High AC and DC Electroconductivity of Scalable and Economic Graphite–Diamond Polylactide Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mngomezulu, M.E.; Luyt, A.S.; Chapple, S.A.; John, M.J. Poly(Lactic Acid)-Starch/Expandable Graphite (PLA-Starch/EG) Flame Retardant Composites. J. Renew. Mater. 2018, 6, 26–37. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) Based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Kang, I.; Schulz, M.J.; Kim, J.H.; Shanov, V.; Shi, D. A Carbon Nanotube Strain Sensor for Structural Health Monitoring. Smart Mater. Struct. 2006, 15, 737–748. [Google Scholar] [CrossRef]
- Khan, S.; Lorenzelli, L. Recent Advances of Conductive Nanocomposites in Printed and Flexible Electronics. Smart Mater. Struct. 2017, 26, 083001. [Google Scholar] [CrossRef]
- Ganguly, S.; Bhawal, P.; Ravindren, R.; Das, N.C. Polymer Nanocomposites for Electromagnetic Interference Shielding: A Review. J. Nanosci. Nanotechnol. 2018, 18, 7641–7669. [Google Scholar] [CrossRef]
- Oguz, O.; Candau, N.; Demongeot, A.; Citak, M.K.; Cetin, F.N.; Stoclet, G.; Michaud, V.; Menceloglu, Y.Z. Poly(Lactide)/Cellulose Nanocrystal Nanocomposites by High-shear Mixing. Polym. Eng. Sci. 2021, 61, 1028–1040. [Google Scholar] [CrossRef]
