Computer Analysis of the Effect of Activation Temperature on the Microporous Structure Development of Activated Carbon Derived from Common Polypody
Abstract
1. Introduction
2. Materials and Methods
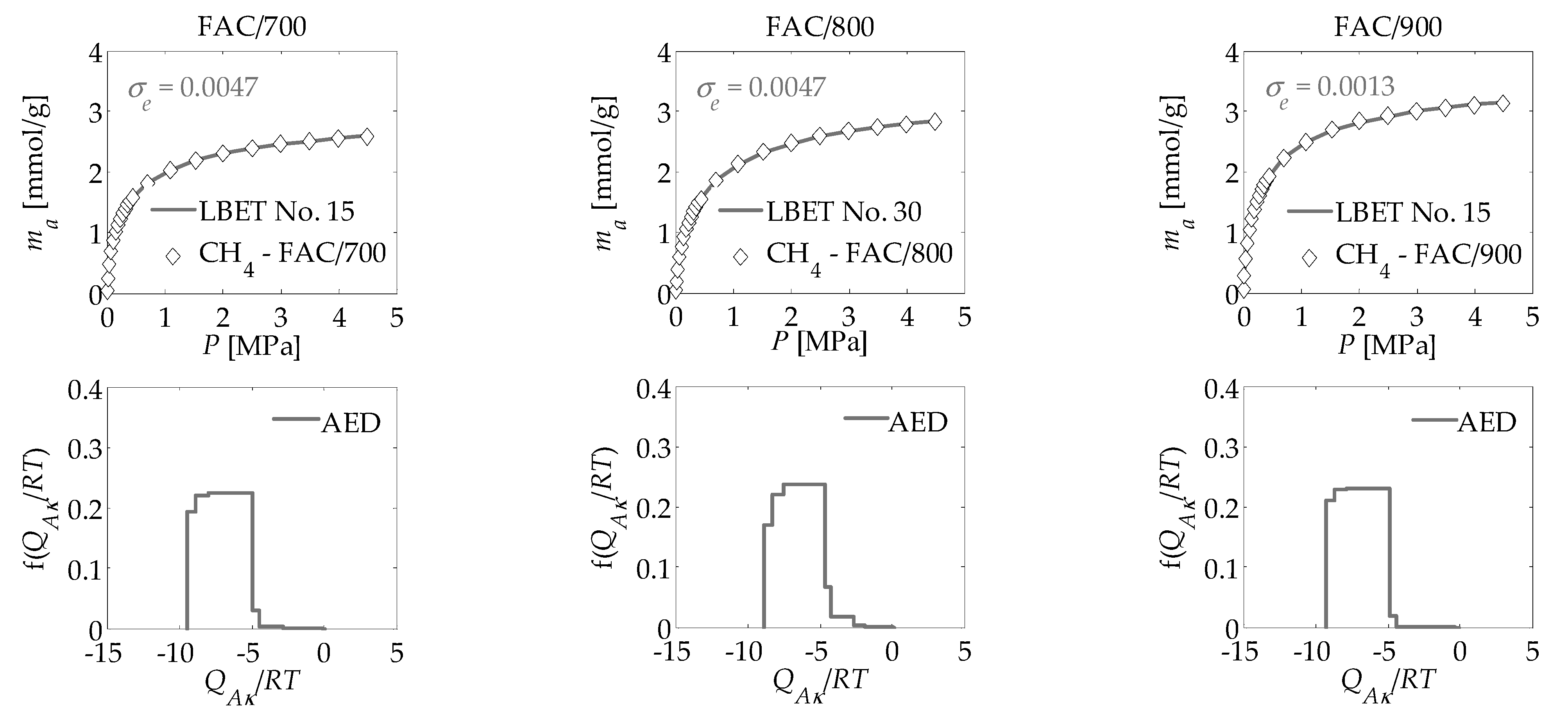
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sreńscek-Nazzal, J.; Narkiewicz, U.; Morawski, A.; Wróbel, R.; Gęsikiewicz-Puchalska, A.; Michalkiewicz, B. Modification of commercial activated carbons for CO₂ adsorption. Acta. Phys. Pol. A 2016, 129, 394–401. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E. An analysis of the porous structure of activated carbons obtained from hazelnut shells by various physical and chemical methods of activation. Colloids and Surfaces A 2017, 529, 443–453. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Ren, S.; Deng, L.; Zhang, B.; Lei, Y.; Ren, H.; Lv, J.; Zhao, R.; Chen, X. Effect of air oxidation on texture, surface properties and dye adsorption of wood-derived porous carbon materials. Materials 2019, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Machrouhi, A.; Alilou, H.; Farnane, M.; El Hamidi, S.; Sadiq, M.; Abdennouri, M.; Tounsadi, H.; Barka, N. Statistical optimization of activated carbon from Thapsia transtagana stems and dyes removal efficiency using central composite design. J. Sci. Adv. Mater. Devices 2019, 4, 544–553. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation-A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Melouki, R.; Ouadah, A.; Llewellyn, P.L. The CO2 adsorption behavior study on activated carbon synthesized from olive waste. J. CO2 Util. 2020, 42, 101292. [Google Scholar] [CrossRef]
