Spark Plasma Sintering of LiFePO4: AC Field Suppressing Lithium Migration
Abstract
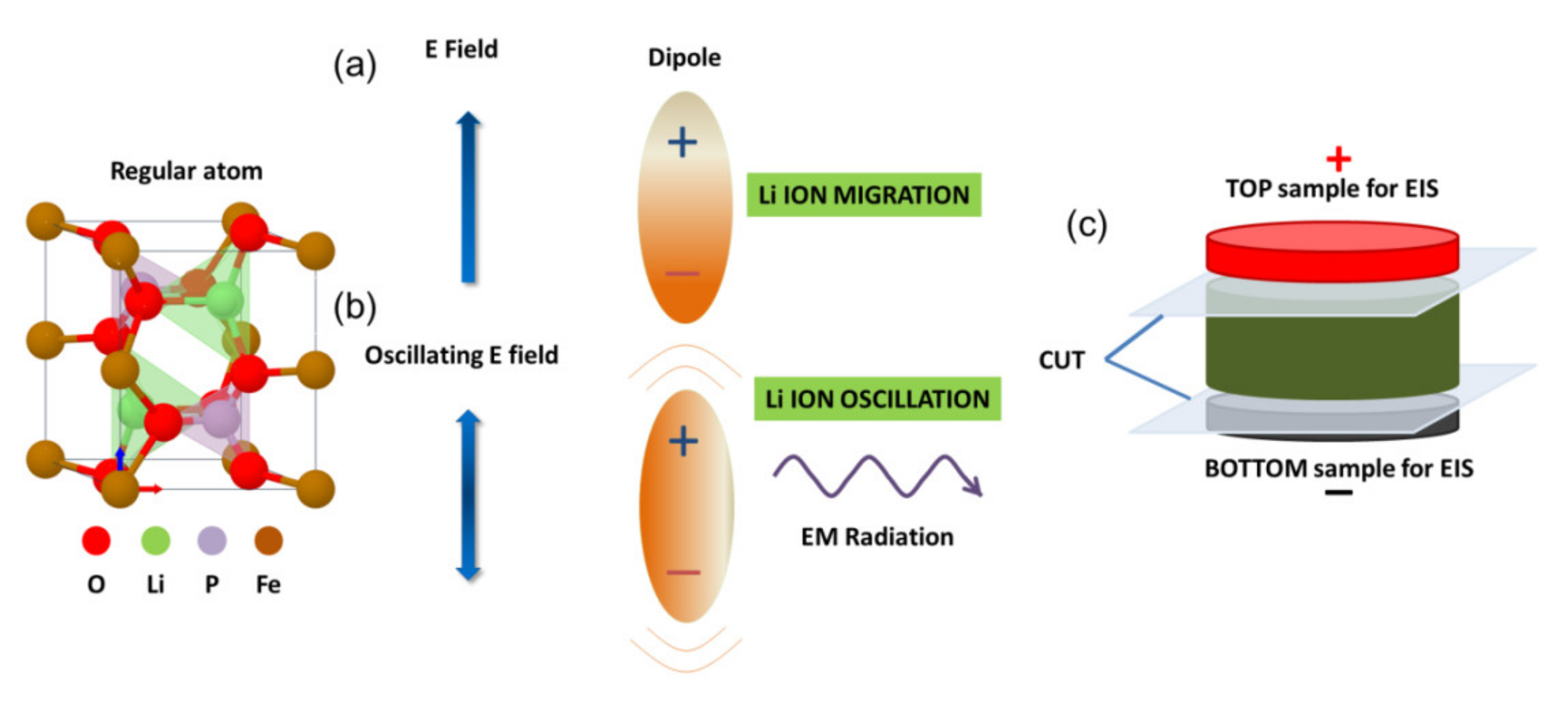
1. Introduction
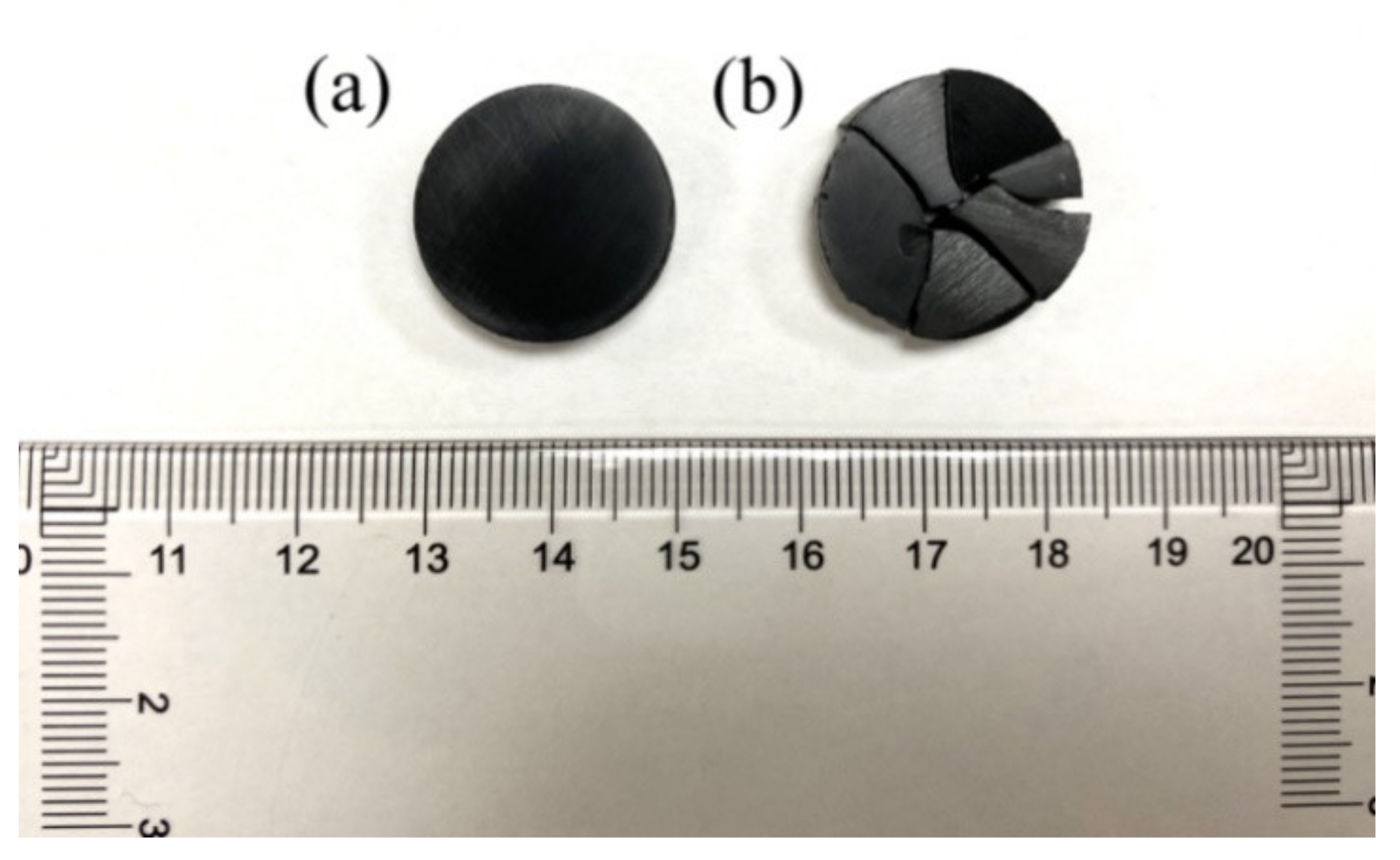
2. Materials and Methods
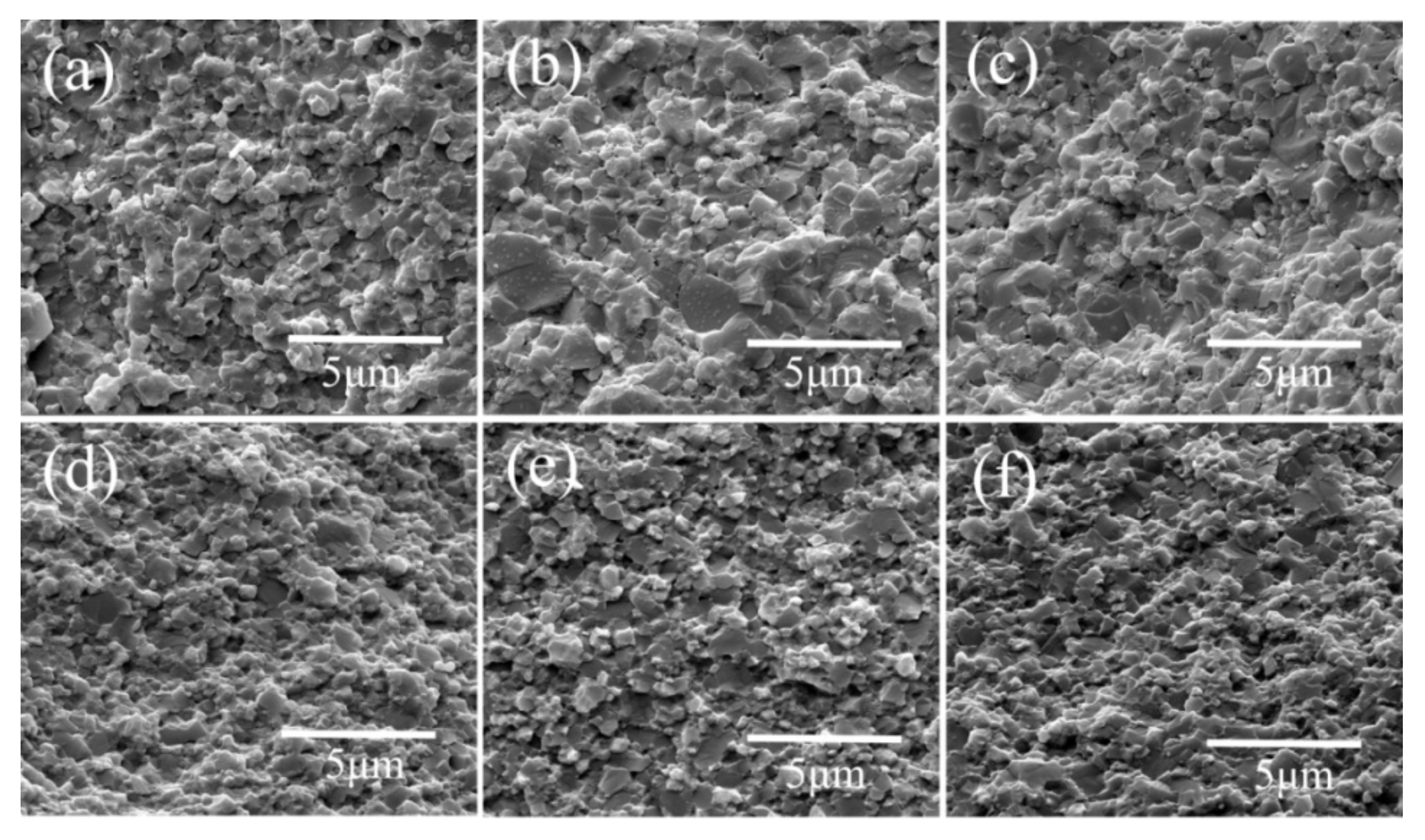
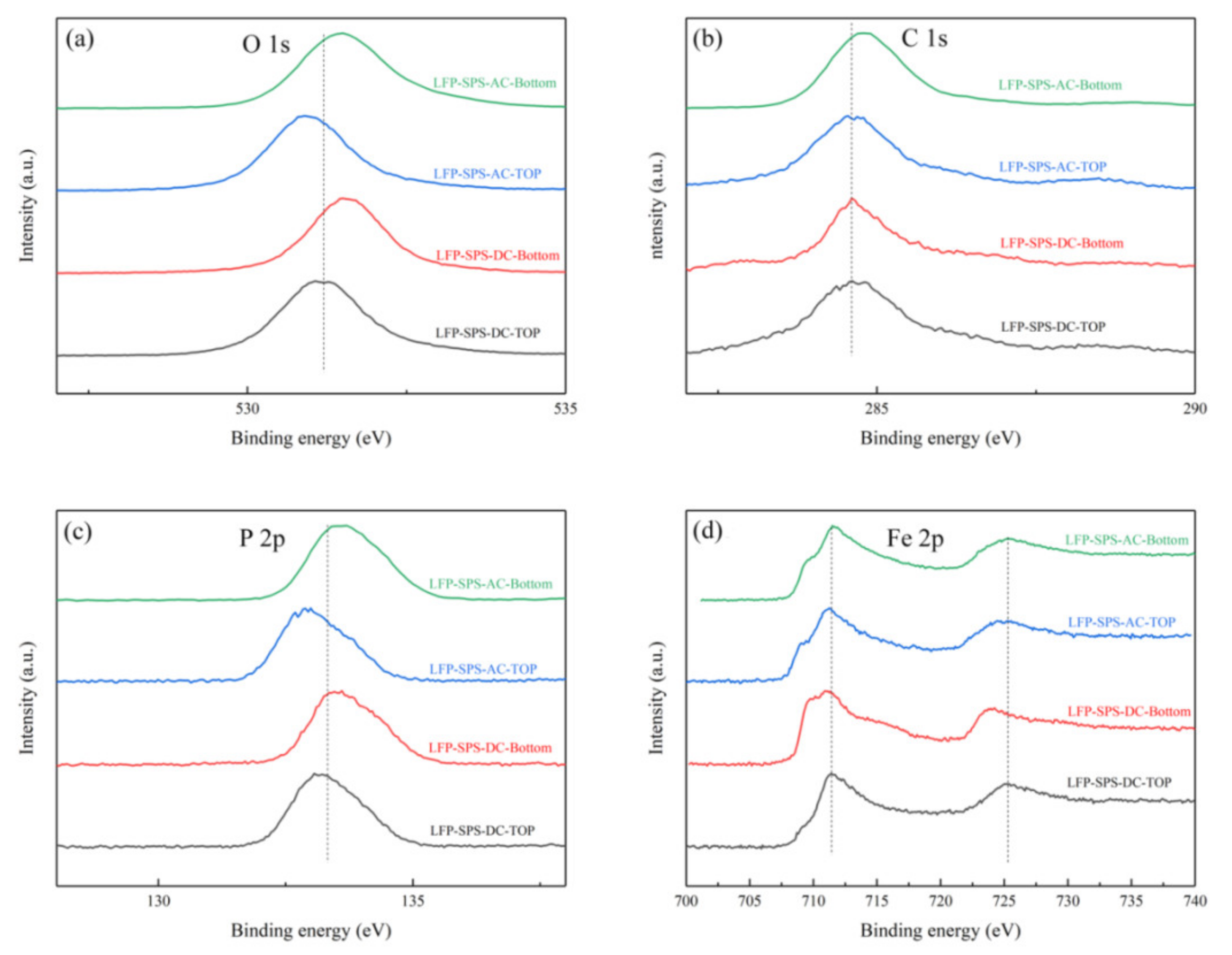
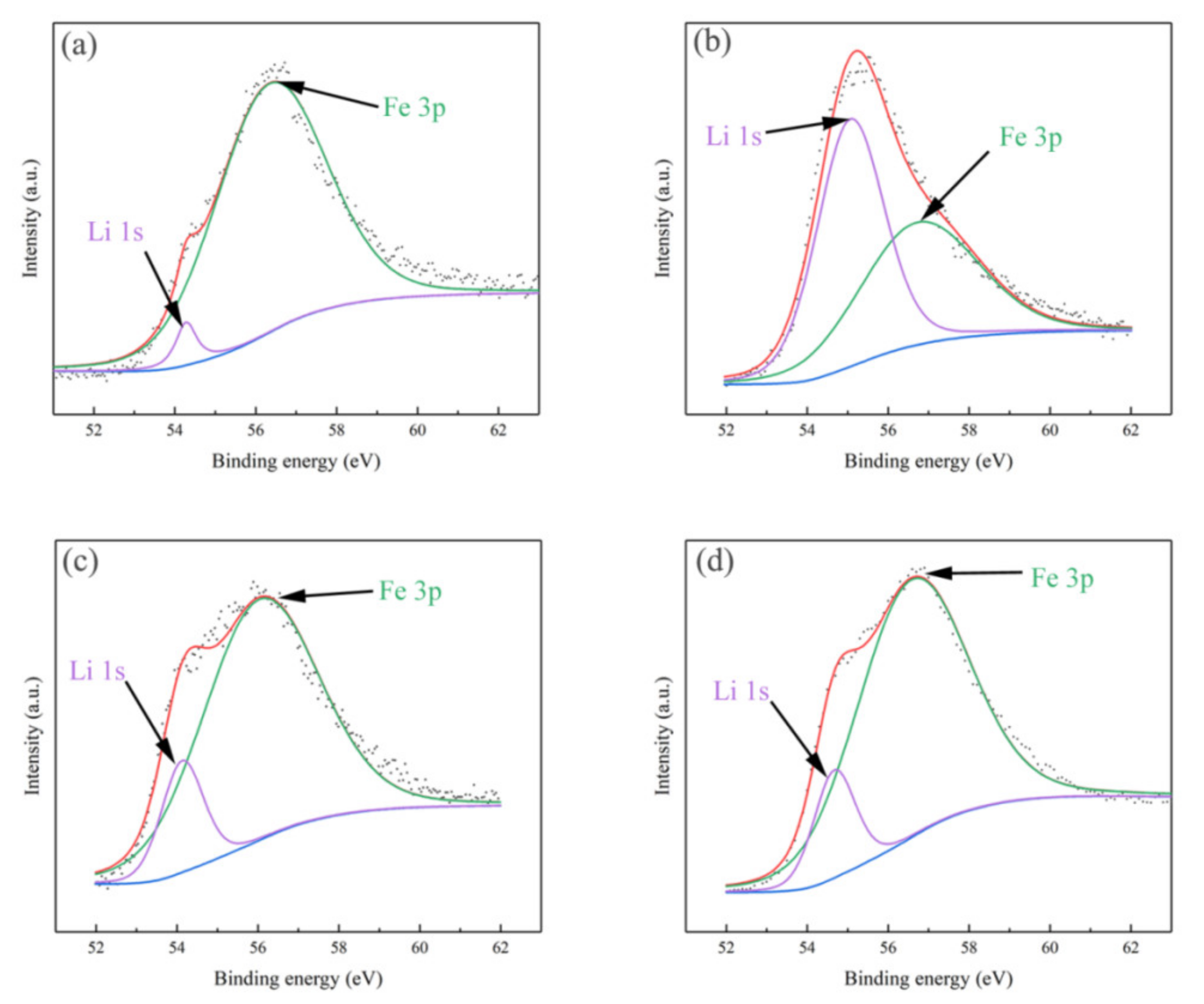
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Liu, N.; Gao, X.; Tian, X.; Zhu, Y.; Zhou, Y.; Zhu, Q. A hydrothermally synthesized LiFePO4/C composite with superior low-temperature performance and cycle life. Appl. Surf. Sci. 2018, 435, 1329–1336. [Google Scholar] [CrossRef]
- Chen, X.; Xie, J.; Lu, Y.; Zhao, X.; Zhu, T. Two-dimensional lithiophilic YFδ enabled lithium dendrite removal for quasi-solid-state lithium batteries. J. Mater. 2021, 7, 355–365. [Google Scholar] [CrossRef]
- Huang, Y.; Manthiram, A.; Chowdari, B.V.R. Solid-state ionic materials for critical applications. J. Mater. 2019, 5, 147–148. [Google Scholar] [CrossRef]
- Seo, J.H.; Verlinde, K.; Guo, J.; Heidary, D.S.B.; Rajagopalan, R.; Mallouk, T.E.; Randall, C.A. Cold sintering approach to fabrication of high rate performance binderless LiFePO4 cathode with high volumetric capacity. Scr. Mater. 2018, 146, 267–271. [Google Scholar] [CrossRef]
- Ravet, N.; Gauthier, M.; Zaghib, K.; Goodenough, J.B.; Mauger, A.; Gendron, F.; Julien, C.M. Mechanism of the Fe3+ reduction at low temperature for LiFePO4 synthesis from a polymeric additive. Chem. Mater. 2007, 19, 2595–2602. [Google Scholar] [CrossRef]
