phys. Chem. C 2007, 111, 4027–4038. [Google Scholar] [CrossRef]
- Stumm, W. Chemistry of the Solid Water Interface; Wiley: New York, NY, USA, 1992; p. 93. [Google Scholar]
- Ho, Y.S. Isotherms for the sorption of lead onto peat: Comparison of linear and non-linear methods. Pol. J. Environ. Stud. 2006, 15, 81–86. [Google Scholar]
- Janusz, W.; Skwarek, E. Comparison of oxalate, citrate and tartrate ions, adsorption in the hydroxyapatite/aqueous electrolyte solution system. Colloids Interfaces 2020, 4, 45. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef]
- Ferrari, M.; Lutterotti, L. Method for the simultaneous determination of anisotropic residual stresses and texture by X-ray diffraction. J. Appl. Phys. 1994, 76, 7246–7255. [Google Scholar] [CrossRef]
- Person, A.; Bocherens, H.; Saliege, J.F.; Paris, F.; Zeitoun, V.; Gerard, M. Early diagenetic evolution of bone phosphate: An x-ray diffractometry analysis. J. Archaeol. Sci. 1995, 22, 211–221. [Google Scholar] [CrossRef]
- Smiciklas, I.; Onjia, A.; Raicevic, S.; Janackovic, D.; Mitric, M. Factors influencing the removal of divalent cations by hydroxyapatite. J. Hazard. Mater. 2008, 152, 876–884. [Google Scholar] [CrossRef]
- Sa, Y.; Guo, Y.; Feng, X.; Wang, M.; Li, P.; Gao, Y.; Yang, X.; Jiang, T. Are different crystallinity-index-calculating methods of hydroxyapatite efficient and consistent? New J. Chem. 2017, 41, 5723–5731. [Google Scholar] [CrossRef]




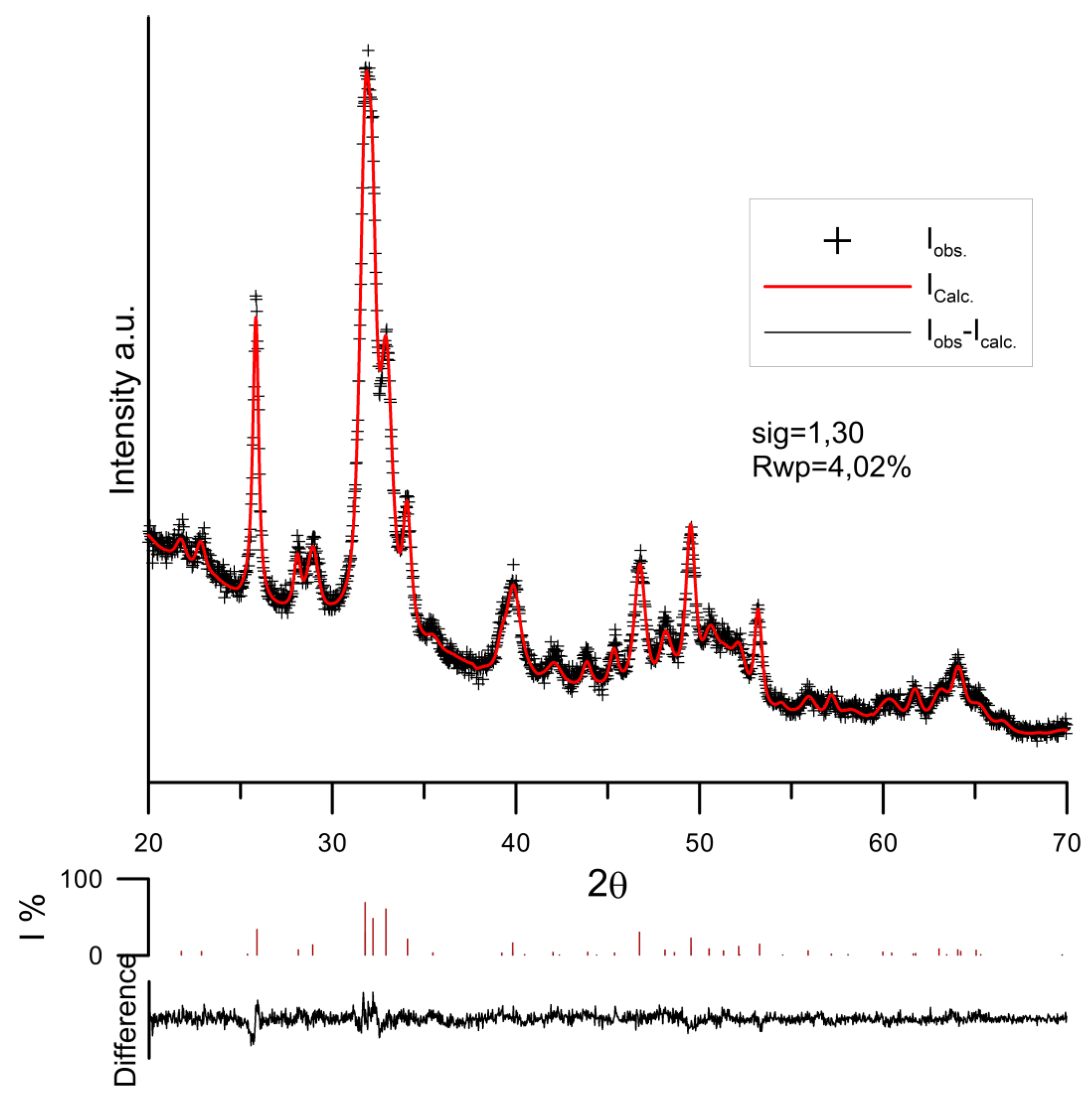
| Sample Code | a [Å] | c [Å] | V [Å3] | Sig | Rwp [%] |
|---|---|---|---|---|---|
| HAp/0.001 mol/dm3 NaCl | 9.41392 | 6.8886 | 528.69 | 0.2 | 5.52 |
| HAp/0.0001 mol/dm3 Tart. | 9.41043 | 6.8848 | 528.01 | 1.86 | 5.78 |
| HAp/0.001 mol/dm3 Tart. | 9.40813 | 6.8850 | 527.77 | 1.30 | 4.02 |
| PDF card no 00-009-0432 | 9.4180 | 6.8840 | 528.80 | - | - |