- Bourbigot, S.; Fontaine, G.; Gallos, A.; Bellayer, S. Reactive Extrusion of PLA and of PLA/Carbon Nanotubes Nanocomposite: Processing, Characterization and Flame Retardancy. Polym. Adv. Technol. 2011, 22, 30–37. [Google Scholar] [CrossRef]
- Keshtkar, M.; Nofar, M.; Park, C.B.; Carreau, P.J. Extruded PLA/Clay Nanocomposite Foams Blown with Supercritical CO2. Polymer 2014, 55, 4077–4090. [Google Scholar] [CrossRef]
- Pluta, M.; Galeski, A.; Alexandre, M.; Paul, M.-A.; Dubois, P. Polylactide/Montmorillonite Nanocomposites and Microcomposites Prepared by Melt Blending: Structure and Some Physical Properties. J. Appl. Polym. Sci. 2002, 86, 1497–1506. [Google Scholar] [CrossRef]
- Costa, R.G.F.; Brichi, G.S.; Ribeiro, C.; Mattoso, L.H.C. Nanocomposite Fibers of Poly(Lactic Acid)/Titanium Dioxide Prepared by Solution Blow Spinning. Polym. Bull. 2016, 73, 2973–2985. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Zucolotto, V.; Mattoso, L.H.C.; Medeiros, E.S. Multi-Walled Carbon Nanotubes and Poly(Lactic Acid) Nanocomposite Fibrous Membranes Prepared by Solution Blow Spinning. J. Nanosci. Nanotechnol. 