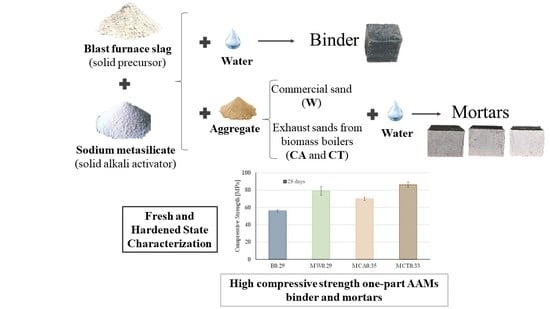
Waste-Based One-Part Alkali Activated Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alkali-Activated Materials
- Homogenization of the solid components (precursor and activator), by mixing in a plastic bag for approximately 1 min;
- Mechanical mixing for 1 min, while adding water;
- Manually mixing for approximately 1 min;
- Mechanical mixing for 1.5 min.
2.3. Characterization Techniques
2.3.1. Raw Materials Characterization
2.3.2. One-Part Alkali-Activated Materials Characterization
3. Results and Discussion
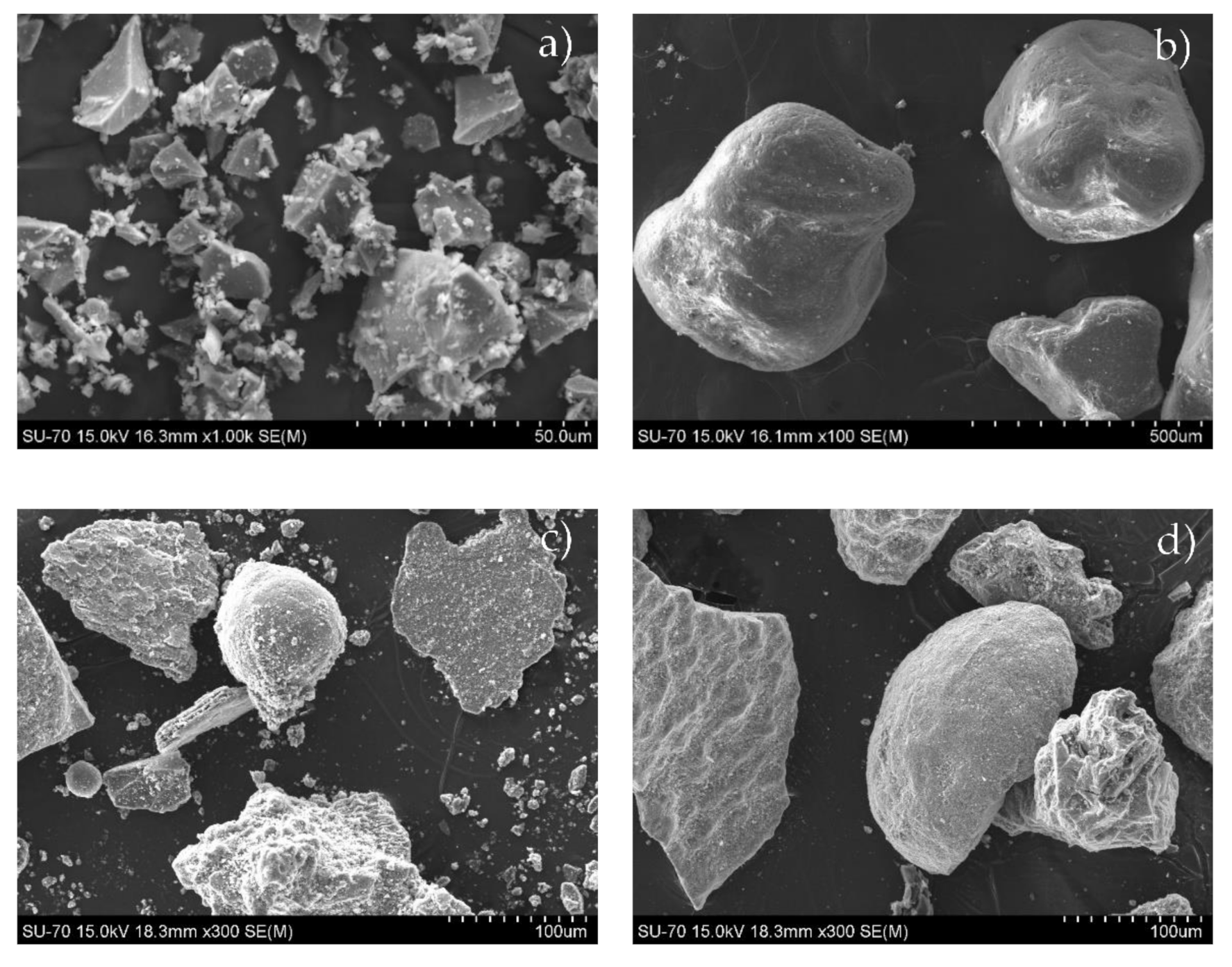
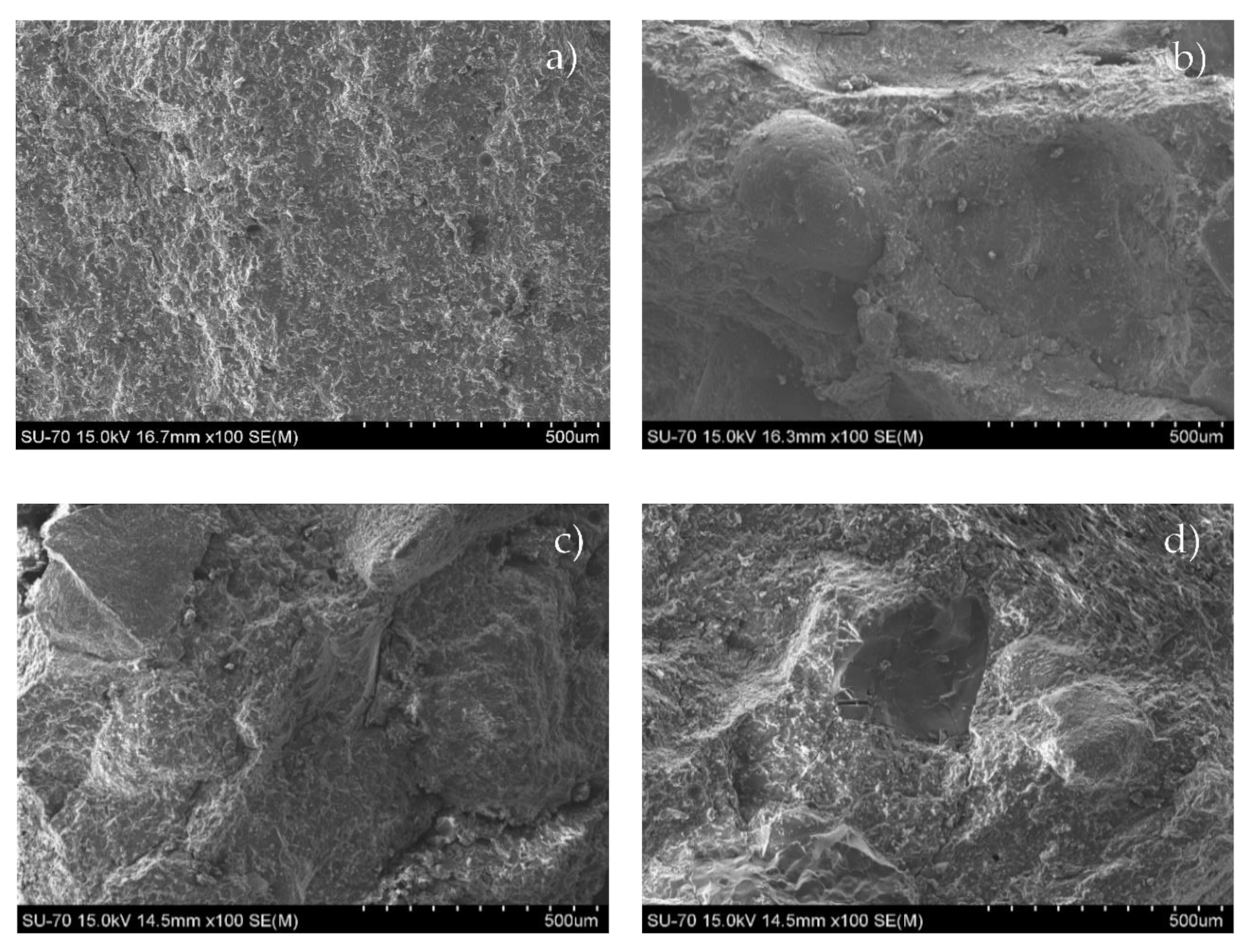