- Gao, S.; Su, Y.; Bao, L.; Li, N.; Chen, L.; Zheng, Y.; Tian, J.; Li, J.; Chen, S.; Wu, F. High-performance LiFePO4/C electrode with polytetrafluoroethylene as an aqueous-based binder. J. Power Sources. 2015, 298, 292–298. [Google Scholar] [CrossRef]
- Yang, S.; Song, Y.; Zavalij, P.Y.; Stanley Whittingham, M. Reactivity, stability and electrochemical behavior of lithium iron phosphates. Electrochem. Commun. 2002, 4, 239–244. [Google Scholar] [CrossRef]
- Jote, B.A.; Beyene, T.T.; Sahalie, N.A.; Weret, M.A.; Olbassa, B.W.; Wondimkun, Z.T.; Berhe, G.B.; Huang, C.J.; Su, W.N.; Hwang, B.J. Effect of diethyl carbonate solvent with fluorinated solvents as electrolyte system for anode free battery. J. Power Sour. 2020, 461, 228102. [Google Scholar] [CrossRef]
- Zugmann, S.; Moosbauer, D.; Amereller, M.; Schreiner, C.; Wudy, F.; Schmitz, R.; Schmitz, R.; Isken, P.; Dippel, C.; Müller, R.; et al. Electrochemical characterization of electrolytes for lithium-ion batteries based on lithium difluoromono(oxalato)borate. J. Power Sour. 2011, 196, 1417–1424. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, R.; Wang, H.; Yang, L.; Hu, J.; Chen, H.; Pan, F. A Metal–Organic-Framework-Based Electrolyte with Nanowetted Interfaces for High-Energy-Density Solid-State Lithium Battery. Adv. Mater. 