2012, 12, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Bharadia, P.; Blaker, J.J.; Bismarck, A. Short Sisal Fibre Reinforced Bacterial Cellulose Polylactide Nanocomposites Using Hairy Sisal Fibres as Reinforcement. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2065–2074. [Google Scholar] [CrossRef]
- Guo, R.; Ren, Z.; Bi, H.; Xu, M.; Cai, L. Electrical and Thermal Conductivity of Polylactic Acid (PLA)-Based Biocomposites by Incorporation of Nano-Graphite Fabricated with Fused Deposition Modeling. Polymers 2019, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Shao, S.; Ya-Li; Wang, L.; Jiang, P.; Liu, B. Polylactide/Graphite Nanosheets/MWCNTs Nanocomposites with Enhanced Mechanical, Thermal and Electrical Properties. Iranian Polym. J. 2012, 21, 109–120. [Google Scholar] [CrossRef]
- Kim, I.-H.; Jeong, Y.G. Polylactide/Exfoliated Graphite Nanocomposites with Enhanced Thermal Stability, Mechanical Modulus, and Electrical Conductivity. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 850–858. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Richert, J.; Rytlewski, P.; Richert, A. Selected Electrical and Thermal Properties of Polylactide/Graphite Composites. Polimery 2011, 56, 489–493. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, C.-S. Preparation and Characterization of Maleated Polylactide-Functionalized Graphite Oxide Nanocomposites. J. Polym. Res. 2014, 21. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Liao, Z.-S.; Huang, J.-J.; Huang, S.-Y.; Fan, W.-L. Incorporation of Supramolecular Polymer-Functionalized Graphene: Towards the Development of Bio-Based High Electrically Conductive Polymeric Nanocomposites. Compos. Sci. Technol. 2017, 148, 89–96. [Google Scholar] [CrossRef]
- Fal, J.; Wanic, M.; Budzik, G.; Oleksy, M.; Żyła, G. Electrical Conductivity and Dielectric Properties of Ethylene Glycol-Based Nanofluids Containing Silicon Oxide–Lignin Hybrid Particles. Nanomaterials 2019, 9, 1008. [Google Scholar] [CrossRef]
- Fal, J.; Bulanda, K.; Traciak, J.; Sobczak, J.; Kuzioła, R.; Grąz, K.M.; Budzik, G.; Oleksy, M.; Żyła, G. Electrical and Optical Properties of Silicon Oxide Lignin Polylactide (SiO2-L-PLA). Molecules 2020, 25, 1354. [Google Scholar] [CrossRef]