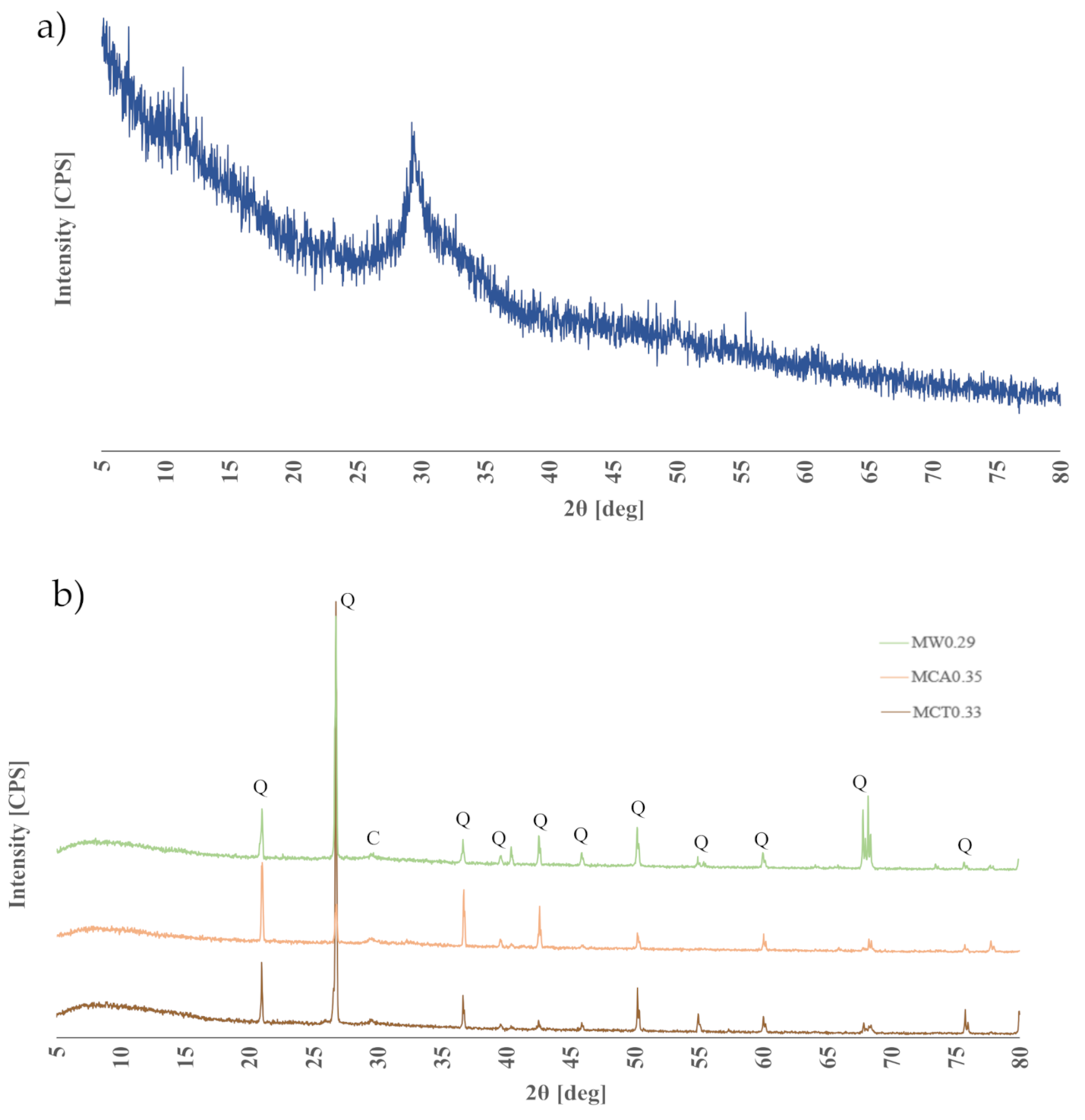
3.1. Characterization of the Materials
3.2. Binder and Mortars Characterization
3.2.1. Fresh State Characterization
3.2.2. Hardened State Characterization
3.3. Comparative Analysis with Previous Works
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huntzinger, D.N.; Eatmon, T.D. A life-cycle assessment of Portland cement manufacturing: Comparing the traditional process with alternative technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Eurostat: Statistics Explained. Climate Change—Driving Forces 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Climate_change_-_driving_forces (accessed on 6 May 2020).