2018, 30, 1704436. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Q.; Li, Y.; Cui, Z.; Guo, X.; Li, H. Sustainable Interfaces between Si Anodes and Garnet Electrolytes for Room-Temperature Solid-State Batteries. ACS Appl. Mater. Interfaces 2018, 10, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Kali, R.; Mukhopadhyay, A. Spark plasma sintered/synthesized dense and nanostructured materials for solid-state Li-ion batteries: Overview and perspective. J. Power Sour. 2014, 247, 920–931. [Google Scholar] [CrossRef]
- Leng, H.; Nie, J.; Luo, J. Combining cold sintering and Bi2O3-Activated liquid-phase sintering to fabricate high-conductivity Mg-doped NASICON at reduced temperatures. J. Mater. 2019, 5, 237–246. [Google Scholar] [CrossRef]
- Wang, S.; Fang, R.; Li, Y.; Liu, Y.; Xin, C.; Richter, F.H.; Nan, C.W. Interfacial challenges for all-solid-state batteries based on sulfide solid electrolytes. J. Mater. 2021, 7, 209–218. [Google Scholar] [CrossRef]
- Liu, R.; Wu, Z.; He, P.; Fan, H.; Huang, Z.; Zhang, L.; Chang, X.; Liu, H.; Wang, C.A.; Li, Y. A self-standing, UV-cured semi-interpenetrating polymer network reinforced composite gel electrolytes for dendrite-suppressing lithium ion batteries. J. Mater. 2019, 5, 185–194. [Google Scholar] [CrossRef]
- Huang, B.; Zhong, S.; Luo, J.; Huang, Z.; Wang, C.A. Highly dense perovskite electrolyte with a high Li+ conductivity for Li–ion batteries. J. Power Sour. 2019, 429, 75–79. [Google Scholar] [CrossRef]