- Fal, J.; Barylyak, A.; Besaha, K.; Bobitski, Y.V.; Cholewa, M.; Zawlik, I.; Szmuc, K.; Cebulski, J.; żyła, G. Experimental Investigation of Electrical Conductivity and Permittivity of SC-TiO2-EG Nanofluids. Nanoscale Res. Lett. 2016, 11. [Google Scholar] [CrossRef]
- Żyła, G.; Fal, J.; Estellé, P. The Influence of Ash Content on Thermophysical Properties of Ethylene Glycol Based Graphite/Diamonds Mixture Nanofluids. Diam. Rel. Mater. 2017. [Google Scholar] [CrossRef]
- Nagata, K.; Iwabuki, H.; Nigo, H. Effect of particle size of graphites on electrical conductivity of graphite/polymer composite. Comp. Interfaces 1998, 6, 483–495. [Google Scholar] [CrossRef]
- Wang, L.; Dang, Z.-M. Carbon Nanotube Composites with High Dielectric Constant at Low Percolation Threshold. Appl. Phys. Lett. 2005, 87, 042903. [Google Scholar] [CrossRef]
- Badia, J.D.; Monreal, L.; Sáenz de Juano-Arbona, V.; Ribes-Greus, A. Dielectric spectroscopy of recycled polylactide. Polym. Degrad. Stab. 2014, 107, 21. [Google Scholar] [CrossRef]