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Reddy, M.S.; Dinakar, P.; Rao, B.H. Mix design development of fly ash and ground granulated blast furnace slag based geopolymer concrete. J. Build. Eng. 2018, 20, 712–722. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Askarian, M.; Tao, Z.; Samali, B.; Adam, G.; Shuaibu, R. Mix composition and characterisation of one-part geopolymers with different activators. Constr. Build. Mater. 2019, 225, 526–537. [Google Scholar] [CrossRef]
- Koloušek, D.; Brus, J.; Urbanova, M.; Andertova, J.; Hulinsky, V.; Vorel, J. Preparation, structure and hydrothermal stability of alternative (sodium silicate-free) geopolymers. J. Mater. Sci. 2007, 42, 9267–9275. [Google Scholar] [CrossRef]
- Yang, K.H.; Song, J.K.; Ashour, A.F.; Lee, E.T. Properties of cementless mortars activated by sodium silicate. Constr. Build. Mater. 2008, 22, 1981–1989. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; de Aguiar, J.B. Eco-concrete: One-part geopolymer mixes. In Proceedings of the TRF Senior Research Scholars Progress II, Khon Kaen University, Khon Kaen, Thailand, 2 August 2013. [Google Scholar]
- Garcia-Lodeiro, I.; Fernández-Jimenez, A.; Palomo, A. Cements with a low clinker content: Versatile use of raw materials. J. Sustain. Cem. Mater. 2015, 4, 140–151. [Google Scholar] [CrossRef]
- Askarian, M.; Tao, Z.; Adam, G.; Samali, B. Mechanical properties of ambient cured one-part hybrid OPC-geopolymer concrete. Constr. Build. Mater. 2018, 186, 330–337. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Ambient temperature cured one-part engineered geopolymer composite: A sustainable alternative to engineered. Constr. Build. Mater. 2017, 2014, 831–838. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of one-part geopolymer binders made from fly ash. Waste Biomass Valorization 2017, 8, 225–233. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Wahab, M.M.A.; Liew, M.S.; Haruna, A. Mechanical and microstructural properties of high calcium fly ash one-part geopolymer cement made with granular activator. Heliyon 2019, 5, e02255. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Sreenivasan, H.; Abdollahnejad, Z.; Yliniemi, J.; Kantola, A.; Telkki, V.V.; Kinnunen, P.; Illikainen, M. Influence of sodium silicate powder silica modulus for mechanical and chemical properties of dry-mix alkali-activated slag mortar. Constr. Build. Mater. 2020, 233, 117354. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Brouwers, H.J.H.; Kühne, H.C. Synthesizing one-part geopolymers from rice husk ash. Constr. Build. Mater. 2016, 124, 961–966. [Google Scholar] [CrossRef]
- Suwan, T.; Fan, M. Effect of manufacturing process on the mechanisms and mechanical properties of fly ash-based geopolymer in ambient curing temperature. Mater. Manuf. Process. 2017, 32, 461–467. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Synthesis of heat and ambient cured one-part geopolymer mixes with different grades of sodium silicate. Ceram. Int. 2015, 41, 5696–5704. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Shakya, C.; Ahmad, M.R.; Shah, S.F.A. Influence of superplasticizers and retarders on the workability and strength of one-part alkali-activated fly ash/slag binders cured at room temperature. Constr. Build. Mater. 2019, 229, 116891. [Google Scholar] [CrossRef]
- Bong, S.H.; Nematollahi, B.; Nazari, A.; Xia, M.; Sanjayan, J. Efficiency of different superplasticizers and retarders on properties of “one-part” fly ash-slag blended geopolymers with different activators. Materials 2019, 12, 3410. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Brandt, J.; Lear, K.; Liu, J. A looming tragedy of the sand commons. Science 2017, 357, 970–971. [Google Scholar] [CrossRef]
- Gholampour, A.; Ho, V.D.; Ozbakkaloglu, T. Ambient-cured geopolymer mortars prepared with waste-based sands: Mechanical and durability-related properties and microstructure. Compos. Part B Eng. 2019, 160, 519–534. [Google Scholar] [CrossRef]
- Norma Portuguesa. NP EN 1097-6: Methods of Test for Aggregates, Part 6: Bulk Density and Water Absorption; Portuguese Institute of Quality: Lisbon, Portugal, 2003. [Google Scholar]
- Norma Portuguesa. NP EN 933-1: Methods of Test for Aggregates, Part 1: Sieve Analysis; Portuguese Institute of Quality: Lisbon, Portugal, 2000; pp. 1–16. [Google Scholar]