- Demuynck, M.; Erauw, J.P.; Van der Biest, O.; Delannay, F.; Cambier, F. Densification of alumina by SPS and HP: A comparative study. J. Eur. Ceram. Soc. 2012, 32, 1957–1964. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.G.; Jung, J.I.; Kang, S.J.L.; Chen, I.W. Enhanced grain boundary mobility in yttria-stabilized cubic zirconia under an electric current. J. Am. Ceram. Soc. 2011, 94, 4231–4238. [Google Scholar] [CrossRef]
- Biesuz, M.; Pinter, L.; Saunders, T.; Reece, M.; Binner, J.; Sglavo, V.M.; Grasso, S. Investigation of electrochemical, optical and thermal effects during flash sintering of 8YSZ. Materials 2018, 11, 1214. [Google Scholar] [CrossRef]
- Yu, M.; Grasso, S.; Mckinnon, R.; Saunders, T.; Reece, M.J. Review of flash sintering: Materials, mechanisms and modelling. Adv. Appl. Ceram. 2017, 116, 24–60. [Google Scholar] [CrossRef]
- Maizza, G.; Mastrorillo, G.D.; Grasso, S.; Ning, H.; Reece, M.J. Peltier effect during spark plasma sintering (SPS) of thermoelectric materials. J. Mater. Sci. 2017, 52, 10341–10352. [Google Scholar] [CrossRef]
- Pinter, L.; Biesuz, M.; Sglavo, V.M.; Saunders, T.; Binner, J.; Reece, M.; Grasso, S. DC-electro softening in soda lime silicate glass: An electro-thermal analysis. Scr. Mater. 2018, 151, 14–18. [Google Scholar] [CrossRef]