- Wu, W.; Liu, T.; Zhang, D.; Sun, Q.; Cao, K.; Zha, J.; Lu, Y.; Wang, B.; Cao, X.; Feng, Y.; et al. Significantly Improved Dielectric Properties of Polylactide Nanocomposites via TiO2 Decorated Carbon Nanotubes. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105650. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Buerschaper, R.A. Thermal and Electrical Conductivity of Graphite and Carbon at Low Temperatures. J. Appl. Phys. 1944, 15, 452. [Google Scholar] [CrossRef]

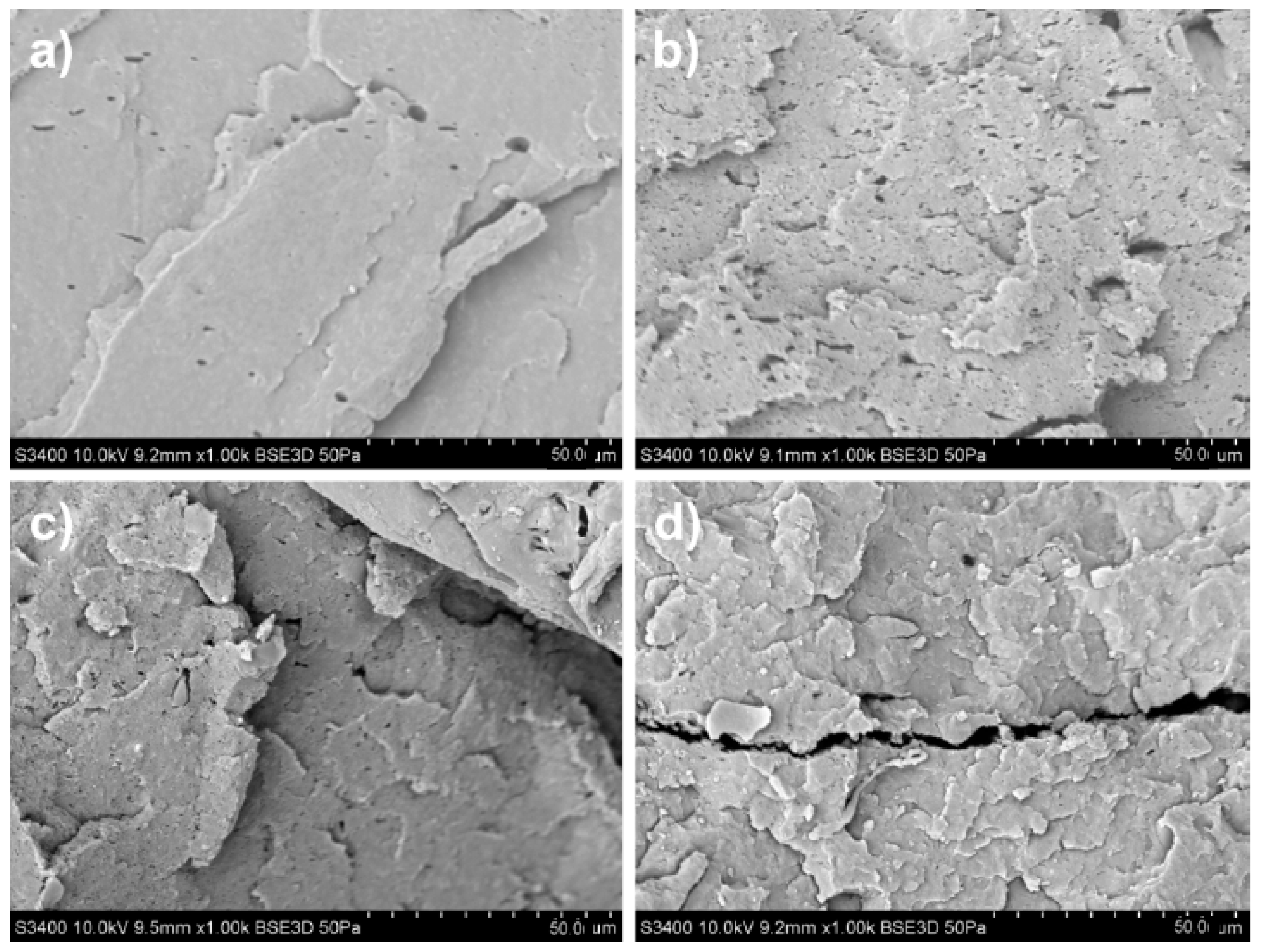

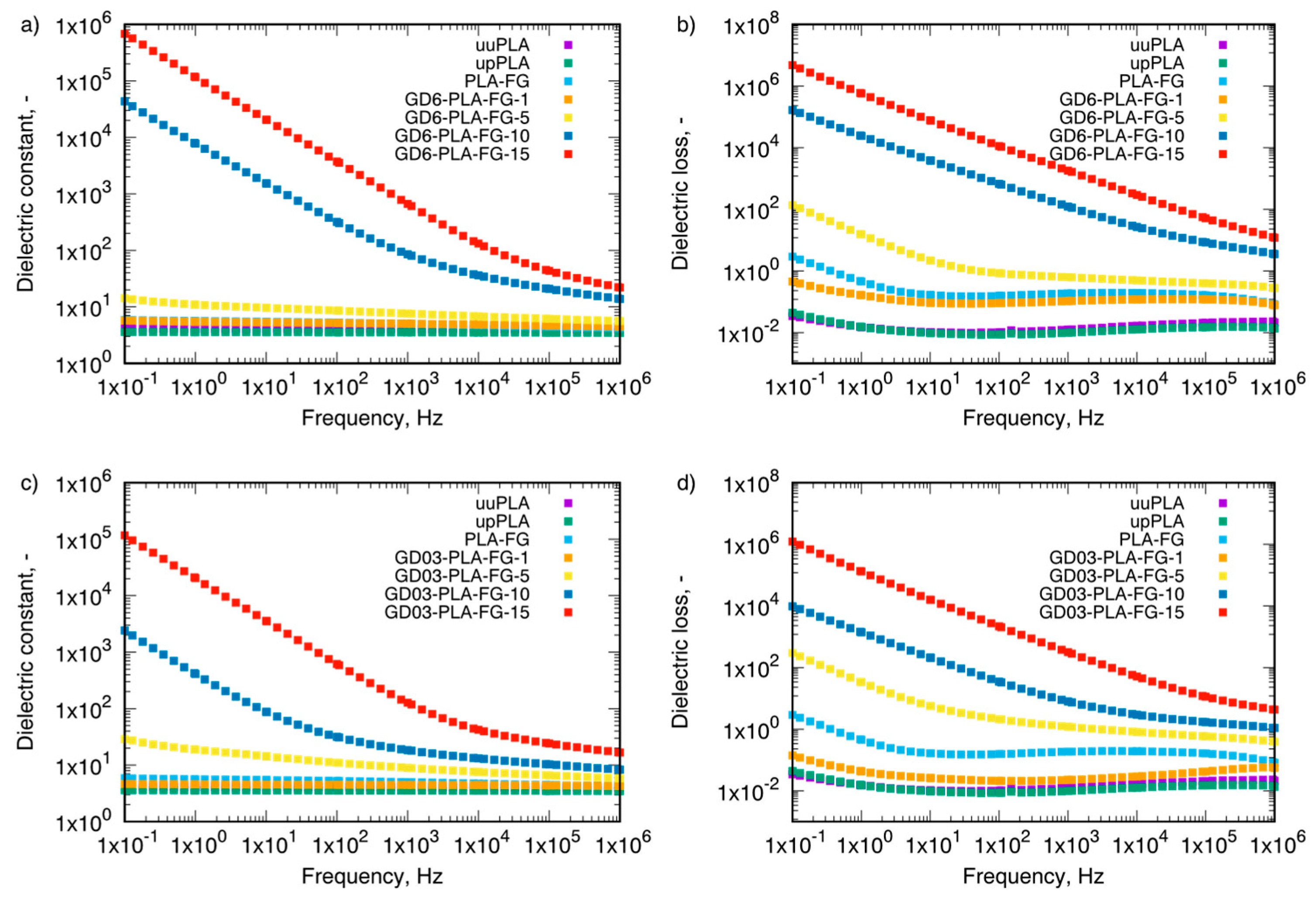
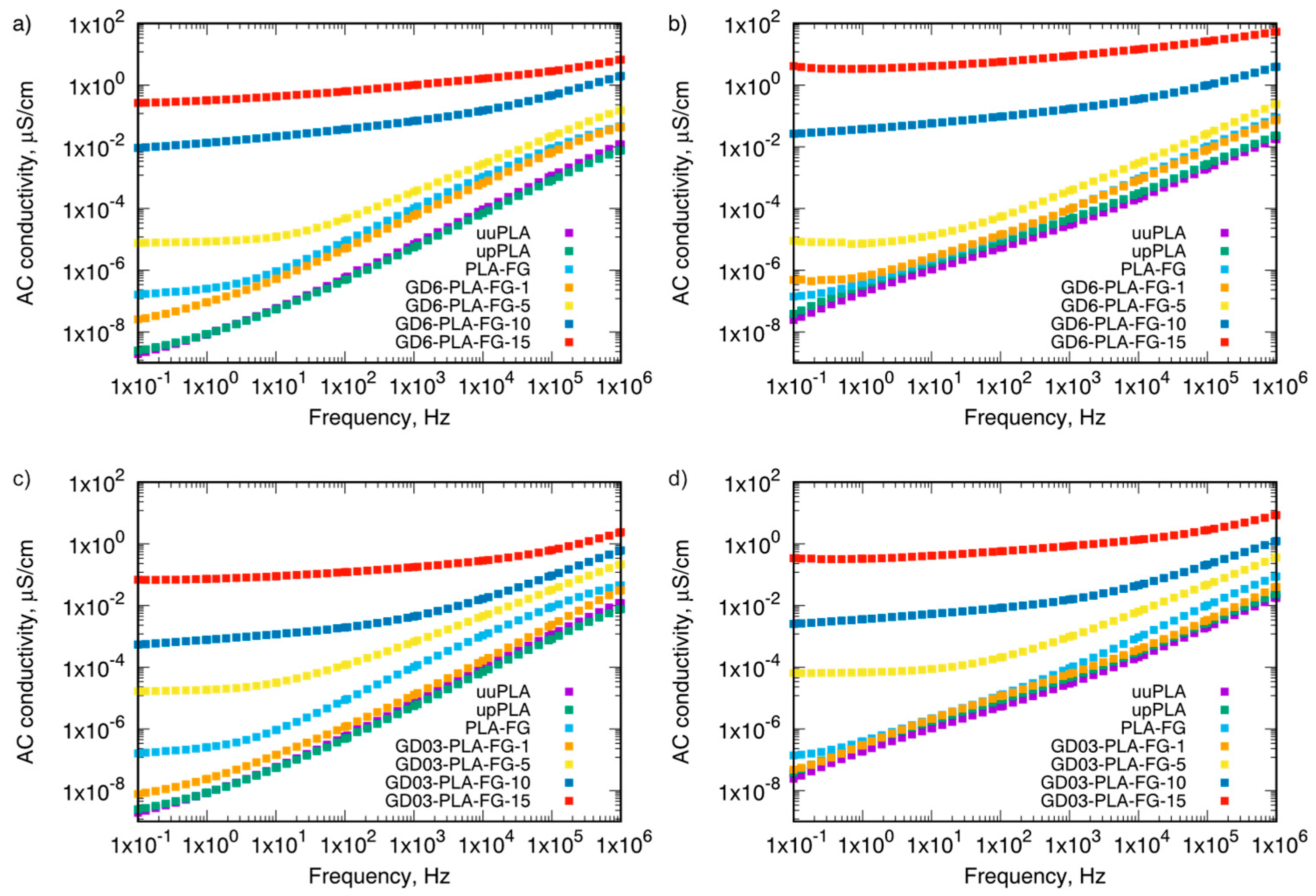
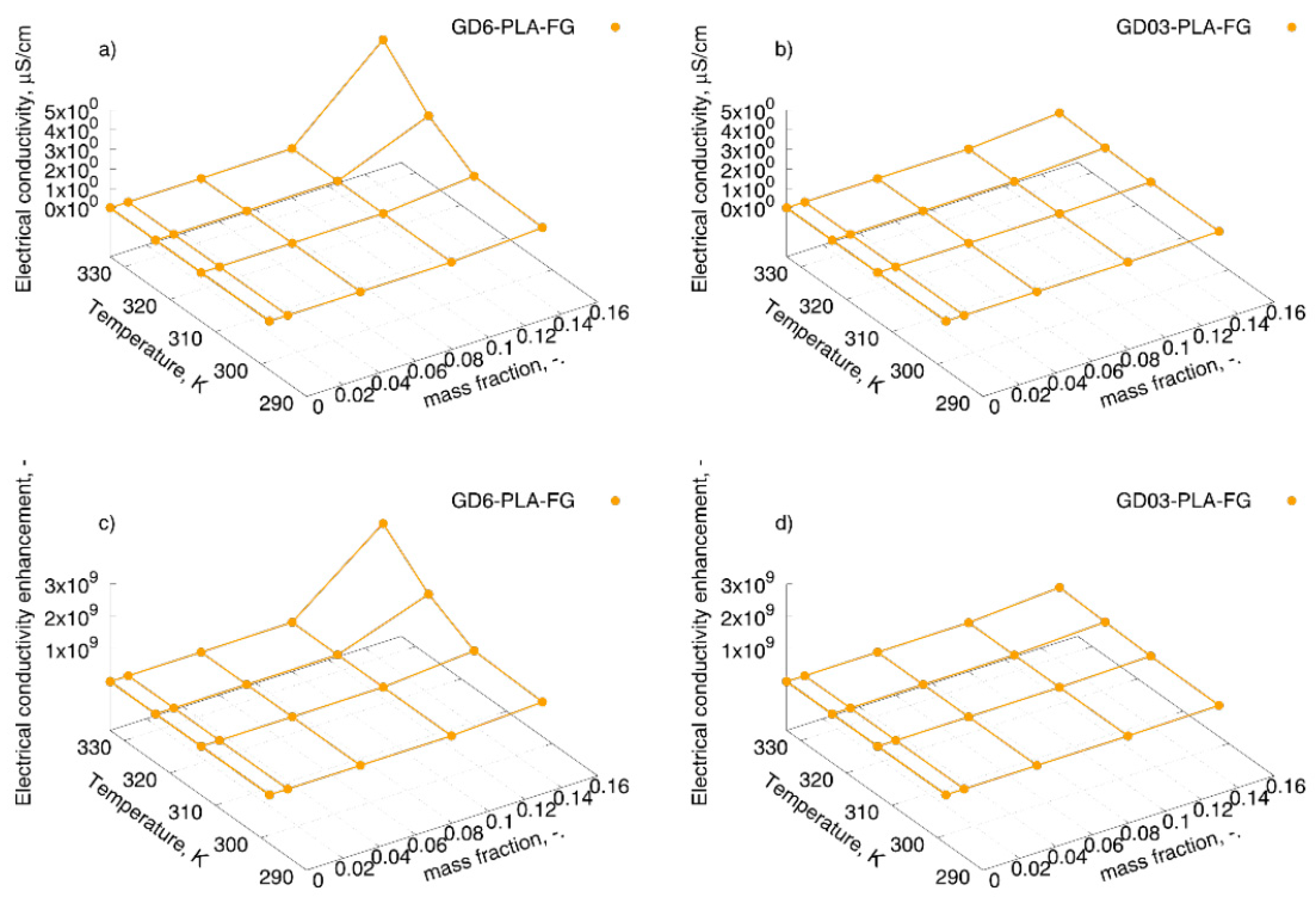
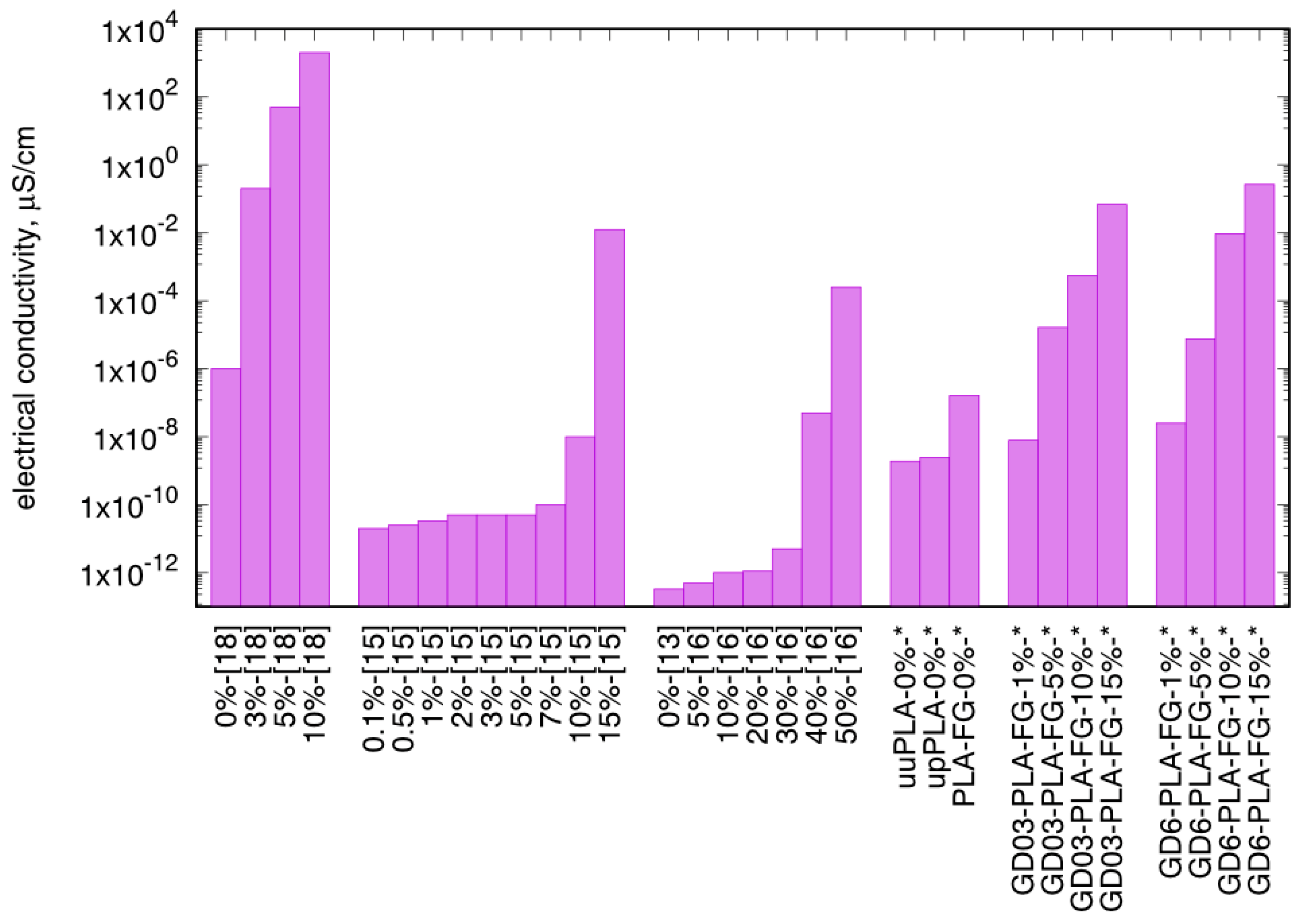
| Sample Type | Mass Fraction, φm | Electrical Conductivity, µS/cm | |||
|---|---|---|---|---|---|
| – | 298.15 K | 313.15 K | 323.15 K | 333.15 K | |
| unfilled PLA | uuPLA (0.0) | 1.91 × 10−9 | 3.22 × 10−9 | 6.31 × 10−9 | 2.43 × 10−8 |
| upPLA (0.0) | 2.45 × 10−9 | 3.74 × 10−9 | 8.75 × 10−9 | 3.84 × 10−8 | |
| PLA-FG (0.0) | 1.62 × 10−7 | 9.66 × 10−8 | 6.39 × 10−8 | 1.39 × 10−7 | |
| GD6-PLA-FG | 0.01 | 2.53 × 10−8 | 5.62 × 10−8 | 9.00 × 10−8 | 4.83 × 10−7 |
| 0.05 | 7.59 × 10−6 | 6.89 × 10−6 | 2.51 × 10−6 | 8.89 × 10−6 | |
| 0.10 | 9.21 × 10−3 | 1.26 × 10−2 | 1.85 × 10−2 | 2.64 × 10−2 | |
| 0.15 | 2.65 × 10−1 | 4.24 × 10−1 | 1.85 × 100 | 4.09 × 100 | |