- Borhan, A.; Yusuf, S. Activation of rubber-seed shell waste by malic acid as potential CO2 removal: Isotherm and kinetics studies. Materials 2020, 13, 4970. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-y.; Chen, Y.; Mo, Y. A review of charge storage in porous carbon-based supercapacitors. New Carbon Mater. 2021, 36, 49–68. [Google Scholar] [CrossRef]
- Chen, S.; Tang, S.; Sun, Y.; Wang, G.; Chen, H.; Yu, X.; Su, Y.; Chen, G. Preparation of a highly porous carbon material based on quinoa husk and its application for removal of dyes by adsorption. Materials 2018, 11, 1407. [Google Scholar] [CrossRef]
- Lendzion-Bielun, Z.; Czekajlo, L.; Sibera, D.; Moszyński, D.; Sreńscek-Nazzal, J.; Morawski, A.W.; Wrobel, R.J.; Michalkiewicz, B.; Arabczyk, W.; Narkiewicz, U. Surface characteristics of KOH-treated commercial carbons applied for CO2 adsorption. Ads Sci. Technol. 2018, 36, 478–492. [Google Scholar] [CrossRef]
- Serafin, J.; Baca, M.; Biegun, M.; Mijowska, E.; Kalenczuk, R.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Direct conversion of biomass to nanoporous activated biocarbons for high CO2 adsorption and supercapacitor applications. Appl. Surf. Sci. 2019, 497, 143722. [Google Scholar] [CrossRef]
- Guo, J.; Song, Y.; Ji, X.; Ji, L.; Cai, L.; Wang, Y.; Zhang, H.; Song, W. Preparation and characterization of nanoporous activated carbon derived from prawn shell and its application for removal of heavy metal ions. Materials 2019, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, R.; Hu, X.; Wang, L.; Wang, X.; Radosz, M.; Fan, M. CO2 adsorption on hazelnut-shell-derived nitrogen-doped porous carbons synthesized by single-step sodium amide activation. Ind. Eng. Chem. Res. 2020, 59, 7046–7053. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Shrestha, T.; Tamrakar, B.M.; Shrestha, R.G.; Maji, S.; Ariga, K.; Shrestha, L.K. Nanoporous carbon materials derived from washnut seed with enhanced supercapacitance. Materials 2020, 13, 2371. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Barreto, D.; Rodriguez Estupiñan, P.; Moreno-Piraján, J.; Ramírez, R.; Giraldo, L. Adsorption and photocatalytic study of phenol using composites of activated carbon prepared from onion leaves (allium fistulosum) and metallic oxides (ZnO and TiO2). Catalysts 2020, 10, 574. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Vo, D.V.N.; Gnana Prakash, D.; Antonysamy, A.J.; Viswanathan, S.; Jayaseelan, A. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Cruz, O.F.; Sreńscek-Nazzal, J. Preparation of low-cost activated carbons from amazonian nutshells for CO2 storage. Biomass Bioenergy 2021, 144, 105925. [Google Scholar] [CrossRef]
- Taslim, T.; Sinaga, F.; Iskandinata, I.; Irawan, F. Furfural synthesis from Mikania mircantha using sulfonated carbon catalyst derived from candlenut shell. Int. J. Eng. Res. Technol. 2020, 13, 2546–2552. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Wang, L.; Chen, F.; Shao, J.; Hu, X. Efficient nitrogen doped porous carbonaceous CO2 adsorbents based on lotus leaf. J. Environ. Sci. 2021, 103, 268–278. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhang, H.; Shao, L.M.; Lü, F.; He, P.J. Preparation and application of hierarchical porous carbon materials from waste and biomass: A review. Waste Biomass Valor. 2021, 12, 1699–1724. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Wei, Q.; Chen, Z.; Cheng, Y.; Wang, X.; Yang, X.; Wang, Z. Preparation and electrochemical performance of orange peel based-activated carbons activated by different activators. Colloids Surf. A Physicochem. Eng. Aspects 2019, 574, 221–227. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, T.; Zhi, J.; Zheng, Q.; Chen, Q.; Zhang, C.; Li, Y. Utilization of Jujube biomass to prepare biochar by pyrolysis and activation: Characterization, adsorption characteristics, and mechanisms for nitrogen. Materials 2020, 13, 5594. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, L.; Tian, H.; Yang, Z.; Luo, X. Adsorption and desorption performance and mechanism of tetracycline hydrochloride by activated carbon-based adsorbents derived from sugar cane bagasse activated with ZnCl2. Molecules 2019, 24, 4534. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Qin, X.; Liu, Z.; Fu, Y. Adsorption property, kinetic and equilibrium studies of activated carbon fiber prepared from liquefied wood by ZnCl2 activation. Materials 2019, 12, 1377. [Google Scholar] [CrossRef] [PubMed]
- Kaewtrakulchai, N.; Faungnawakij, K.; Eiad-Ua, A. Parametric study on microwave-assisted pyrolysis combined KOH activation of oil palm male flowers derived nanoporous carbons. Materials 2020, 13, 2876. [Google Scholar] [CrossRef] [PubMed]
- Benítez, A.; Morales, J.; Caballero, Á. Pistachio shell-derived carbon activated with phosphoric acid: A more efficient procedure to improve the performance of Li–S batteries. Nanomaterials 2020, 10, 840. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Sreńscek-Nazzal, J.; Michalkiewicz, B. An analysis of the effect of the additional activation process on the formation of the porous structure and pore size distribution of the commercial activated carbon WG-12. Adsorption 2017, 23, 551–561. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E. An evaluation of the reliability of the results obtained by the LBET, QSDFT, BET, and DR methods for the analysis of the porous structure of activated carbons. Materials 2020, 13, 3929. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E.; Fierro, V.; Celzard, A. An Evaluation of the Impact of the Amount of Potassium Hydroxide on the Porous Structure Development of Activated Carbons. Materials 2021, 14, 2045. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, M.; Delgadillo, D.P.V. Computer analysis of the effect of the type of activating agent on the formation of the porous structure of activated carbon monoliths. J. Mater. Res. Technol. 2019, 8, 4457–4463. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micromesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
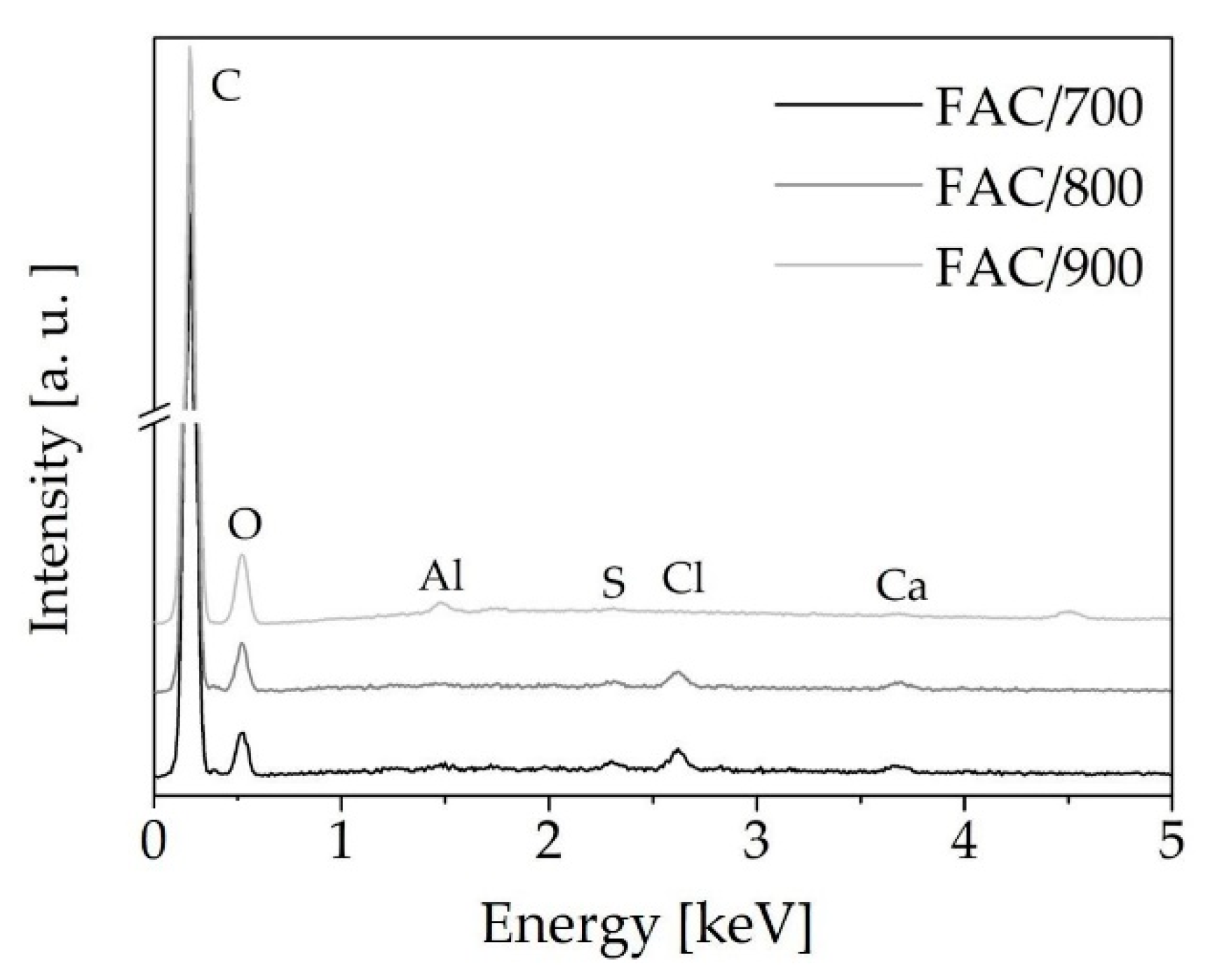
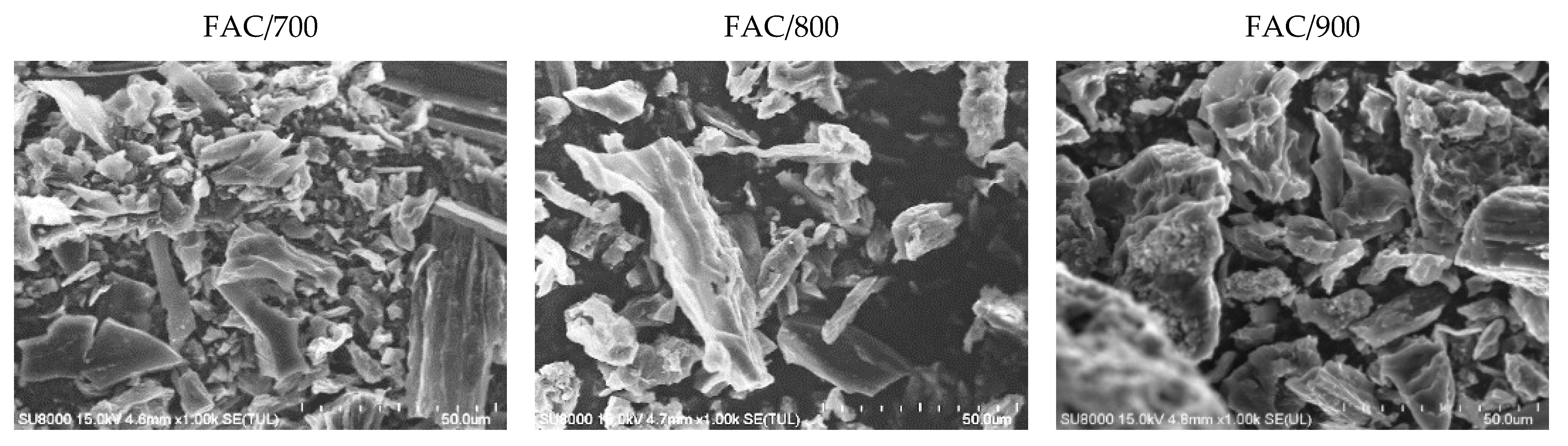

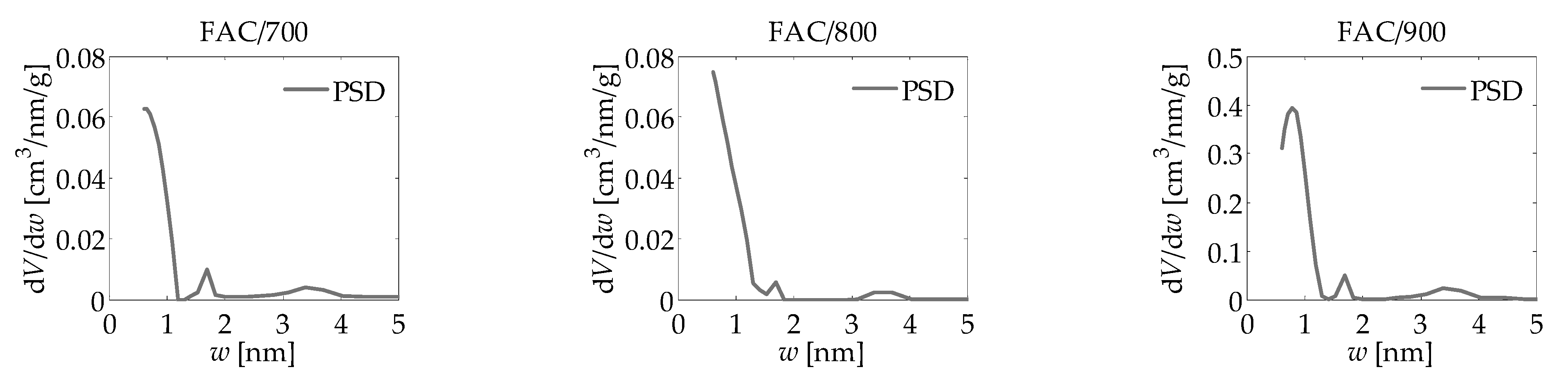
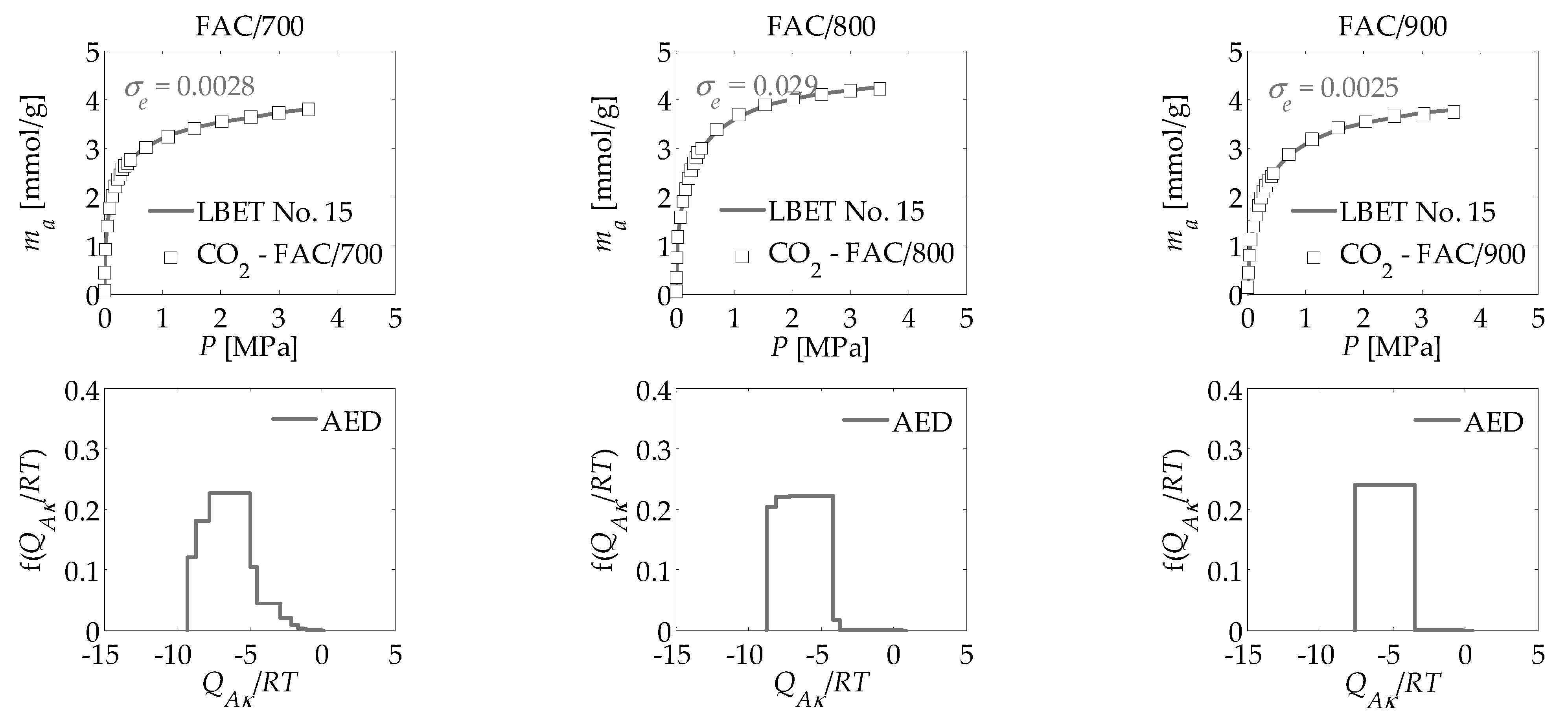
| Adsorbate | LBET No. | VhA [cm3/g] | QA/RT | BC | ZA | h | α | β | σe | wid |
|---|---|---|---|---|---|---|---|---|---|---|
| FAC/700 | ||||||||||
| N2 | 15 | 0.167 | −12.85 | 4.82 | 0.536 | 9 | 0.25 | 2.28 | 0.032 | 0.40 |
| CO2 | 15 | 0.241 | −9.26 | 0.64 | 0.807 | 9 | 0.48 | 1.14 | 0.003 | 0.76 |
| CH4 | 15 | 0.154 | −9.45 | 0.96 | 0.904 | 9 | 0.13 | 1.00 | 0.005 | 0.64 |
| FAC/800 | ||||||||||
| N2 | 15 | 0.198 | −11.29 | 6.91 | 0.496 | 9 | 0.10 | 3.04 | 0.022 | 0.24 |
| CO2 | 15 | 0.220 | −8.73 | 1.51 | 0.818 | 9 | 0.29 | 1.00 | 0.029 | 0.34 |
| CH4 | 30 | 0.179 | −8.87 | 0.92 | 0.854 | 9 | 0.08 | 1.00 | 0.012 | 0.36 |
| FAC/900 | ||||||||||
| N2 | 9 | 0.211 | −7.85 | 3.35 | 0.406 | 5 | 0.15 | 1.76 | 0.021 | 0.51 |