- Mishra, T.P.; Laptev, A.M.; Ziegner, M.; Sistla, S.K.; Kaletsch, A.; Broeckmann, C.; Guillon, O.; Bram, M. Field-assisted sintering/spark plasma sintering of gadolinium-doped ceria with controlled re-oxidation for crack prevention. Materials 2020, 13, 3184. [Google Scholar] [CrossRef]
- Grasso, S.; Sakka, Y. Electric field in SPS: Geometry and pulsed current effects. J. Ceram. Soc. Japan 2013, 121, 524–526. [Google Scholar] [CrossRef]
- Stamps, M.A.; Eischen, J.W.; Huang, H.Y.S. Particle- and crack-size dependency of lithium-ion battery materials LiFePO4. AIMS Mater. Sci. 2016, 3, 190–203. [Google Scholar] [CrossRef]
- Wang, D.; Wu, X.; Wang, Z.; Chen, L. Cracking causing cyclic instability of LiFePO4 cathode material. J. Power Sour. 2005, 140, 125–128. [Google Scholar] [CrossRef]
- Qin, W.; Majidi, H.; Yun, J.; van Benthem, K. Electrode Effects on Microstructure Formation During FLASH Sintering of Yttrium-Stabilized Zirconia. J. Am. Ceram. Soc. 2016, 99, 2253–2259. [Google Scholar] [CrossRef]
- Huan, Y.; Fan, Y.; Li, Y.; Yin, B.; Hu, X.; Dong, D.; Wei, T. Factors influencing Li+ migration in garnet-type ceramic electrolytes. J. Mater. 2019, 5, 214–220. [Google Scholar] [CrossRef]
- Biesuz, M.; Sedlák, R.; Saunders, T.; Kovalčíková, A.; Dusza, J.; Reece, M.; Zhu, D.; Hu, C.; Grasso, S. Flash spark plasma sintering of 3YSZ. J. Eur. Ceram. Soc. 2019, 39, 1932–1937. [Google Scholar] [CrossRef]