| GD03-PLA-FG | 0.01 | 7.83 × 10-9 | 2.05 × 10−8 | 1.24 × 10−7 | 4.74 × 10−8 |
| 0.05 | 1.65 × 10-5 | 2.90 × 10−5 | 3.09 × 10−5 | 6.47 × 10−5 | |
| 0.10 | 5.43 × 10-4 | 1.18 × 10−3 | 1.70 × 10−3 | 2.54 × 10−3 | |
| 0.15 | 6.85 × 10-2 | 1.19 × 10−1 | 2.15 × 10−1 | 3.41 × 10−1 | |
| Mass Fraction,φm | Electrical Conductivity Enhancement, – | ||||
| – | 298.15 K | 313.15 K | 323.15 K | 333.15 K | |
| unfilled PLA | uuPLA (0.0) | 1.00 × 100 | 1.00 × 100 | 1.00 × 100 | 1.00 × 100 |
| upPLA (0.0) | 1.28 × 100 | 1.16 × 100 | 1.39 × 100 | 1.58 × 100 | |
| PLA-FG (0.0) | 8.50 × 101 | 3.00 × 101 | 1.01 × 101 | 5.72 × 100 | |
| GD6-PLA-FG | 0.01 | 1.33 × 101 | 1.75 × 101 | 1.43 × 101 | 1.99 × 101 |
| 0.05 | 3.98 × 103 | 2.14 × 103 | 3.98 × 102 | 3.66 × 102 | |
| 0.10 | 4.83 × 106 | 3.91 × 106 | 2.93 × 106 | 1.09 × 106 | |
| 0.15 | 1.39 × 108 | 1.32 × 108 | 2.92 × 108 | 1.68 × 108 | |
| GD03-PLA-FG | 0.01 | 4.11 × 100 | 6.37 × 100 | 1.97 × 101 | 1.95 × 100 |
| 0.05 | 8.66 × 103 | 9.01 × 103 | 4.89 × 103 | 2.67 × 103 | |
| 0.10 | 2.85 × 105 | 3.66 × 105 | 2.70 × 105 | 1.05 × 105 | |