- CEN: European Committee for Standardization. EN 1015-3: Methods of Test for Mortar for Masonry Part 3—Determination of Consistence of Fresh Mortar (by Flow Table); CEN: Brussels, Belgium, 1998. [Google Scholar]
- CEN: European Committee for Standardization. EN 196-3: Methods of Testing Cement Part 3—Determination of Setting Times and Soundness; CEN: Brussels, Belgium, 2005. [Google Scholar]
- CEN: European Committee for Standardization. EN 1015-11: Methods of Test for Mortar for Masonry Part 11—Determination of Flexural and Compressive Strength of Hardened Mortar; CEN: Brussels, Belgium, 1999. [Google Scholar]
- CEN: European Committee for Standardization. EN 1015-18: Methods of Test for Mortar for Masonry Part 18—Determination of water- Absorption Coefficient Due to Capillary Action of Hardened Mortar; CEN: Brussels, Belgium, 2002. [Google Scholar]
- Pourkhorshidi, A.R.; Najimi, M.; Parhizkar, T.; Jafarpour, F.; Hillemeier, B. Applicability of the standard specifications of ASTM C618 for evaluation of natural pozzolans. Cem. Concr. Compos. 2010, 32, 794–800. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. Influence of curing conditions on properties of high calcium fly ash geopolymer containing Portland cement as additive. Mater. Des. 2014, 53, 269–274. [Google Scholar] [CrossRef]
- Suwan, T.; Fan, M. Influence of OPC replacement and manufacturing procedures on the properties of self-cured geopolymer. Constr. Build. Mater. 2014, 73, 551–561. [Google Scholar] [CrossRef]
- Venkatarama Reddy, B.V.; Gupta, A. Influence of sand grading on the characteristics of mortars and soil-cement block masonry. Constr. Build. Mater. 2008, 22, 1614–1623. [Google Scholar] [CrossRef]
- Criado, M.; Walkley, B.; Ke, X.; Provis, J.L.; Bernal, S.A. Slag and activator chemistry control the reaction kinetics of sodium metasilicate-activated slag cements. Sustainability 2018, 10, 4709. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; De Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Comparison of alkali and silica sources in one-part alkali-activated blast furnace slag mortar. J. Clean. Prod. 2018, 187, 171–179. [Google Scholar] [CrossRef]