- Balakrishna, A.R.; Chiang, Y.M.; Carter, W.C. Li-diffusion accelerates grain growth in intercalation electrodes: A phase-field study. Phys. Rev. Mater. 2018, 3, 065404. [Google Scholar] [CrossRef]
- Castro, L.; Dedryvère, R.; El Khalifi, M.; Lippens, P.E.; Bréger, J.; Tessier, C.; Gonbeau, D. The spin-polarized electronic structure of LiFePO4 and FePO4 evidenced by in-lab XPS. J. Phys. Chem. C 2010, 114, 17995–18000. [Google Scholar] [CrossRef]
- Bhuvaneswari, M.S.; Bramnik, N.N.; Ensling, D.; Ehrenberg, H.; Jaegermann, W. Synthesis and characterization of Carbon Nano Fiber/LiFePO4 composites for Li-ion batteries. J. Power Sour. 2008, 180, 553–560. [Google Scholar] [CrossRef]
- Rajoba, S.J.; Jadhav, L.D.; Kalubarme, R.S.; Patil, P.S.; Varma, S.; Wani, B.N. Electrochemical performance of LiFePO4/GO composite for Li-ion batteries. Ceram. Int. 2018, 44, 6886–6893. [Google Scholar] [CrossRef]
- Li, J.; Dong, S.; Wang, C.; Hu, Z.; Zhang, Z.; Zhang, H.; Cui, G. A study on the interfacial stability of the cathode/polycarbonate interface: Implication of overcharge and transition metal redox. J. Mater. Chem. A 2018, 6, 11846–11852. [Google Scholar] [CrossRef]
- Xiong, W.; Hu, Q.; Liu, S. A novel and accurate analytical method based on X-ray photoelectron spectroscopy for the quantitative detection of the lithium content in LiFePO4. Anal. Methods 2014, 6, 5708–5711. [Google Scholar] [CrossRef]