| CO2 | 15 | 0.243 | −7.59 | 2.53 | 0.704 | 9 | 0.08 | 1.00 | 0.025 | 0.30 |
| CH4 | 15 | 0.190 | −9.26 | 0.92 | 0.884 | 9 | 0.01 | 1.00 | 0.013 | 0.43 |
| Sample | SBET [m2/g] | SDR [m2/g] | SQSDFT [m2/g] | VTotal [cm3/g] | VDR [cm3/g] | VQSDFT [cm3/g] | DDR [nm] | DQSDFT [nm] |
|---|---|---|---|---|---|---|---|---|
| FAC/700 | 366.7 | 519.9 | 615.7 | 0.230 | 0.185 | 0.212 | 1.179 | 1.556 |
| FAC/800 | 423.5 | 610.7 | 643.9 | 0.236 | 0.212 | 0.217 | 1.204 | 1.786 |
| FAC/900 | 448.5 | 644.0 | 664.3 | 0.262 | 0.229 | 0.241 | 1.235 | 1.725 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, M.; Serafin, J.; Booth, A.M.; Michalkiewicz, B. Computer Analysis of the Effect of Activation Temperature on the Microporous Structure Development of Activated Carbon Derived from Common Polypody. Materials 2021, 14, 2951. https://doi.org/10.3390/ma14112951
Kwiatkowski M, Serafin J, Booth AM, Michalkiewicz B. Computer Analysis of the Effect of Activation Temperature on the Microporous Structure Development of Activated Carbon Derived from Common Polypody. Materials. 2021; 14(11):2951. https://doi.org/10.3390/ma14112951
Chicago/Turabian StyleKwiatkowski, Mirosław, Jarosław Serafin, Andy M. Booth, and Beata Michalkiewicz. 2021. "Computer Analysis of the Effect of Activation Temperature on the Microporous Structure Development of Activated Carbon Derived from Common Polypody" Materials 14, no. 11: 2951. https://doi.org/10.3390/ma14112951
APA StyleKwiatkowski, M., Serafin, J., Booth, A. M., & Michalkiewicz, B. (2021). Computer Analysis of the Effect of Activation Temperature on the Microporous Structure Development of Activated Carbon Derived from Common Polypody. Materials, 14(11), 2951. https://doi.org/10.3390/ma14112951