- Singh, S.B.; Munjal, P.; Thammishetti, N. Role of water/cement ratio on strength development of cement mortar. J. Build. Eng. 2015, 4, 94–100. [Google Scholar] [CrossRef]
- Yahya, Z.; Abdullah, M.M.A.B.; Talib, S.Z.A.; Razak, R.A. Comparative study on early strength of sodium hydroxide (NaOH) activated fly ash based geopolymer. In Proceedings of the AIP Conference, East Java, Indonesia, 8–9 August 2017. [Google Scholar] [CrossRef]
- Gulbe, L.; Vitina, I.; Setina, J. The influence of cement on properties of lime mortars. Procedia Eng. 2017, 172, 325–332. [Google Scholar] [CrossRef]
- Mermerdaş, K.; Manguri, S.; Nassani, D.E.; Oleiwi, S.M. Effect of aggregate properties on the mechanical and absorption characteristics of geopolymer mortar. Eng. Sci. Technol. Int. J. 2017, 20, 1642–1652. [Google Scholar] [CrossRef]
- Novais, R.M.; Carvalheiras, J.; Senff, L.; Labrincha, J.A. Upcycling unexplored dregs and biomass fly ash from the paper and pulp industry in the production of eco-friendly geopolymer mortars: A preliminary assessment. Constr. Build. Mater. 2018, 184, 464–472. [Google Scholar] [CrossRef]
- Mobili, A.; Telesca, A.; Marroccoli, M.; Tittarelli, F. Calcium sulfoaluminate and alkali-activated fly ash cements as alternative to Portland cement: Study on chemical, physical-mechanical, and durability properties of mortars with the same strength class. Constr. Build. Mater. 2020, 246, 118436. [Google Scholar] [CrossRef]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Natural pozzolana addition effect on compressive strength and capillary water absorption of Mortar. Energy Procedia 2017, 139, 689–695. [Google Scholar] [CrossRef]
- Dong, M.; Elchalakani, M.; Karrech, A. Development of high strength one-part geopolymer mortar using sodium metasilicate. Constr. Build. Mater. 2020, 236, 117611. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Effect of synthesis parameters on compressive strength of fly ash-slag blended geopolymer. Constr. Build. Mater. 2018, 170, 225–234. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag-fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
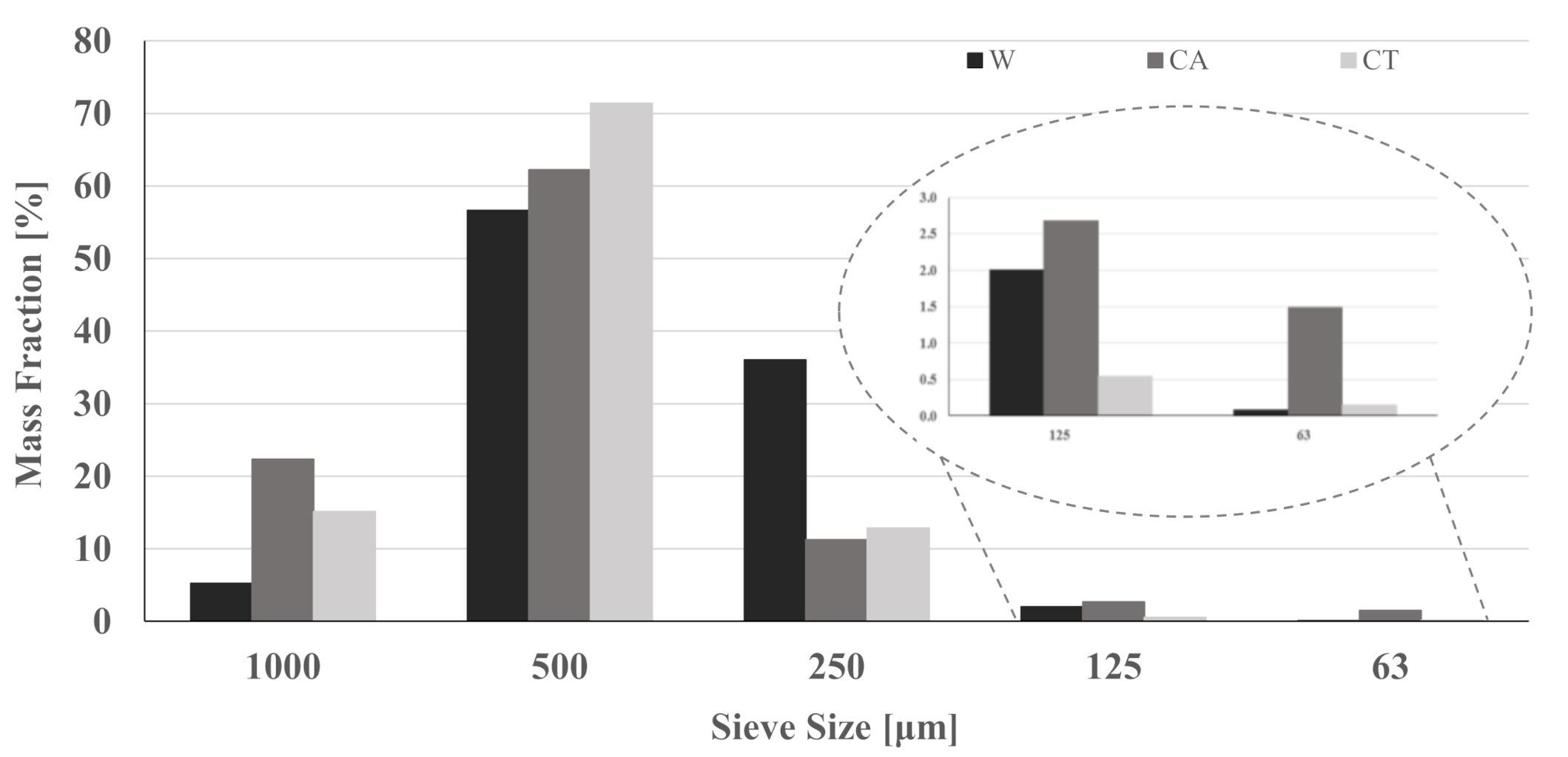
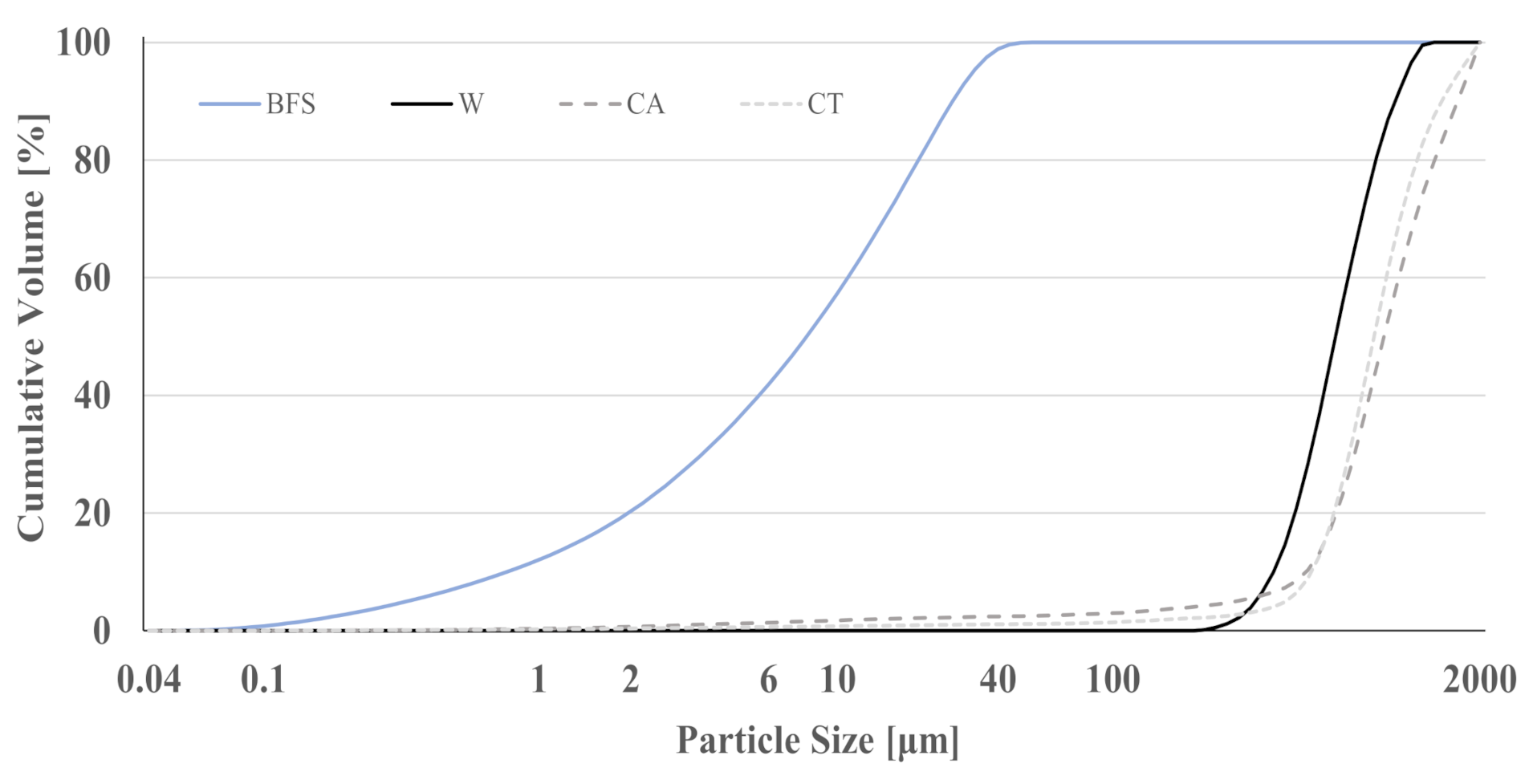



| Sample Name | Mixture Proportion [g] | W/B | ||