- Wang, C.; Hong, J. Ionic/electronic conducting characteristics of LiFePO4 cathode materials. Electrochem. Solid-State Lett. 2007, 10, 65–69. [Google Scholar] [CrossRef]
- Wang, S.; Yan, M.; Li, Y.; Vinado, C.; Yang, J. Separating electronic and ionic conductivity in mix-conducting layered lithium transition-metal oxides. J. Power Sour. 2018, 393, 75–82. [Google Scholar] [CrossRef]
- Elango, R.; Nadeina, A.; Cadiou, F.; De Andrade, V.; Demortière, A.; Morcrette, M.; Seznec, V. Impact of electrode porosity architecture on electrochemical performances of 1 mm-thick LiFePO4 binder-free Li-ion electrodes fabricated by Spark Plasma Sintering. J. Power Sour. 2021, 488, 229402. [Google Scholar] [CrossRef]
- Yang, X.; Tu, J.; Lei, M.; Zuo, Z.; Wu, B.; Zhou, H. Selection of Carbon Sources for Enhancing 3D Conductivity in the Secondary Structure of LiFePO4/C Cathode. Electrochim. Acta 2016, 193, 206–215. [Google Scholar] [CrossRef]
- Elango, R.; Demortière, A.; de Andrade, V.; Morcrette, M.; Seznec, V. Thick Binder-Free Electrodes for Li–Ion Battery Fabricated Using Templating Approach and Spark Plasma Sintering Reveals High Areal Capacity. Adv. Energy Mater. 2018, 8, 1703031. [Google Scholar] [CrossRef]

| Samples | Re (Ω.cm2) | σe(S/cm) | Ri (Ω.cm2) | σi(S/cm) | ICP Li (wt%) | |
|---|---|---|---|---|---|---|
| DC | Top surface (+) | 9879 | 1.01 × 10−5 | 708 | 1.41 × 10−4 | 3.85 |