| Sample | D(002) [nm] | D(300) [nm] | D(002)/D(300) | CIXRD [46] |
|---|---|---|---|---|
| HAp/0.001 mol/dm3 NaCl | 26.5 ± 0.3 | 14.0 ± 0.3 | 1.89 | 10.19 |
| HAp/0.0001 mol/dm3 Tart. | 26.5 ± 0.4 | 14.1 ± 0.4 | 1.89 | 9.95 |
| HAp/0.001 mol/dm3 Tart. | 26.6 ± 0.5 | 13.4 ± 0.3 | 1.99 | 9.86 |
| Property | Sample | |||
|---|---|---|---|---|
| HAP (Initial) | HAP/0.001 mol/dm3 NaCl | HAP/0.0001 mol/dm3 Tartr. | HAP/0.001 mol/dm3 Tartr. | |
| BET Surface Area [m2/g] | 87.7 | 69.6 | 73.7 | 73.6 |
| Langmuir Surface Area [m2/g] | 128.2 | 101.9 | 107.8 | 107.5 |
| BJH Adsorption cumulative volume of pores 1.7 nm < d < 300 nm diameter [cm3/g] | 0.53 | 0.46 | 0.51 | 0.50 |
| BJH Desorption cumulative volume of pores 1.7 nm < d <300 nm diameter [cm3/g] | 0.53 | 0.47 | 0.51 | 0.51 |
| Adsorption average pore width (4 V/A by BET): [nm] | 24.2 | 26.7 | 27.9 | 27.6 |
| BJH Adsorption average pore diameter (4 V/A): [nm] | 24.0 | 27.2 | 28.6 | 28.4 |
| BJH Desorption average pore diameter (4 V/A): [nm] | 22.6 | 24.8 | 26.0 | 26.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janusz, W.; Skwarek, E. Adsorption of the Tartrate Ions in the Hydroxyapatite/Aqueous Solution of NaCl System. Materials 2021, 14, 3039. https://doi.org/10.3390/ma14113039
Janusz W, Skwarek E. Adsorption of the Tartrate Ions in the Hydroxyapatite/Aqueous Solution of NaCl System. Materials. 2021; 14(11):3039. https://doi.org/10.3390/ma14113039
Chicago/Turabian StyleJanusz, Władysław, and Ewa Skwarek. 2021. "Adsorption of the Tartrate Ions in the Hydroxyapatite/Aqueous Solution of NaCl System" Materials 14, no. 11: 3039. https://doi.org/10.3390/ma14113039
APA StyleJanusz, W., & Skwarek, E. (2021). Adsorption of the Tartrate Ions in the Hydroxyapatite/Aqueous Solution of NaCl System. Materials, 14(11), 3039. https://doi.org/10.3390/ma14113039