|---|---|---|---|---|
| BFS | Sodium Metasilicate | Sand | ||
| B0.29 B0.31 B0.33 | 100 | 10 | - | 0.29 0.31 0.33 |
| MW0.29 MCA0.29 MCT0.29 | 100 | 10 | 200 | 0.29 |
| MW0.33 MCA0.33 MCT0.33 | 0.33 | |||
| MW0.35 MCA0.35 MCT0.35 | 0.35 | |||
| Component | BFS | W | CA | CT |
|---|---|---|---|---|
| [wt.%] | ||||
| Na2O | 0.29 | 0.07 | 1.69 | 0.42 |
| MgO | 6.77 | 0.06 | 2.16 | 0.69 |
| Al2O3 | 9.21 | 1.15 | 2.86 | 3.83 |
| SiO2 | 32.43 | 97.15 | 64.57 | 80.80 |
| P2O5 | 0.01 | 0.02 | 1.03 | 0.34 |
| SO3 | 1.81 | - | 0.55 | 0.05 |
| Cl | 0.02 | - | 0.22 | 0.08 |
| K2O | 0.55 | 0.79 | 2.01 | 2.03 |
| CaO | 46.55 | 0.11 | 20.00 | 7.30 |
| TiO2 | 0.95 | 0.03 | 0.22 | 0.15 |
| MnO | 0.18 | - | 0.39 | 0.09 |
| Fe2O3 | 0.36 | 0.09 | 1.24 | 1.54 |
| Sr | 0.09 | - | 0.05 | - |
| Zr | 0.04 | - | - | - |
| Ba | 0.06 | - | 0.03 | 0.01 |
| LOI | 0.65 | 0.28 | 2.92 | 2.62 |
| Sample | Apparent Density [g/cm3] | |
|---|---|---|
| 7 days | 28 days | |
| MW0.29 | 2.20 ± 0.06 | 2.22 ± 0.03 |
| MW0.33 | 2.16 ± 0.02 | 2.14 ± 0.05 |
| MW0.35 | 2.14 ± 0.19 | 2.15 ± 0.09 |
| MCA0.29 | 1.98 ± 0.08 | 1.94 ± 0.07 |
| MCA0.33 | 1.84 ± 0.24 | 1.85 ± 0.23 |
| MCA0.35 | 2.11 ± 0.06 | 2.10 ± 0.06 |
| MCT0.29 | 2.09 ± 0.11 | 2.09 ± 0.13 |
| MCT0.33 | 2.10 ± 0.05 | 2.07 ± 0.05 |
| MCT0.35 | 2.15 ± 0.05 | 2.07 ± 0.01 |
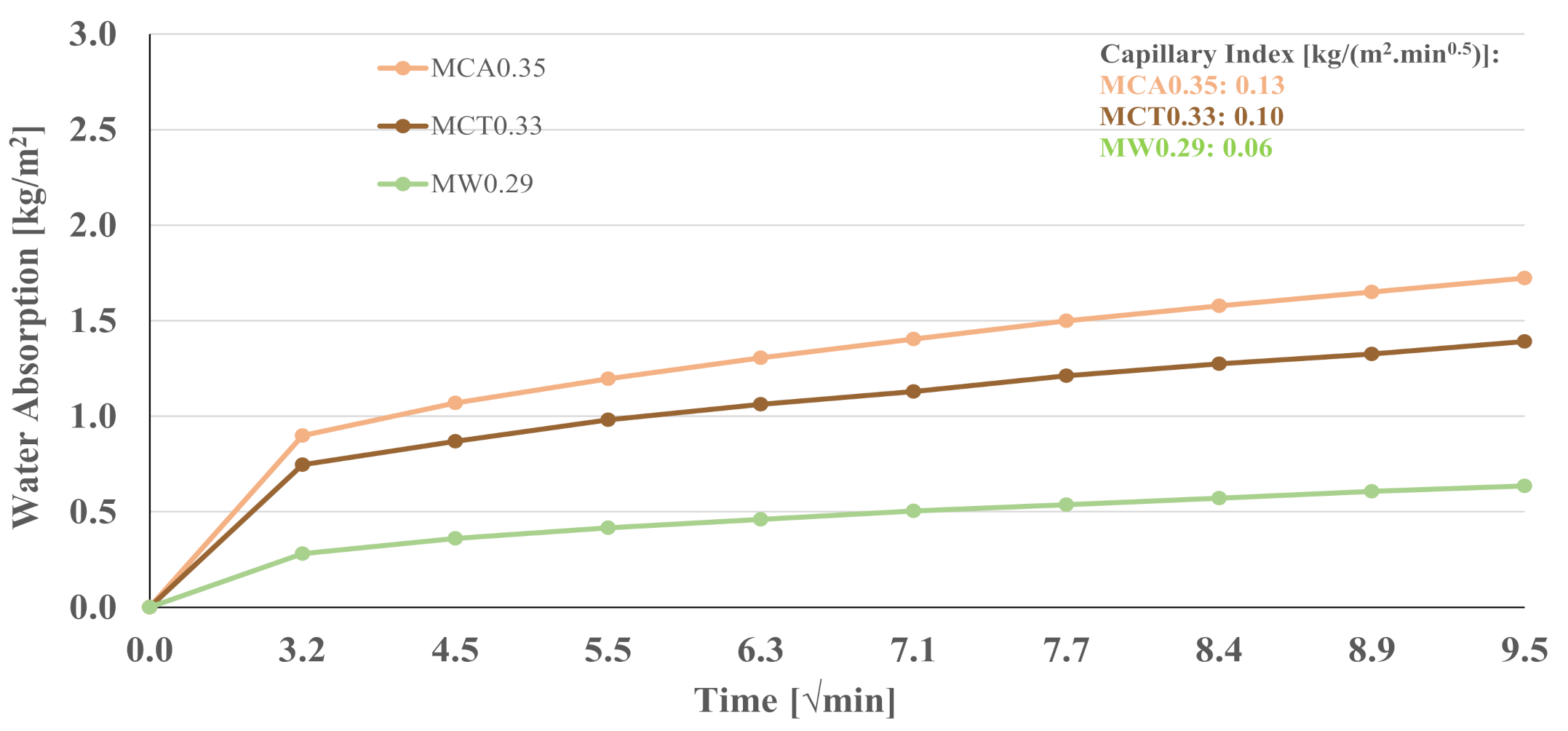
| Sample | Water Absorption [%] |
|---|---|
| B0.29 | 0.70 ± 0.04 |
| MW0.29 | 0.77 ± 0.03 |
| MCA0.35 | 1.36 ± 0.03 |
| MCT0.33 | 1.37 ± 0.15 |
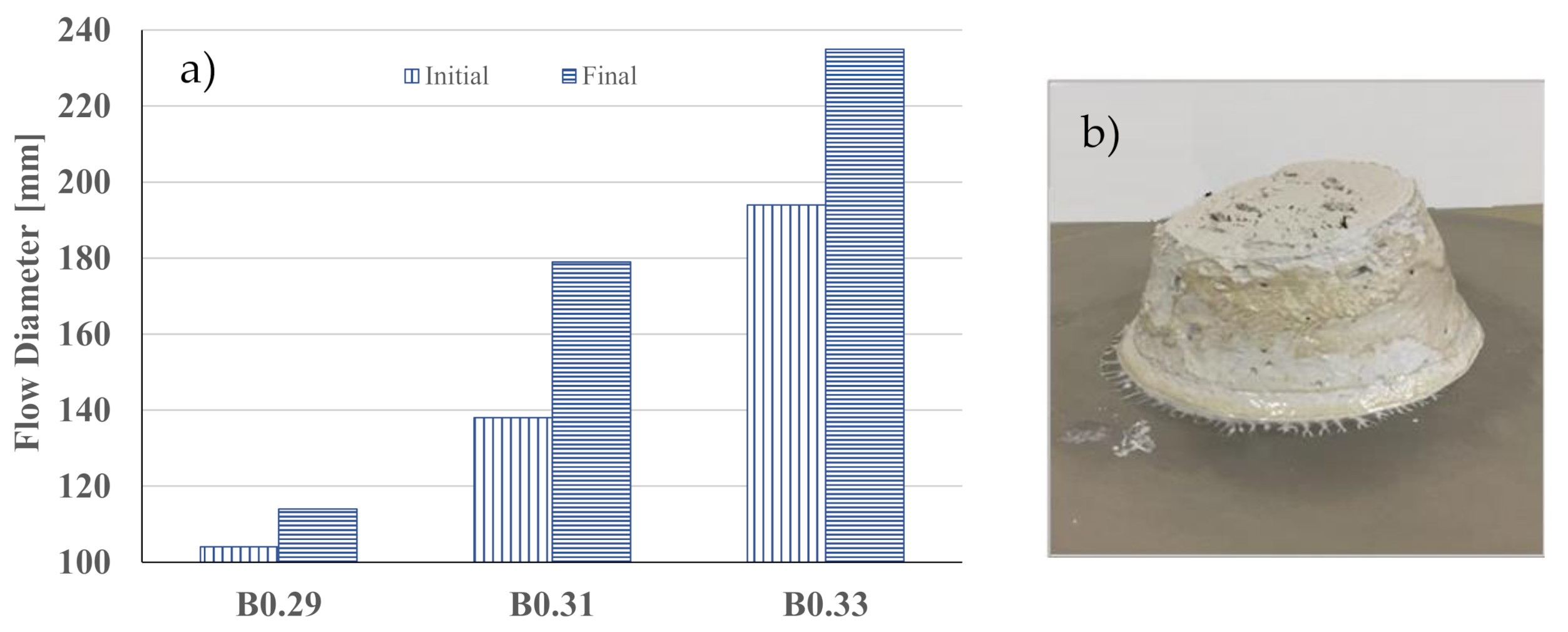
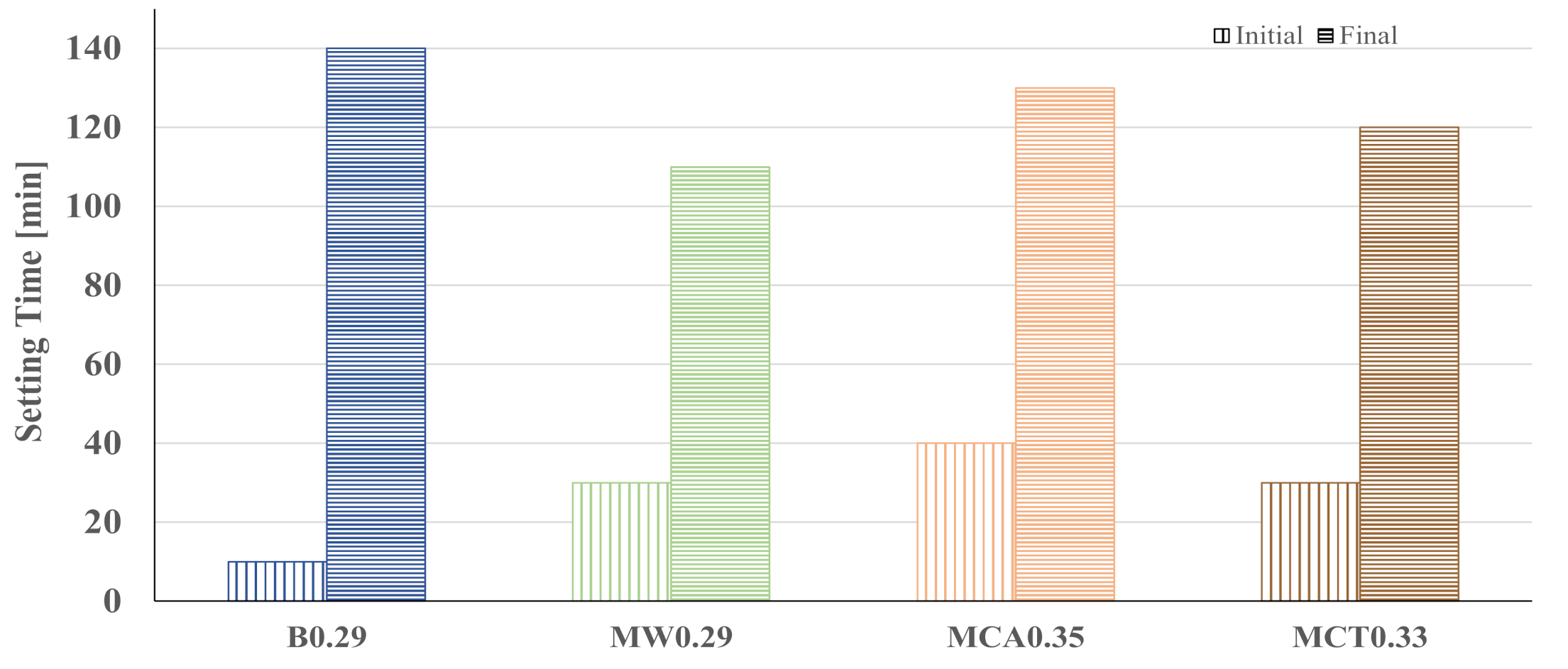
| Precursors | Solid Activator | W/B | Curing Condition | Workability [mm] | Final Setting Time [min] | Apparent Density [kg/m3] | Rc(+) [MPa] | Water Abs. [%] | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| FA | Na2SiO3 and NaOH | 0.34 | 40 °C | - | - | - | 65 | - | [14] |
| BFS | Na2SiO3 | 0.29 | Ambient | 114 | 140 | 1820 | 56 | 0.7 | This work |
| FA | Na2SiO3 | 0.25 | 25 °C | 241 | 70 | 2130 | 50 | - | [15] |
| FA and BFS | Na2SiO3 | 0.37 | 23 °C | 164 | 255 | - | 45 | - | [21] |
| FA and BFS | Na2SiO3 | 0.3 | 23 °C 70%RH | 240 | - | - | 30 | - | [5] |