| Bottom surface (−) | 14626 | 6.84 × 10−6 | 372.2 | 2.69× 10−4 | 3.89 | |
| AC | Top surface | 3941 | 2.54 × 10−5 | 22.61 | 4.42 × 10−3 | 3.92 |
| Bottom surface | 3908 | 2.56 × 10−5 | 21.19 | 4.71 × 10−3 | 3.94 | |
| Literature | DC SPS | (1~4) × 10−5 [38] | (3.4~14) × 10−5 [38] | |||
| Cold dry pressing | (3~10) × 10−5 [39] | (7~12) × 10−6 [39] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, N.; Lin, Y.; Guo, J.; Quattrocchi, E.; Deng, H.; Dong, J.; Ciucci, F.; Boi, F.; Hu, C.; Grasso, S. Spark Plasma Sintering of LiFePO4: AC Field Suppressing Lithium Migration. Materials 2021, 14, 2826. https://doi.org/10.3390/ma14112826
Luo N, Lin Y, Guo J, Quattrocchi E, Deng H, Dong J, Ciucci F, Boi F, Hu C, Grasso S. Spark Plasma Sintering of LiFePO4: AC Field Suppressing Lithium Migration. Materials. 2021; 14(11):2826. https://doi.org/10.3390/ma14112826
Chicago/Turabian StyleLuo, Nan, Yong Lin, Jian Guo, Emanuele Quattrocchi, Huaijiu Deng, Jian Dong, Francesco Ciucci, Filippo Boi, Chunfeng Hu, and Salvatore Grasso. 2021. "Spark Plasma Sintering of LiFePO4: AC Field Suppressing Lithium Migration" Materials 14, no. 11: 2826. https://doi.org/10.3390/ma14112826
APA StyleLuo, N., Lin, Y., Guo, J., Quattrocchi, E., Deng, H., Dong, J., Ciucci, F., Boi, F., Hu, C., & Grasso, S. (2021). Spark Plasma Sintering of LiFePO4: AC Field Suppressing Lithium Migration. Materials, 14(11), 2826. https://doi.org/10.3390/ma14112826