| 0.15 | 3.59 × 107 | 3.71 × 107 | 3.41 × 107 | 1.40 × 107 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fal, J.; Bulanda, K.; Oleksy, M.; Sobczak, J.; Shi, J.; Liu, M.; Boncel, S.; Żyła, G. High AC and DC Electroconductivity of Scalable and Economic Graphite–Diamond Polylactide Nanocomposites. Materials 2021, 14, 2835. https://doi.org/10.3390/ma14112835
Fal J, Bulanda K, Oleksy M, Sobczak J, Shi J, Liu M, Boncel S, Żyła G. High AC and DC Electroconductivity of Scalable and Economic Graphite–Diamond Polylactide Nanocomposites. Materials. 2021; 14(11):2835. https://doi.org/10.3390/ma14112835
Chicago/Turabian StyleFal, Jacek, Katarzyna Bulanda, Mariusz Oleksy, Jolanta Sobczak, Jinwen Shi, Maochang Liu, Sławomir Boncel, and Gaweł Żyła. 2021. "High AC and DC Electroconductivity of Scalable and Economic Graphite–Diamond Polylactide Nanocomposites" Materials 14, no. 11: 2835. https://doi.org/10.3390/ma14112835
APA StyleFal, J., Bulanda, K., Oleksy, M., Sobczak, J., Shi, J., Liu, M., Boncel, S., & Żyła, G. (2021). High AC and DC Electroconductivity of Scalable and Economic Graphite–Diamond Polylactide Nanocomposites. Materials, 14(11), 2835. https://doi.org/10.3390/ma14112835