| Rice husk | NaAlO2 | 0.5 | 80 °C 80%RH | - | - | - | 30(*) | - | [17] |
| FA and slag | Na2SiO3 and Ca(OH)2 | 0.45 | Ambient | 200 | - | 1730 | 27 | - | [6] |
| FA and slag | Na2SiO3 | 0.30 | Ambient | 225 | - | 1760 | 24 | - | [6] |
| FA | Na2SiO3 | 0.30 | 40 °C | - | - | - | 21 | - | [14] |
| FA | NaOH and Na2SiO3 | 0.40 | 20 °C | - | - | - | 11 | - | [18] |
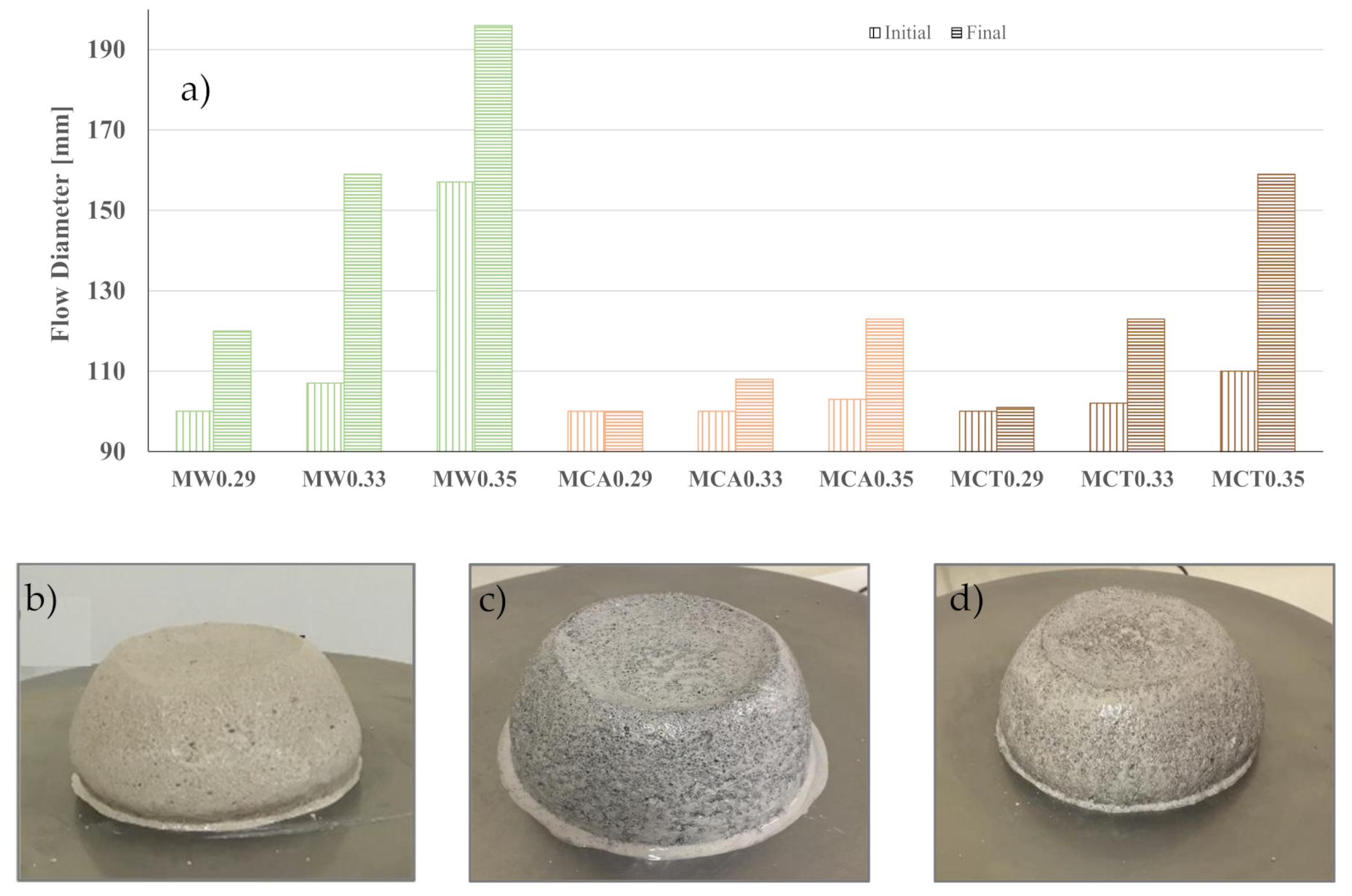
| Precursors | Solid Activator | W/B | Curing Condition | Aggregate | Workability [mm] | Final Setting Time [min] | Apparent Density [kg/m3] | Rc(+) [MPa] | Water Abs. [%] | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| BFS | Na2SiO3 | 0.34 | 22 °C 100%RH | Sand | - | 30 | - | 105 | - | [16] |
| BFS | Na2SiO3 | 0.33 | Ambient | Waste sand (CT) | 123 | 120 | 1950 | 86 | 1.37 | This work |
| FA and BFS | Na2SiO3 | 0.31 | 23 °C 95%RH | Dune sand | - | - | - | 84 | - | [45] |
| BFS | Na2SiO3 | 0.29 | Ambient | Sand | 120 | 110 | 2220 | 79 | 0.77 | This work |
| BFS | Na2SiO3 | 0.35 | Ambient | Waste sand (CA) | 123 | 130 | 1990 | 70 | 1.36 | This work |
| BFS and microsilica | Na2SiO3 | 0.34 | 23 °C 95%RH | Dune sand | - | - | - | 43 | - | [45] |
| Kaolin, FA and OPC | Ca(OH)2 | 0.29 | 58%RH | Sand | - | - | - | 43 | - | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.; Vilarinho, I.S.; Capela, M.; Caetano, A.; Novais, R.M.; Labrincha, J.A.; Seabra, M.P. Waste-Based One-Part Alkali Activated Materials. Materials 2021, 14, 2911. https://doi.org/10.3390/ma14112911
Gonçalves M, Vilarinho IS, Capela M, Caetano A, Novais RM, Labrincha JA, Seabra MP. Waste-Based One-Part Alkali Activated Materials. Materials. 2021; 14(11):2911. https://doi.org/10.3390/ma14112911
Chicago/Turabian StyleGonçalves, Margarida, Inês Silveirinha Vilarinho, Marinélia Capela, Ana Caetano, Rui Miguel Novais, João António Labrincha, and Maria Paula Seabra. 2021. "Waste-Based One-Part Alkali Activated Materials" Materials 14, no. 11: 2911. https://doi.org/10.3390/ma14112911
APA StyleGonçalves, M., Vilarinho, I. S., Capela, M., Caetano, A., Novais, R. M., Labrincha, J. A., & Seabra, M. P. (2021). Waste-Based One-Part Alkali Activated Materials. Materials, 14(11), 2911. https://doi.org/10.3390/ma14112911